Structural, Optical, and Electrical Parameters of Doped PVA/PVP Blend with TPAI or THAI Salt
Abstract
1. Introduction
2. Methods and Materials
3. Results and Discussion
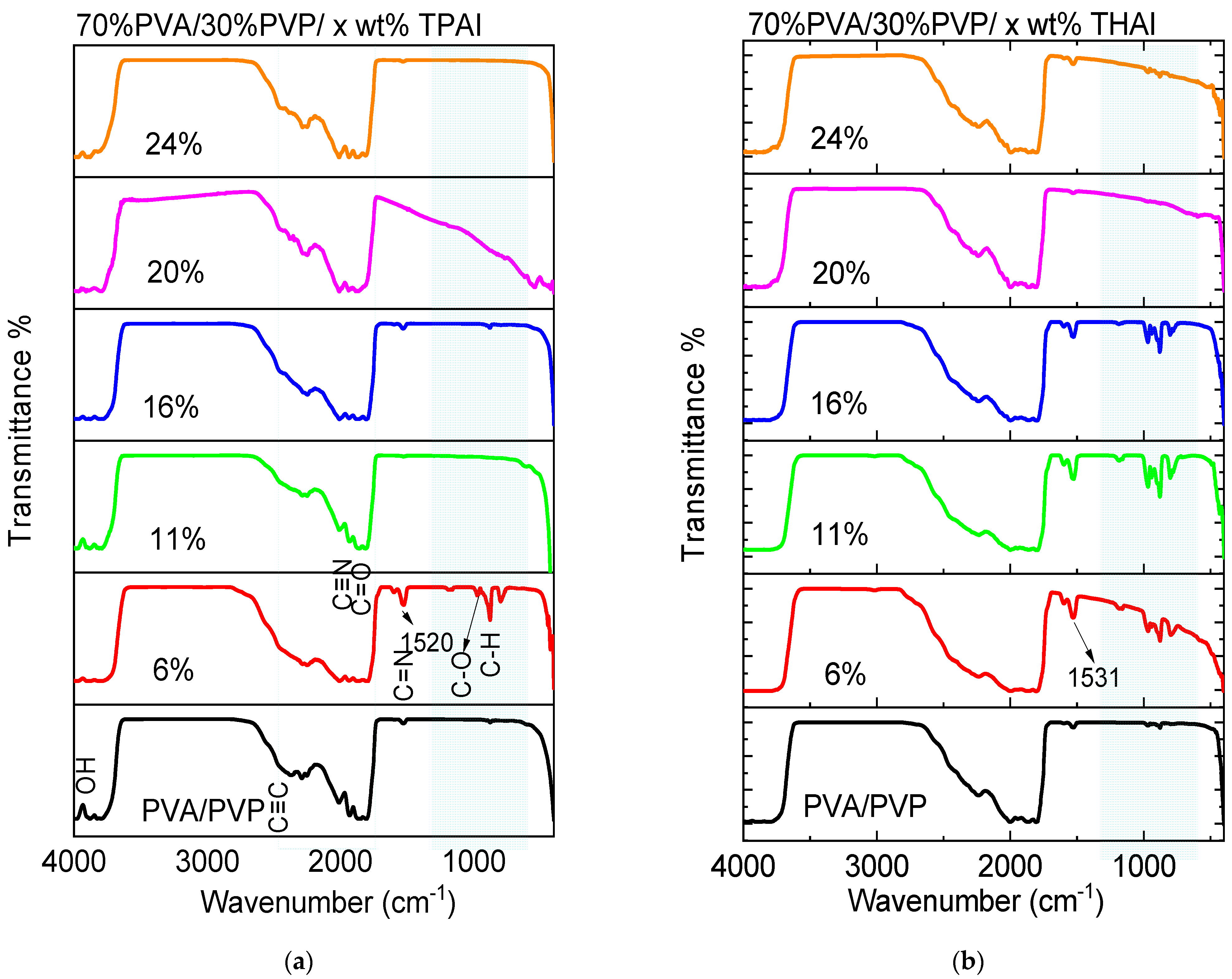
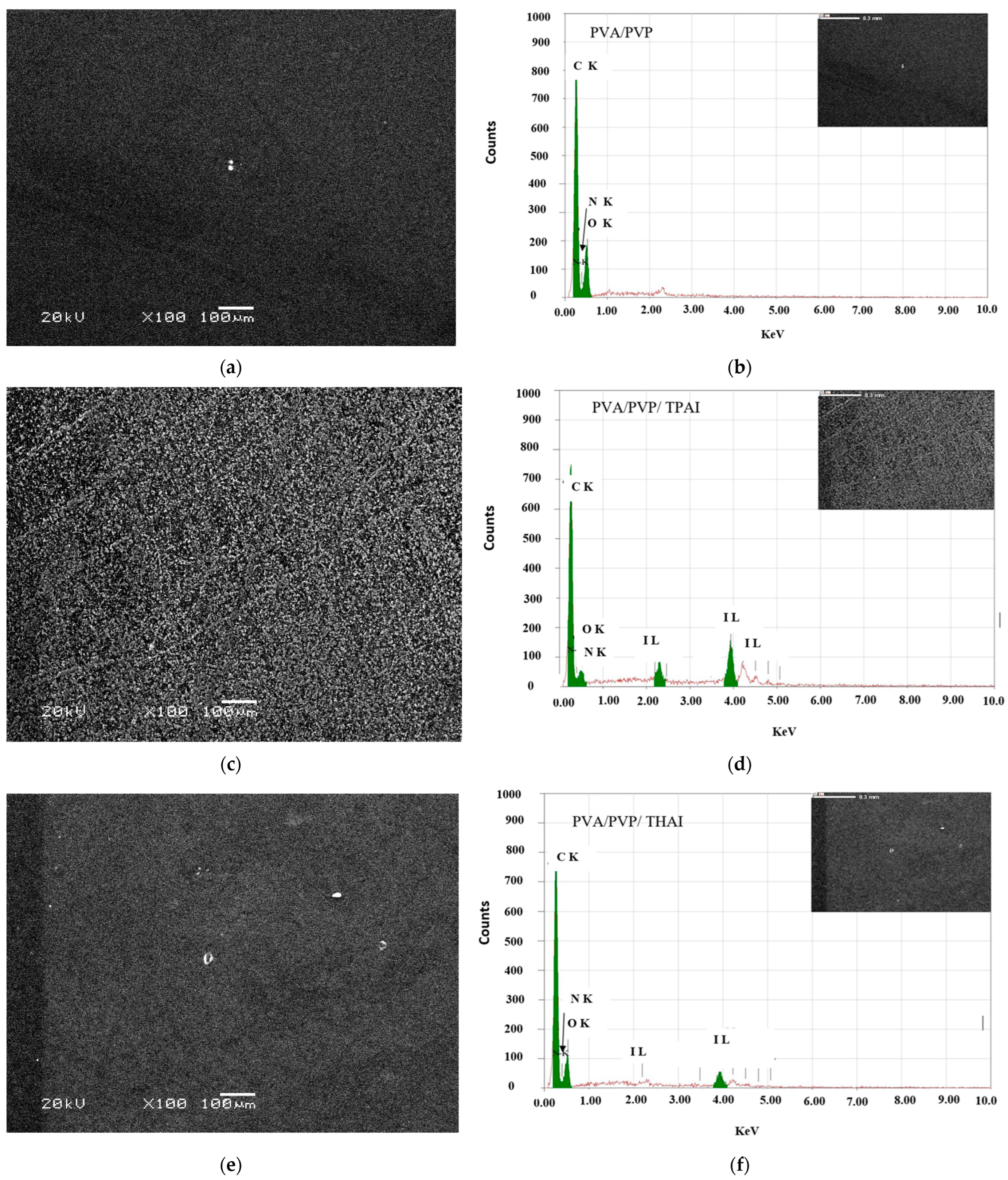
3.1. Structural Investigation
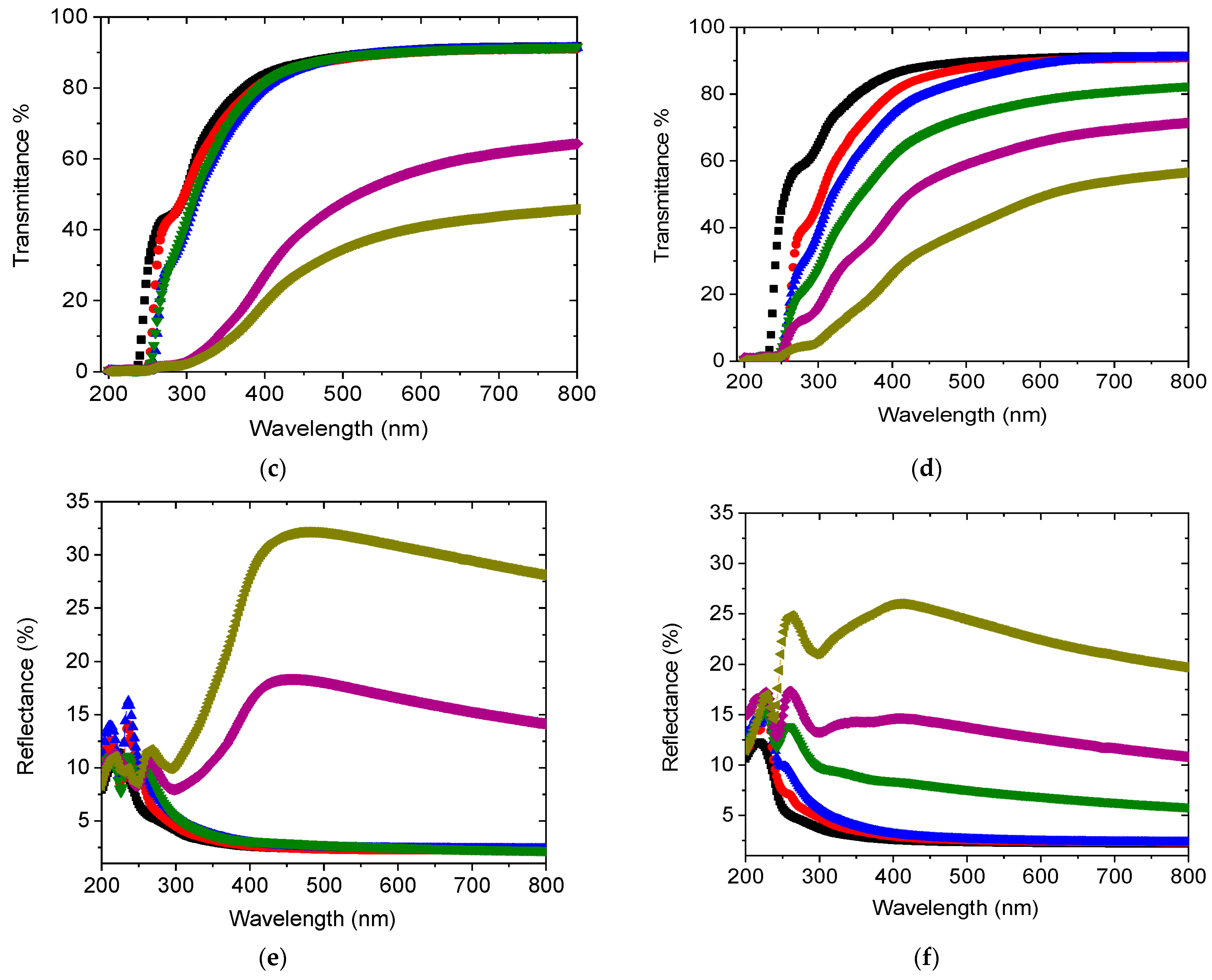
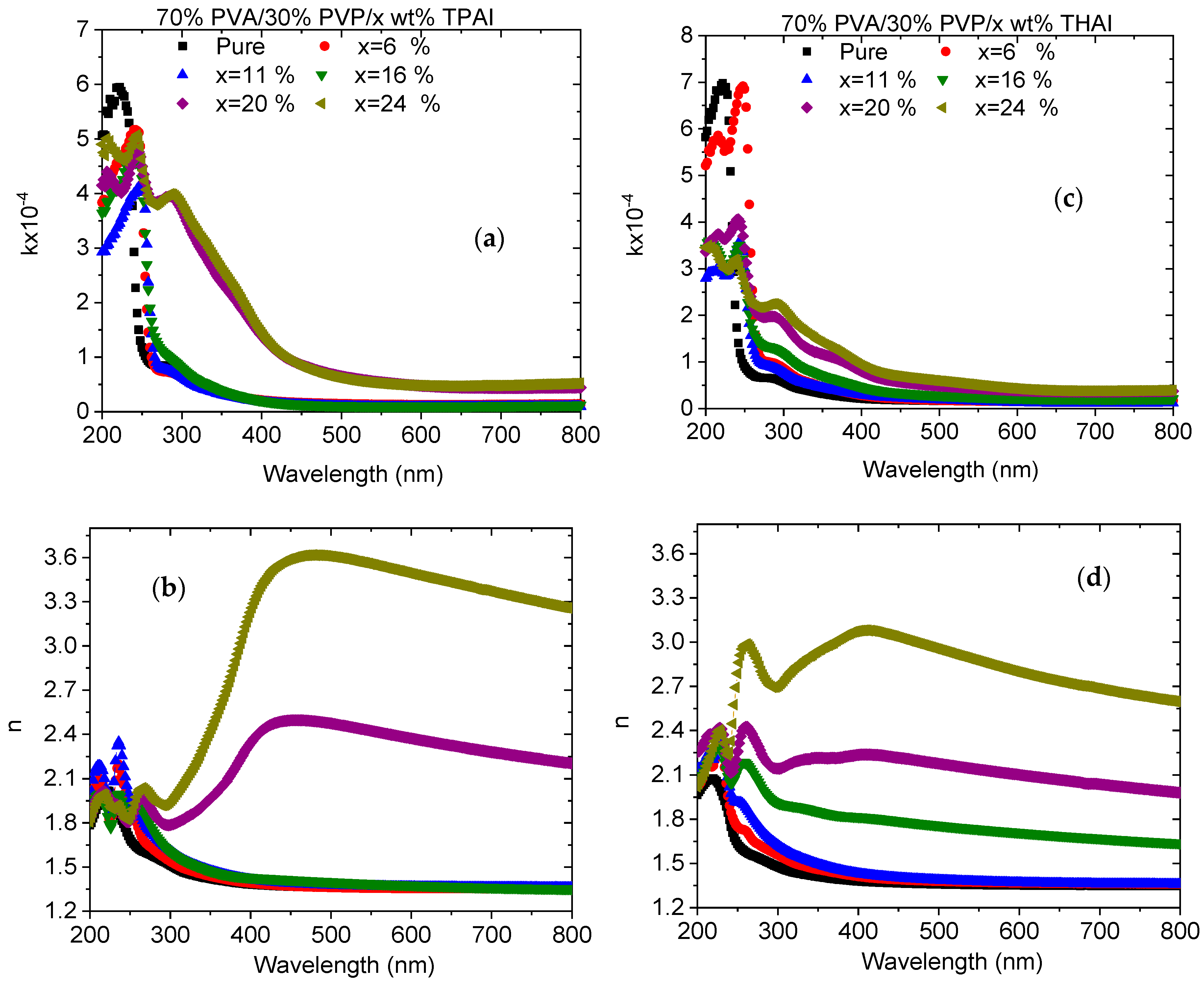
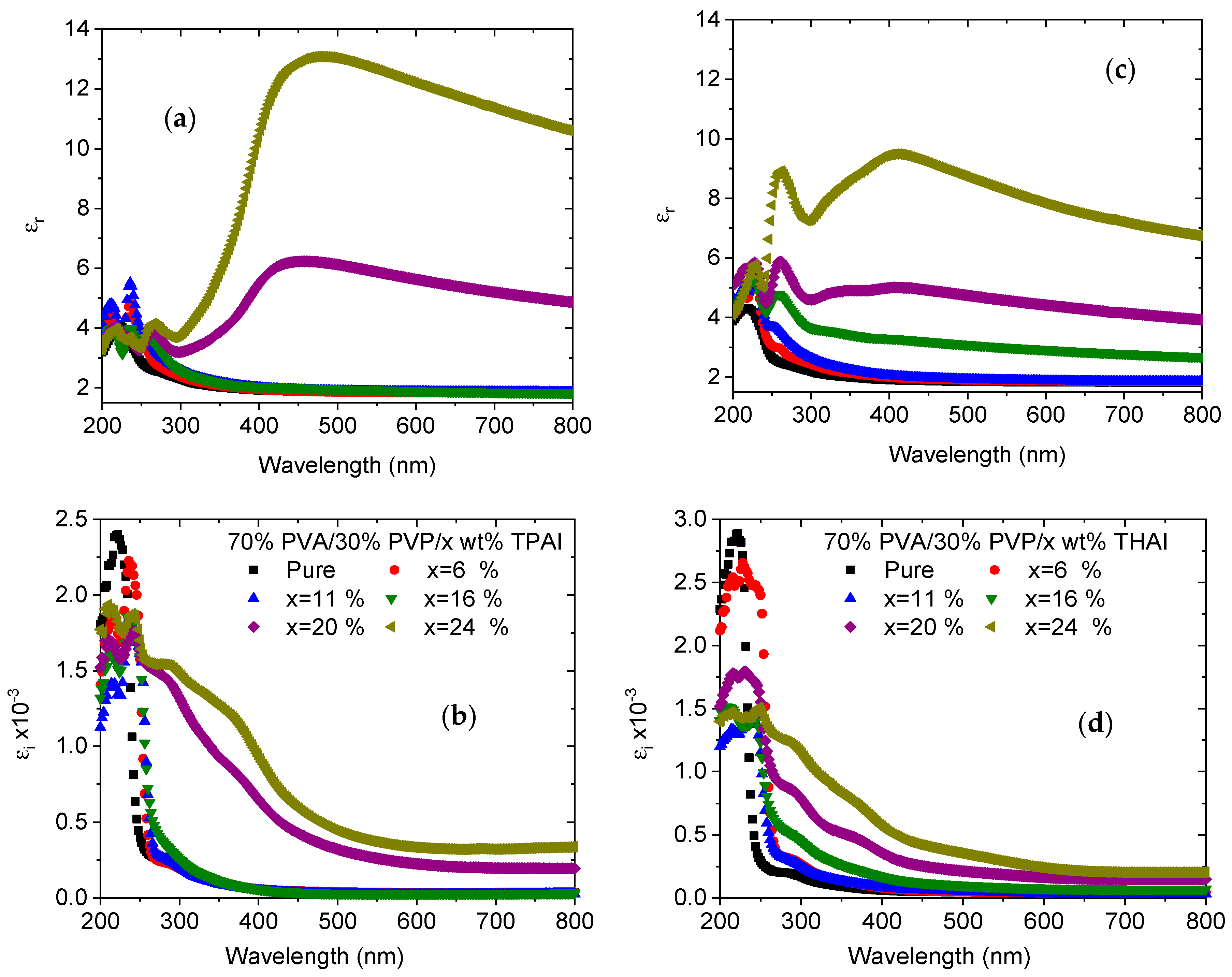
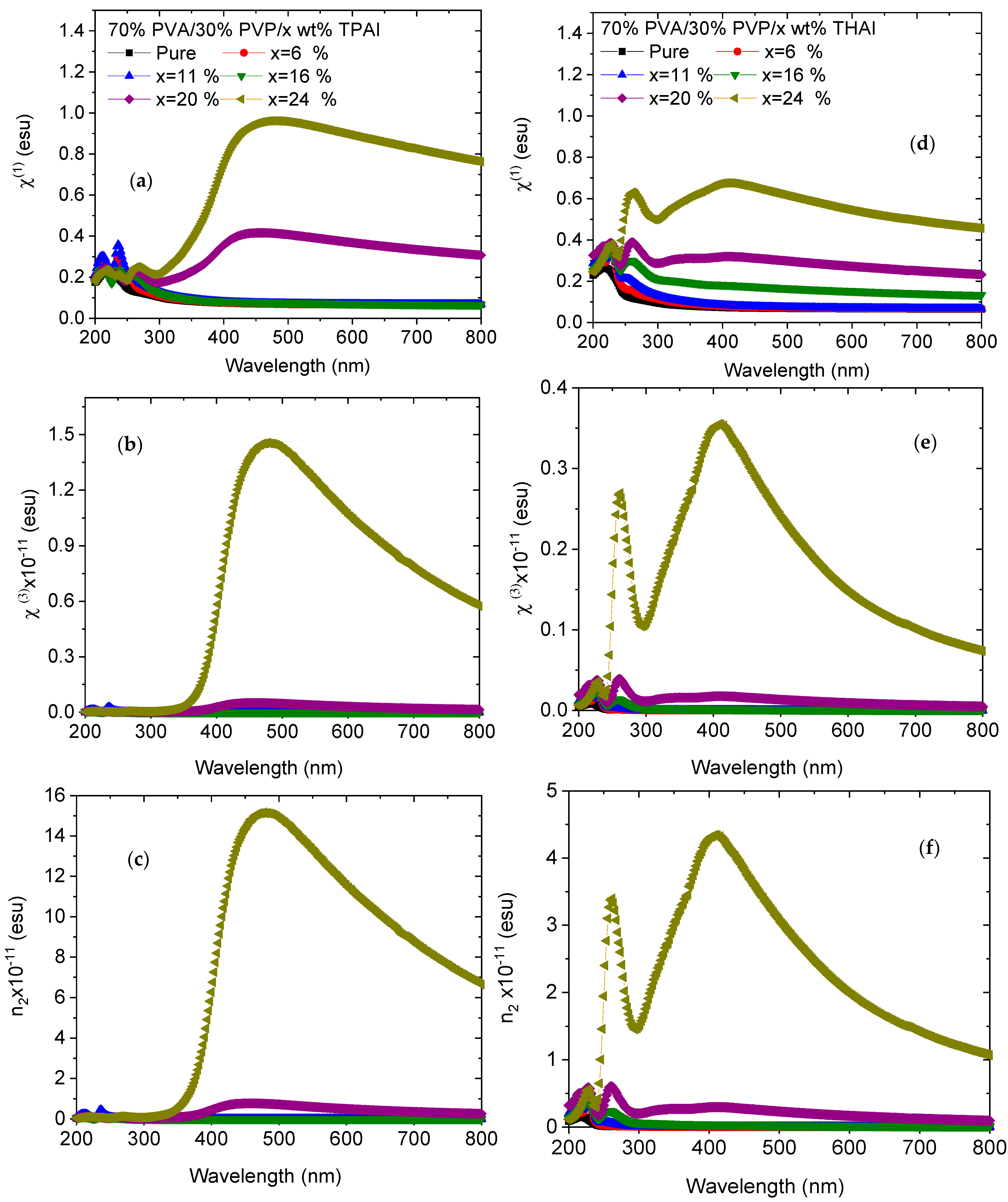
3.2. UV Spectra Analysis
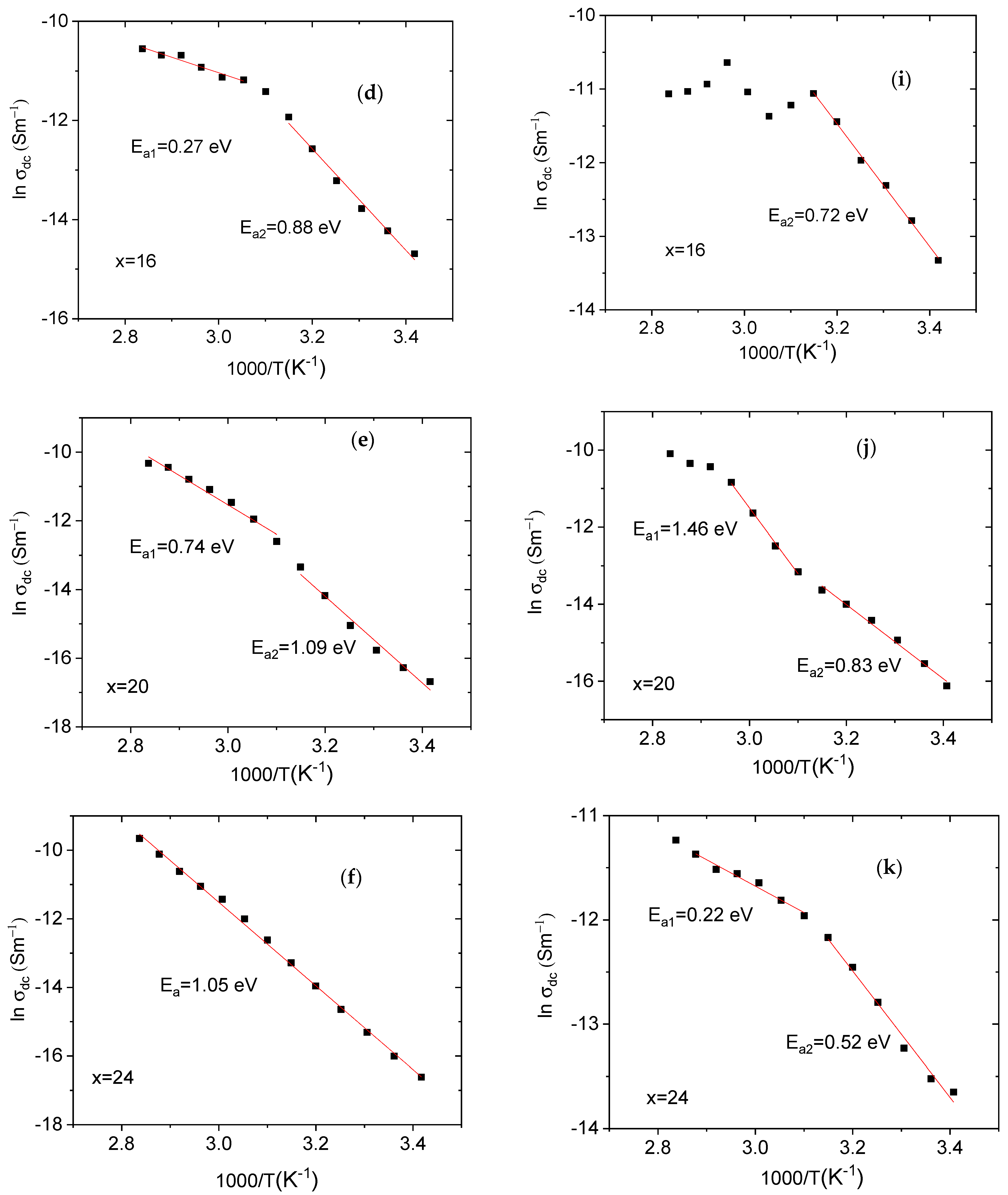
3.3. DC Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Abd-Elkader, O.H.; Lakshminarayana, G.; Mohamed, M.B. Structural, thermal, and linear/nonlinear optical performance of PVA/CMC polymer blend doped with ZnS/V prepared at different temperatures. J. Polym. Res. 2022, 29, 475. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Alsaggaf, A.; Heiba, Z.K.; Kamal, A.M.; Aldhafiri, A.M.; Mohamed, M.B. Impact of MWCNTs on the structural, electrical, and optical characteristics of PVA/PANi blends. J. Taibah Univ. Sci. 2023, 17, 2207769. [Google Scholar] [CrossRef]
- Singh, R.; Kulkarni, S.G.; Naik, N.H. Effect Of Nano Sized Transition Metal Salts And Metals On Thermal decomposition Behavior Of Polyvinyl Alcohol. Adv. Mater. Lett. 2013, 4, 82. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Shah, S.; Teo, L.P.; Chowdhury, F.I.; Careem, M.A.; Albinsson, I.; Mellander, B.-E.; Arof, A.K. High efficient dye sensitized solar cells using phthaloylchitosan based gel polymer electrolytes. Electrochim. Acta 2017, 245, 846–853. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekerapandian, S.; Hirankumar, G.; Sakunthala, A.; Arunkumar, D.; Nithya, H. Structural and thermal studies of PVA:NH4I. J. Phys. Chem. Solids 2009, 70, 1098. [Google Scholar] [CrossRef]
- Aziz, M.F.; Noor, I.; Buraidah, M.; Careem, M.; Arof, A. PVA-based gel polymer electrolytes doped with (CH3)4NI/KI for application in dye-sensitized solar cells. In Proceedings of the 15th International Conference on Transparent Optical Networks ICTON 2013, Cartagena, Spain, 23–27 June 2013; pp. 1–4. [Google Scholar]
- Bandara, T.M.W.J.; Dissanayake, M.A.K.L.; Jayasundara, W.J.M.J.S.R.; Albinsson, I.; Mellander, B.-E. Efficiency enhancement in dye sensitized solar cells using gel polymer electrolytes based on a tetrahexylammonium iodide and MgI2 binary iodide system. Phys. Chem. Chem. Phys. 2012, 14, 8620–8627. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Mahesha, M.G.; Devadiga, D.; Ahipa, T.N.; Kumar, S.S. Novel photosensitizer for dye-sensitized solar cell based on ionic liquid–doped blend polymer electrolyte. J. Solid State Electrochem. 2021, 25, 1461–1478. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Buraidah, M.H.; Madhavan, J.; Arof, A.K. Effect of tetrabutylammonium iodide content on PVDF-PMMA polymer blend electrolytes for dye-sensitized solar cells. Ionics 2015, 21, 2889–2896. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Ekanayake, E.M.B.S.; Bandara, L.R.A.K.; Seneviratne, V.A.; Thotawatthage, C.A.; Jayaratne, S.L.; Senadeera, G.K.R. Efficiency enhancement by mixed cation effect in polyethylene oxide (PEO)-based dye-sensitized solar cells. J. Solid State Electrochem. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Ebnalwaled, A.A.; Ismaiel, A.M. Developing novel UV shielding films based on PVA/Gelatin/0.01CuO nanocomposite: On the properties optimization using c-irradiation. Measurement 2019, 134, 89–100. [Google Scholar] [CrossRef]
- Devadiga, D.; Tn, A. Cyanopyridone doped PMMA films as UV and blue light filters: Preparation and characterization. Optik 2021, 229, 166233. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Wang, H.; Tong, J.; Zhang, J.; Wang, X.; Zeng, Y. High-efficiency ultraviolet shielding and high transparency of Ti3C2Tx MXene/poly(vinyl alcohol) nanocomposite films. Compos. Commun. 2022, 33, 101235. [Google Scholar] [CrossRef]
- Posoknistakul, P.; Tangkrakul, C.; Chaosuanphae, P.; Deepentham, S.; Techasawong, W.; Phonphirunrot, N.; Bairak, S.; Sakdaronnarong, C.; Laosiripojana, N. Fabrication and Characterization of Lignin Particles and Their Ultraviolet Protection Ability in PVA Composite Film. ACS Omega 2020, 5, 20976–20982. [Google Scholar] [CrossRef] [PubMed]
- Alsaada, A.M.; Al-Bataineha, Q.M.; Ahmad, A.A.; Jum’h, I.; Alaqtash, N.; Bani-Salameh, A.A. Optical properties of transparent PMMA-PS/ZnO NPs polymeric nanocomposite films: UV-Shielding applications. Mater. Res. Express 2019, 6, 126446. [Google Scholar] [CrossRef]
- Zidan, H.M.; Abdelrazek, E.M.; Abdelghany, A.M.; Tarabiah, A.E. Characterization and some physical studies of PVA/PVP filled with MWCNTs. J. Mater. Res. Technol. 2019, 8, 904–913. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Helberg, R.M.L.; Dai, Z.; Ansaloni, L.; Deng, L. PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: Synergistic enhancement of CO2 separation performance. Green Energy Environ. 2020, 5, 59–68. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.; Kenawy, E.R. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Ghanem, S. Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. J. Polym. Res. 2009, 16, 1–10. [Google Scholar] [CrossRef]
- Deepa, M.; Sharma, N.; Varshney, P.; Chandra, S.; Agnihotry, S.A. Ion Conducting Materials: Theory and Applications; Kulkarni, A.R., Gopalan, P., Eds.; Narosa Publishing Company: New Delhi, India, 2001. [Google Scholar]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Aldwayyan, A.S. Solvent Effects on the Structural and Optical Properties of MAPbI3 Perovskite Thin Film for Photovoltaic Active Layer. Coatings 2022, 12, 549. [Google Scholar] [CrossRef]
- El-Naggar, A.; Heiba, Z.K.; Kamal, A.; Altowairqi, Y.; Mohamed, M.B. Impact of loading PVA/CMC/PVP blend with CdS0.9M0.1 non-stoichiometrically doped by transition metals (M). Opt. Mater. 2022, 133, 113085. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Altowairqi, Y.; Mohamed, M.B. Enhancing the linear and nonlinear optical properties by ZnS/V-doped polyvinyl alcohol/carboxymethyl cellulose/polyethylene glycol polymeric nanocomposites for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2022, 33, 25127–25138. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, A.K.; Kailath, A.; Mishra, T.; Guha, A.; Nayar, S.; Sinha, A. Composition dependent structural modulations in transparent poly(vinyl alcohol) hydrogels. Colloids Surf. B Biointerfaces 2009, 74, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray Diffraction Analysis of Poly(vinyl alcohol) Hydrogels, Obtained by Freezing and Thawing Techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Li, X.-G.; Kresse, I.; Springer, J.; Nissen, J.; Yang, Y.-L. Morphology and gas permselectivity of blend membranes of polyvinylpyridine with ethylcellulose. Polymer 2001, 42, 6859–6869. [Google Scholar] [CrossRef]
- Maheshwari, S.U.; Govindan, K.; Raja, M.; Raja, A.; Pravin, M.B.S.; Kumar, S.V. Synthesis and Characterization of Calcium Phosphate Ceramic/(Poly(vinyl alcohol)–Polycaprolactone) Bilayer Nanocomposites-A Bone Tissue Regeneration Scaffold. Biomed. Mater. Eng. 2017, 28, 401. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, X.J.; Chen, S.J.; Ma, W.Z.; Zhang, J.; Wang, X.L. Effect of solution-blended poly(styrene-co-acrylonitrile) copolymer on crystallization of poly (vinylidene fluoride). Express Polym. Lett. 2010, 4, 284. [Google Scholar] [CrossRef]
- Farah, A.M.; Thema, F.T.; Dikio, E.D. Electrochemical Detection of Hydrogen Peroxide Based on Graphene Oxide/Prussian Blue Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2012, 7, 5069–5083. [Google Scholar]
- Abdelrazek, E.; Elashmawi, I.; El-Khodary, A.; Yassin, A. Structural, optical, thermal and electrical studies on PVA/PVP blends filled with lithium bromide. Curr. Appl. Phys. 2010, 10, 607–613. [Google Scholar] [CrossRef]
- Nasalapure, V.K.A.; Chalannavar, R.K.; Gani, R.S.; Kasai, D.R. Preparation and characterization of polyvinyl alcohol and carboxy methyl cellulose hydrogel film for biomedical application. AIP Conf. Proc. 2020, 2244, 080001. [Google Scholar] [CrossRef]
- Kumar, G.H.; Rao, J.L.; Gopal, N.; Narasimhulu, K.; Chakradhar, R.; Rajulu, A.V. Spectroscopic investigations of Mn2+ ions doped polyvinylalcohol films. Polymer 2004, 45, 5407–5415. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Abdel-Kader, M. Effect of annealed ZnS nanoparticles on the structural and optical properties of PVA polymer nanocomposite. Mater. Chem. Phys. 2020, 241, 122285. [Google Scholar] [CrossRef]
- Hassanien, A.; Akl, A.A. Optical characterizations and refractive index dispersion parameters of annealed TiO2 thin films synthesized by RF-sputtering technique at different flow rates of the reactive oxygen gas. Phys. B Condens. Matter 2020, 576, 411718. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Bouzidi, A.; Zahran, H.Y.; Jalalah, M.; Harraz, F.A.; Yahia, I.S. Ammonium iodide salt-doped polyvinyl alcohol polymeric electrolyte for UV-shielding filters: Synthesis, optical and dielectric characteristics. J. Mater. Sci. Mater. Electron. 2021, 32, 4416–4436. [Google Scholar] [CrossRef]
- Kincl, M.; Tichy, L. Thermally and optically induced irreversible changes in some Ge–As–S amorphous thin films. Chem. Phys. 2008, 110, 322. [Google Scholar] [CrossRef]
- Hassanien, A.S. Studies on dielectric properties, opto-electrical parameters and electronic polarizability of thermally evaporated amorphous Cd50S50−xSex thin films. J. Alloys Compd. 2016, 671, 566. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, D.W.; Prakash, J.; Sun, Y.K. Electrochemical stability and conductivity enhancement of composite polymer electrolytes. Solid State Ion. 2003, 159, 111. [Google Scholar] [CrossRef]
- Pan, S.; Yuan, J.; Zhang, P.; Sokoluk, M.; Yao, G.; Li, X. Effect of electron concentration on electrical conductivity in in situ Al-TiB2 nanocomposites. Appl. Phys. Lett. 2020, 116, 014102. [Google Scholar] [CrossRef]
- Pan, S.; Wang, T.; Jin, K.; Cai, X. Understanding and designing metal matrix nanocomposites with high electrical conductivity: A review. J. Mater. Sci. 2022, 57, 6487–6523. [Google Scholar] [CrossRef]
- Makled, M.; Sheha, E.; Shanap, T.; El-Mansy, M. Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 2013, 4, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Wadatkar, N.; Waghuley, S. Characterizing the electro-optical properties of polyaniline/poly(vinyl acetate) composite films as-synthesized through chemical route. Results Surf. Interfaces 2021, 4, 100016. [Google Scholar] [CrossRef]


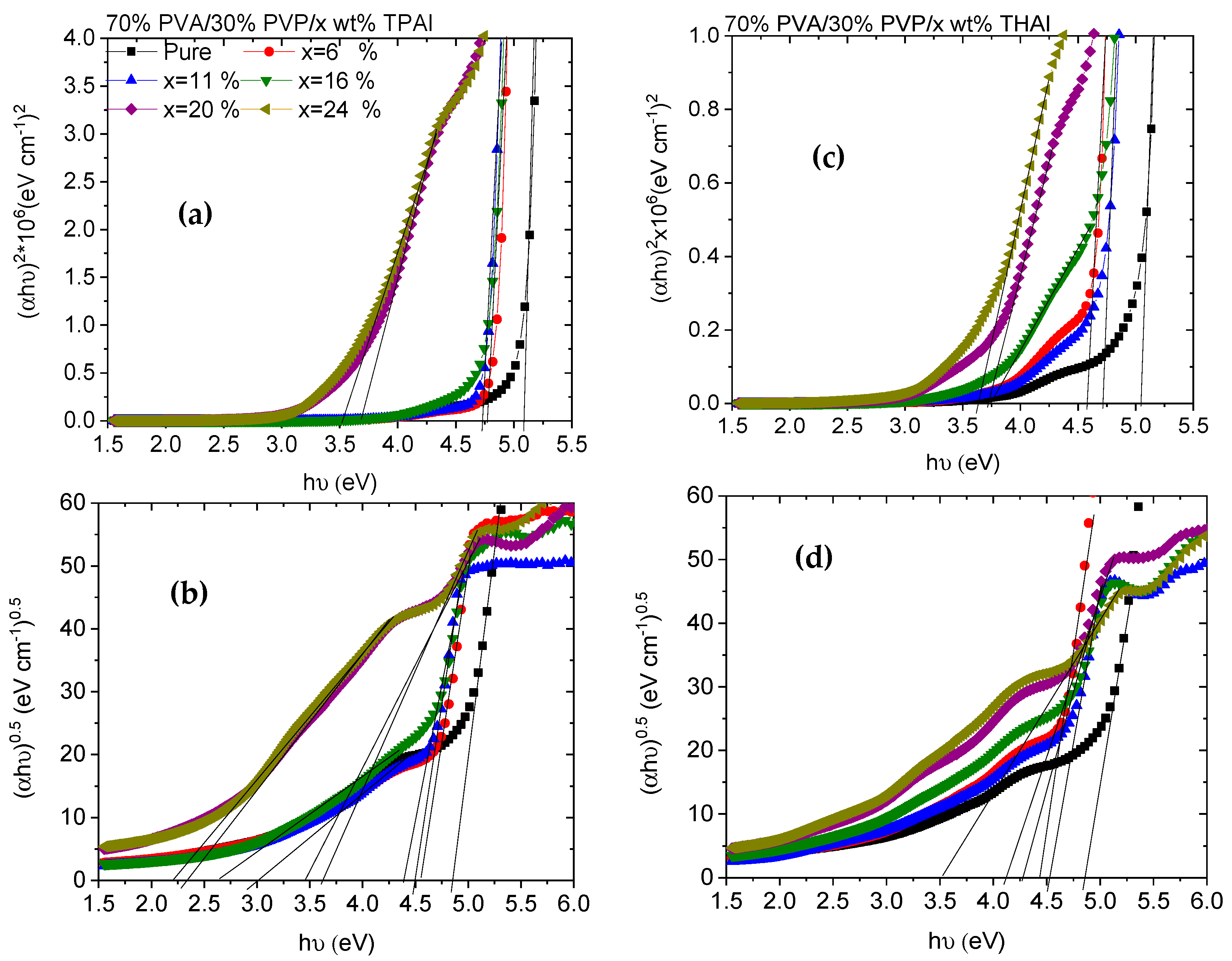


| Eg (eV) | |||||
|---|---|---|---|---|---|
| wt % TPAI | Direct | Indirect | wt % THAI | Direct | Indirect |
| Pure | 5.1 | 4.86 | Pure | 5.03 | 4.86 |
| x = 6% | 4.78 | 4.56 | x = 6% | 4.72 | 4.53 |
| x = 11% | 4.74 | 4.49, 3.01 | x = 11% | 4.59 | 4.44 |
| x = 16% | 4.72 | 4.39, 2.62 | x = 16% | 3.77 | 4.29 |
| x = 20% | 3.68 | 3.62, 2.32 | x = 20% | 3.73 | 4.12 |
| x = 24% | 3.52 | 3.45, 2.21 | x = 24% | 3.63 | 3.51 |
| wt % TPAI | Ea1 (eV) | Ea2 (eV) | wt % THAI | Ea1 (eV) | Ea2 (eV) |
|---|---|---|---|---|---|
| Pure | 0.32 | 0.12 | Pure | 0.30 | 0.59 |
| x = 6% | --- | 0.92 | x = 6% | 0.13 | 0.86 |
| x = 11% | --- | 0.58 | x = 11% | --- | 1.13 |
| x = 16% | 0.27 | 0.88 | x = 16% | --- | 0.72 |
| x = 20% | 0.74 | 1.09 | x = 20% | 1.46 | 0.83 |
| x = 24% | 1.05 | 1.05 | x = 24% | 0.22 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, A.M.; Brnawi, S.Z.; Kamal, A.M.; Albassam, A.A.; Heiba, Z.K.; Mohamed, M.B. Structural, Optical, and Electrical Parameters of Doped PVA/PVP Blend with TPAI or THAI Salt. Polymers 2023, 15, 2661. https://doi.org/10.3390/polym15122661
El-Naggar AM, Brnawi SZ, Kamal AM, Albassam AA, Heiba ZK, Mohamed MB. Structural, Optical, and Electrical Parameters of Doped PVA/PVP Blend with TPAI or THAI Salt. Polymers. 2023; 15(12):2661. https://doi.org/10.3390/polym15122661
Chicago/Turabian StyleEl-Naggar, A. M., Shadia Z. Brnawi, A. M. Kamal, A. A. Albassam, Zein K. Heiba, and Mohamed Bakr Mohamed. 2023. "Structural, Optical, and Electrical Parameters of Doped PVA/PVP Blend with TPAI or THAI Salt" Polymers 15, no. 12: 2661. https://doi.org/10.3390/polym15122661
APA StyleEl-Naggar, A. M., Brnawi, S. Z., Kamal, A. M., Albassam, A. A., Heiba, Z. K., & Mohamed, M. B. (2023). Structural, Optical, and Electrical Parameters of Doped PVA/PVP Blend with TPAI or THAI Salt. Polymers, 15(12), 2661. https://doi.org/10.3390/polym15122661






