Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria of the Articles
2.3. Inclusion and Exclusion Criteria
2.4. Quality Assessment
3. Results
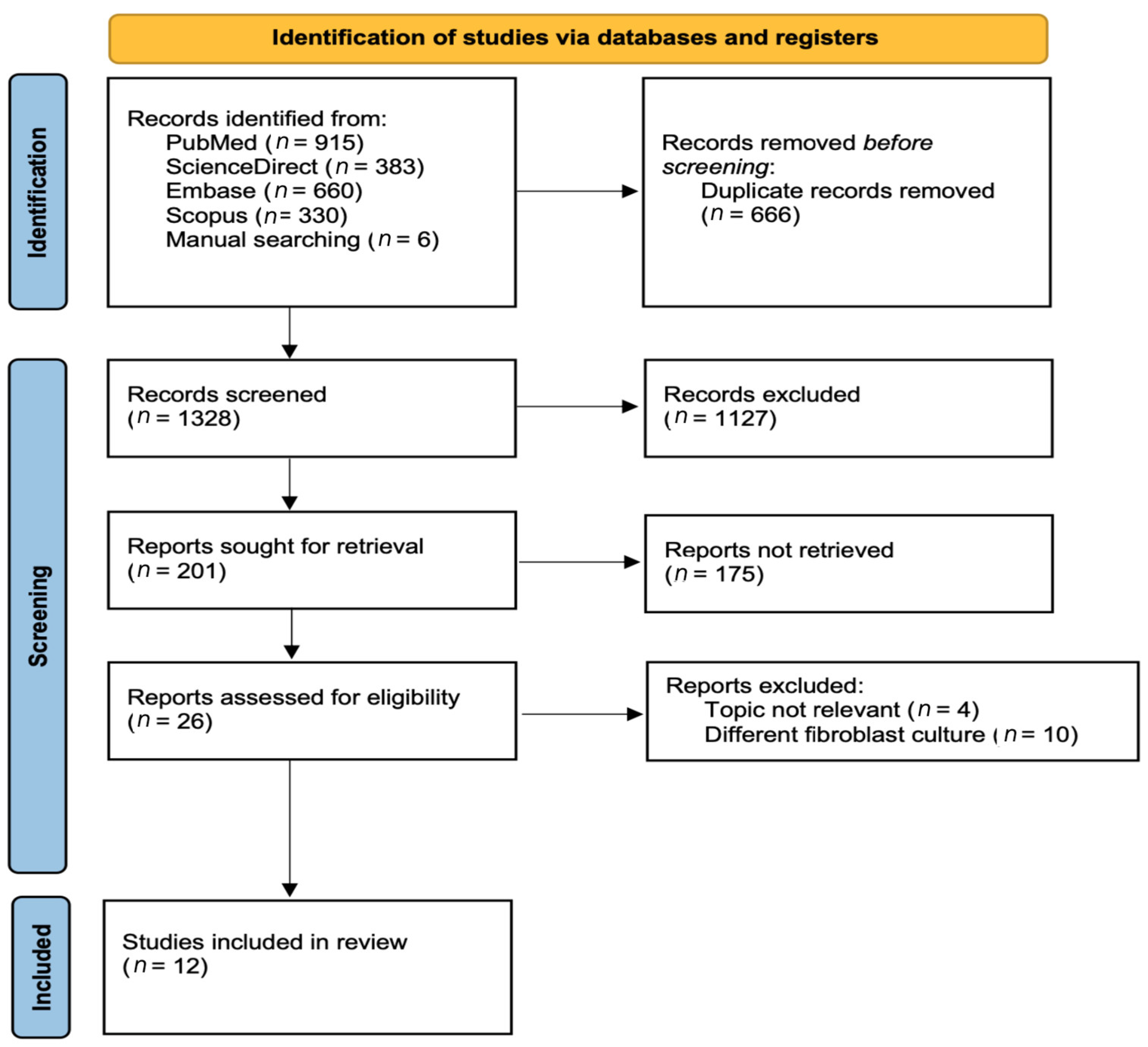
3.1. Selection of Studies
3.2. Characteristics of Studies
3.3. Result of Studies Included
3.3.1. Physical Property Analyses
3.3.2. Biological Property Analyses
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zangrando, M.S.; Valle, L.A.; Stuani, V.D.; Costa, M.R.; Shibuy, S.K.; Damante, C.A. Clinical Outcomes of Root Cov-erage with Subepithelial Connective Tissue Graft According to Site Specific Factors—Longitudinal Retrospective Clinical Study. J. Int. Acad. Periodontol. 2019, 21, 159–167. [Google Scholar] [PubMed]
- Adam, K.; Staufenbiel, I.; Geurtsen, W.; Günay, H. Root coverage using a connective tissue graft with epithelial striation in combination with enamel matrix derivatives—A long-term retrospective clinical interventional study. BMC Oral Health 2019, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Romanos, G.E.; Geurs, N.C.; Sullivan, A.; Suárez-López Del Amo, F.; Eber, R.M. Comparison of Two Differently Processed Acellular Dermal Matrix Products for Root Coverage Procedures: A Prospective, Randomized Multicenter Study. J. Periodontol. 2014, 85, 1693–1701. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Di Gianfilippo, R.; Modarressi, M.; Cairo, F.; Rasperini, G.; Wang, H.-L. Acellular Dermal Matrix and Coronally Advanced Flap or Tunnel Technique in the Treatment of Multiple Adjacent Gingival Recessions. A 12-Year Follow-up from a Randomized Clinical Trial. J. Clin. Periodontol. 2019, 46, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2019, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, S.; Han, J.; Lin, H.; Zhang, X. Design and fabrication of a chitosan hydrogel with gradient structures via a step-by-step cross-linking process. Carbohydr. Polym. 2017, 176, 195–202. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Re-view. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering 2020, 7, 85. [Google Scholar] [CrossRef]
- Du, Y.; Yu, M.; Lu, W.; Kong, J. Three-dimensional (3D), macroporous, elastic, and biodegradable nanocomposite scaffold for in situ bone regeneration: Toward structural, biophysical, and biochemical cues integration. Compos. Part B Eng. 2021, 225, 109270. [Google Scholar] [CrossRef]
- Mao, Z.; Fan, B.; Wang, X.; Huang, X.; Guan, J.; Sun, Z.; Xu, B.; Yang, M.; Chen, Z.; Jiang, D.; et al. A Systematic Review of Tissue Engineering Scaffold in Tendon Bone Healing in vivo. Front. Bioeng. Biotechnol. 2021, 9, 621483. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ham, A.; López-Gutierrez, J.; Bermúdez, M.; Aguilar-Medina, M.; Sarmiento-Sánchez, J.I.; López-Camarillo, C.; Sanchez-Schmitz, G.; Ramos-Payan, R. Hydrogel-Based Scaffolds in Oral Tissue Engineering. Front. Mater. 2021, 8, 708945. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef]
- Yacob, N.; Hashim, K. Morphological effect on swelling behaviour of hydrogel. AIP Conf. Proc. 2014, 1584, 153–159. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, Á.; Vallecillo, C.; Lynch, C.D.; Osorio, M.T.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part I: Natural Polymers-Based Biomaterials. Polymers 2020, 12, 1850. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent Advances of Injectable Hydrogels for Drug Delivery and Tissue Engineering Applica-tions. Polym. Test 2020, 81, 106283. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Systematic Reviews of Effectiveness. In Joanna Briggs Institute Reviewer’s Manual; The Joanna Briggs Institute: Adelaide, Australia, 2017; pp. 1–7. [Google Scholar]
- Cheung, J.W.; Rose, E.E.; Santerre, J.P. Perfused culture of gingival fibroblasts in a degradable/polar/hydrophobic/ionic polyurethane (D-PHI) scaffold leads to enhanced proliferation and metabolic activity. Acta Biomater. 2013, 9, 6867–6875. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, Y.; Gilad, A.; Kim, T.; Oh, M.H.; Hyeon, T.; Park, H.; Lee, K.H. Engineered collagen hydrogels for the sustained release of biomolecules and imaging agents: Promoting the growth of human gingival cells. Int. J. Nanomed. 2014, 9, 5189–5201. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.T.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes Wound Healing in a Primary Gingival Fibroblast Model. Appl. Sci. 2021, 11, 4405. [Google Scholar] [CrossRef]
- Cozens, E.J.; Roohpour, N.; Gautrot, J.E. Comparative Adhesion of Chemically and Physically Crosslinked Poly(Acrylic Acid)-Based Hydrogels to Soft Tissues. Eur. Polym. J. 2021, 146, 110250. [Google Scholar] [CrossRef]
- Kang, H.J.; Ko, N.; Oh, S.J.; An, S.Y.; Hwang, Y.S.; Kim, S.Y. Injectable Human Hair Keratin–Fibrinogen Hydrogels for En-gineering 3d Microenvironments to Accelerate Oral Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 14538–14547. [Google Scholar] [CrossRef]
- Laird, D.; El-Baba, M.D.; Hamri, G.C.-E.; Eberwein, P.; Nelson, K.; Tomakidi, P.; Steinberg, T. In vitro and in vivo biocompatibility evaluation of a novobiocin stimulus-responsive poly(ethylene glycol)-based hydrogel designed for soft tissue regeneration. J. Bioact. Compat. Polym. 2015, 30, 319–339. [Google Scholar] [CrossRef]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1691–1702. [Google Scholar] [CrossRef]
- Moatary, A.; Teimouri, A.; Bagherzadeh, M.; Chermahini, A.N.; Razavizadeh, R. Design and fabrication of novel chitin hydrogel/chitosan/nano diopside composite scaffolds for tissue engineering. Ceram. Int. 2017, 43, 1657–1668. [Google Scholar] [CrossRef]
- Montalbano, G.; Toumpaniari, S.; Popov, A.; Duan, P.; Chen, J.; Dalgarno, K.; Scott, W.E.; Ferreira, A.M. Synthesis of Bioin-spired Collagen/Alginate/Fibrin Based Hydrogels for Soft Tissue Engineering. Mater. Sci. Eng. 2018, 91, 236–246. [Google Scholar] [CrossRef]
- Rosdiani, A.F.; Widiyanti, P.; Rudyarjo, D.I. Synthesis and Characterization Biocomposite Collagen-Chitosan- Glycerol as Scaffold for Gingival Recession Therapy. J. Int. Dent. Med. Res. 2017, 10, 118–122. [Google Scholar]
- Tabatabaei, F.; Moharamzadeh, K.; Tayebi, L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J. Oral Biol. Craniofacial Res. 2020, 10, 573–577. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, K.; Sui, B.; Boccaccini, A.R.; Sun, J. In Vitro Evaluation of Poly (Vinyl Alcohol)/Collagen Blended Hydrogels for Regulating Human Periodontal Ligament Fi Broblasts and Gingival Fi Broblasts. Int. J. Biol. Macromol. 2020, 163, 1938–1946. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2018, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of Synthesis of Hydrogels…A Review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable Scaffolds: Preparation and Application in Dental and Craniofacial Regenera-tion. Mater. Sci. Eng. R 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Choi, D.J.; Park, S.J.; Gu, B.K.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Effect of the pore size in a 3D bioprinted gelatin scaffold on fibroblast proliferation. J. Ind. Eng. Chem. 2018, 67, 388–395. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Bácskay, I.; Nemes, D.; Fenyvesi, F.; Váradi, J.; Vasvári, G.; Fehér, P.; Vecsernyés, M.; Ujhelyi, Z. Role of Cytotoxicity Experi-ments in Pharmaceutical Development. In Cytotoxicity; InTech Open: Apulia, Italy, 2018. [Google Scholar]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Peña, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yuheng, J.; Chu, W. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, M.; Entezam, M.; Masaeli, E.; Ejeian, F.; Nasr-Esfahani, M.H. Physical modification approaches to enhance cell supporting potential of poly (vinyl alcohol)-based hydrogels. J. Appl. Polym. Sci. 2021, 139, 51485. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Islam, R.; Tanveer, S.; Chen, C.-C. Modeling swelling behavior of hydrogels in aqueous organic solvents. Chem. Eng. Sci. 2021, 242, 116744. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Li, W.; Du, Z.; Qin, S. Preparation and Characterization of Novel Poly (Vinyl Alcohol)/Collagen Double-Network Hydrogels. Int. J. Biol. Macromol. 2018, 118, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hy-drogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells in Vitro. Biol. Biomacromol. 2009, 10, 541–546. [Google Scholar] [CrossRef]
- Fraczyk, J.; Wasko, J.; Walczak, M.; Kaminski, Z.J.; Puchowicz, D.; Kaminska, I.; Bogun, M.; Kolasa, M.; Stodolak-Zych, E.; Scislowska-Czarnecka, A.; et al. Conjugates of Copper Alginate with Arginine-Glycine-Aspartic Acid (RGD) for Potential Use in Regenerative Medicine. Materials 2020, 13, 337. [Google Scholar] [CrossRef]
- Chamkouri, H. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Londono, R.; Badylak, S.F. Biomaterials from Decellularized Tissues. In Biomaterials from Nature for Advanced Devices and Therapies; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 190–210. ISBN 9781119126218. [Google Scholar]
- Yamaguchi-Ueda, K.; Akazawa, Y.; Kawarabayashi, K.; Sugimoto, A.; Nakagawa, H.; Miyazaki, A.; Kurogoushi, R.; Iwata, K.; Kitamura, T.; Yamada, A.; et al. Combination of Ions Promotes Cell Migration via Extracellular Signal-Regulated Kinase 1/2 Signaling Pathway in Human Gingival Fibroblasts. Mol. Med. Rep. 2019, 19, 5039–5045. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Monsalve, E.J.; Alvarez, M.M.; Hosseini, S.; Espinosa-Hernandez, M.A.; Ceballos-González, C.F.; Sanchez-Dominguez, M.; Shin, S.R.; Cecen, B.; Hassan, S.; Di Maio, E.; et al. Engineering bioactive synthetic polymers for biomedical applications: A review with emphasis on tissue engineering and controlled release. Mater. Adv. 2021, 2, 4447–4478. [Google Scholar] [CrossRef]
- Arora, R.; Narula, S.C.; Sharma, R.K.; Tewari, S. Evaluation of Supracrestal Gingival Tissue After Surgical Crown Lengthening: A 6-Month Clinical Study. J. Periodontol. 2013, 84, 934–940. [Google Scholar] [CrossRef]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef]
- Kaigler, D.; Cirelli, J.A.; Giannobile, W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, J.; Wang, S. Multifunctional Hydrogels Based on Chitosan, Hyaluronic Acid and Other Biological Macro-molecules for the Treatment of Inflammatory Bowel Disease: A Review. Int. J. Biol. Macromol. 2023, 227, 505–523. [Google Scholar] [CrossRef]
- Jiao, W.; Li, X.; Shan, J.; Wang, X. Study of Several Alginate-Based Hydrogels for In Vitro 3D Cell Cultures. Gels 2022, 8, 147. [Google Scholar] [CrossRef]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress To. Gels 2021, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mavlyanova, R.; Yang, R.; Tao, T.; Aquib, M.; Kesse, S.; Maviah, M.B.J.; Boakye-Yiadom, K.O.; Farooq, M.A.; Wang, B. In-jectable Hydrogels for Targeted Delivering of Therapeutic Molecules for Tissue Engineering and Disease Treatment. Polym. Adv.Technol. 2020, 31, 192–203. [Google Scholar] [CrossRef]
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef]
- Unangolla, J.M.; Jayasuriya, A.C. Hydrogel-Based 3D Bioprinting: A Comprehensive Review on Cell- Laden Hydrogels, Bioink Formulations, and Future Perspectives. Appl. Mater. Today 2020, 176, 139–148. [Google Scholar]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels with Tunable Structures and Properties for Tissue Engi-neering Applications. Front Chem. 2018, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Wang, S.-S.; Wang, Y.-Z.; Hsieh, C.-H.; Fu, E. Enhancing growth and proliferation of human gingival fibroblasts on chitosan grafted poly (ε-caprolactone) films is influenced by nano-roughness chitosan surfaces. J. Mater. Sci. Mater. Med. 2008, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Schreier, T.; Degen, E.; Baschong, W. Fibroblast migration and proliferation during in vitro wound healing. Res. Exp. Med. 1993, 193, 195–205. [Google Scholar] [CrossRef]
- Sterczała, B.; Grzech-Leśniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of Human Gingival Fibroblast Proliferation after Laser Stimulation In Vitro Using Different Laser Types and Wavelengths (1064, 980, 635, 450, and 405 nm)—Preliminary Report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef]
- Amberg, R.; Elad, A.; Rothamel, D.; Fienitz, T.; Szakacs, G.; Heilmann, S.; Witte, F. Design of a migration assay for human gingival fibroblasts on biodegradable magnesium surfaces. Acta Biomater. 2018, 79, 158–167. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.R.; Soeroso, Y.M.; Fatma, D.; Sunarto, H.; Sulijaya, B.; Idrus, E.; Rahdewati, H.; Tjokrovonco, A.M.; Izumi, K.; Abbas, B.; et al. Periodontal Ligament Cell Sheets and RGD-Modified Chitosan Improved Regeneration in the Horizontal Periodontal Defect Model. Eur. J. Dent. 2020, 14, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.M.P.; Pohlit, H.; Sjögren, F.; Shi, L.; Ossipov, D.; Antfolk, M.; Tenje, M. A simplified approach to control cell adherence on biologically derived in vitro cell culture scaffolds by direct UV-mediated RGD linkage. J. Mater. Sci. Mater. Med. 2020, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2013, 35, 49–62. [Google Scholar] [CrossRef]
- Pathan, S.G.; Fitzgerald, L.M.; Ali, S.M.; Damrauer, S.M.; Bide, M.J.; Nelson, D.W.; Ferran, C.; Phaneuf, T.M.; Phaneuf, M.D. Cytotoxicity associated with electrospun polyvinyl alcohol. J. Biomed. Mater. Res. Part B 2015, 103, 1652–1662. [Google Scholar] [CrossRef]
- Allena, R. Cell Migration with Multiple Pseudopodia: Temporal and Spatial Sensing Models. Cell Migr. Mult. Pseudopodia 2013, 75, 288–316. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal Res. 2018, 54, 33–45. [Google Scholar] [CrossRef]
- De Souza, V.Z.; Manfro, R.; Joly, J.C.; Elias, C.N.; Peruzzo, D.C.; Napimoga, M.H.; Martinez, E.F. Viability and Collagen Se-cretion by Fibroblasts on Titanium Surfaces with Different Acid-Etching Protocols. Int. J. Implant. Dent. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin Promotes Collagen Type I, Keratinocyte Growth Factor-1, and Epidermal Growth Factor Receptor Expressions in the In Vitro Wound Healing Model of Human Gingival Fibroblasts. Eur. J. Dent. 2020, 15, 063–070. [Google Scholar] [CrossRef]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, C.; Li, L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Donderwinkel, I.; van Hest, J.C.M.; Cameron, N.R. Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef]
- Nesic, D.; Durual, S.; Marger, L.; Mekki, M.; Sailer, I.; Scherrer, S.S. Could 3D Printing Be the Future for Oral Soft Tissue Re-generation? Bioprinting 2020, 20, e00100. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Yang, Q.; Zhang, S.; Lin, C.; Zhang, G.; Tang, Z. Three-dimensional cell printing of gingival fibroblast/acellular dermal matrix/gelatin–sodium alginate scaffolds and their biocompatibility evaluation in vitro. RSC Adv. 2020, 10, 15926–15935. [Google Scholar] [CrossRef]

| Database | Search Strategy |
|---|---|
| PubMed | (hydrogel) AND (injectable)) OR (gingival [MeSH Terms])) OR (periodontal [MeSH Terms])) AND (regeneration [MeSH Terms])) AND (natural polymer)) OR (synthetic polymer)) AND (in vitro [MeSH Terms]) |
| Science Direct | hydrogel OR injectable AND natural polymer OR synthetic polymer AND gingival regeneration OR periodontal regeneration AND in vitro |
| Embase and Scopus | (hydrogel:ti OR injectable:ti) AND (‘natural polymers’ OR (‘natural’/exp OR natural) AND (‘polymers’/exp OR polymers)) OR ‘hyaluronic acid’/exp OR ‘hyaluronic acid’ OR (hyaluronic AND (‘acid’/exp OR acid)) OR collagen OR ‘chitosan’/exp OR chitosan OR ‘gelatin’/exp OR gelatin OR ‘cellulose’/exp OR cellulose OR ‘hyaluronan’/exp OR hyaluronan OR ‘agarose’/exp OR agarose OR ‘natural scaffold’ OR ((‘natural’/exp OR natural) AND (‘scaffold’/exp OR scaffold)) OR ‘synthetic polymer’/exp OR ‘synthetic polymer’ OR (synthetic AND (polymer’/exp OR polymer)) OR ‘polyester’/exp OR polyester OR ‘poly aci’ OR (poly AND lactic AND aciD) OR ‘poly glycolic acid’/exp OR ‘poly glycolic acid’ OR ‘plga’/exp OR plga OR ‘pga’/exp OR pga OR ‘peg’/exp OR peg OR (poly AND (‘ethylene glycol’/exp OR ‘ethylene glycol’ OR ((‘ethylene’/exp OR ethylene) AND (‘glycol’/exp OR alcohol)) |) OR (poly AND (‘vinyl alcohol’/exp OR ‘vinyl alcohol’ OR ((‘vinyl’/exp OR vinyl) AND (‘alcohol’/exp OR alcohol)) AND ‘gingival regeneration’ OR ‘gingival fibroblasts’ OR (gingival AND (‘regeneration’/exp OF regeneration)) |
| Authors | Joanna Briggs Institute Items | Raw Score and % | Risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | |||
| Cheung [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Choi [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Colangelo [24] | 1 | 1 | U | 1 | 1 | 1 | 1 | 1 | U | 78% | Low |
| Cozens [25] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | U | 78% | Low |
| Kang [26] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | U | 78% | Low |
| Laird [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Miranda [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Moatary [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Montalbano [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Rosdiani [31] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | U | 78% | Low |
| Tabatabaei [32] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | U | 89% | Low |
| Zhou [33] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | U | 67% | Moderate |
| No. | Author | Biomaterial | Crosslinking Method | Fabrication |
|---|---|---|---|---|
| 1 | Cheung [22] | DVO, PEG | Chemical | D-PHI flat films were generated using a divinyl oligomer (DVO); PEG was used as porogen |
| 2 | Choi [23] | Collagen, growth factor (TGF-b1), nanoparticles (gold, TaO, dextran, or ferritin) | Chemical | Collagen type I from rat tail tendons was loaded with either TGF-b1 or other nanoparticles such as gold, TaO, dextran, or ferritin |
| 3 | Colangelo [24] | PN, HyA | Chemical | PN extraction from salmon trout gonads mixed with HyA |
| 4 | Cozens [25] | PAA, Tyr, Cys, BA, BP | Chemical | Coupling amine to PAA using 4-(4,6-dime- thoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) and then crosslinking with Tyr/Cys/BA/BP |
| 5 | Kang [26] | KRT, FIB | Chemical | Keratin from human hair mixed with fibrinogen from human plasma |
| 6 | Laird [27] | PEG, gyrase B (with/without RGD motifs), coumermycin, novobiocin | Chemical | PEG added to mixture of Novobiocin (antibiotic) or Coumermycin (dimeric form of novobiocin) and GyrB with/without RGD motifs (protein expressed by Eschericia coli) |
| 7 | Miranda [28] | Chitosan, HyA | Chemical | Medium-molecular-weight chitosan was succinylated, and HyA oxides were mixed |
| 8 | Moatary [29] | Nano diopside, b-chitin, chitosan | Chemical | Nano diopside ceramic was prepared by a modified sol–gel method then added to sulfate derivative of chitin and chitosan |
| 9 | Montalbano [30] | Collagen, alginate, fibrin | Chemical | Collagen type I from calf skin, low-viscosity alginate from brown algae, fibrinogen from bovine plasma |
| 10 | Tabatabaei [32] | Collagen, GelMA | Chemical | Cell-embedded collagen and collagen-embedded gelatin methacryloyl (GelMA) |
| 11 | Rosdiani [31] | Collagen, chitosan, glycerol | Chemical | Collagen and chitosan powders from tissue banks were dissolved into the solvents, and then glycerol was added |
| 12 | Zhou [33] | PVA, collagen | Chemical | Tilapia collagen type I mixed with PVA |
| No. | Authors | Type of Scaffold | Physical Property Analyses | Main Findings |
|---|---|---|---|---|
| 1 | Cheung et al. [22] |
|
|
|
| 2 | Choi et al. [23] |
|
|
|
| 3 | Cozens et al. [25] |
|
|
|
| 4 | Kang et al. [26] |
|
|
|
| 5 | Miranda et al. [28] |
|
|
|
| 6 | Moatary et al. [29] |
|
|
|
| 7 | Montalbano et al. [30] |
|
|
|
| 8 | Rosdiani et al. [31] |
|
|
|
| 9 | Zhou et al. [33] |
|
|
|
| No. | Author | Type of Scaffold | HGF Cell Culture | Biological Property Analyses | Main Findings |
|---|---|---|---|---|---|
| 1 | Cheung et al. [22] |
| ATCC |
|
|
| 2 | Choi et al. [23] |
| Primary HGF |
|
|
| 3 | Colangelo et al. [24] |
| ATCC |
|
|
| 4 | Kang et al. [26] |
| Primary HGF from ScienCell |
|
|
| 5 | Laird et al. [27] |
| Primary HGF |
|
|
| 6 | Miranda et al. [28] |
| NIH3T3 |
|
|
| 7 | Moatary et al. [29] |
| ESK-1 |
|
|
| 8 | Montalbano et al. [30] |
| L929 |
|
|
| 9 | Tabatabaei et al. [32] |
| Primary HGF |
|
|
| 10 | Zhou et al. [33] |
| HGFs #2620 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutomo, D.I.; Amir, L.; Suniarti, D.F.; Bachtiar, E.W.; Soeroso, Y. Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers 2023, 15, 2591. https://doi.org/10.3390/polym15122591
Hutomo DI, Amir L, Suniarti DF, Bachtiar EW, Soeroso Y. Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers. 2023; 15(12):2591. https://doi.org/10.3390/polym15122591
Chicago/Turabian StyleHutomo, Dimas Ilham, Lisa Amir, Dewi Fatma Suniarti, Endang Winiati Bachtiar, and Yuniarti Soeroso. 2023. "Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies" Polymers 15, no. 12: 2591. https://doi.org/10.3390/polym15122591
APA StyleHutomo, D. I., Amir, L., Suniarti, D. F., Bachtiar, E. W., & Soeroso, Y. (2023). Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers, 15(12), 2591. https://doi.org/10.3390/polym15122591









