Preparation and Characterization of Light-Colored Polyimide Nanocomposite Films Derived from a Fluoro-Containing Semi-Alicyclic Polyimide Matrix and Colloidal Silica with Enhanced High-Temperature Dimensionally Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. CPI Resin Synthesis and the Film Preparation
3. Results and Discussion
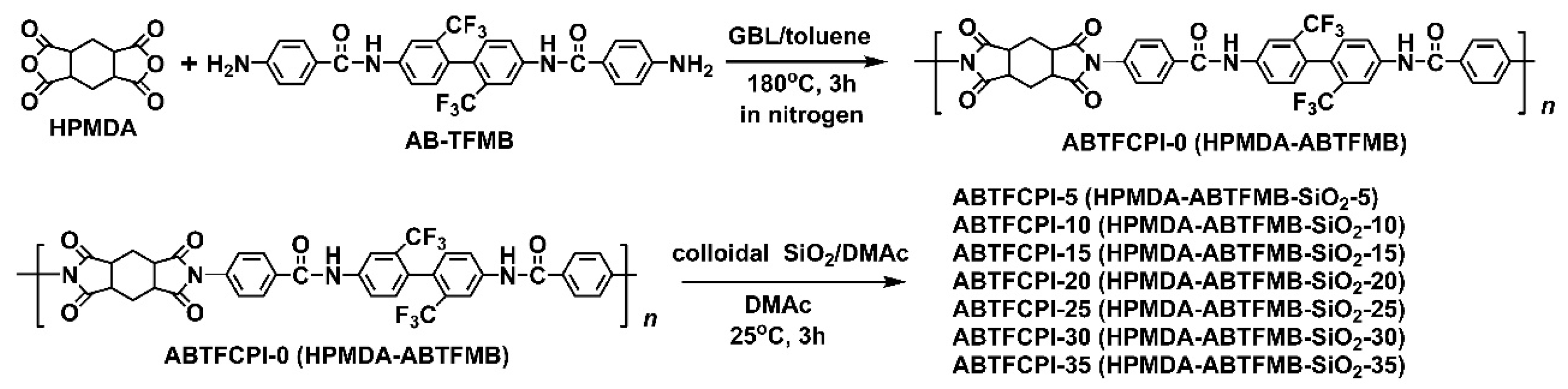
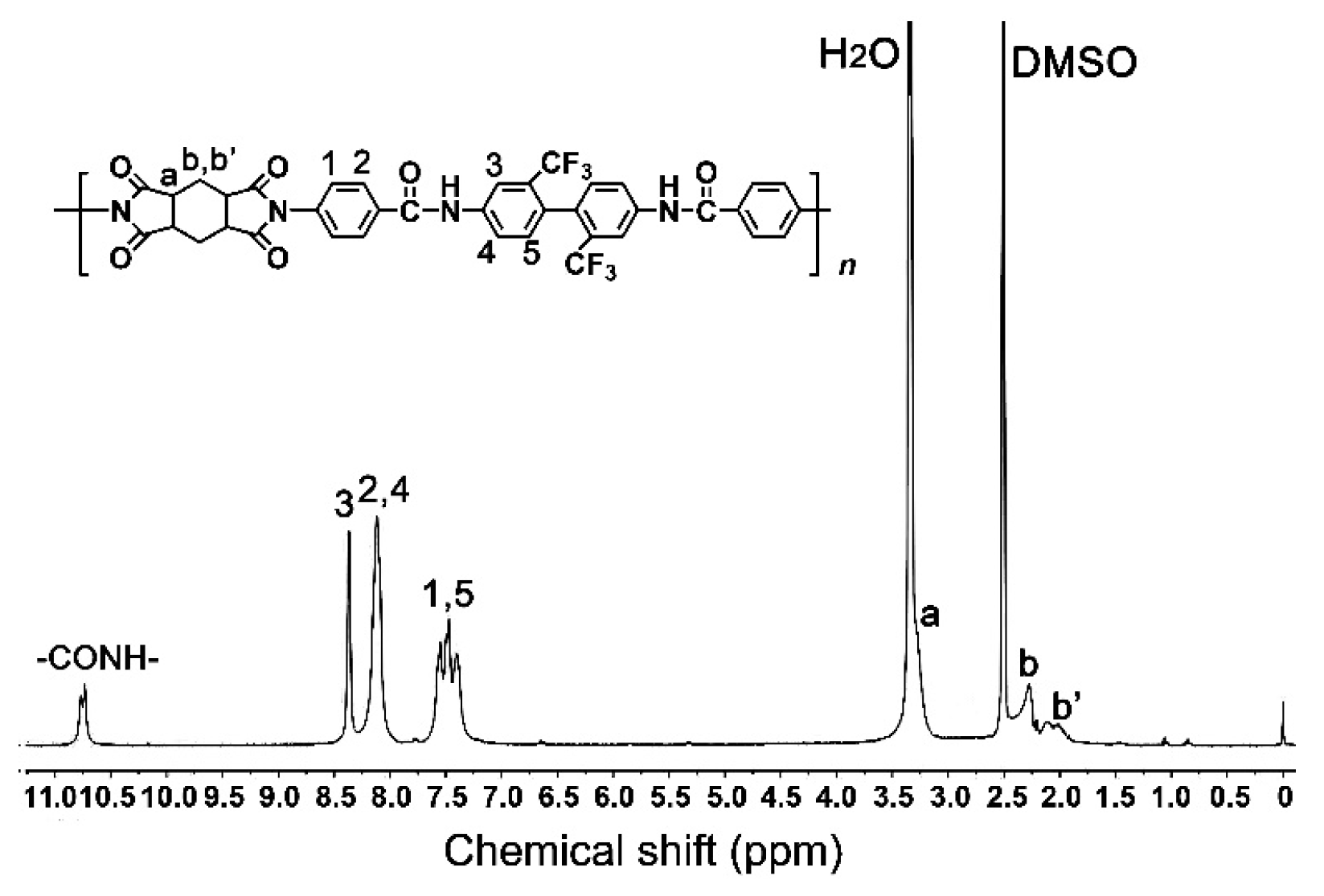
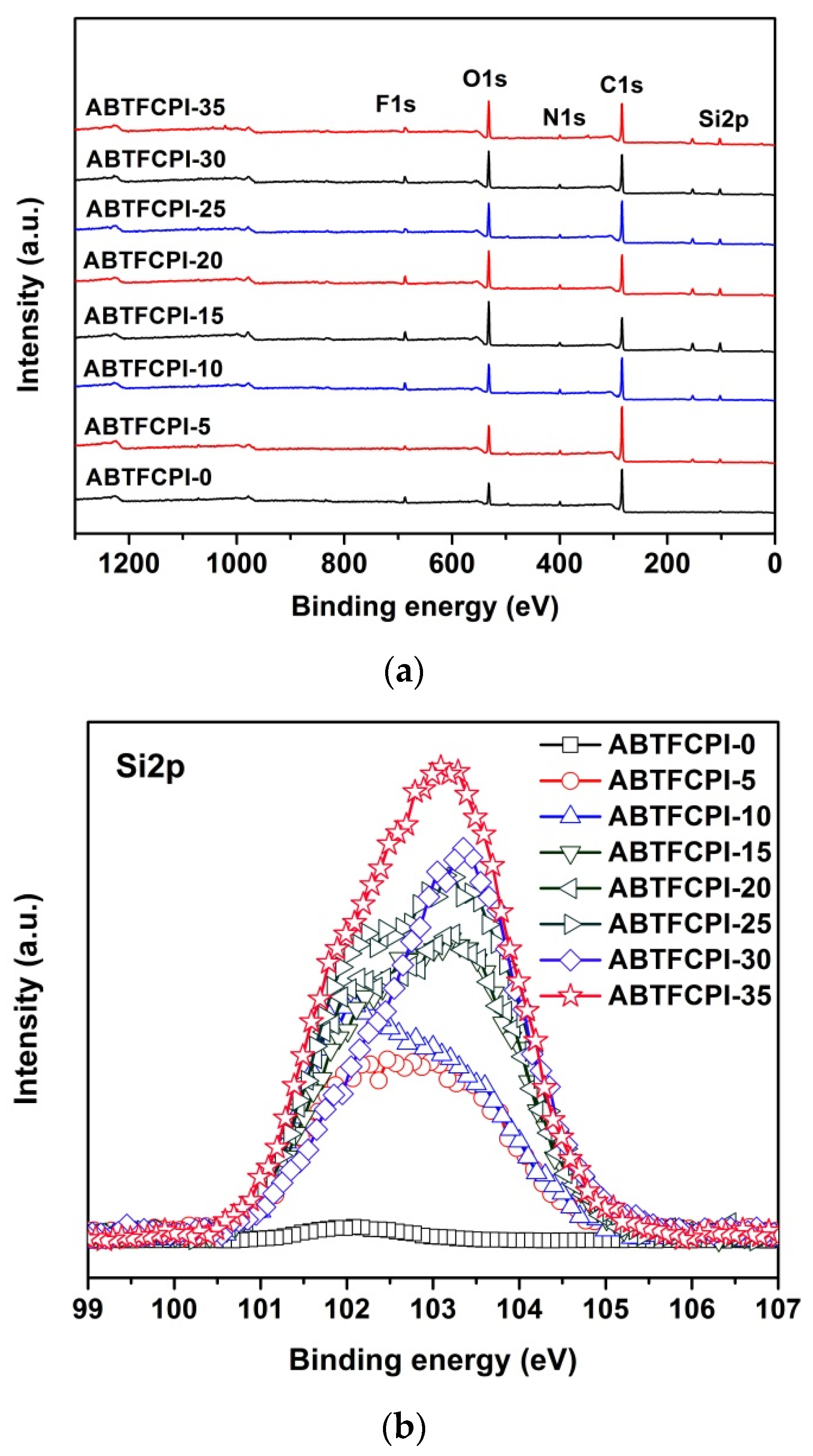
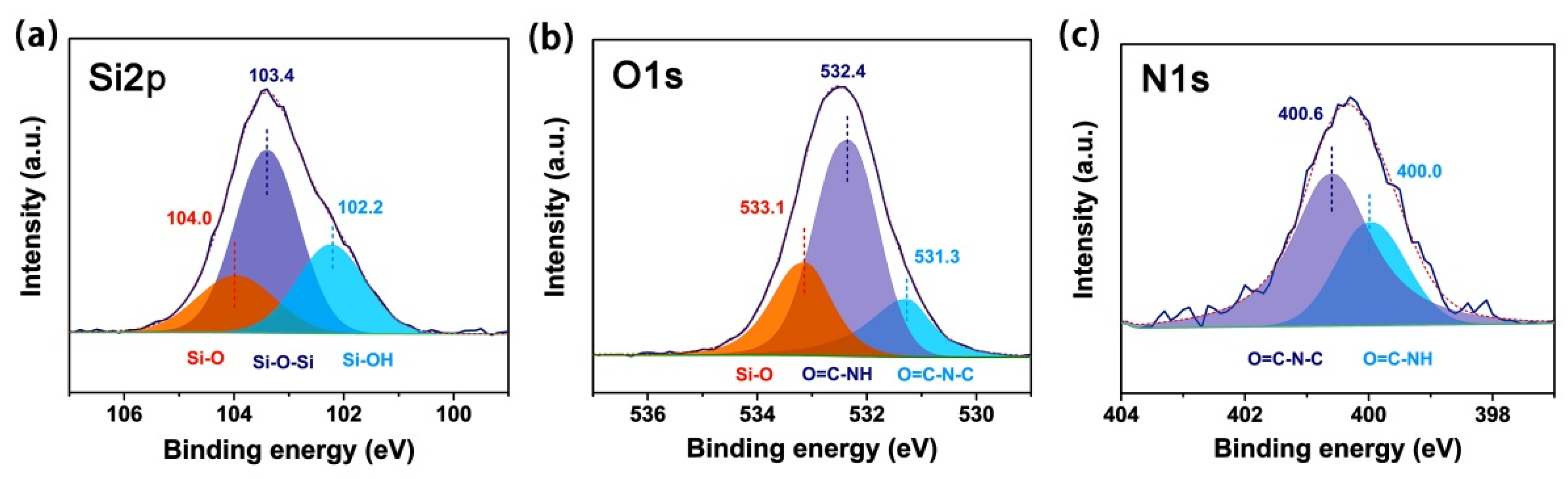
3.1. CPI Resin Synthesis and Film Preparation
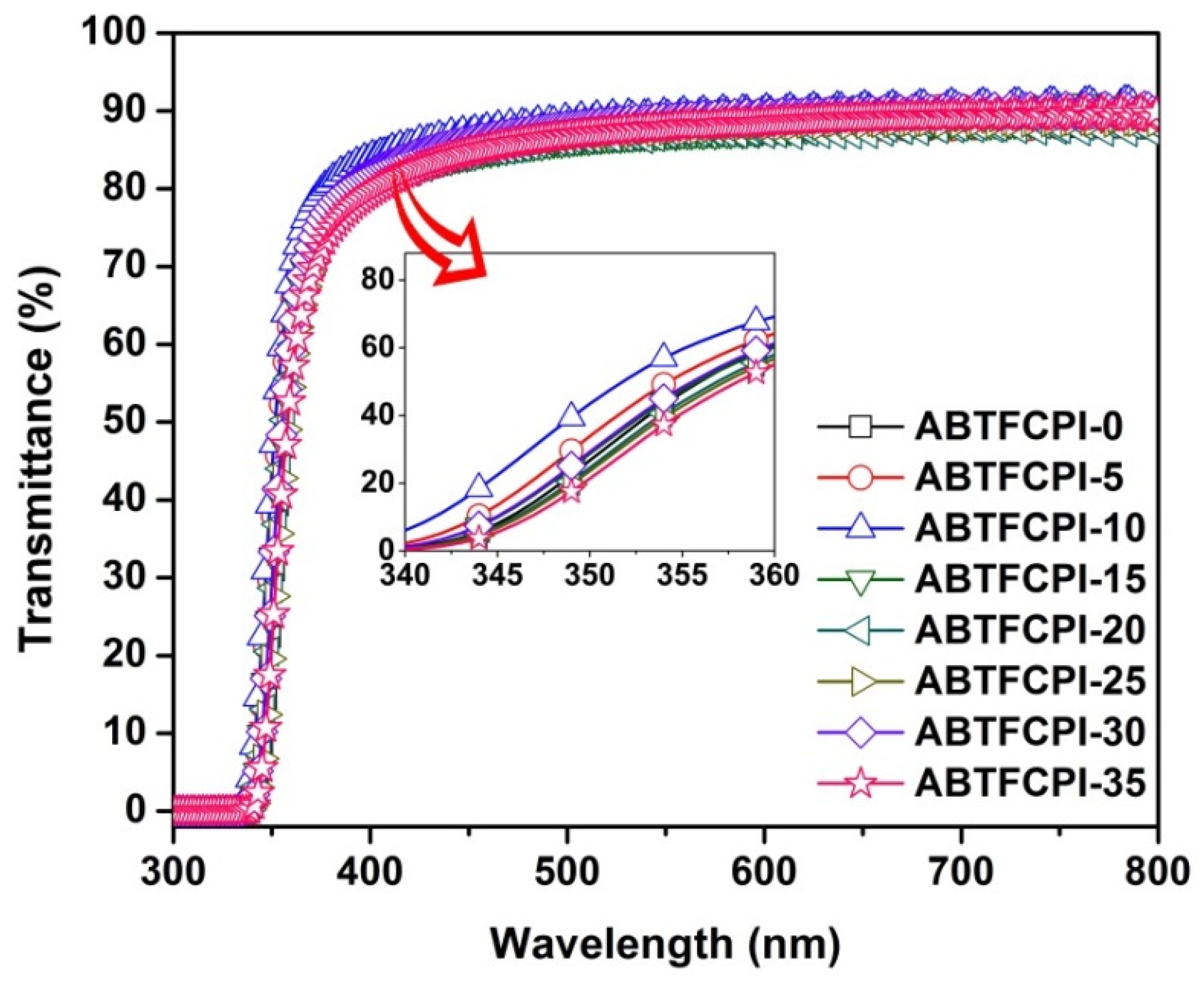
3.2. Optical Properties
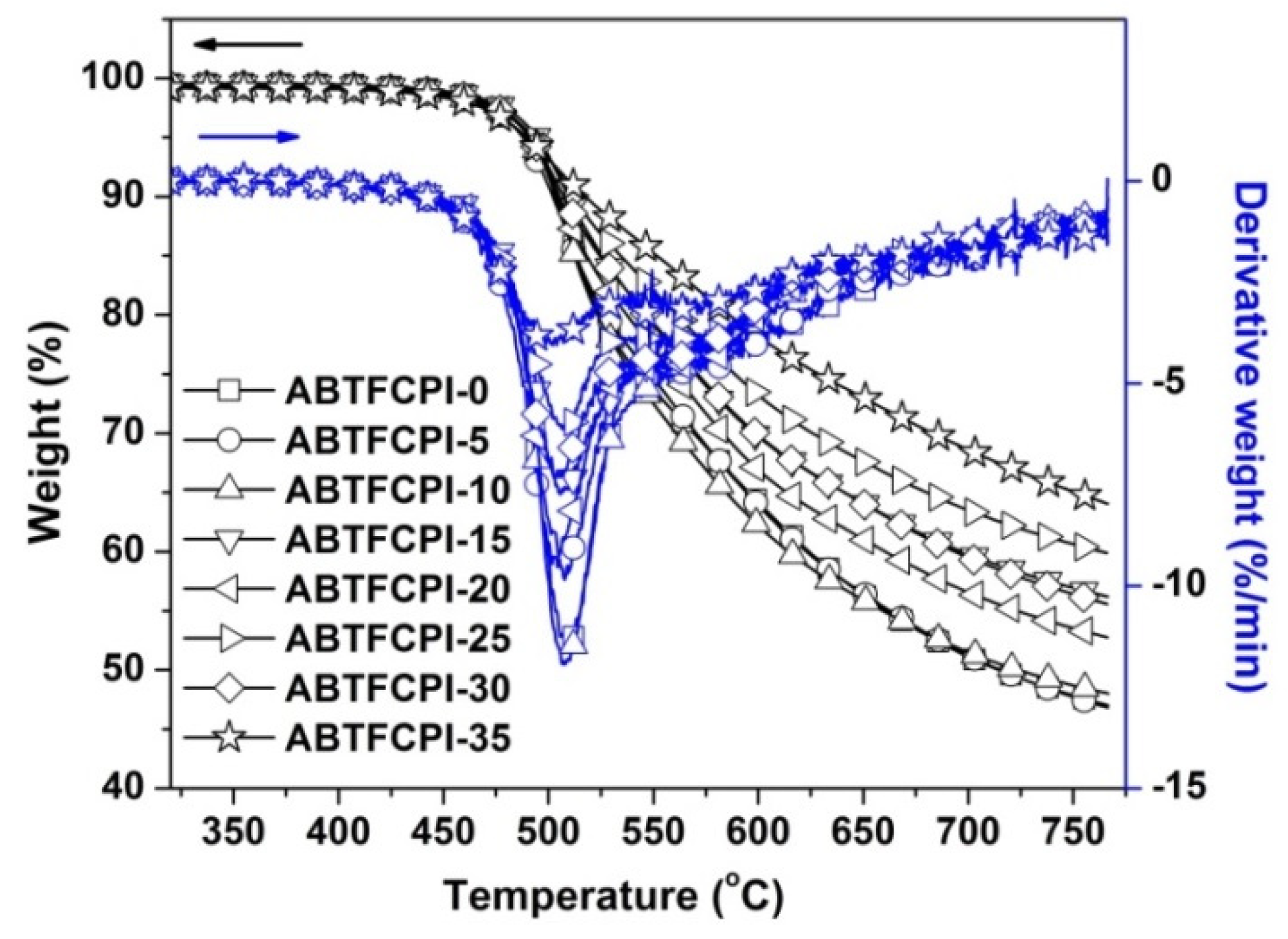
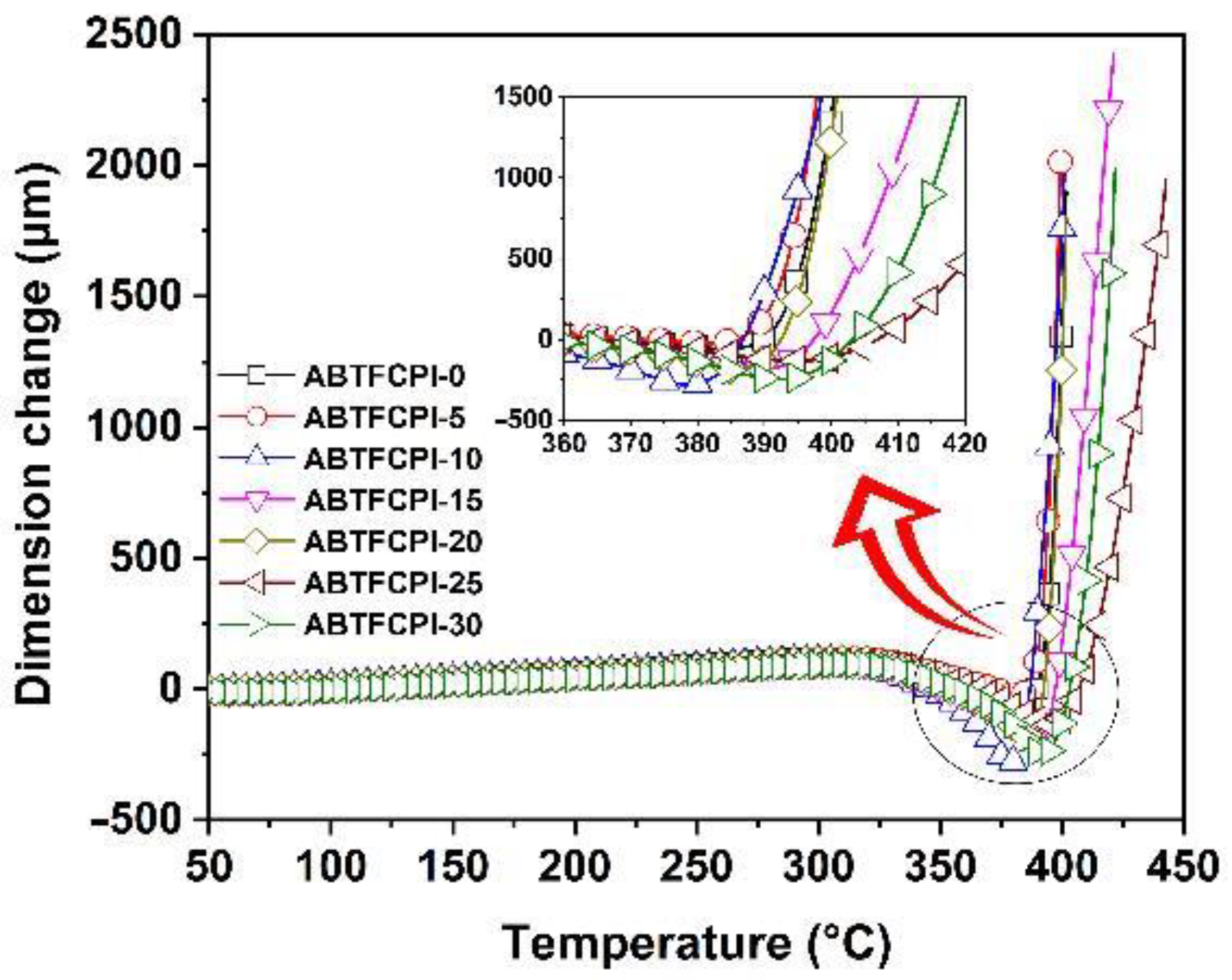
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, C.; Liu, P.; Yue, J.; Huan, H.; Yang, Z.; Yang, K.; Guo, H.; Zhao, J. High-transparency and colorless polyimide film prepared by inhibiting the formation of chromophores. Polymers 2022, 14, 4242. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Y.; Qin, X.; Lv, Y.; Huang, Y.; Yang, Q.; Li, G.; Kong, M. Revealing molecular mechanisms of colorless transparent polyimide films under photo-oxidation. Polym. Degrad. Stab. 2023, 210, 110294. [Google Scholar] [CrossRef]
- Jeon, H.; Kwac, L.K.; Kim, H.G.; Chang, J.H. Comparison of properties of colorless and transparent polyimide films using various diamine monomers. Rev. Adv. Mater. Sci. 2022, 61, 394–404. [Google Scholar] [CrossRef]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamada, N. Polyimide derived from 2,2-bis(trifluoromethyl)-4,4-diaminobiphenyl. 1. Synthesis and characterization of polyimides prepared with 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Sha, C.H.; Lee, C.C. Low-temperature solid-state silver bonding of silicon chips to alumina substrates. IEEE Trans. Compon. Packag. Manuf. Technol. 2011, 1, 1983–1987. [Google Scholar] [CrossRef]
- Moran, B.; Wu, F.; Romanov, A.E.; Mishra, U.K.; Denbaars, S.P.; Speck, J.S. Structural and morphological evolution of GaN grown by metalorganic chemical vapor deposition on SiC substrates using an AlN initial layer. J. Cryst. Growth 2004, 273, 38–47. [Google Scholar] [CrossRef]
- Gerthoffer, A.; Poulain, C.; Roux, F.; Emieux, F.; Grenet, L.; Perraud, S. CIGS solar cells on ultra-thin glass substrates: Determination of mechanical properties by nanoindentation and application to bending-induced strain calculation. Solar Energy Mater. Solar Cells 2017, 166, 254–261. [Google Scholar] [CrossRef]
- Seo, K.; Nam, K.H.; Lee, S.; Han, H. Low stress polyimide/silica nanocomposites as dielectrics for wafer level chip scale packaging. Mater. Lett. 2020, 263, 127204. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wu, D. Synthetic strategies for highly transparent and colorless polyimide film. J. Appl. Polym. Sci. 2022, 139, e52604. [Google Scholar] [CrossRef]
- Yang, Y.; Jung, Y.; Cho, M.D.; Lee, S.G.; Kwon, S. Transient color changes in oxidative-stable fluorinated polyimide film for flexible display substrates. RSC Adv. 2015, 5, 57339–57345. [Google Scholar] [CrossRef]
- Zhi, X.; Jiang, G.; Zhang, Y.; Jia, Y.; Wu, L.; An, Y.; Liu, J.; Liu, Y. Preparation and properties of colorless and transparent semi-alicyclic polyimide films with enhanced hightemperature dimensional stability via incorporation of alkyl-substituted benzanilide units. J. Appl. Polym. Sci. 2022, 139, 51544. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Shen, J.; Zhao, J.; Tu, G. Synthesis of a novel rigid semi-alicyclic dianhydride and its copolymerized transparent polyimide films’ properties. Polymers 2022, 14, 4132. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; He, Z.B.; Yuan, S.Q.; Wu, H.; Zhi, X.X.; Zhang, Y.; Chen, S.J.; Liu, J.G. Enhancement of ultraviolet light resistance of colorless and transparent semi-alicyclic polyimide nanocomposite films via the incorporation of hindered amine light stabilizers for potential applications in flexible optoelectronics. Polymers 2022, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Dai, F.; Ke, Z.; Yan, K.; Chen, C.; Qian, G.; Li, H. Synthesis of colorless polyimides with high Tg from asymmetric twisted benzimidazole diamines. Eur. Polym. J. 2022, 164, 110975. [Google Scholar] [CrossRef]
- Sawada, R.; Ando, S. Colorless, low dielectric, and optically active semialicyclic polyimides incorporating a biobased isosorbide moiety in the main chain. Macromolecules 2022, 55, 6787–6800. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless polyimides derived from an alicyclic tetracarboxylic dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sato, H.; Hishino, K.; Arao, Y.; Ishii, J. Colorless polyimides derived from octahydro-2,3,6,7-anthracenetetracarboxylic dianhydride. Polymers 2023, 3, 175–199. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ichikawa, K.; Takahashi, S.; Ishii, J. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride: Strategies to reduce the coefficients of thermal expansion by maximizing the spontaneous chain orientation behavior during solution casting. Polymers 2022, 14, 1131. [Google Scholar] [CrossRef]
- Xia, X.; He, X.; Zhang, S.; Zheng, F.; Lu, Q. Short-side-chain regulation of colorless and transparent polyamide-imides for flexible transparent displays. Eur. Polym. J. 2023, 191, 112030. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Wang, Z.; Yan, J. Colorless poly(amide-imide) copolymers for flexible display applications. J. Appl. Polym. Sci. 2022, 139, e53082. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, D.; Du, H.; Wu, X.; Zhang, Y.; Tan, Y.; Wu, L.; Liu, J.; Zhang, X. Reduced coefficients of linear thermal expansion of colorless and transparent semi-alicyclic polyimide films via incorporation of rigid-rod amide moiety: Preparation and properties. Polymers 2020, 12, 413. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Watanabe, Y.; Ishii, J. Solution-processable colorless polyimides with ultralow coefficients of thermal expansion for optoelectronic applications. Polym. Int. 2016, 65, 1063–1073. [Google Scholar] [CrossRef]
- Ren, X.; Wang, H.; Du, X.; Qi, H.; Pan, Z.; Wang, X.; Dai, S.; Yang, C.; Liu, J. Synthesis and properties of optically transparent fluoro-containing polyimide films with reduced linear coefficients of thermal xxpansion from organo-soluble resins derived from aromatic diamine with benzanilide units. Materials 2022, 15, 6346. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, S.; He, X.; Zheng, F.; Lu, Q. Molecular necklace strategy for enhancing modulus and toughness of colorless transparent polyimides for cover window application. Polymer 2022, 259, 125358. [Google Scholar] [CrossRef]
- Morikawa, A.; Iyoku, Y.; Kakimoto, M.; Imai, Y. Preparation of a new class of polyimide-silica hybrid films by sol-gel process. Polym. J. 1992, 24, 107–113. [Google Scholar] [CrossRef]
- Bae, W.J.; Kovalev, M.K.; Kalinina, F.; Kim, M.; Cho, C. Towards colorless polyimide/silica hybrids for flexible substrates. Polymer 2016, 105, 124–132. [Google Scholar] [CrossRef]
- Moon, K.H.; Chae, B.; Kim, K.S.; Lee, S.W.; Jung, Y.M. Preparation and characterization of transparent polyimide-silica composite films using polyimide with carboxylic acid groups. Polymers 2019, 11, 489. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chien, W.C.; Tsai, T.W. High transparent soluble polyimide/silica hybrid optical thin films. Polym. Test. 2010, 29, 33–40. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chien, W.C.; Lai, C.L. Synthesis and characterization of polyimide/monodispersed colloidal silica hybrid thin films. J. Nanosci. Nanotechnol. 2009, 9, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, P.; Li, F.; Guo, H.; Kang, C.; Gao, L. Ultrahigh thermal-stability polyimides with low CTE and required flexibility by formation of hydrogen bonds between poly(amic acid)s. Eur. Polym. J. 2021, 148, 110369. [Google Scholar] [CrossRef]
- Moaddeb, M.; Koros, W.J. Gas transport properties of thin polymeric membranes in the presence of silicon dioxide particles. J. Membr. Sci. 1997, 125, 143–163. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Ding, H.; Li, J. Microstructure and properties of organosoluble polyimide/silica hybrid films. Eur. Polym. J. 2006, 42, 2921–2930. [Google Scholar] [CrossRef]
- Iida, K.; Imamura, Y.; Liao, C.; Nakamura, S.; Sawa, G. Evaluation of molecular orientation in aromatic polyimide films by FT-IR reflection absorption spectroscopy. Polym. J. 1996, 28, 352–356. [Google Scholar] [CrossRef]
- Luo, L.; Pang, Y.; Jiang, X.; Wang, X.; Zhang, P.; Chen, Y.; Peng, C.; Liu, X. Preparation and characterization of novel polyimide films containing amide groups. J. Polym. Res. 2012, 19, 9783. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Feng, X.; Yu, X.; Li, S.; Zhao, J.; Fan, Z.; Sun, X.; Zhang, P.; Cui, J.; et al. Polyimide films containing trifluoromethoxy groups with high comprehensive performance for flexible circuitry substrates. ACS Appl. Polym. Mater. 2022, 4, 5831–5839. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, W.C. Synthesis and optical properties of polyimide-silica hybrid thin films. Chem. Mater. 2002, 14, 4242–4248. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Z.; Piao, F.; Guo, H. Transparent polyimide films with ultra-low coefficient of thermal expansion. High Perform. Polym. 2022, 34, 871–879. [Google Scholar] [CrossRef]





| Samples | ABTFCPI-0, DMAc (g, g) | cSiO2 (20 wt% in DMAc, g) | MSiO2/Mtotal (wt%) |
|---|---|---|---|
| ABTFCPI-0 | 20.0, 80.0 | 0 | 0 |
| ABTFCPI-5 | 19.0, 76.0 | 5.0 | 5 |
| ABTFCPI-10 | 18.0, 72.0 | 10.0 | 10 |
| ABTFCPI-15 | 17.0, 68.0 | 15.0 | 15 |
| ABTFCPI-20 | 16.0, 64.0 | 20.0 | 20 |
| ABTFCPI-25 | 15.0, 60.0 | 25.0 | 25 |
| ABTFCPI-30 | 14.0, 56.0 | 30.0 | 30 |
| ABTFCPI-35 | 13.0, 52.0 | 35.0 | 35 |
| Wavenumber (cm−1) | Assignment | Reference |
|---|---|---|
| 1785 | C=O symmetric stretching | [35] |
| 1710 | C=O asymmetric stretching | [35] |
| 1670 | amide C=O (amide I) | [36] |
| 1504 | C=C stretching | [35] |
| 1381 | C-N stretching | [35] |
| 1319 | C-F stretching | [37] |
| 1049 | Si-O stretching | [38] |
| PI | Optical Properties a | Thermal Properties b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ (nm) | T400 (%) | L* | a* | b* | haze (%) | Tg (°C) | T5% (°C) | Rw750 (%) | CTE (×10−6/K) | |
| ABTFCPI-0 | 338 | 81.8 | 95.19 | −0.10 | 1.77 | 0.25 | 390.3 | 492 | 47.8 | 32.7 |
| ABTFCPI-5 | 336 | 82.0 | 95.21 | −0.11 | 1.77 | 0.66 | 389.9 | 488 | 47.7 | 28.9 |
| ABTFCPI-10 | 338 | 80.3 | 95.08 | −0.18 | 2.33 | 0.24 | 387.2 | 491 | 48.7 | 28.1 |
| ABTFCPI-15 | 337 | 80.4 | 95.24 | −0.17 | 2.11 | 0.27 | 391.4 | 494 | 56.9 | 25.8 |
| ABTFCPI-20 | 338 | 80.1 | 95.10 | −0.25 | 2.82 | 0.54 | 389.2 | 492 | 53.5 | 27.5 |
| ABTFCPI-25 | 333 | 83.8 | 95.54 | −0.18 | 2.03 | 0.13 | 388.5 | 493 | 60.6 | 28.3 |
| ABTFCPI-30 | 338 | 79.6 | 95.37 | −0.19 | 2.15 | 0.50 | 386.1 | 491 | 56.4 | 25.4 |
| ABTFCPI-35 | 339 | 77.2 | 95.70 | −0.23 | 2.24 | 0.42 | 388.3 | 494 | 64.7 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Ren, X.; Wang, Z.; Pan, Z.; Qi, Y.; Han, S.; Yu, H.; Liu, J. Preparation and Characterization of Light-Colored Polyimide Nanocomposite Films Derived from a Fluoro-Containing Semi-Alicyclic Polyimide Matrix and Colloidal Silica with Enhanced High-Temperature Dimensionally Stability. Polymers 2023, 15, 3015. https://doi.org/10.3390/polym15143015
He Z, Ren X, Wang Z, Pan Z, Qi Y, Han S, Yu H, Liu J. Preparation and Characterization of Light-Colored Polyimide Nanocomposite Films Derived from a Fluoro-Containing Semi-Alicyclic Polyimide Matrix and Colloidal Silica with Enhanced High-Temperature Dimensionally Stability. Polymers. 2023; 15(14):3015. https://doi.org/10.3390/polym15143015
Chicago/Turabian StyleHe, Zhibin, Xi Ren, Zhenzhong Wang, Zhen Pan, Yuexin Qi, Shujun Han, Haifeng Yu, and Jingang Liu. 2023. "Preparation and Characterization of Light-Colored Polyimide Nanocomposite Films Derived from a Fluoro-Containing Semi-Alicyclic Polyimide Matrix and Colloidal Silica with Enhanced High-Temperature Dimensionally Stability" Polymers 15, no. 14: 3015. https://doi.org/10.3390/polym15143015
APA StyleHe, Z., Ren, X., Wang, Z., Pan, Z., Qi, Y., Han, S., Yu, H., & Liu, J. (2023). Preparation and Characterization of Light-Colored Polyimide Nanocomposite Films Derived from a Fluoro-Containing Semi-Alicyclic Polyimide Matrix and Colloidal Silica with Enhanced High-Temperature Dimensionally Stability. Polymers, 15(14), 3015. https://doi.org/10.3390/polym15143015










