Anthraquinone-Polyaniline-Integrated Textile Platforms for In Situ Electrochemical Production of Hydrogen Peroxide for Microbial Deactivation
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. CNT/CNC H2O2 Sensor Electrode Fabrication
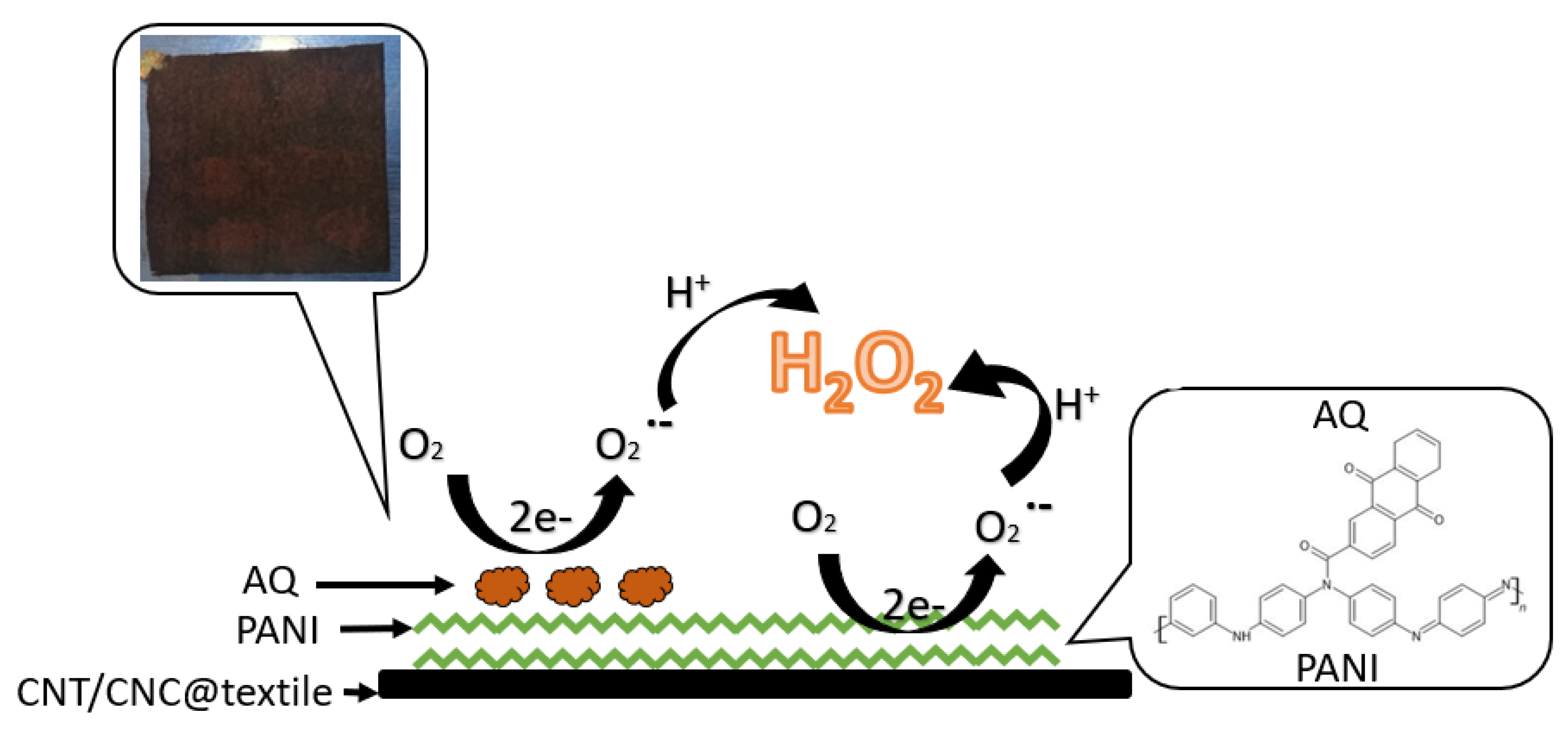
2.3. AQ@PANI@CNT/CNC@Textile Antibacterial Patch Fabrication
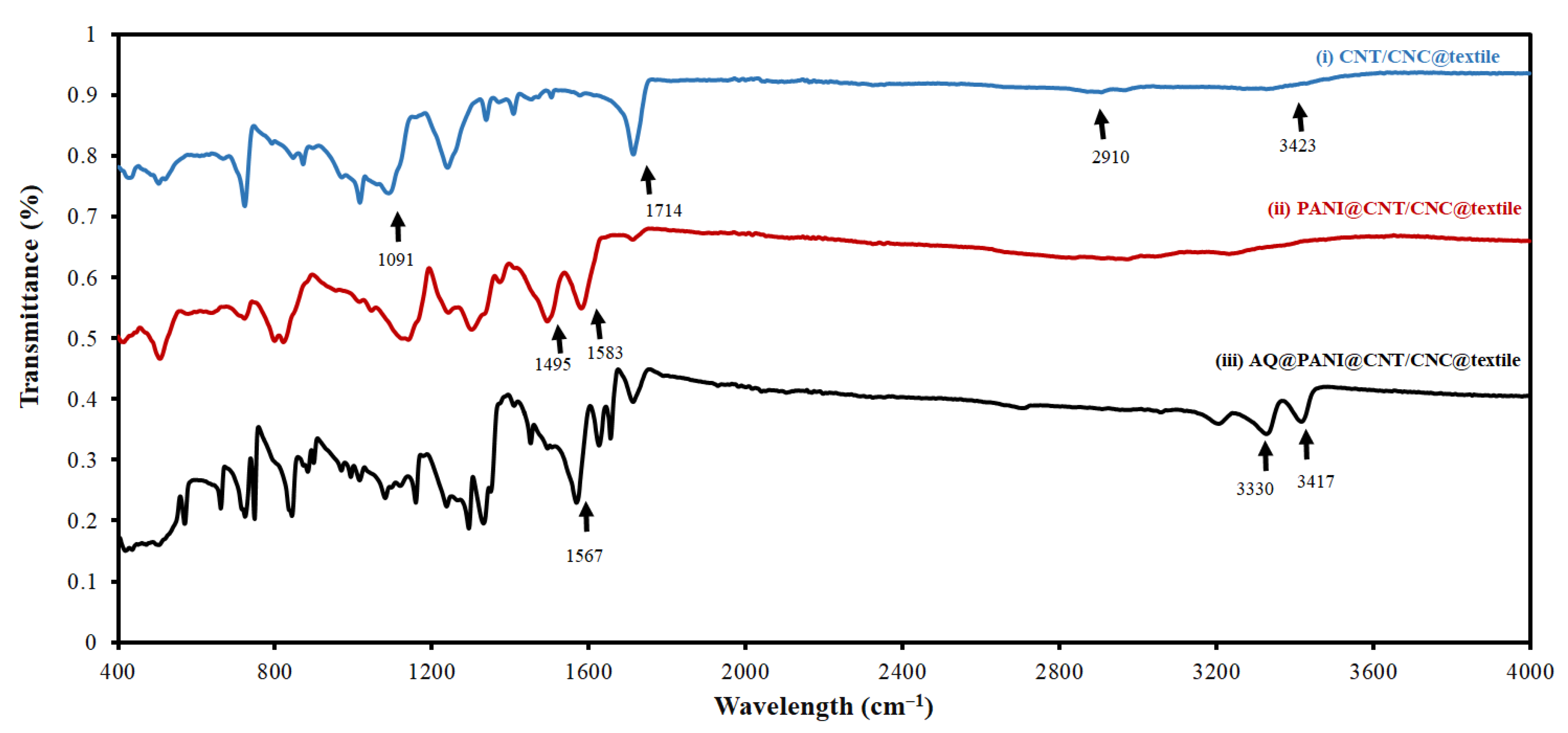
2.4. Characterization of AQ@PANI@CNT/CNC@Textile Antibacterial Patch
2.5. In Situ Electrochemical Generation of H2O2, and Electrochemical and Colorimetric Quantification
2.6. E. coli Deactivation Test Using the Antibacterial Patches
3. Results and Discussion
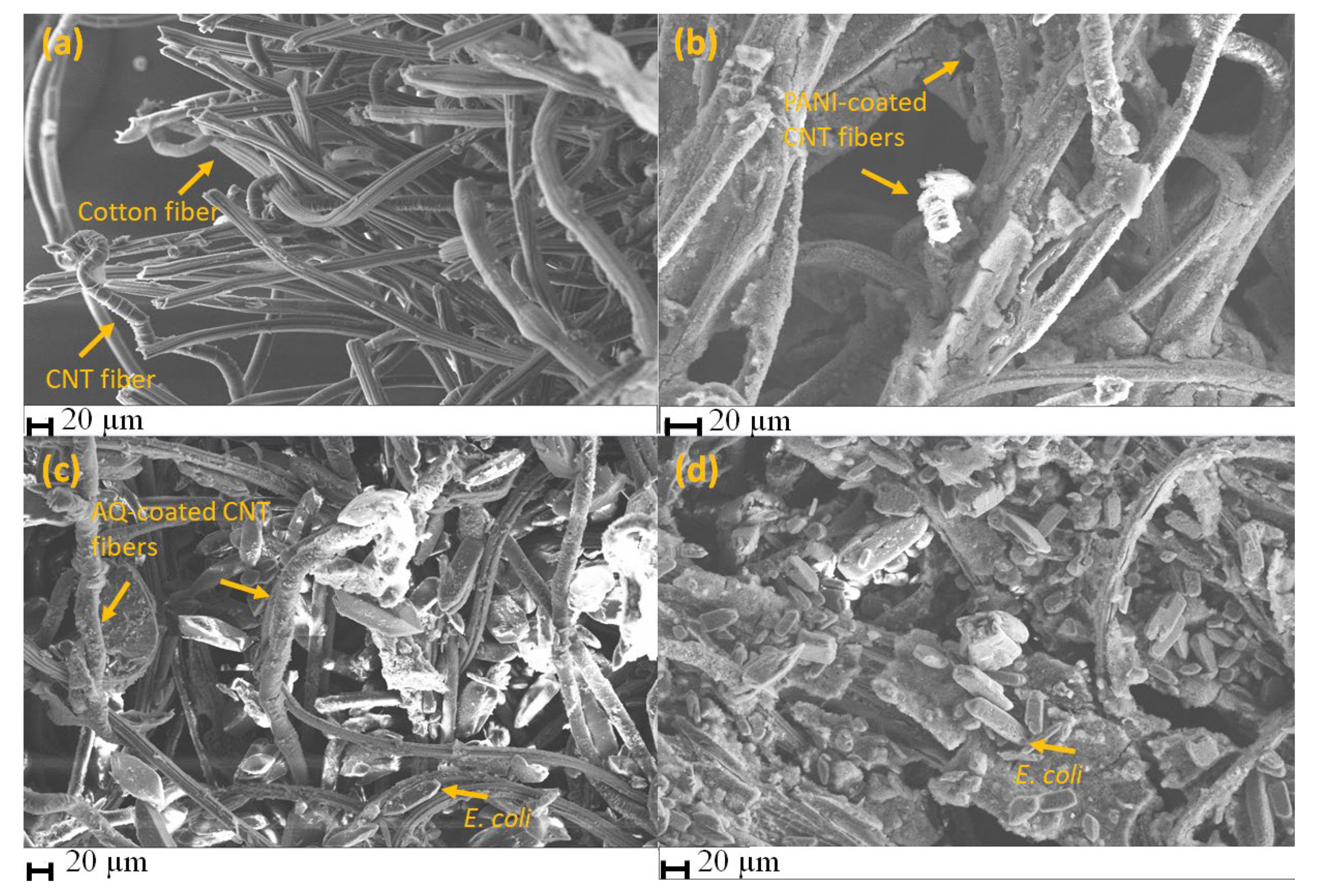
3.1. Morphological Characterization of Antibacterial Textile Patches
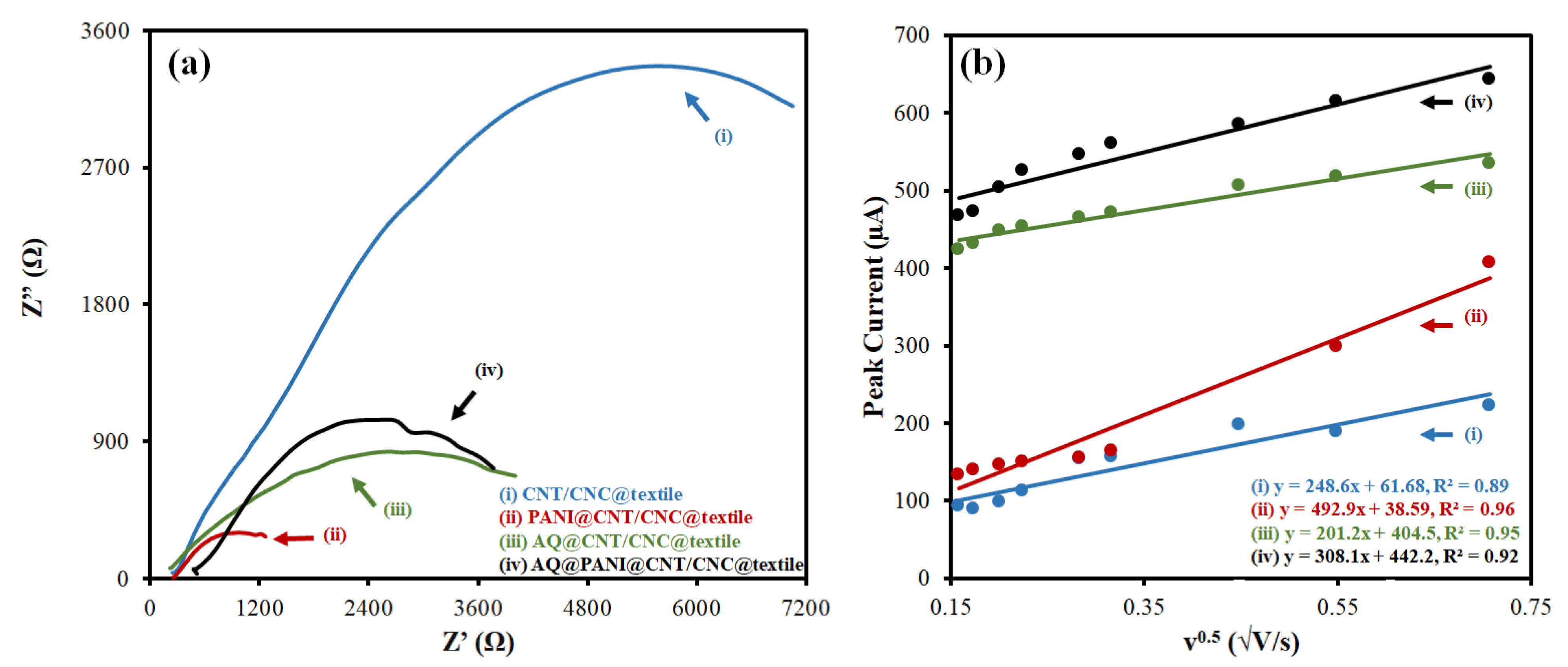
3.2. Electrochemical Characterization of Antibacterial Patches
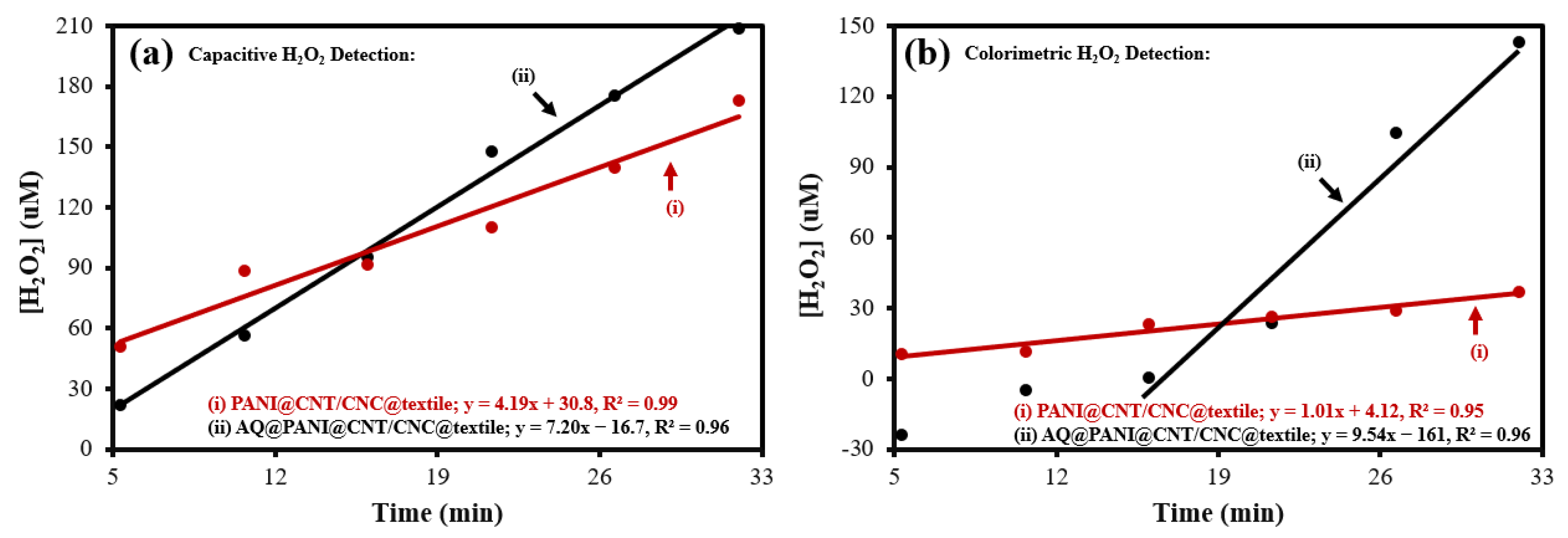
3.3. Electrochemical and Colorimetric Quantification of Antibacterial Textile Patch H2O2 Production
3.4. Antibacterial Efficacy of AQ@PANI@CNT/CNC@Textile Antibacterial Patch
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Han, L.; Strasser, P. A comparative perspective of electrochemical and photochemical approaches for catalytic H2O2 production. Chem. Soc. Rev. 2020, 49, 6605–6631. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-G.; Tang, L.; Tang, Y.-K.; Li, C.-X.; Li, M.-L.; Zhou, J.-J.; Chen, W.; Zhu, F.; Jiang, J. Selective Electrocatalytic Water Oxidation to Produce H2O2 Using a C,N Codoped TiO2 Electrode in an Acidic Electrolyte. ACS Appl. Mater. Interfaces 2019, 12, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-N.; Chen, S.-T. Bactericidal Effects of Oxidative Stress Generated by EDTA-Fe and Hydrogen Peroxide. Biocontrol Sci. 2019, 24, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.; Andruchow, J.E.; Conly, J.; Lee, D.D.; Mazurik, L.; Atkinson, P.; Lang, E. Personal protective equipment preservation strategies in the covid-19 era: A narrative review. Infect. Prev. Pract. 2021, 3, 100146. [Google Scholar] [CrossRef]
- Grossman, J.; Pierce, A.; Mody, J.; Gagne, J.; Sykora, C.; Sayood, S.; Cook, S.; Shomer, N.; Liang, S.Y.; Eckhouse, S.R. Institution of a Novel Process for N95 Respirator Disinfection with Vaporized Hydrogen Peroxide in the Setting of the COVID-19 Pandemic at a Large Academic Medical Center. J. Am. Coll. Surg. 2020, 231, 275–280. [Google Scholar] [CrossRef]
- Pradhan, D.; Biswasroy, P.; Kumar Naik, P.; Ghosh, G.; Rath, G. A Review of Current Interventions for COVID-19 Prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef]
- Berger, D.; Gundermann, G.; Sinha, A.; Moroi, M.; Goyal, N.; Tsai, A. Review of aerosolized hydrogen peroxide, vaporized hydrogen peroxide, and hydrogen peroxide gas plasma in the decontamination of filtering facepiece respirators. Am. J. Infect. Control 2021, 50, 203–213. [Google Scholar] [CrossRef]
- Basso, I.B.; Schroder, A.G.D.; Santos, R.S.; Ravazzi, G.C.; Gonçalves, F.M.; Stechman-Neto, J.; Zeigelboim, B.S.; Povh, B.; de Araujo, C.M.; Guariza-Filho, O. Efficacy of disinfecting inanimate surfaces against coronavirus: A systematic review. Res. Soc. Dev. 2021, 10, e452101019100. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Anantharaj, S.; Pitchaimuthu, S.; Noda, S. A review on recent developments in electrochemical hydrogen peroxide synthesis with a critical assessment of perspectives and strategies. Adv. Colloid Interface Sci. 2020, 287, 102331. [Google Scholar] [CrossRef]
- Zhu, Q.; Hinkle, M.; Kim, D.J.; Kim, J.-H. Modular Hydrogen Peroxide Electrosynthesis Cell with Anthraquinone-Modified Polyaniline Electrocatalyst. ACS EST Eng. 2020, 1, 446–455. [Google Scholar] [CrossRef]
- Khomenko, V.; Barsukov, V.; Katashinskii, A. The catalytic activity of conducting polymers toward oxygen reduction. Electrochim. Acta 2004, 50, 1675–1683. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, W.; Li, Y.; Zhang, Q.; Chen, H.; Zhang, J.; Huang, T. Anthraquinone (AQS)/polyaniline (PANI) modified carbon felt (CF) cathode for selective H2O2 generation and efficient pollutant removal in electro-Fenton. J. Environ. Manag. 2021, 304, 114315. [Google Scholar] [CrossRef] [PubMed]
- Kajita, M.; Hikosaka, K.; Iitsuka, M.; Kanayama, A.; Toshima, N.; Miyamoto, Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic. Res. 2007, 41, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Dhanjai; Mugo, S.M.; Lu, W. Modified stainless steel microneedle electrode for polyphenolics detection. Anal. Bioanal. Chem. 2020, 412, 7063–7072. [Google Scholar] [CrossRef] [PubMed]
- Mugo, S.M.; Lu, W.; Wood, M.; Lemieux, S. Wearable microneedle dual electrochemical sensor for simultaneous pH and cortisol detection in sweat. Electrochem. Sci. Adv. 2021, 2, e2100039. [Google Scholar] [CrossRef]
- Mugo, S.M.; Berg, D.; Bharath, G. Integrated Microcentrifuge Carbon Entrapped Glucose Oxidase Poly (n-Isopropylacrylamide) (pNIPAm) Microgels for Glucose Amperometric Detection. Anal. Lett. 2018, 52, 825–838. [Google Scholar] [CrossRef]
- Metwally, N.H.; Saad, G.R.; El-Wahab, E.A.A. Grafting of multiwalled carbon nanotubes with pyrazole derivatives: Characterization, antimicrobial activity and molecular docking study. Int. J. Nanomed. 2019, 14, 6645–6659. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Kale, B.B. Studies of conducting polyaniline (PANI) wrapped-multiwalled carbon nanotubes (MWCNTs) nanocomposite and its application for optical pH sensing. Sens. Actuators B Chem. 2013, 187, 407–412. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.-X. Carbon nanotube–polyaniline composites. Prog. Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Wu, H.P.; Yang, Q.; Meng, Q.H.; Ahmad, A.; Zhang, M.; Zhu, L.Y.; Liu, Y.G.; Wei, Z.X. A polyimide derivative containing different carbonyl groups for flexible lithium ion batteries. J. Mater. Chem. A 2015, 4, 2115–2121. [Google Scholar] [CrossRef]
- Habibi, B.; Jahanbakhshi, M. A novel nonenzymatic hydrogen peroxide sensor based on the synthesized mesoporous carbon and silver nanoparticles nanohybrid. Sens. Actuators B Chem. 2014, 203, 919–925. [Google Scholar] [CrossRef]
- Xu, H.; Guo, H.; Chai, C.; Li, N.; Lin, X.; Xu, W. Anodized graphite felt as an efficient cathode for in-situ hydrogen peroxide production and Electro-Fenton degradation of rhodamine B. Chemosphere 2021, 286, 131936. [Google Scholar] [CrossRef]
- Khoram, M.M.; Nematollahi, D.; Khazalpour, S.; Zarei, M.; Zolfigol, M.A. Electrocatalytic generation of hydrogen peroxide using carbon electrode modified with 5H-dibenzo[b,i]xanthene-5,7,12,14(13H)-tetraone derivative. A green and efficient method. Electrochim. Acta 2022, 407, 139885. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Ma, T.; Zhang, L. Nanointerface engineering Z-scheme CuBiOS@CuBi2O4 heterojunction with O S interpenetration for enhancing photocatalytic hydrogen peroxide generation and accelerating chromium(VI) reduction. J. Colloid Interface Sci. 2022, 611, 760–770. [Google Scholar] [CrossRef]
- Sánchez-Montes, I.; Santos, G.O.S.; Silva, T.O.; Colombo, R.; Lanza, M.R.V. An innovative approach to the application of electrochemical processes based on the in-situ generation of H2O2 for water treatment. J. Clean. Prod. 2023, 392, 136242. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R.; Bennett, J.R.; Swift, S.; Easteal, A.J.; Ambrose, M. Broad spectrum antimicrobial activity of functionalized polyanilines. Acta Biomater. 2011, 7, 4204–4209. [Google Scholar] [CrossRef]

| Patch Type | Rct (Ω) | Electroactive Surface Area (cm2) |
|---|---|---|
| CNT/CNC@textile | 1.63 × 104 | 13.3 |
| PANI@CNT/CNC@textile | 1.45 × 103 | 24.7 |
| AQ@CNT/CNC@textile | 5.13 × 103 | 10.8 |
| AQ@PANI@CNT/CNC@textile | 4.65 × 103 | 16.5 |
| H2O2 Production Method | Rate of Production (µM·min−1) | Total H2O2 Produced (µM) | Reference |
|---|---|---|---|
| Electrochemical production using H2SO4-anodized graphite felt as a cathode | 27.1 | 3.25 × 103 | [24] |
| Electrochemical production using 13-(4-nitrophenyl)-5H-dibenzo[b,i]xanthene-5,7,12,14(13H)-tetraone–modified carbon electrode | 113 | 1.36 × 104 | [25] |
| Photocatalytic production using a catalytic CuBiOS@CuBi2O4 heterojunction with O-S interpenetration | 1.12 | 202 | [26] |
| Electrochemical production using a carbon-polytetrafluoroethylene-modified carbon cloth gas diffusion electrode | 141–329 | 1.41–3.29 × 103 | [27] |
| Electrochemical production using AQ@PANI@CNT/CNC@textile patch | 7.20 | 209 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugo, S.M.; Lu, W.; Robertson, S. Anthraquinone-Polyaniline-Integrated Textile Platforms for In Situ Electrochemical Production of Hydrogen Peroxide for Microbial Deactivation. Polymers 2023, 15, 2859. https://doi.org/10.3390/polym15132859
Mugo SM, Lu W, Robertson S. Anthraquinone-Polyaniline-Integrated Textile Platforms for In Situ Electrochemical Production of Hydrogen Peroxide for Microbial Deactivation. Polymers. 2023; 15(13):2859. https://doi.org/10.3390/polym15132859
Chicago/Turabian StyleMugo, Samuel M., Weihao Lu, and Scott Robertson. 2023. "Anthraquinone-Polyaniline-Integrated Textile Platforms for In Situ Electrochemical Production of Hydrogen Peroxide for Microbial Deactivation" Polymers 15, no. 13: 2859. https://doi.org/10.3390/polym15132859
APA StyleMugo, S. M., Lu, W., & Robertson, S. (2023). Anthraquinone-Polyaniline-Integrated Textile Platforms for In Situ Electrochemical Production of Hydrogen Peroxide for Microbial Deactivation. Polymers, 15(13), 2859. https://doi.org/10.3390/polym15132859







