Development of Light-Polymerized Dental Composite Resin Reinforced with Electrospun Polyamide Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. FTIR Analysis
2.3. Morphological Analyses
2.4. Mechanical Testing
2.5. Thermal Properties
3. Results and Discussion
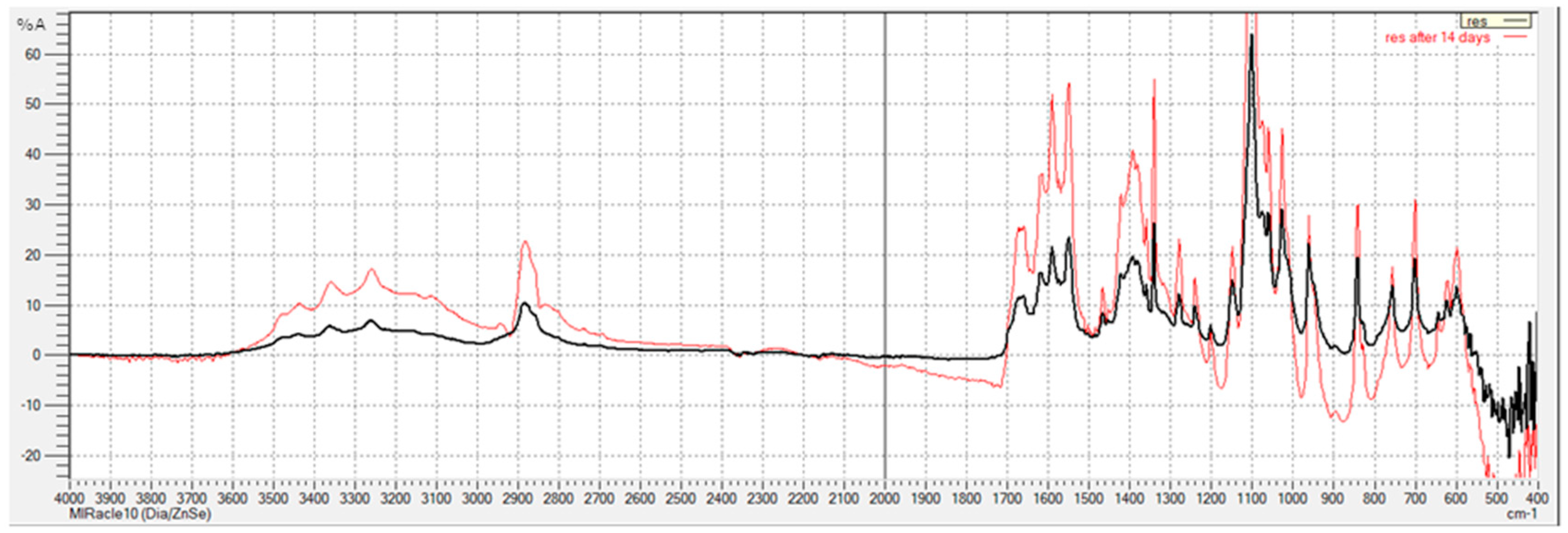
3.1. FTIR Analysis
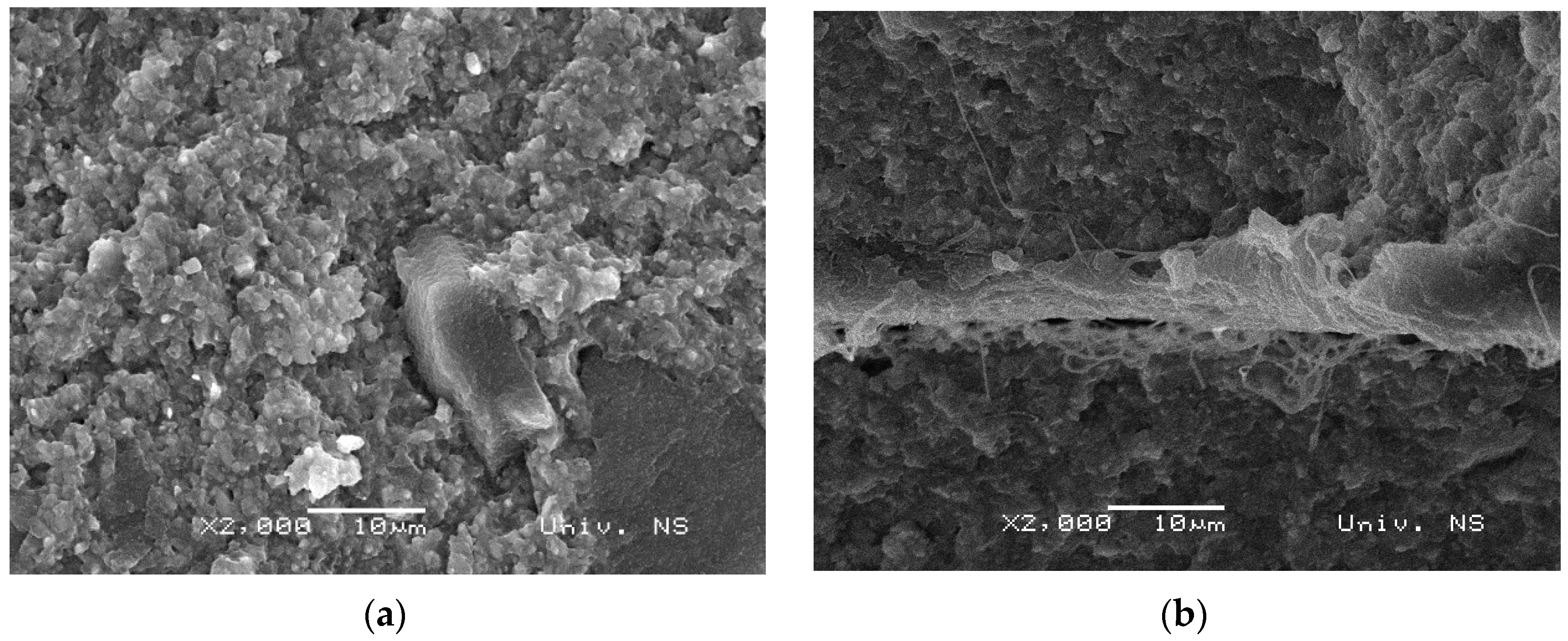
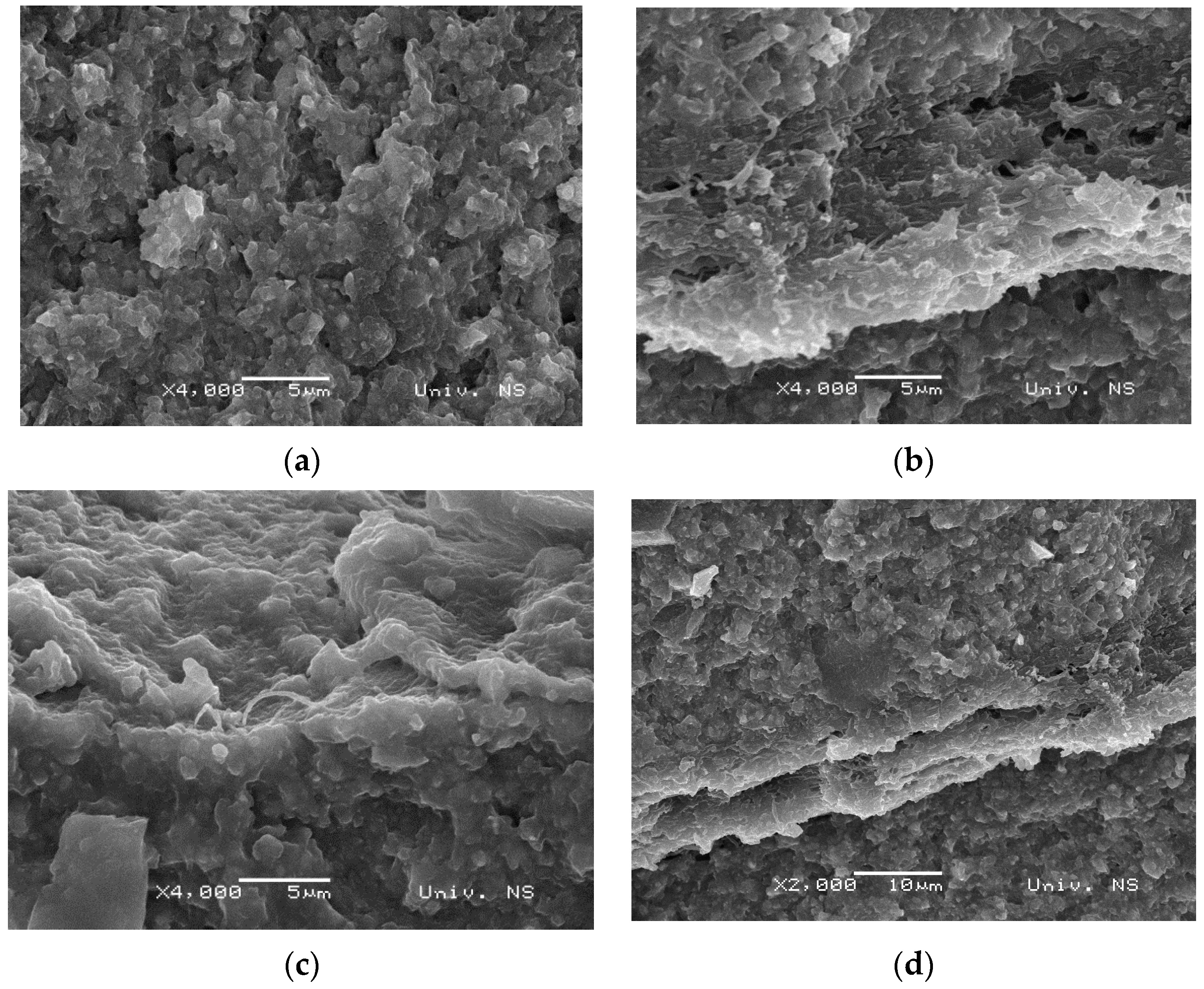
3.2. Morphology of Samples
3.3. Mechanical Properties
3.4. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzaki, N.; Yamaguchi, S.; Hirose, N.; Tanaka, R.; Takahashi, Y.; Imazato, S.; Hayashi, M. Evaluation of physical properties of fiber-reinforced composite resin. Dent. Mater. 2020, 36, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, M. Dental restorative composite materials: A review. J. Oral Biosci. 2019, 61, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Song, F.V.; Yang, B.; Di Tommaso, D.; Donnan, R.S.; Chass, G.A.; Yada, R.Y.; Farrar, D.H.; Tian, K.V. Resolving nanoscopic structuring and interfacial THz dynamics in setting cements. Mater. Adv. 2022, 3, 4982–4990. [Google Scholar] [CrossRef]
- Vidotti, H.A.; Manso, A.P.; Leung, V.; Valle, A.L.D.; Ko, F.; Carvalho, R.M. Flexural properties of experimental nanofiber reinforced composite are affected by resin composition and nanofiber/resin ratio. Dent. Mater. 2015, 31, 1132–1141. [Google Scholar] [CrossRef]
- Vallittu, P.K. An overview of development and status of fiber-reinforced composites as dental and medical biomaterials. Acta Biomater. Odontol. Scand. 2018, 4, 44–55. [Google Scholar] [CrossRef]
- Dodiuk-Kenig, H.; Lizenboim, K.; Roth, S.; Zalsman, B.; McHale, W.A.; Jaffe, M.; Griswold, K. Performance Enhancement of Dental Composites Using Electrospun Nanofibers. J. Nanomater. 2008, 2008, 840254. [Google Scholar] [CrossRef]
- Chen, M.-H. Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Y.; Liu, Y.; Liao, Y.; Xu, R.; Hedin, N.E.; Fong, H. Bis-GMA/TEGDMA dental composites reinforced with electrospun nylon 6 nanocomposite nanofibers containing highly aligned fibrillar silicate single crystals. Polymer 2007, 48, 2720–2728. [Google Scholar] [CrossRef]
- Fong, H. Electrospun nylon 6 nanofiber reinforced BIS-GMA/TEGDMA dental restorative composite resins. Polymer 2004, 45, 2427–2432. [Google Scholar] [CrossRef]
- Borges, A.L.; Münchow, E.A.; Souza, A.C.d.O.; Yoshida, T.; Vallittu, P.K.; Bottino, M.C. Effect of random/aligned nylon-6/MWCNT fibers on dental resin composite reinforcement. J. Mech. Behav. Biomed. Mater. 2015, 48, 134–144. [Google Scholar] [CrossRef]
- Szalewski, L.; Kamińska, A.; Wallner, E.; Batkowska, J.; Warda, T.; Wójcik, D.; Borowicz, J. Degradation of a Micro-Hybrid Dental Composite Reinforced with Polyaramide Fiber under the Influence of Cyclic Loads. Appl. Sci. 2020, 10, 7296. [Google Scholar] [CrossRef]
- Al-Jawoosh, S.; Ireland, A.; Su, B. Characterisation of mechanical and surface properties of novel biomimetic interpenetrating alumina-polycarbonate composite materials. Dent. Mater. 2020, 36, 1595–1607. [Google Scholar] [CrossRef]
- Nakanishi, L.; Kaizer, M.R.; Brandeburski, S.; Cava, S.S.; Della Bona, A.; Zhang, Y.; Moraes, R.R. Non-silicate nanoparticles for improved nanohybrid resin composites. Dent. Mater. 2020, 36, 1314–1321. [Google Scholar] [CrossRef]
- Marovic, D.; Tarle, Z.; Hiller, K.-A.; Mueller, R.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Reinforcement of experimental composite materials based on amorphous calcium phosphate with inert fillers. Dent. Mater. 2014, 30, 1052–1060. [Google Scholar] [CrossRef]
- Kumar, S.R.; Bhat, I.K.; Patnaik, A. Novel dental composite material reinforced with silane functionalized microsized gypsum filler particles. Polym. Compos. 2017, 38, 404–415. [Google Scholar] [CrossRef]
- Lassila, L.; Keulemans, F.; Vallittu, P.K.; Garoushi, S. Characterization of restorative short-fiber reinforced dental composites. Dent. Mater. J. 2020, 39, 992–999. [Google Scholar] [CrossRef]
- Sadr, A.; Bakhtiari, B.; Hayashi, J.; Luong, M.N.; Chen, Y.-W.; Chyz, G.; Chan, D.; Tagami, J. Effects of fiber reinforcement on adaptation and bond strength of a bulk-fill composite in deep preparations. Dent. Mater. 2020, 36, 527–534. [Google Scholar] [CrossRef]
- Tanaka, C.B.; Lopes, D.P.; Kikuchi, L.N.; Moreira, M.S.; Catalani, L.H.; Braga, R.R.; Kruzic, J.J.; Gonçalves, F. Development of novel dental restorative composites with dibasic calcium phosphate loaded chitosan fillers. Dent. Mater. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Guimarães, G.M.D.F.; Bronze-Uhle, E.S.; Lisboa-Filho, P.N.; Fugolin, A.P.P.; Borges, A.F.S.; Gonzaga, C.C.; Pfeifer, C.S.; Furuse, A.Y. Effect of the addition of functionalized TiO2 nanotubes and nanoparticles on properties of experimental resin composites. Dent. Mater. 2020, 36, 1544–1556. [Google Scholar] [CrossRef]
- Bijelic-Donova, J.; Garoushi, S.; Vallittu, P.K.; Lassila, L.V. Mechanical properties, fracture resistance, and fatigue limits of short fiber reinforced dental composite resin. J. Prosthet. Dent. 2015, 115, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dafar, M.O.; Grol, M.W.; Canham, P.B.; Dixon, S.J.; Rizkalla, A.S. Reinforcement of flowable dental composites with titanium dioxide nanotubes. Dent. Mater. 2016, 32, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Sfondrini, M.F.; Massironi, S.; Pieraccini, G.; Scribante, A.; Vallittu, P.K.; Lassila, L.V.; Gandini, P. Flexural strengths of conventional and nanofilled fiber-reinforced composites: A three-point bending test. Dent. Traumatol. 2013, 30, 32–35. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, X.; Li, M.; Peng, X.; Wang, S.; Zhou, X.; Cheng, L. Development and status of resin composite as dental restorative materials. J. Appl. Polym. Sci. 2019, 136, 48180. [Google Scholar] [CrossRef]
- Mohammadzadehmoghadam, S.; Dong, Y.; Davies, I.J. Recent progress in electrospun nanofibers: Reinforcement effect and mechanical performance. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1171–1212. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Sun, G.; Wang, M.; Yu, J. Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 2013, 58, 1173–1243. [Google Scholar] [CrossRef]
- Peres, B.U.; Manso, A.P.; Carvalho, L.D.; Ko, F.; Troczynski, T.; Vidotti, H.A.; Carvalho, R.M. Experimental composites of polyacrilonitrile-electrospun nanofibers containing nanocrystal cellulose. Dent. Mater. 2019, 35, e286–e297. [Google Scholar] [CrossRef]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Jiang, Y.; Hu, J.; Peng, Q.; Lin, Y. Electrospun Fibers for Dental and Craniofacial Applications. Curr. Stem Cell Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef]
- Ristić, I.; Miletic, A.; Vukić, N.; Marinović-Cincović, M.; Smits, K.; Cakić, S.; Pilić, B. Characterization of electrospun poly(lactide) composites containing multiwalled carbon nanotubes. J. Thermoplast. Compos. Mater. 2021, 34, 695–706. [Google Scholar] [CrossRef]
- Ivanoska-Dacikj, A.; Makreski, P.; Geskovski, N.; Karbowniczek, J.; Stachewicz, U.; Novkovski, N.; Tanasić, J.; Ristić, I.; Bogoeva-Gaceva, G. Electrospun PEO/rGO Scaffolds: The Influence of the Concentration of rGO on Overall Properties and Cytotoxicity. Int. J. Mol. Sci. 2022, 23, 988. [Google Scholar] [CrossRef]
- Ferracane, J.; Hilton, T.; Stansbury, J.; Watts, D.; Silikas, N.; Ilie, N.; Heintze, S.; Cadenaro, M.; Hickel, R. Academy of Dental Materials guidance—Resin composites: Part II—Technique sensitivity (handling, polymerization, dimensional changes). Dent. Mater. 2017, 33, 1171–1191. [Google Scholar] [CrossRef]
- Neisiany, R.E.; Khorasani, S.N.; Naeimirad, M.; Lee, J.K.Y.; Ramakrishna, S. Improving Mechanical Properties of Carbon/Epoxy Composite by Incorporating Functionalized Electrospun Polyacrylonitrile Nanofibers. Macromol. Mater. Eng. 2017, 302, 1600551. [Google Scholar] [CrossRef]
- Jin, S.; Yeung, A.W.K.; Zhang, C.; Tsoi, J.K.-H. A Bibliometric Analysis of Electrospun Nanofibers for Dentistry. J. Funct. Biomater. 2022, 13, 90. [Google Scholar] [CrossRef]
- Karatepe, U.Y.; Ozdemir, T. Improving mechanical and antibacterial properties of PMMA via polyblend electrospinning with silk fibroin and polyethyleneimine towards dental applications. Bioact. Mater. 2020, 5, 510–515. [Google Scholar] [CrossRef]
- Maletin, A.; Ristic, I.; Veljovic, T.; Ramic, B.; Puskar, T.; Jeremic-Knezevic, M.; Koprivica, D.D.; Milekic, B.; Vukoje, K. Influence of Dimethacrylate Monomer on the Polymerization Efficacy of Resin-Based Dental Cements—FTIR Analysis. Polymers 2022, 14, 247. [Google Scholar] [CrossRef]
- Ramić, B.D.; Stojanac, I.L.; Drobac, M.R.; Kantardžić, I.R.; Maletin, A.Z.; Cvjetićanin, M.T.; Otašević, K.S.; Petrović, L.M. Application of Scanning Electron Microscopy in the observation of dentin-adhesive interface. Microsc. Res. Tech. 2020, 84, 602–607. [Google Scholar] [CrossRef]
- Nakano, L.; Lopes, G.; Firmino, A.; de Matos, J.; Tango, R.; Paes-Junior, T. Analysis of bond strength between a nylon reinforcement structure and dental resins. J. Clin. Exp. Dent. 2021, 13, e505–e510. [Google Scholar] [CrossRef]
- Velo, M.M.; Nascimento, T.R.; Scotti, C.K.; Bombonatti, J.F.; Furuse, A.Y.; Silva, V.D.; Simões, T.A.; Medeiros, E.S.; Blaker, J.J.; Silikas, N.; et al. Improved mechanical performance of self-adhesive resin cement filled with hybrid nanofibers-embedded with niobium pentoxide. Dent. Mater. 2019, 35, e272–e285. [Google Scholar] [CrossRef]




| DiaFil Constituent | Synonym | Content (%) |
|---|---|---|
| Urethane dimethacrylate | UDMA | 8−12 |
| Bisphenol A glycidyl methacrylate | Bis-GMA | 6−10 |
| Triethylene glycol dimethacrylate | TEGDMA | 1−4 |
| Barium alumino silicate | Barium aluminosilicate | 65−75 |
| Silicon dioxide | Silicon dioxide | 5−10 |
| Camphorquinone | 7,7-trimethyl-3-dione(+/−)-bicyclo[2.2.1]heptane-1(C amphorquinone) | 0.01−0.02 |
| Dibutylhydroxy toluene | 2,6-di-butyl-4-Methylphenol(BHT) | 0.001−0.002 |
| Iron oxide | Iron Oxide | 0.0001−0.01 |
| Sample | Number of Resin Layers | Thickness of PA Layer | Number of PA Layers |
|---|---|---|---|
| res | 1 | - | - |
| res-PA8-res * | 2 | 8 μm | 1 |
| res-PA16-res ** | 2 | 16 μm | 1 |
| res-PA8-res-PA8-res | 3 | 8 μm | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maletin, A.; Ristić, I.; Nešić, A.; Knežević, M.J.; Koprivica, D.Đ.; Cakić, S.; Ilić, D.; Milekić, B.; Puškar, T.; Pilić, B. Development of Light-Polymerized Dental Composite Resin Reinforced with Electrospun Polyamide Layers. Polymers 2023, 15, 2598. https://doi.org/10.3390/polym15122598
Maletin A, Ristić I, Nešić A, Knežević MJ, Koprivica DĐ, Cakić S, Ilić D, Milekić B, Puškar T, Pilić B. Development of Light-Polymerized Dental Composite Resin Reinforced with Electrospun Polyamide Layers. Polymers. 2023; 15(12):2598. https://doi.org/10.3390/polym15122598
Chicago/Turabian StyleMaletin, Aleksandra, Ivan Ristić, Aleksandra Nešić, Milica Jeremić Knežević, Daniela Đurović Koprivica, Suzana Cakić, Dušica Ilić, Bojana Milekić, Tatjana Puškar, and Branka Pilić. 2023. "Development of Light-Polymerized Dental Composite Resin Reinforced with Electrospun Polyamide Layers" Polymers 15, no. 12: 2598. https://doi.org/10.3390/polym15122598
APA StyleMaletin, A., Ristić, I., Nešić, A., Knežević, M. J., Koprivica, D. Đ., Cakić, S., Ilić, D., Milekić, B., Puškar, T., & Pilić, B. (2023). Development of Light-Polymerized Dental Composite Resin Reinforced with Electrospun Polyamide Layers. Polymers, 15(12), 2598. https://doi.org/10.3390/polym15122598









