Efficient and Green Isolation of Keratin from Poultry Feathers by Subcritical Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Subcritical Water Extraction
2.3. SDS-PAGE Electrophoresis
2.4. Analysis of Amino Acids
2.5. FTIR-Analysis
2.6. Elemental Analysis
2.7. Particle Size and Zeta Potential Measurements
2.8. Cytotoxicity Assay
3. Results and Discussion
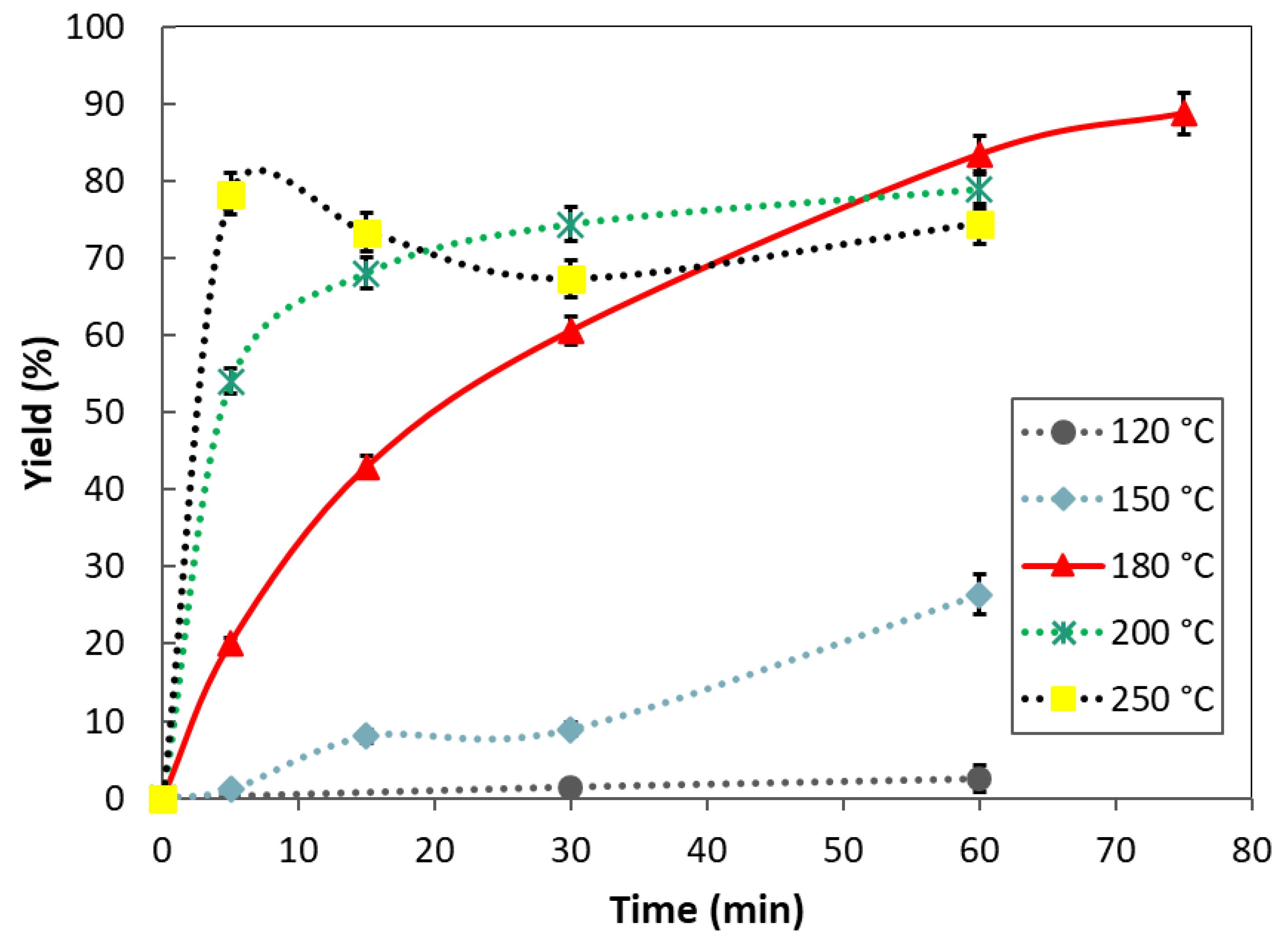
3.1. Yield of Extraction
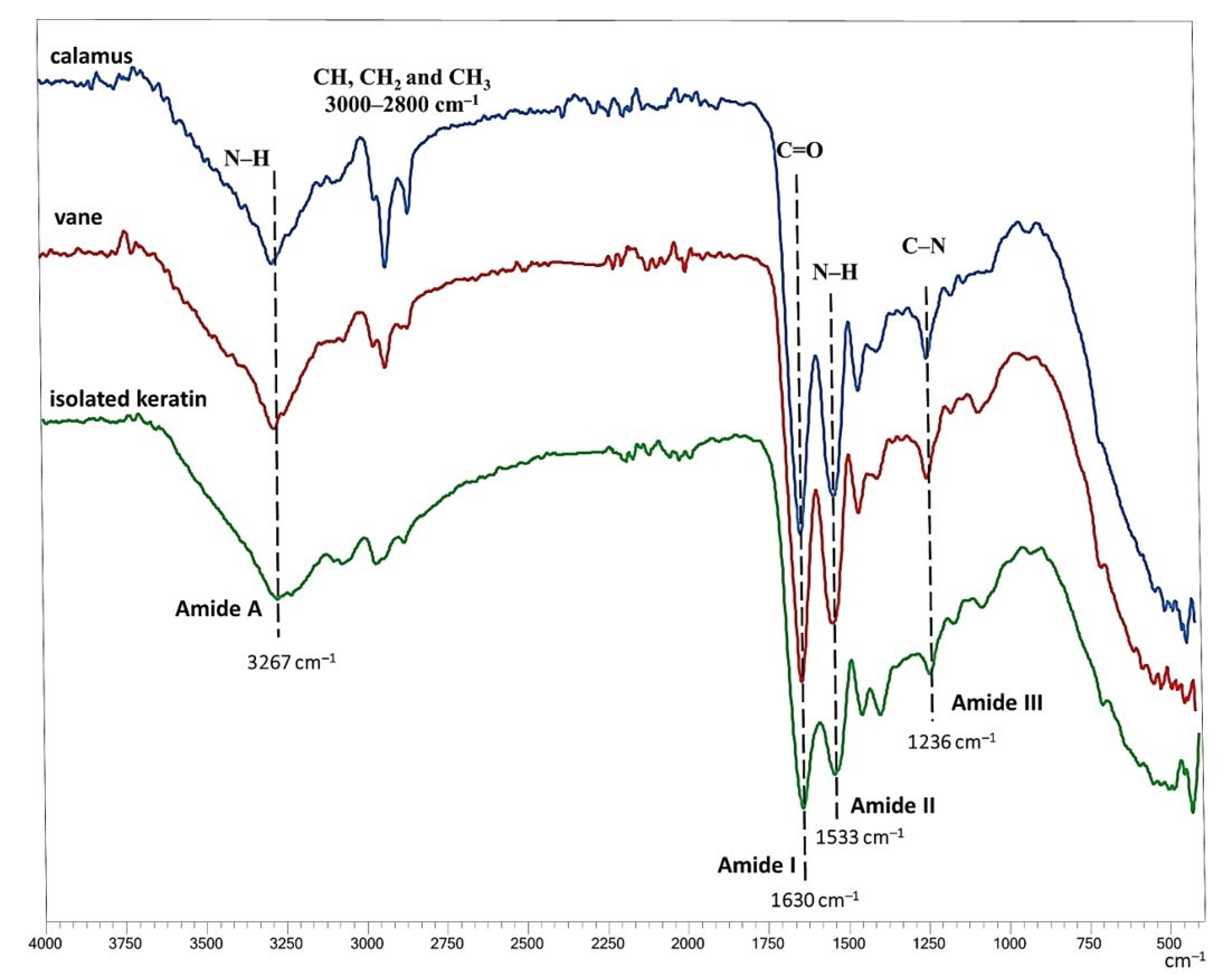
3.2. FTIR Analysis of Feathers and Hydrolysate
3.3. CHNS Elemental Analysis of Feathers and Hydrolysate
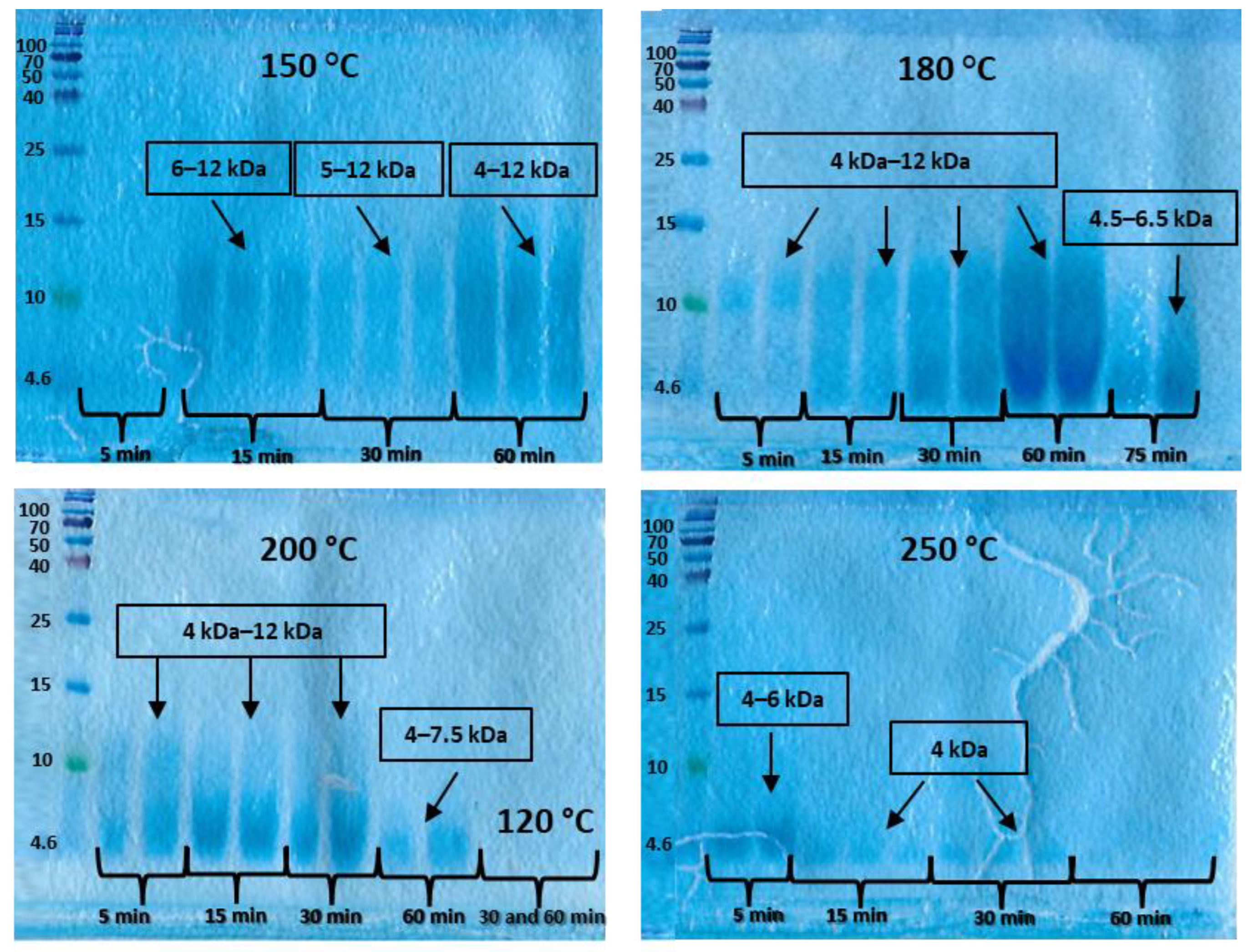
3.4. Molecular Weight of Hydrolysate
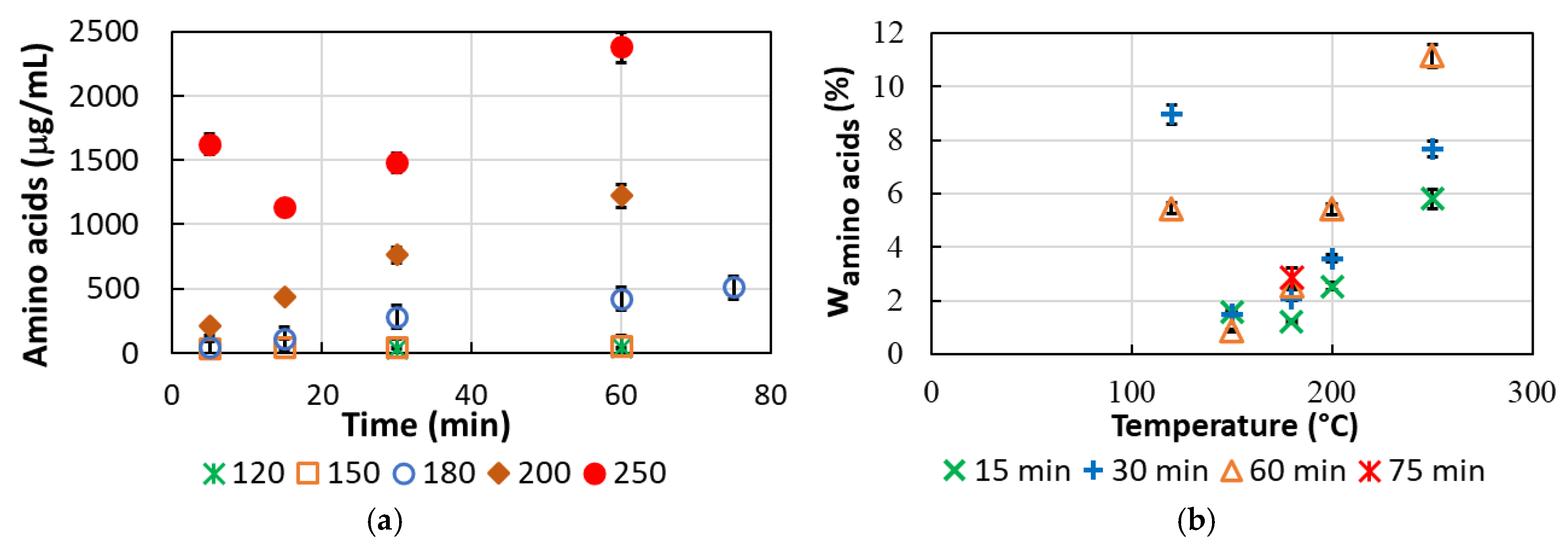
3.5. Content of Free Amino Acids in the Hydrolysate
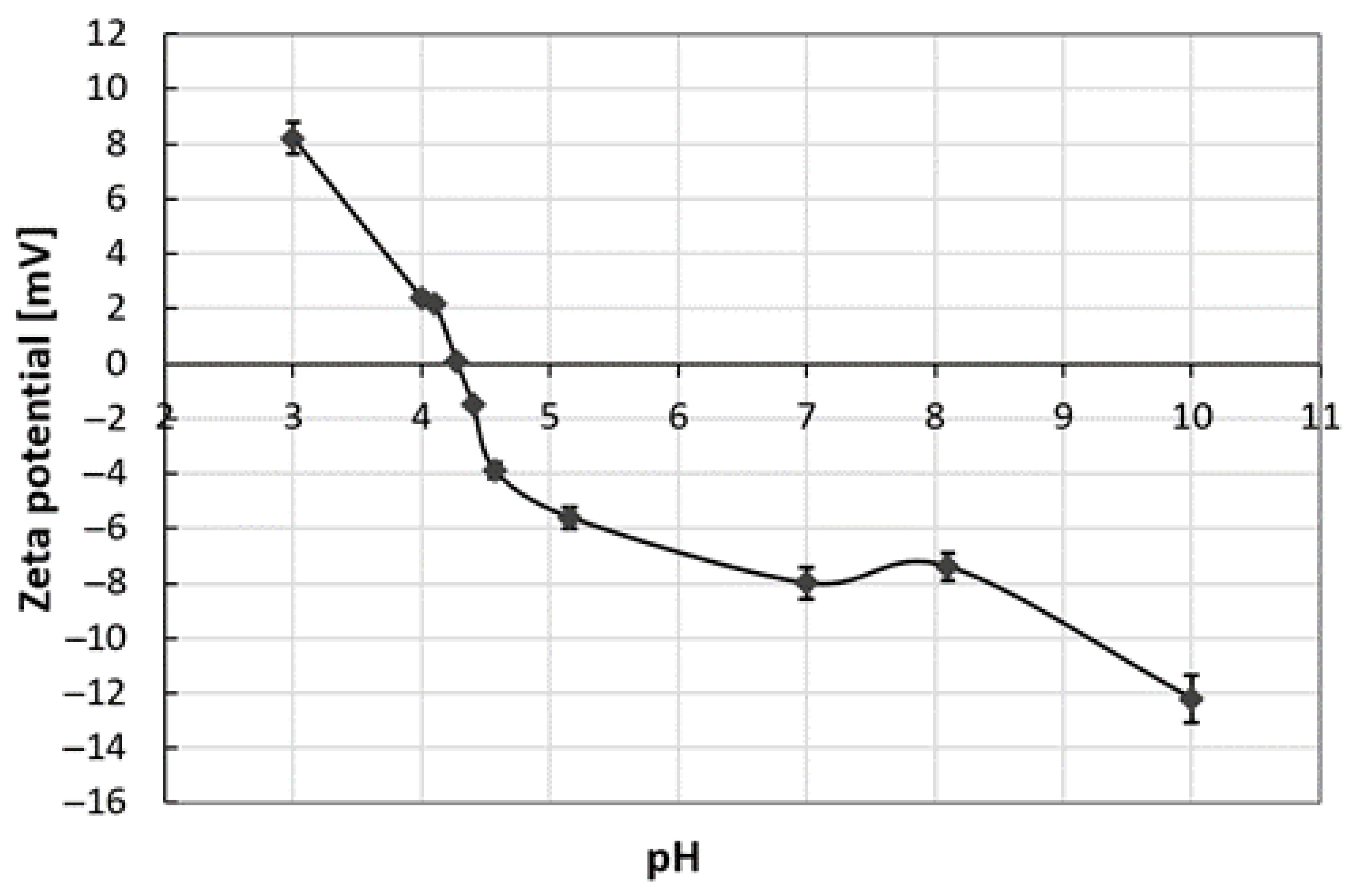
3.6. Particle Size and Zeta Potential of Hydrolysate
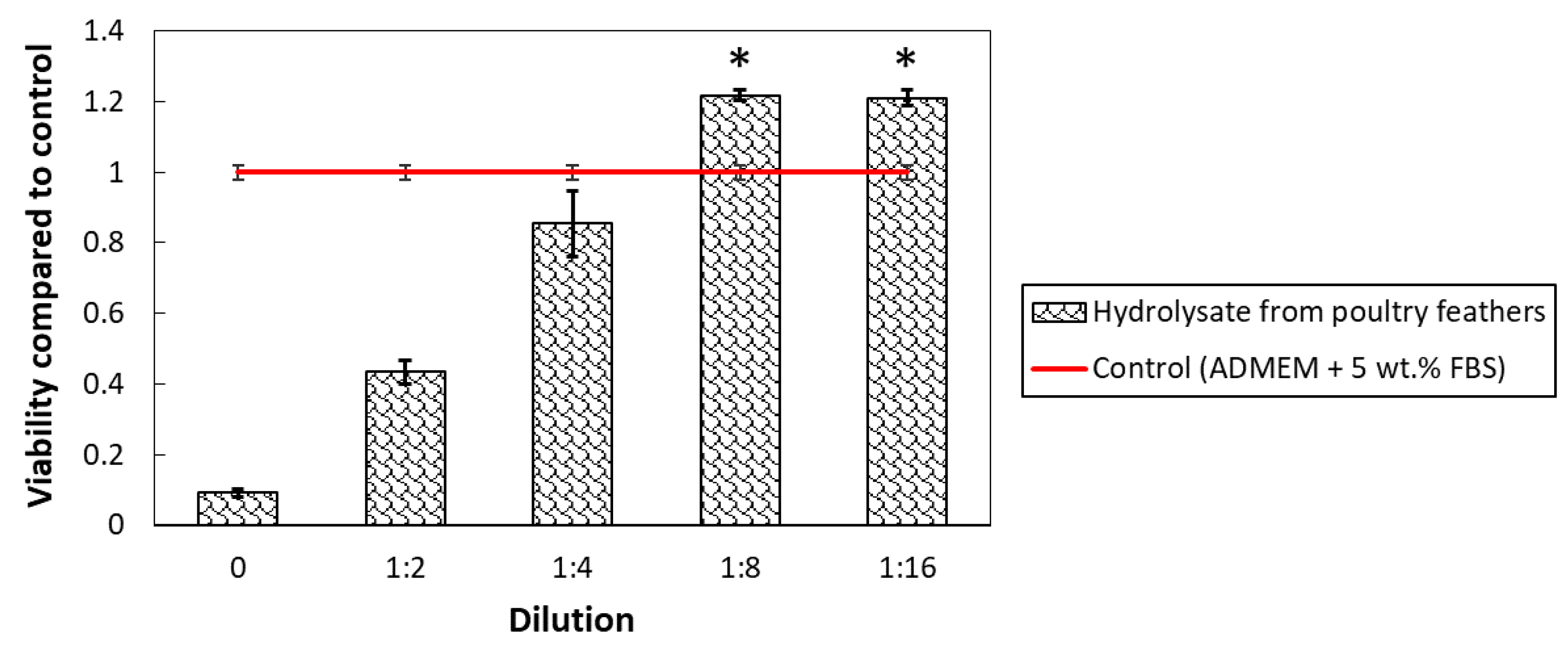
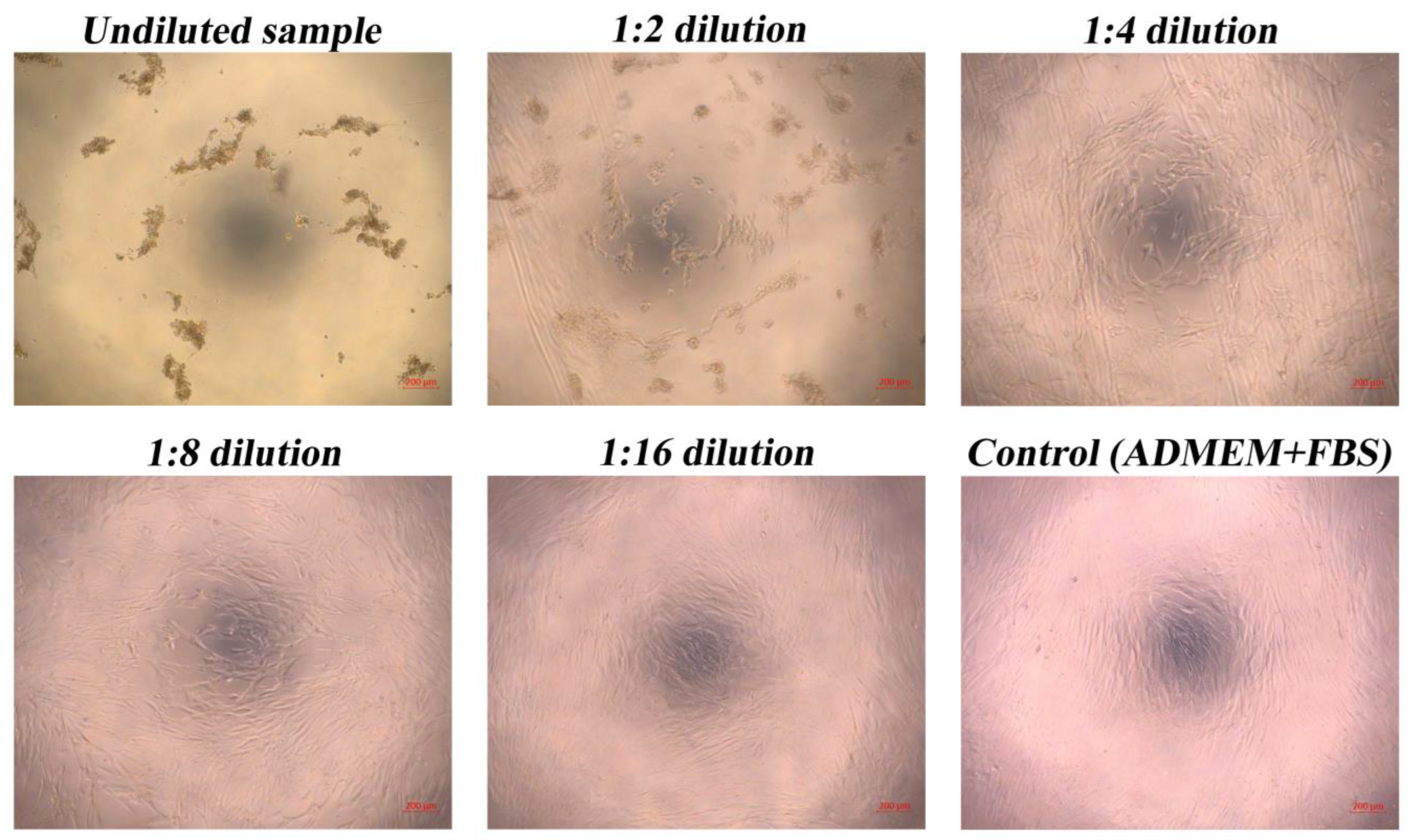
3.7. Biocompatibility of Hydrolysate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond Plucking: Feathers Bioprocessing into Valuable Protein Hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and Application of Keratin from Natural Resources: A Review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.K.; Mija, A. Keratin Associations with Synthetic, Biosynthetic and Natural Polymers: An Extensive Review. Polymers 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin—Based Materials for Biomedical Applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Progress in Microbial Degradation of Feather Waste. Front. Microbiol. 2019, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, A. Keratin as a Protein Biopolymer: Extraction from Waste Biomass and Applications; Sharma, S., Kumar, A., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-02900-5. [Google Scholar]
- Staroń, P.; Banach, M.; Kowalski, Z. Keratin-Origins, Properties, Application. Chemik 2011, 65, 1019–1026. [Google Scholar]
- Rajabi, M.; Ali, M.; McConnell, M.; Cabral, J. Keratinous Materials: Structures and Functions in Biomedical Applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D. Valorisation of Chicken Feathers: A Review on Recycling and Recovery Route—Current Status and Future Prospects. Clean Technol. Environ. Policy 2017, 19, 2363–2378. [Google Scholar] [CrossRef]
- Chojnacka, K.; Górecka, H.; Michalak, I.; Górecki, H. A Review: Valorization of Keratinous Materials. Waste Biomass Valorization 2011, 2, 317–321. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of Chicken By-Products as Sources of Useful Biological Resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A. Sustainable Management of Keratin Waste Biomass: Applications and Future Perspectives. Braz. Arch. Biol. Technol. 2016, 59, 1–14. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Chowdhury, S.; Sanpui, P. Extraction of Keratin from Keratinous Wastes: Current Status and Future Directions. J. Mater. Cycles Waste Manag. 2023, 25, 1–16. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, X.; Zhu, C.; Qian, J.; Zhu, N.; Zhao, L.; Chen, J. Hydrolysis Technology of Biomass Waste to Produce Amino Acids in Sub-Critical Water. Bioresour. Technol. 2008, 99, 3337–3341. [Google Scholar] [CrossRef] [PubMed]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of Protein and Amino Acids from Deoiled Rice Bran by Subcritical Water Hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Xiao, Z.; Zhou, R.; Feng, N.; Niu, Y. A Review of Amino Acids Extraction from Animal Waste Biomass and Reducing Sugars Extraction from Plant Waste Biomass by a Clean Method. Biomass Convers. Biorefinery 2015, 5, 309–320. [Google Scholar] [CrossRef]
- Zhu, G.-Y.; Zhu, X.; Wan, X.-L.; Fan, Q.; Ma, Y.-H.; Qian, J.; Liu, X.-L.; Shen, Y.-J.; Jiang, J.-H. Hydrolysis Technology and Kinetics of Poultry Waste to Produce Amino Acids in Subcritical Water. J. Anal. Appl. Pyrolysis 2010, 88, 187–191. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of Slaughterhouse Waste in Value-Added Applications: Recent Advances in the Development of Wood Adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef]
- Bhavsar, P.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Comparative Study on the Effects of Superheated Water and High Temperature Alkaline Hydrolysis on Wool Keratin. Text. Res. J. 2017, 87, 1696–1705. [Google Scholar] [CrossRef]
- Di Domenico Ziero, H.; Ampese, L.C.; Sganzerla, W.G.; Torres-Mayanga, P.C.; Timko, M.T.; Mussatto, S.I.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Poultry Feathers for Amino Acids Production. J. Supercrit. Fluids 2022, 181, 105492. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Suherman, C.; Maxiselly, Y.; Akbar, M.A.; Purwoko, B.A.; Prawisudha, P.; Yoshikawa, K. Liquid Feather Protein Hydrolysate as a Potential Fertilizer to Increase Growth and Yield of Patchouli (Pogostemon Cablin Benth) and Mung Bean (Vigna Radiata). Int. J. Recycl. Org. Waste Agric. 2019, 8, 221–232. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Nakhshiniev, B.; Zaini, I.N.; Saidov, N.; Takahashi, F.; Yoshikawa, K. Characterization of Potential Liquid Fertilizers Obtained by Hydrothermal Treatment of Chicken Feathers. Environ. Prog. Sustain. Energy 2018, 37, 375–382. [Google Scholar] [CrossRef]
- Yin, J.; Rastogi, S.; Terry, A.E.; Popescu, C. Self-Organization of Oligopeptides Obtained on Dissolution of Feather Keratins in Superheated Water. Biomacromolecules 2007, 8, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, K. A Novel Thermal Hydrolysis Process for Extraction of Keratin from Hog Hair for Commercial Applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef]
- Verdnik, A.; Čolnik, M.; Knez, Ž.; Škerget, M. Isolation of Keratin from Waste Wool Using Hydrothermal Processes. Acta Chim. Slov. 2021, 68, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Strnad, S.; Kreže, T. Poultry Feather Wastes—Optimization of Cleaning Procedure for New Products Development. In Proceedings of the International Conference on Innovative Technologies, Bratislava, Slovakia, 1 September 2011; pp. 532–535. [Google Scholar]
- Badawy, A.A.-B.; Morgan, C.J.; Turner, J.A. Application of the Phenomenex EZ: FaastTM Amino Acid Analysis Kit for Rapid Gas-Chromatographic Determination of Concentrations of Plasma Tryptophan and Its Brain Uptake Competitors. Amino Acids 2008, 34, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Reakasame, S.; Dranseikiene, D.; Schrüfer, S.; Zheng, K.; Schubert, D.W.; Boccaccini, A.R. Development of Alginate Dialdehyde-Gelatin Based Bioink with Methylcellulose for Improving Printability. Mater. Sci. Eng. C 2021, 128, 112336. [Google Scholar] [CrossRef]
- Rajabinejad, H.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Physicochemical Properties of Keratin Extracted from Wool by Various Methods. Text. Res. J. 2018, 88, 2415–2424. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta BBA—Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Bower, D.I.; Maddams, W.F. The Vibrational Spectroscopy of Polymers; Cambridge University Press: Cambridge, UK, 1992; ISBN 978-0-521-42195-9. [Google Scholar]
- Shavandi, A.; Bekhit, A.E.-D.A.; Carne, A.; Bekhit, A. Evaluation of Keratin Extraction from Wool by Chemical Methods for Bio-Polymer Application. J. Bioact. Compat. Polym. 2017, 32, 163–177. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Hernández-Montoya, V.; Montes-Morán, M.A.; Reynel-Avila, H.E. Batch and Column Studies of Zn2+ Removal from Aqueous Solution Using Chicken Feathers as Sorbents. Chem. Eng. J. 2011, 167, 67–76. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Z.-T.; Liu, Z.-W. Chemically Modified Chicken Feather as Sorbent for Removing Toxic Chromium (VI) Ions. Ind. Eng. Chem. Res. 2009, 48, 6882–6889. [Google Scholar] [CrossRef]
- Tuna, A.; Okumuş, Y.; Çelebi, H.; Seyhan, A.T. Thermochemical Conversion of Poultry Chicken Feather Fibers of Different Colors into Microporous Fibers. J. Anal. Appl. Pyrolysis 2015, 115, 112–124. [Google Scholar] [CrossRef]
- Horwitz, W. AOAC (2000) Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Schrooyen, P.M.M.; Dijkstra, P.J.; Oberthür, R.C.; Bantjes, A.; Feijen, J. Partially Carboxymethylated Feather Keratins. 1. Properties in Aqueous Systems. J. Agric. Food Chem. 2000, 48, 4326–4334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, R.; Zhao, W. Improving Digestibility of Feather Meal by Steam Flash Explosion. J. Agric. Food Chem. 2014, 62, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Klingler, D.; Berg, J.; Vogel, H. Hydrothermal Reactions of Alanine and Glycine in Sub- and Supercritical Water. J. Supercrit. Fluids 2007, 43, 112–119. [Google Scholar] [CrossRef]
- Sato, N.; Quitain, A.T.; Kang, K.; Daimon, H.; Fujie, K. Reaction Kinetics of Amino Acid Decomposition in High-Temperature and High-Pressure Water. Ind. Eng. Chem. Res. 2004, 43, 3217–3222. [Google Scholar] [CrossRef]
- John, A.; Črešnar, K.P.; Bikiaris, D.N.; Zemljič, L.F. Colloidal Solutions as Advanced Coatings for Active Packaging Development: Focus on PLA Systems. Polymers 2023, 15, 273. [Google Scholar] [CrossRef]
- Jaiswar, G.; Modak, S.; Singh, R.; Dabas, N. 8—Functionalization of Biopolymer Keratin-Based Biomaterials and Their Absorption Properties for Healthcare Application. In Polymeric Biomaterials for Healthcare Applications; Varaprasad, K., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2022; pp. 257–270. ISBN 978-0-323-85233-3. [Google Scholar]
- Horinek, D. DLVO Theory. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 343–346. ISBN 978-1-4419-6996-5. [Google Scholar]
- Shanmugasundaram, O.L.; Syed Zameer Ahmed, K.; Sujatha, K.; Ponnmurugan, P.; Srivastava, A.; Ramesh, R.; Sukumar, R.; Elanithi, K. Fabrication and Characterization of Chicken Feather Keratin/Polysaccharides Blended Polymer Coated Nonwoven Dressing Materials for Wound Healing Applications. Mater. Sci. Eng. C 2018, 92, 26–33. [Google Scholar] [CrossRef]

| pH | Particle Size-Average Hydrodynamic Diameter [nm] |
|---|---|
| 3 | 5164 |
| 4.4 | 6720 |
| 7 | 7644 |
| 10 | 2227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škerget, M.; Čolnik, M.; Zemljič, L.F.; Gradišnik, L.; Semren, T.Ž.; Lovaković, B.T.; Maver, U. Efficient and Green Isolation of Keratin from Poultry Feathers by Subcritical Water. Polymers 2023, 15, 2658. https://doi.org/10.3390/polym15122658
Škerget M, Čolnik M, Zemljič LF, Gradišnik L, Semren TŽ, Lovaković BT, Maver U. Efficient and Green Isolation of Keratin from Poultry Feathers by Subcritical Water. Polymers. 2023; 15(12):2658. https://doi.org/10.3390/polym15122658
Chicago/Turabian StyleŠkerget, Mojca, Maja Čolnik, Lidija Fras Zemljič, Lidija Gradišnik, Tanja Živković Semren, Blanka Tariba Lovaković, and Uroš Maver. 2023. "Efficient and Green Isolation of Keratin from Poultry Feathers by Subcritical Water" Polymers 15, no. 12: 2658. https://doi.org/10.3390/polym15122658
APA StyleŠkerget, M., Čolnik, M., Zemljič, L. F., Gradišnik, L., Semren, T. Ž., Lovaković, B. T., & Maver, U. (2023). Efficient and Green Isolation of Keratin from Poultry Feathers by Subcritical Water. Polymers, 15(12), 2658. https://doi.org/10.3390/polym15122658











