Thermal and Dielectric Investigations of Polystyrene Nanoparticles as a Viable Platform—Toward the Next Generation of Fillers for Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
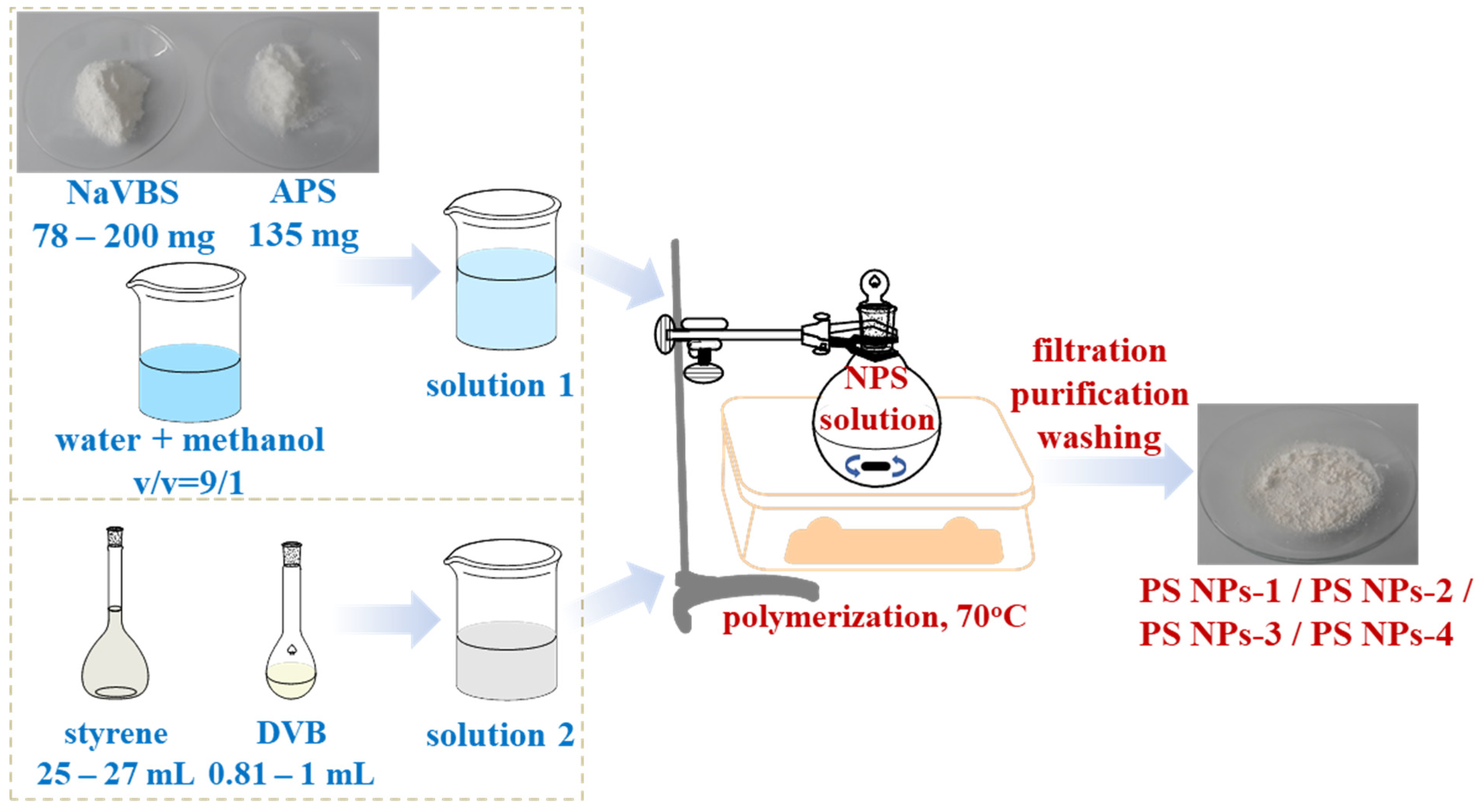
2.2. Synthesis of Polystyrene Nanoparticles
2.3. Characterization Methods
3. Results and Discussions
3.1. Preparation of Polystyrene Nanoparticles with Different Diameters
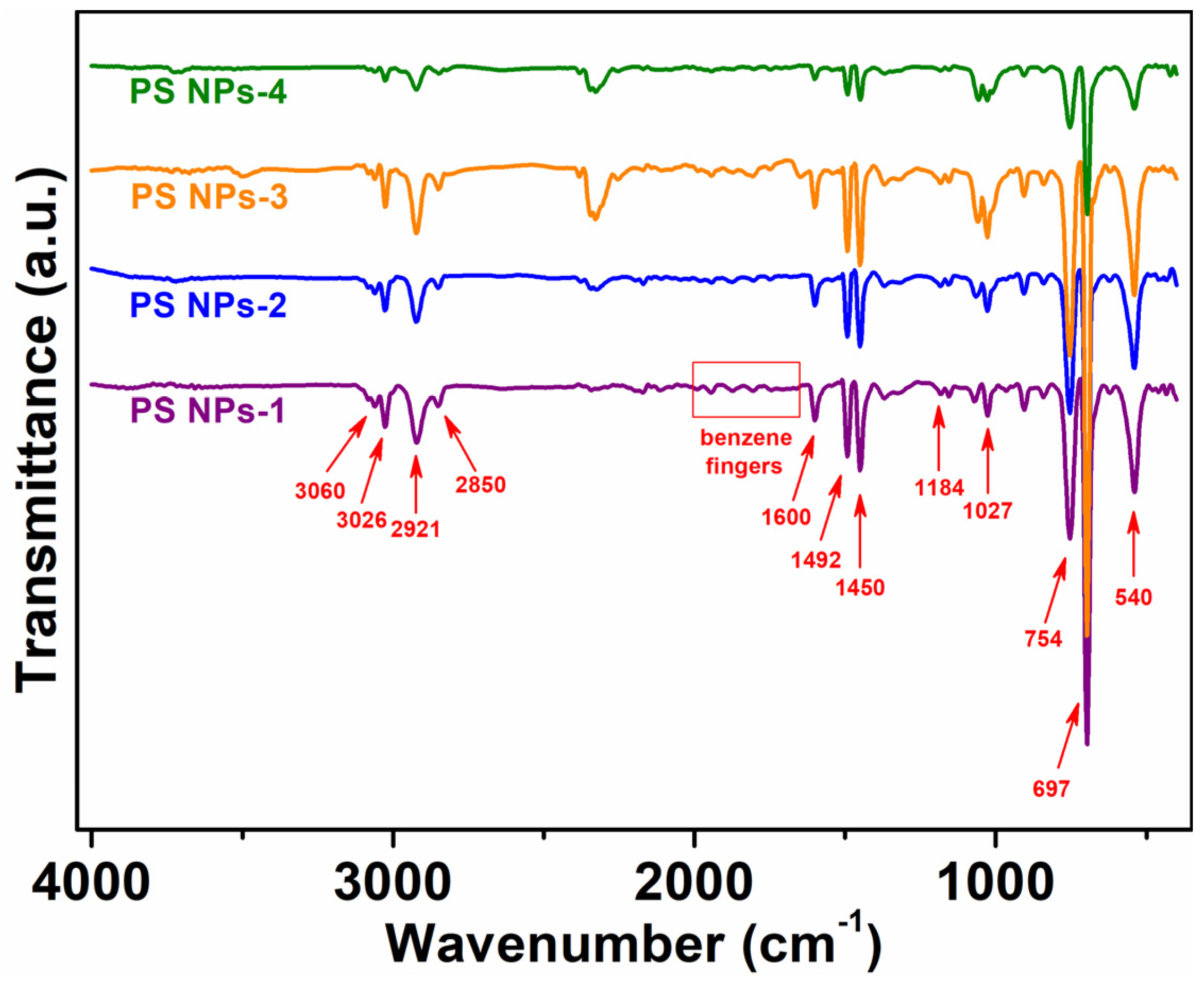
3.2. Chemical Structure
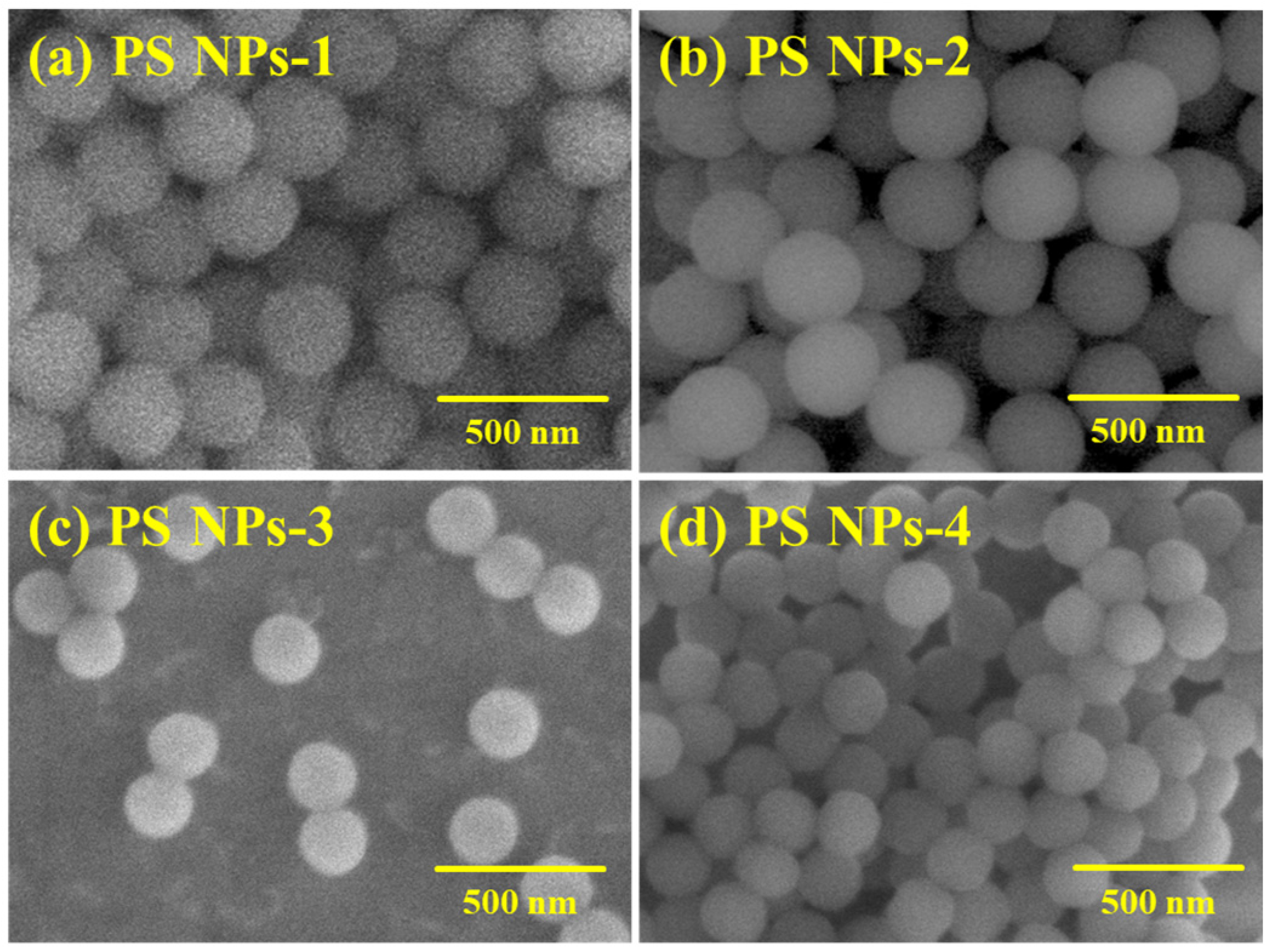
3.3. Morphology
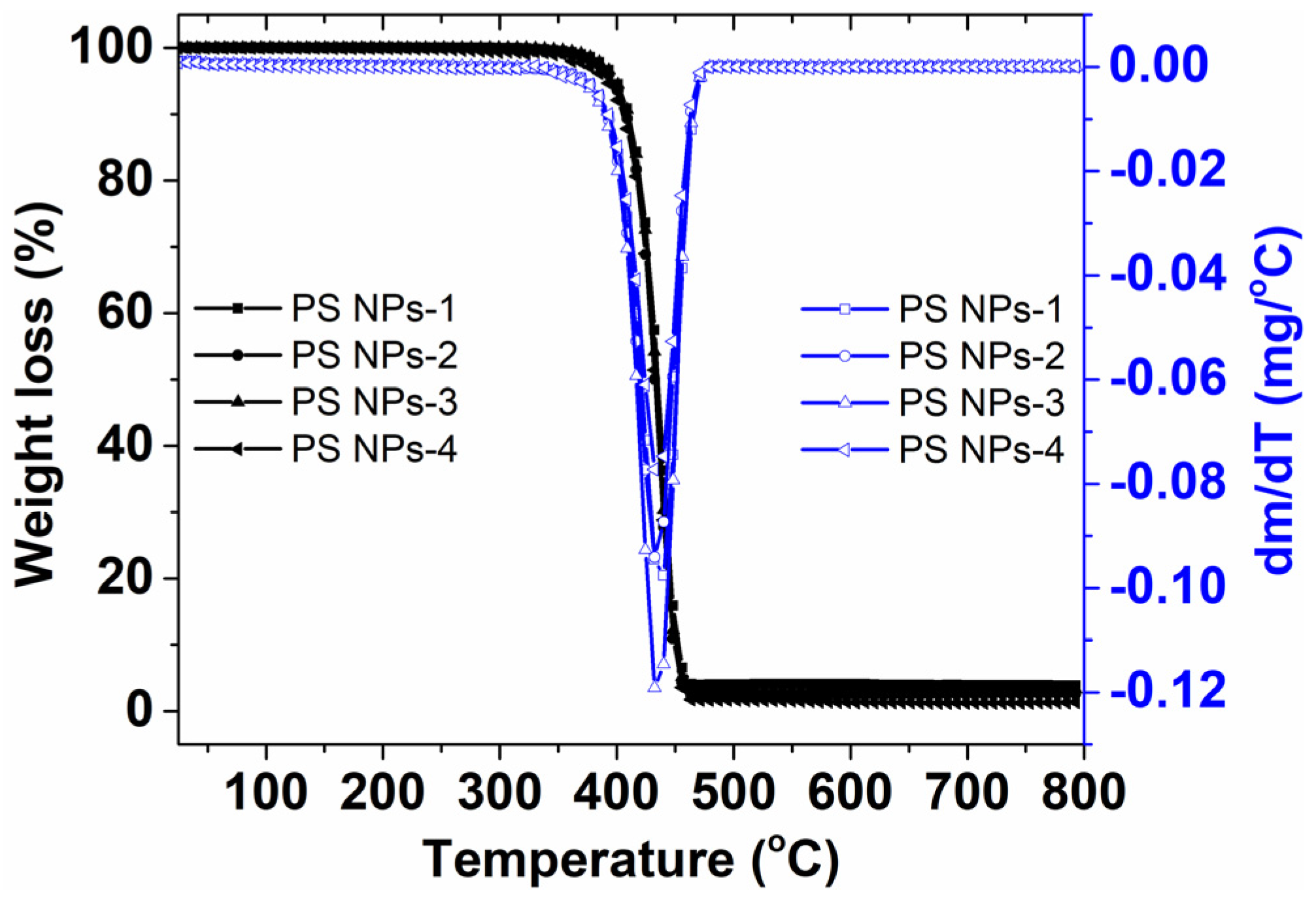
3.4. Thermal Stability
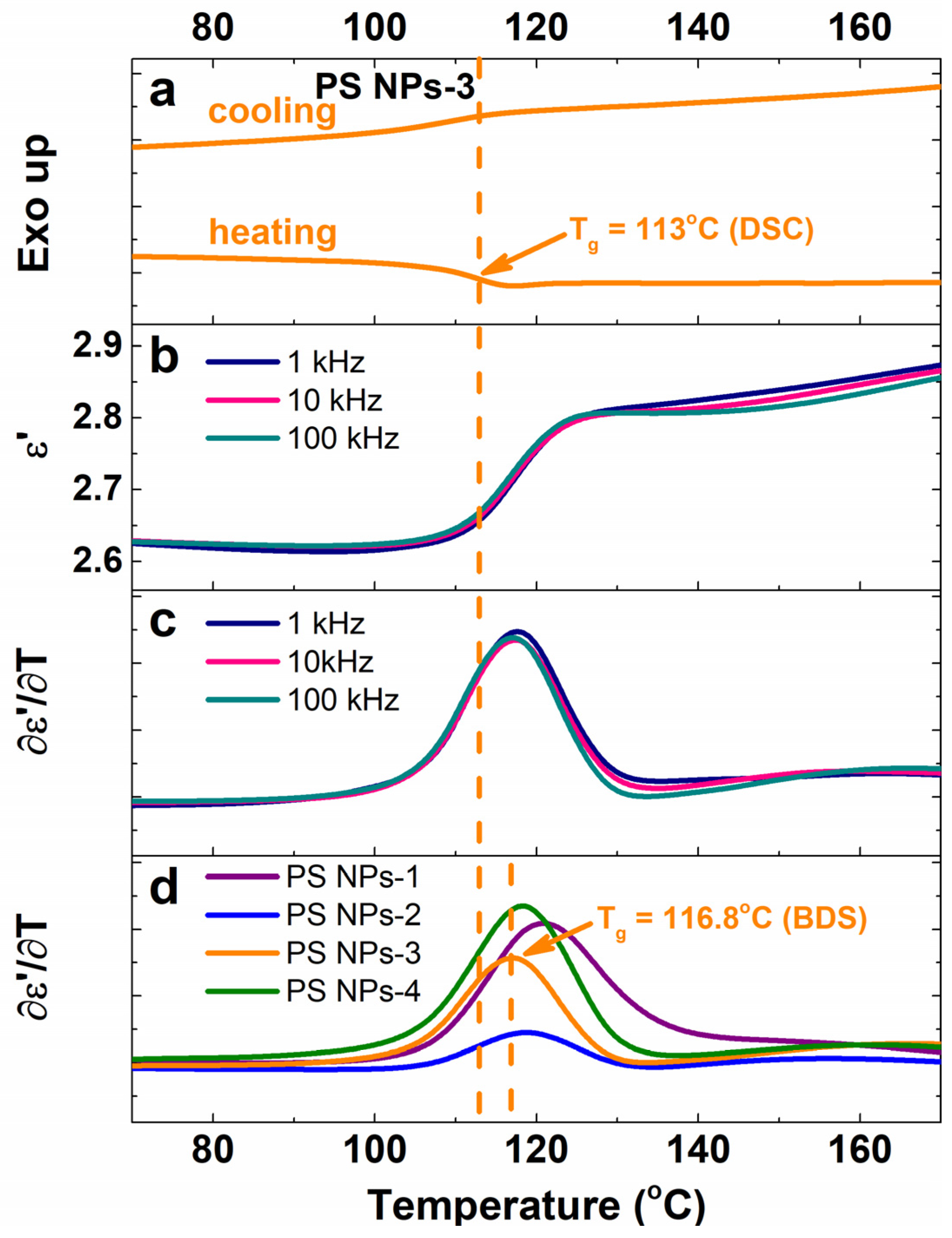
3.5. Glass Transition Temperature
3.6. Assessment of the Dielectric Behavior of Polystyrene Nanoparticles
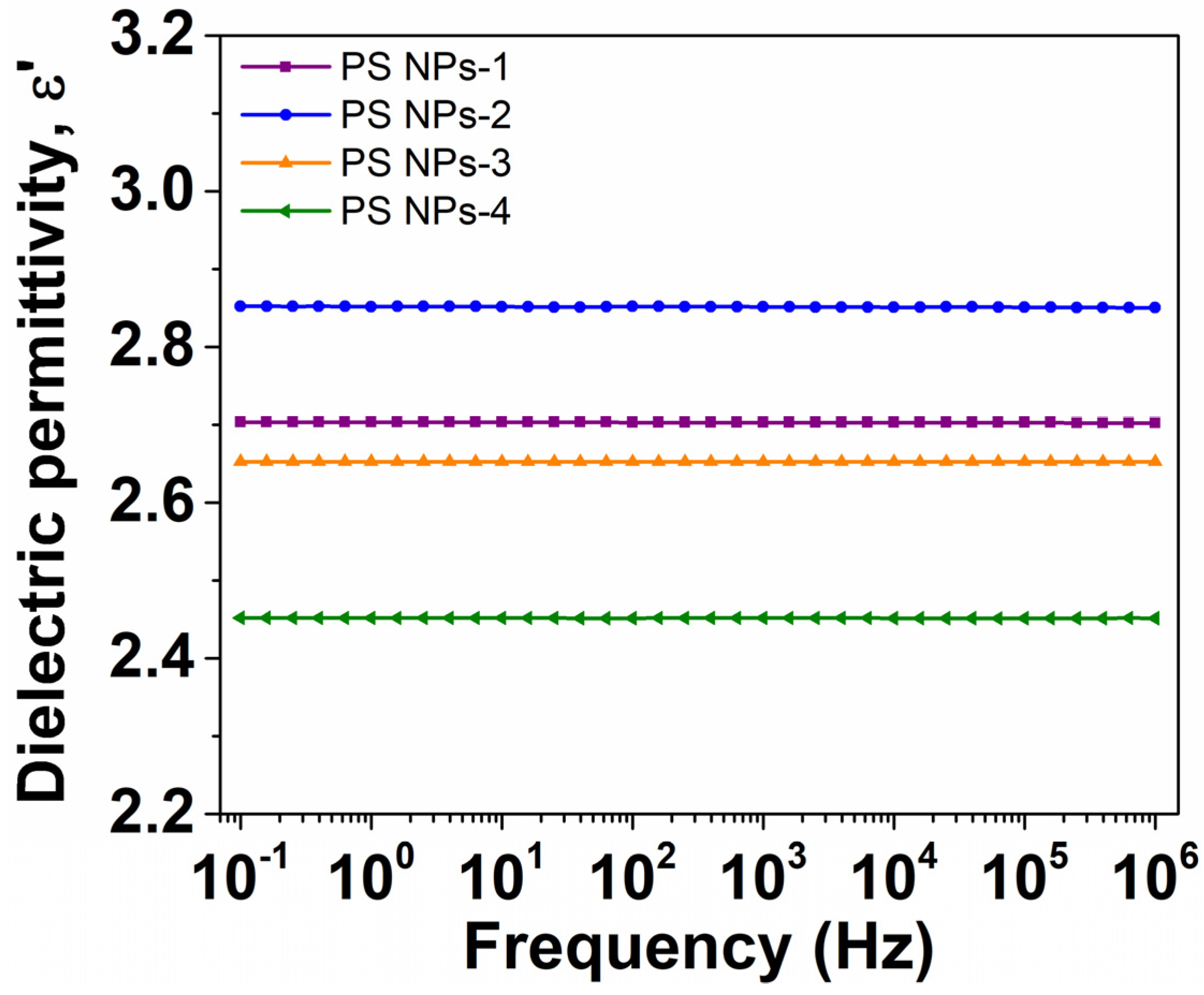
3.6.1. Evolution of Dielectric Permittivity with Frequency
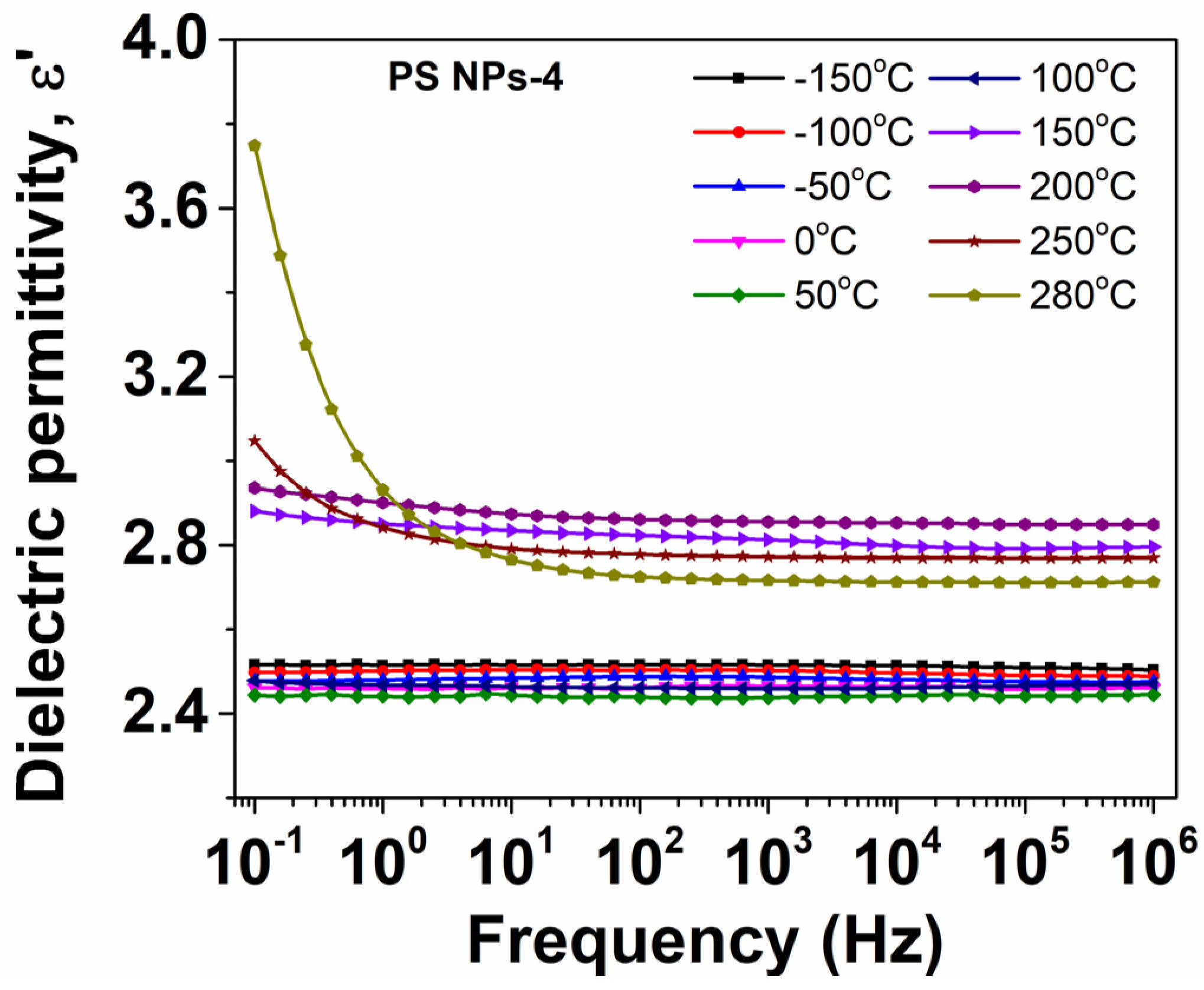
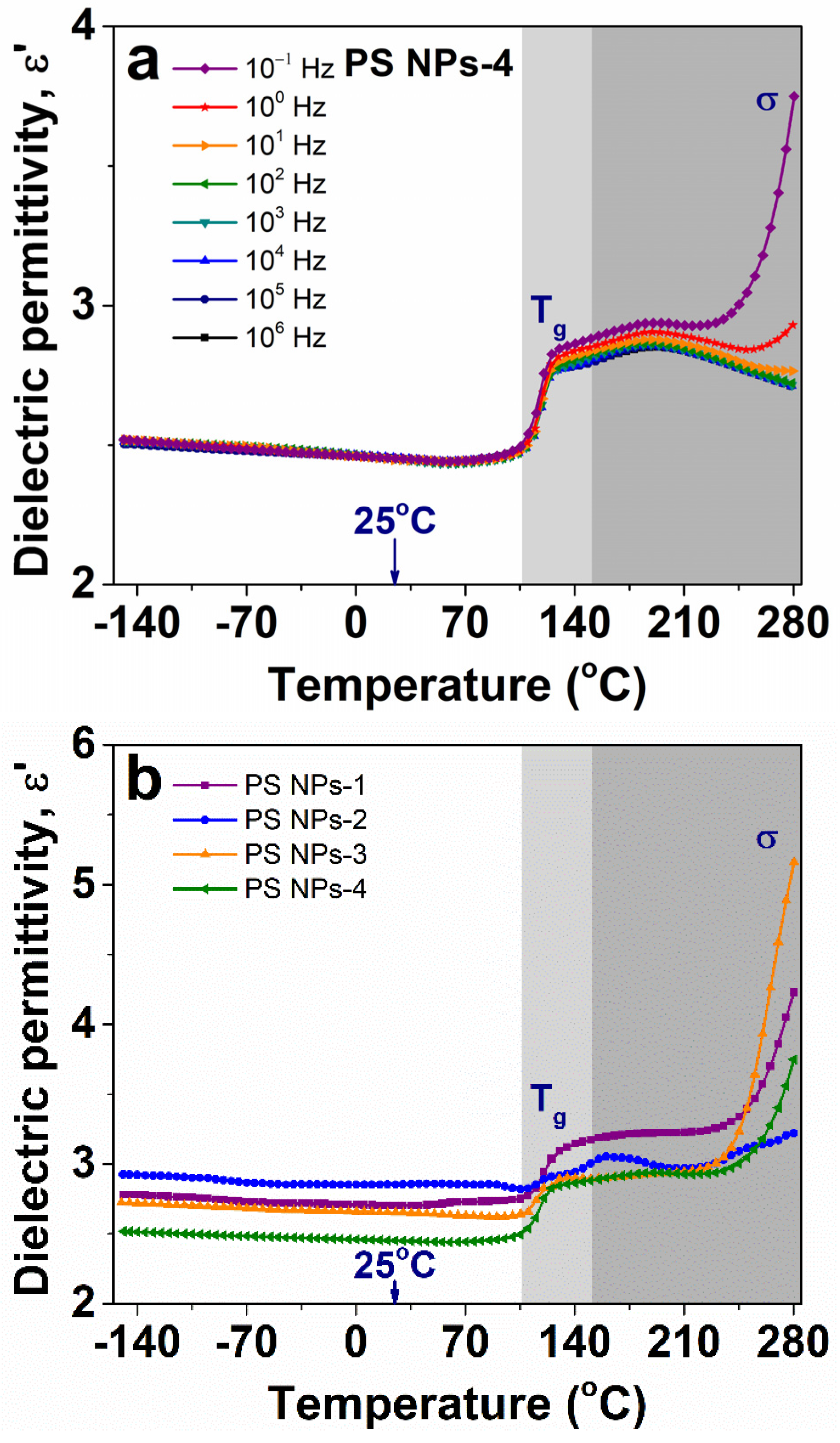
3.6.2. Evolution of Dielectric Permittivity with Temperature
3.6.3. Synthesis Parameters and Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- West, J.L.; Halas, N.J. Applications of Nanotechnology to Biotechnology. Curr. Opin. Biotechnol. 2000, 11, 215–217. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, P. Nanomaterials and Nanotechnology for Biomedical Soft Robots. Mater. Today Adv. 2023, 17, 100338. [Google Scholar] [CrossRef]
- Egorov, E.; Pieters, C.; Korach-Rechtman, H.; Shklover, J.; Schroeder, A. Robotics, Microfluidics, Nanotechnology and AI in the Synthesis and Evaluation of Liposomes and Polymeric Drug Delivery Systems. Drug Deliv. Transl. Res. 2021, 11, 345–352. [Google Scholar] [CrossRef]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental Trends in Polymer Nanocomposites—A Review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer Nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Carbon Nanotubes-Polymer Nanocomposite Membranes for Pervaporation. In Polymer Nanocomposite Membranes for Pervaporation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 105–133. ISBN 978-0-12-816785-4. [Google Scholar]
- Khan, I.; Khan, I.; Saeed, K.; Ali, N.; Zada, N.; Khan, A.; Ali, F.; Bilal, M.; Akhter, M.S. Polymer Nanocomposites: An Overview. In Smart Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–184. ISBN 978-0-323-91611-0. [Google Scholar]
- Tavares, M.I.B.; Silva, E.O.D.; Silva, P.R.C.D.; Menezes, L.R.D. Polymer Nanocomposites. In Nanostructured Materials-Fabrication to Applications; Seehra, M.S., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3371-1. [Google Scholar]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, A.; Murthy, A.A.; Thipperudrappa, S.; Bharath, K.N. Nanoparticles Filled Polymer Nanocomposites: A Technological Review. Cogent Eng. 2021, 8, 1991229. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M. Composites of Intrinsically Conducting Polymers as Sensing Nanomaterials. Chem. Rev. 2008, 108, 746–769. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A Review of the Interfacial Characteristics of Polymer Nanocomposites Containing Carbon Nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef]
- Loos, C.; Syrovets, T.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Nienhaus, G.U.; Simmet, T. Functionalized Polystyrene Nanoparticles as a Platform for Studying Bio–Nano Interactions. Beilstein J. Nanotechnol. 2014, 5, 2403–2412. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene Nanoparticles: Sources, Occurrence in the Environment, Distribution in Tissues, Accumulation and Toxicity to Various Organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Abitaev, K.; Qawasmi, Y.; Atanasova, P.; Dargel, C.; Bill, J.; Hellweg, T.; Sottmann, T. Adjustable Polystyrene Nanoparticle Templates for the Production of Mesoporous Foams and ZnO Inverse Opals. Colloid Polym. Sci. 2021, 299, 243–258. [Google Scholar] [CrossRef]
- Wu, D.; Chew, J.W.; Honciuc, A. Polarity Reversal in Homologous Series of Surfactant-Free Janus Nanoparticles: Toward the Next Generation of Amphiphiles. Langmuir 2016, 32, 6376–6386. [Google Scholar] [CrossRef]
- Safaie, N.; Ferrier, R.C. Janus Nanoparticle Synthesis: Overview, Recent Developments, and Applications. J. Appl. Phys. 2020, 127, 170902. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Synthesis, Properties and Applications of Janus Nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Mihali, V.; Honciuc, A. Evolution of Self-Organized Microcapsules with Variable Conductivities from Self-Assembled Nanoparticles at Interfaces. ACS Nano 2019, 13, 3483–3491. [Google Scholar] [CrossRef]
- Mihali, V.; Honciuc, A. Semiconductive Materials with Tunable Electrical Resistance and Surface Polarity Obtained by Asymmetric Functionalization of Janus Nanoparticles. Adv. Mater. Interfaces 2017, 4, 1700914. [Google Scholar] [CrossRef]
- Popov, I.; Cheng, S.; Sokolov, A.P. Broadband Dielectric Spectroscopy and Its Application in Polymeric Materials. In Macromolecular Engineering; Hadjichristidis, N., Gnanou, Y., Matyjaszewski, K., Muthukumar, M., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 1–39. ISBN 978-3-527-34455-0. [Google Scholar]
- Ciomaga, C.E.; Guzu, A.; Airimioaei, M.; Curecheriu, L.P.; Lukacs, V.A.; Avadanei, O.G.; Stoian, G.; Grigoras, M.; Lupu, N.; Asandulesa, M.; et al. Comparative Study of Magnetoelectric BaTiO3–Co0.8Zn0.2Fe2O4 Bi-Tunable Ceramics Sintered by Spark Plasma Sintering and Classical Method. Ceram. Int. 2019, 45, 24168–24175. [Google Scholar] [CrossRef]
- Klonos, P.; Terzopoulou, Z.; Koutsoumpis, S.; Zidropoulos, S.; Kripotou, S.; Papageorgiou, G.Z.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Rigid Amorphous Fraction and Segmental Dynamics in Nanocomposites Based on Poly(l–Lactic Acid) and Nano-Inclusions of 1–3D Geometry Studied by Thermal and Dielectric Techniques. Eur. Polym. J. 2016, 82, 16–34. [Google Scholar] [CrossRef]
- Butnaru, I.; Constantin, C.-P.; Asandulesa, M.; Wolińska-Grabczyk, A.; Jankowski, A.; Szeluga, U.; Damaceanu, M.-D. Insights into Molecular Engineering of Membranes Based on Fluorinated Polyimide-Polyamide Miscible Blends Which Do Not Obey the Trade-off Rule. Sep. Purif. Technol. 2020, 233, 116031. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-62809-2. [Google Scholar]
- Fanari, F.; Iacob, C.; Carboni, G.; Desogus, F.; Grosso, M.; Wilhelm, M. Broadband Dielectric Spectroscopy (BDS) Investigation of Molecular Relaxations in Durum Wheat Dough at Low Temperatures and Their Relationship with Rheological Properties. LWT 2022, 161, 113345. [Google Scholar] [CrossRef]
- Racles, C.; Dascalu, M.; Bele, A.; Tiron, V.; Asandulesa, M.; Tugui, C.; Vasiliu, A.-L.; Cazacu, M. All-Silicone Elastic Composites with Counter-Intuitive Piezoelectric Response, Designed for Electromechanical Applications. J. Mater. Chem. C 2017, 5, 6997–7010. [Google Scholar] [CrossRef]
- Racles, C.; Ursu, C.; Dascalu, M.; Asandulesa, M.; Tiron, V.; Bele, A.; Tugui, C.; Teodoroff-Onesim, S. Multi-Stimuli Responsive Free-Standing Films of DR1- Grafted Silicones. Chem. Eng. J. 2020, 401, 126087. [Google Scholar] [CrossRef]
- Serghei, A.; Lutkenhaus, J.L.; Miranda, D.F.; McEnnis, K.; Kremer, F.; Russell, T.P. Density Fluctuations and Phase Transitions of Ferroelectric Polymer Nanowires. Small 2010, 6, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Samet, M.; Levchenko, V.; Boiteux, G.; Seytre, G.; Kallel, A.; Serghei, A. Electrode Polarization vs. Maxwell-Wagner-Sillars Interfacial Polarization in Dielectric Spectra of Materials: Characteristic Frequencies and Scaling Laws. J. Chem. Phys. 2015, 142, 194703. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers III: Hydrocarbon Polymers. Spectroscopy 2021, 36, 22–25. [Google Scholar] [CrossRef]
- Asandulesa, M.; Topala, I.; Pohoata, V.; Legrand, Y.M.; Dobromir, M.; Totolin, M.; Dumitrascu, N. Chemically Polymerization Mechanism of Aromatic Compounds under Atmospheric Pressure Plasma Conditions: Plasma Polymerization Mechanism of Aromatic Compounds. Plasma Process. Polym. 2013, 10, 469–480. [Google Scholar] [CrossRef]
- Lin, J.; Wu, X.; Liu, Y.; Fu, J.; Chen, Y.; Ou, H. Sinking Behavior of Polystyrene Microplastics after Disinfection. Chem. Eng. J. 2022, 427, 130908. [Google Scholar] [CrossRef]
- Xu, Z.P.; Braterman, P.S. High Affinity of Dodecylbenzene Sulfonate for Layered Double Hydroxide and Resulting Morphological Changes. J. Mater. Chem. 2003, 13, 268–273. [Google Scholar] [CrossRef]
- Gurman, J.L.; Baier, L.; Levin, B.C. Polystyrenes: A Review of the Literature on the Products of Thermal Decomposition and Toxicity. Fire Mater. 1987, 11, 109–130. [Google Scholar] [CrossRef]
- Hermán, V.; Takacs, H.; Duclairoir, F.; Renault, O.; Tortai, J.H.; Viala, B. Core Double–Shell Cobalt/Graphene/Polystyrene Magnetic Nanocomposites Synthesized by in Situ Sonochemical Polymerization. RSC Adv. 2015, 5, 51371–51381. [Google Scholar] [CrossRef]
- Davodi, B.; Lashkenari, M.S.; Eisazadeh, H. Fabrication and Thermal Degradation Behavior of Polystyrene Nanoparticles Coated with Smooth Polyaniline. Synth. Met. 2011, 161, 1207–1210. [Google Scholar] [CrossRef]
- Pargen, S.; Willems, C.; Keul, H.; Pich, A.; Möller, M. Surfactant-Free Synthesis of Polystyrene Nanoparticles Using Oligoglycidol Macromonomers. Macromolecules 2012, 45, 1230–1240. [Google Scholar] [CrossRef]
- Resmerita, A.; Asandulesa, M.; Farcas, A. Evaluation of the Chemical, Morphological and Dielectric Properties of Supramolecular Networks Consisting of Polyethylene Glycol Polyrotaxanes and Polystyrene/Semi-Rotaxane with Hydroxypropyl-β-Cyclodextrins. Macro Chem. Phys. 2022, 223, 2100383. [Google Scholar] [CrossRef]
- Asandulesa, M.; Kostromin, S.; Podshivalov, A.; Tameev, A.; Bronnikov, S. Relaxation Processes in a Polymer Composite for Bulk Heterojunction: A Dielectric Spectroscopy Study. Polymer 2020, 203, 122785. [Google Scholar] [CrossRef]
- Sasabe, H.; Saito, S. Effects of Temperature and Pressure on the Dielectric Constant in Non-Polar Polymers. Polym. J. 1972, 3, 749–755. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Yuan, S.; Wang, D.; Hu, P.-H.; Dang, Z.-M. Low Dielectric Loss and Weak Frequency Dependence of Dielectric Permittivity of the CeO2/Polystyrene Nanocomposite Films. Appl. Phys. Lett. 2014, 105, 052905. [Google Scholar] [CrossRef]
- He, F.; Lam, K.; Ma, D.; Fan, J.; Chan, L.H.; Zhang, L. Fabrication of Graphene Nanosheet (GNS)–Fe3O4 Hybrids and GNS–Fe3O4/Syndiotactic Polystyrene Composites with High Dielectric Permittivity. Carbon 2013, 58, 175–184. [Google Scholar] [CrossRef]
- Honciuc, A. Chemistry of Functional Materials Surfaces and Interfaces; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-821059-8. [Google Scholar]

| Sample | mNaVBS (mg) | VStyrene (mL) | VDVB (mL) |
|---|---|---|---|
| PS NPs-1 | 78 | 25 | 0.81 |
| PS NPs-2 | 78 | 27 | 0.81 |
| PS NPs-3 | 200 | 26.2 | 0.85 |
| PS NPs-4 | 200 | 26.2 | 1.0 |
| Sample | Mole Fraction of Crosslinker (%) | Diameter (mm) | Tg (DSC) (°C) | Tg (BDS) (°C) | ε∞ |
|---|---|---|---|---|---|
| PS NPs-1 | 2.536 | 271 | 116 | 120.8 | 2.7 |
| PS NPs-2 | 2.353 | 286 | 115 | 118.7 | 2.85 |
| PS NPs-3 | 2.533 | 191 | 113 | 116.8 | 2.65 |
| PS NPs-4 | 2.967 | 178 | 114 | 118.2 | 2.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asandulesa, M.; Solonaru, A.-M.; Resmerita, A.-M.; Honciuc, A. Thermal and Dielectric Investigations of Polystyrene Nanoparticles as a Viable Platform—Toward the Next Generation of Fillers for Nanocomposites. Polymers 2023, 15, 2899. https://doi.org/10.3390/polym15132899
Asandulesa M, Solonaru A-M, Resmerita A-M, Honciuc A. Thermal and Dielectric Investigations of Polystyrene Nanoparticles as a Viable Platform—Toward the Next Generation of Fillers for Nanocomposites. Polymers. 2023; 15(13):2899. https://doi.org/10.3390/polym15132899
Chicago/Turabian StyleAsandulesa, Mihai, Ana-Maria Solonaru, Ana-Maria Resmerita, and Andrei Honciuc. 2023. "Thermal and Dielectric Investigations of Polystyrene Nanoparticles as a Viable Platform—Toward the Next Generation of Fillers for Nanocomposites" Polymers 15, no. 13: 2899. https://doi.org/10.3390/polym15132899
APA StyleAsandulesa, M., Solonaru, A.-M., Resmerita, A.-M., & Honciuc, A. (2023). Thermal and Dielectric Investigations of Polystyrene Nanoparticles as a Viable Platform—Toward the Next Generation of Fillers for Nanocomposites. Polymers, 15(13), 2899. https://doi.org/10.3390/polym15132899








