Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and 3D-Printing
2.2. Flexural Strength Test
2.3. Biocompatibility Test
2.3.1. In Vitro Cell Culture
2.3.2. Eluent Preparation
2.3.3. MTT Assay
2.3.4. Live and Dead Assay
2.4. Material Characterizations
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Energy-Dispersive X-ray Spectrometer (EDS)
2.5. Statistical Analyses
3. Results
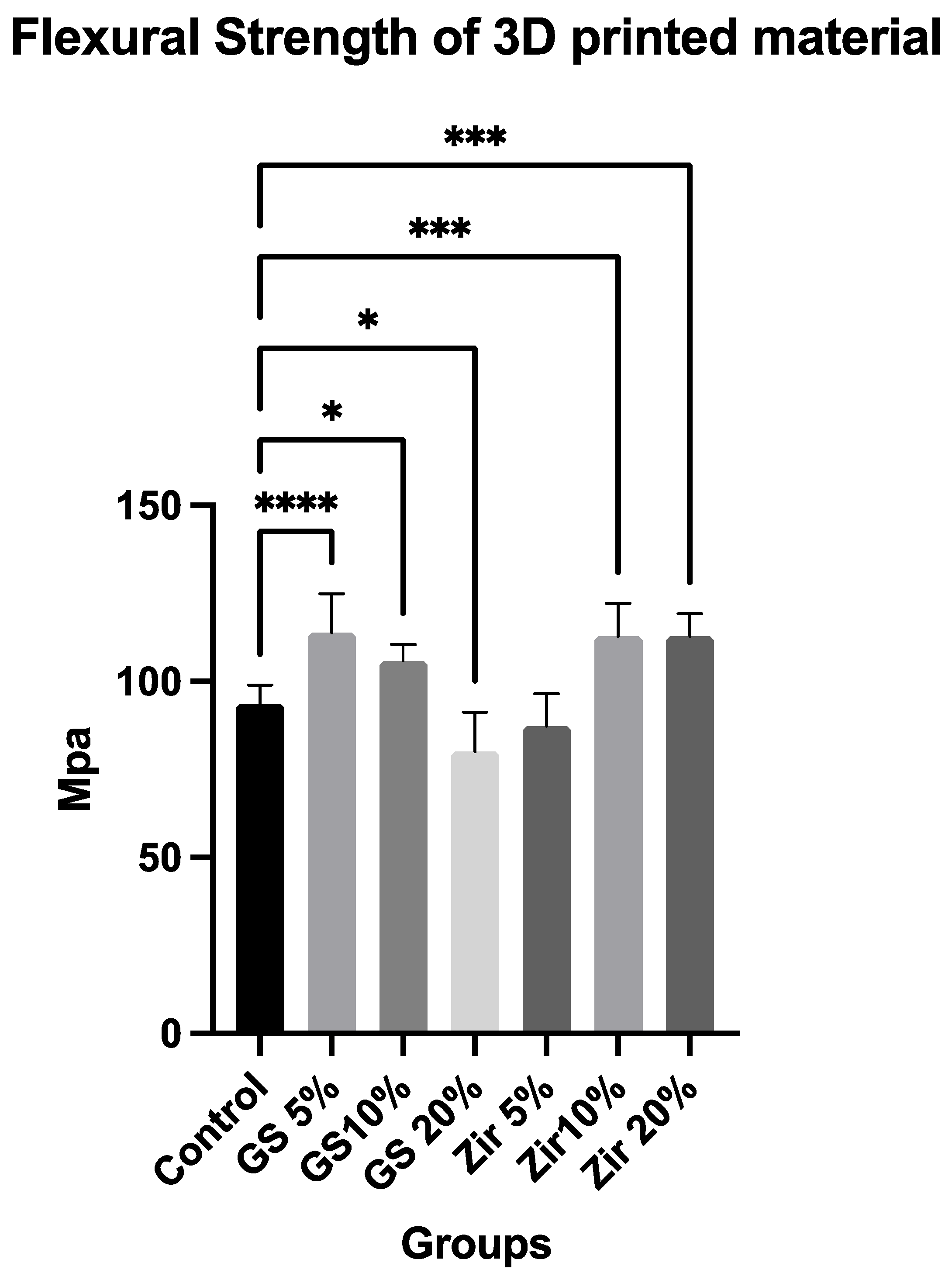
3.1. Flexural Strength
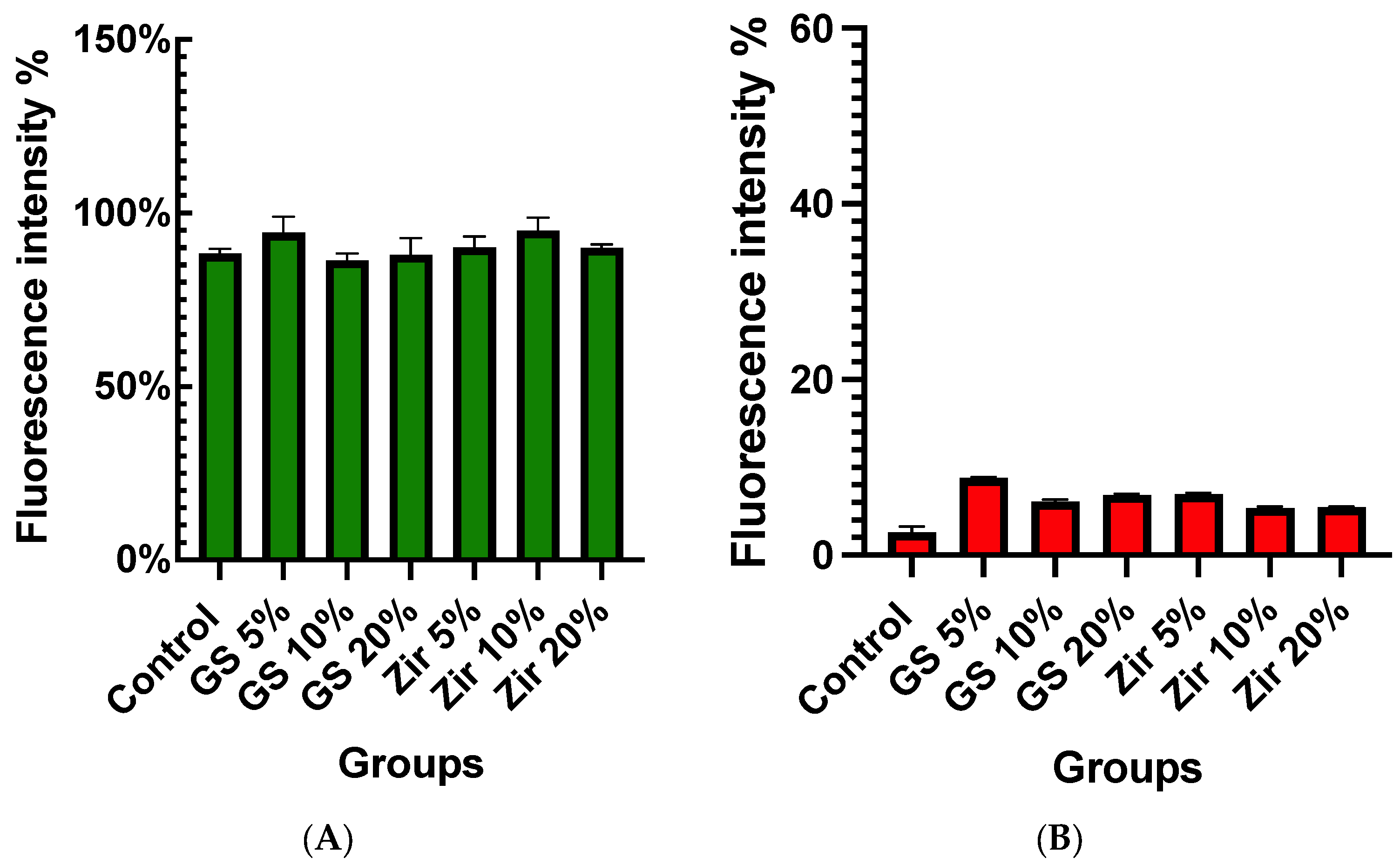
3.2. Cell Viability (MTT Assay)
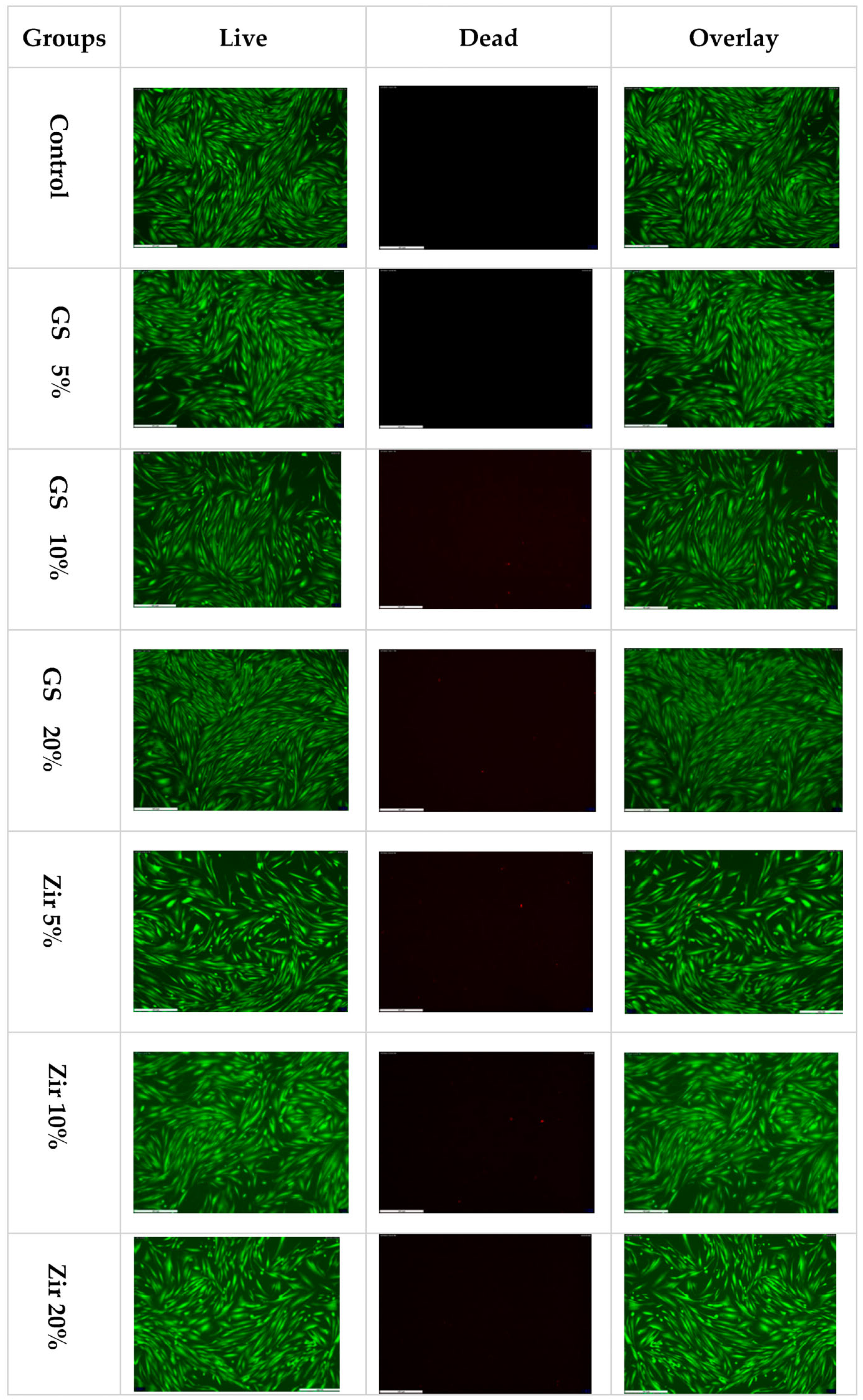
3.3. LIVE/DEAD Staining for Live-Cell Imaging
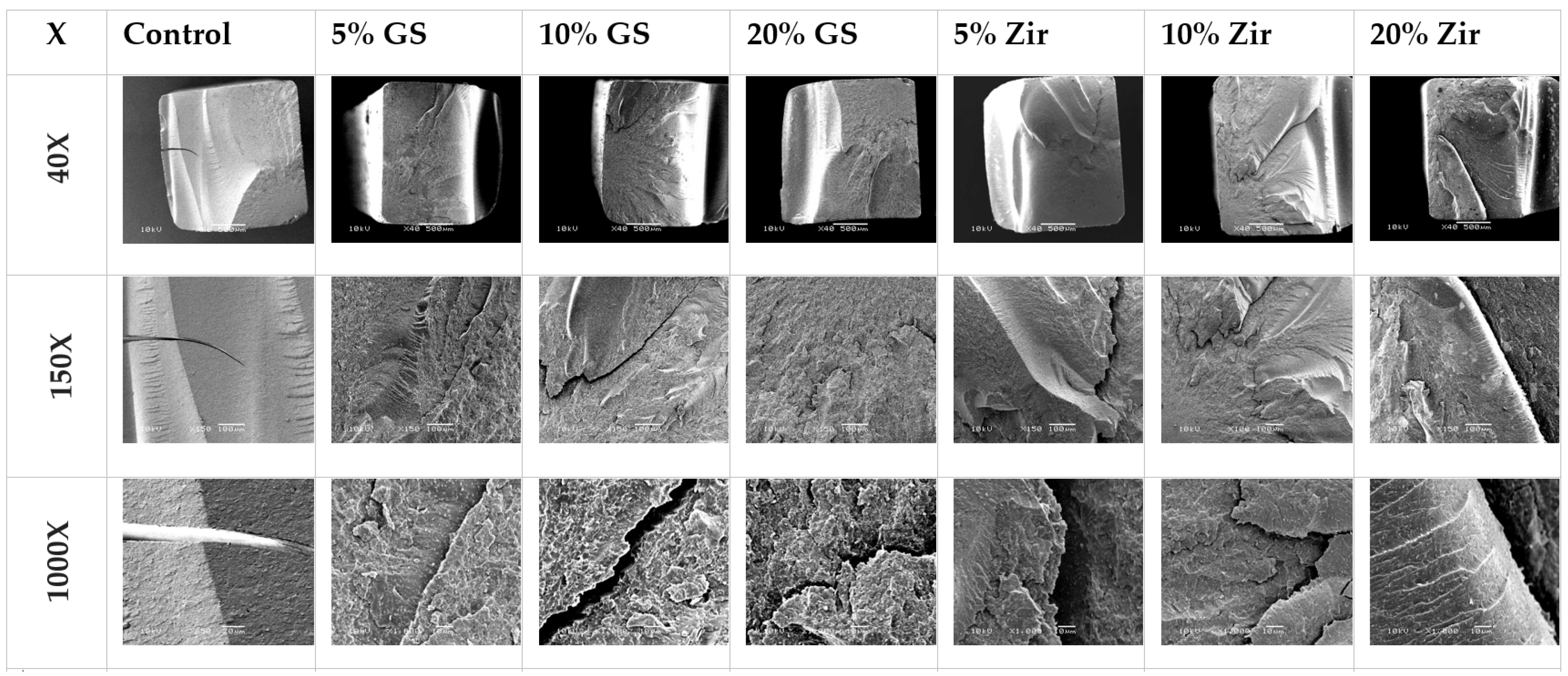
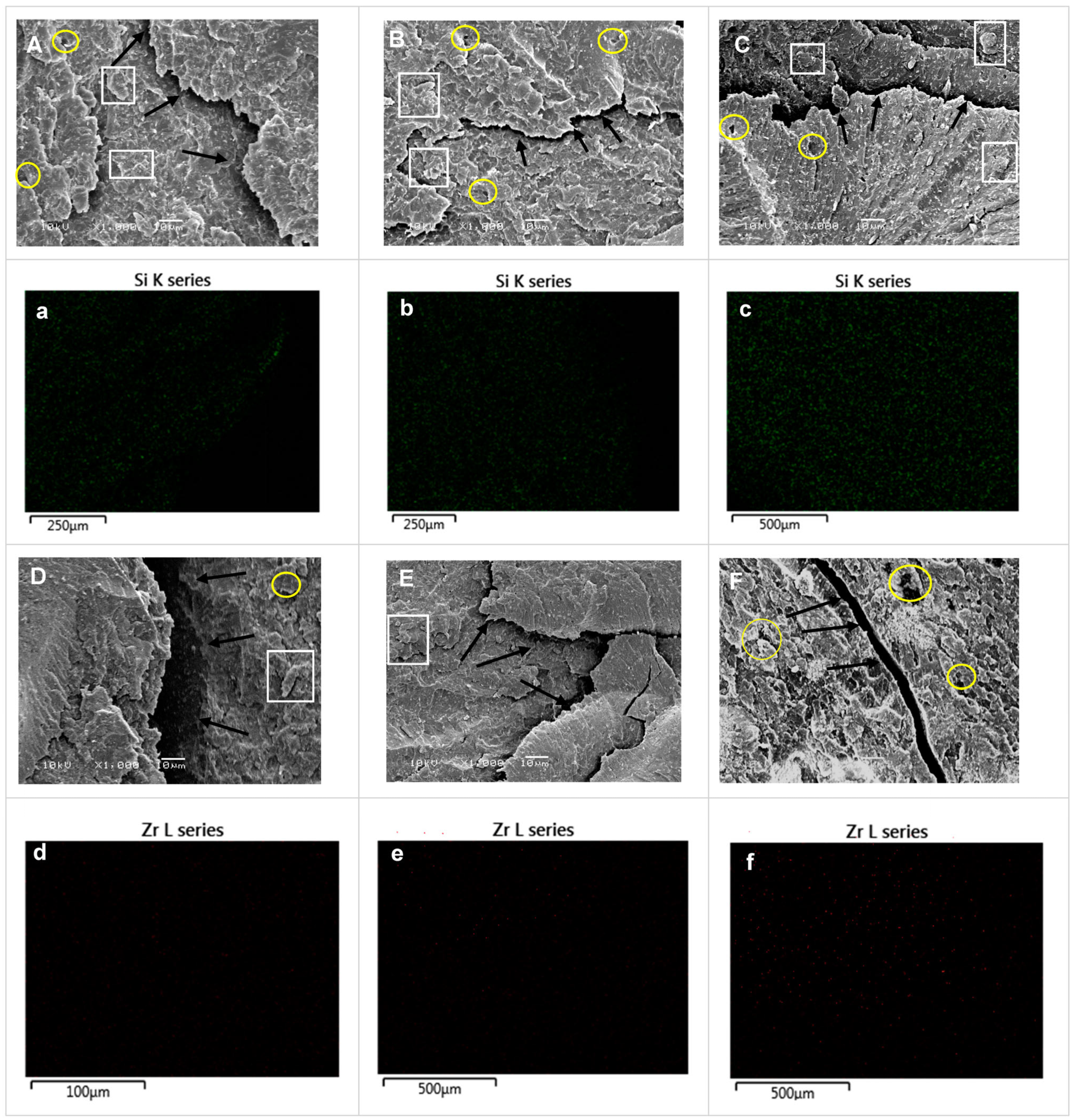
3.4. SEM Analyses
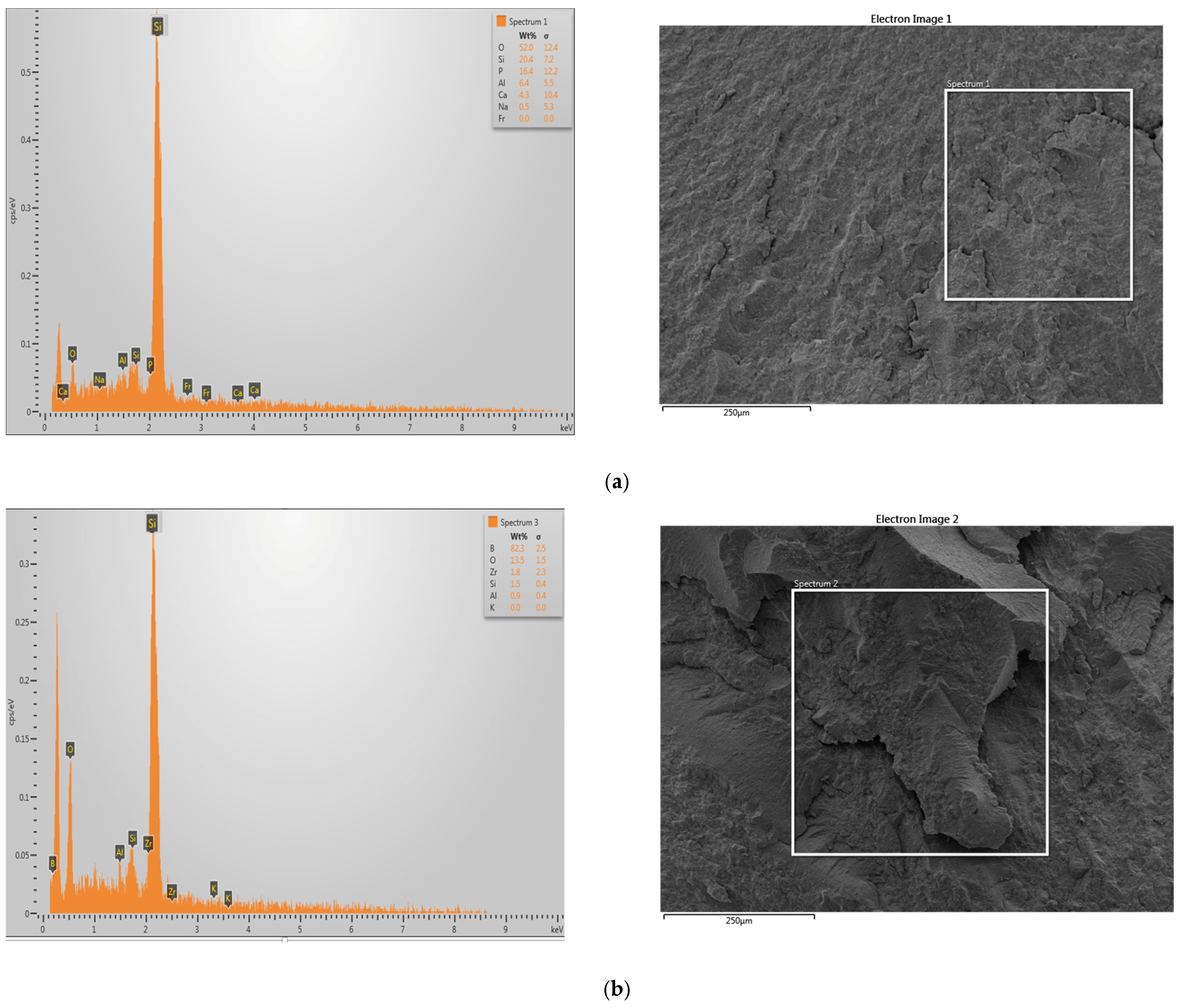
3.5. EDS Analyses
4. Discussion
5. Conclusions
- The addition of ZrO2 nanoparticles and 5 and 10 wt% glass fillers shows increased flexural strength of 3D-printed resin;
- Incorporating zirconia and glass fillers nanoparticles creates a biocompatible 3D-printed resin that does not negatively affect cell viability.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witkowski, S. (CAD-)/CAM in dental technology. Quintessence Dent. Technol. 2005, 28, 169–184. [Google Scholar]
- Revilla-León, M.; Özcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, A.; Maragathavalli, G. Applications of 3D Printing in Dentistry—A Review. J. Pharm. Sci. Res. 2019, 11, 1670–1675. [Google Scholar]
- Konidena, A. 3D Printing: Future of dentistry? J. Indian. Acad. Oral. Med. Radiol. 2016, 28, 109. [Google Scholar] [CrossRef]
- Liu, Q.; Leu, M.C.; Schmitt, S.M. Rapid prototyping in dentistry: Technology and application. Int. J. Adv. Manuf. Technol. 2006, 29, 317–335. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Additive technology: Update on current materials and applications in dentistry. J. Prosthodont. 2017, 26, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.E. Factors essential for successful all-ceramic restorations. J. Am. Dent. Assoc. 2008, 139, S14–S18. [Google Scholar] [CrossRef]
- Fugolin, A.P.P.; Pfeifer, C.S. New resins for dental composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef]
- Gutteridge, D.L. Reinforcement of poly (methyl methacrylate) with ultra-high-modulus polyethylene fibre. J. Dent. 1992, 20, 50–54. [Google Scholar] [CrossRef]
- Protopapa, P.; Kontonasaki, E.; Bikiaris, D.; Paraskevopoulos, K.M.; Koidis, P. Reinforcement of a PMMA resin for fixed interim prostheses with nanodiamonds. Dent. Mater. J. 2011, 30, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Panyayong, W.; Oshida, Y.; Andres, C.J.; Barco, T.M.; Brown, D.T.; Hovijitra, S. Reinforcement of acrylic resins for provisional fixed restorations. Part III: Effects of addition of titania and zirconia mixtures on some mechanical and physical properties. Biomed. Mater. Eng. 2002, 12, 353–366. [Google Scholar] [PubMed]
- Asar, N.V.; Albayrak, H.; Korkmaz, T.; Turkyilmaz, I. Influence of various metal oxides on mechanical and physical properties of heat-cured polymethyl methacrylate denture base resins. J. Adv. Prosthodont. 2013, 5, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Rhee, S.-H. The mechanical properties and bioactivity of poly (methyl methacrylate)/SiO2–CaO nanocomposite. Biomaterials 2009, 30, 3444–3449. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D printed resin reinforced with modified ZrO2 nanoparticles for long-term provisional dental restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef]
- Alshaikh, A.A.; Khattar, A.; Almindil, I.A.; Alsaif, M.H.; Akhtar, S.; Khan, S.Q.; Gad, M.M. 3D-printed nanocomposite denture-base resins: Effect of ZrO2 nanoparticles on the mechanical and surface properties in vitro. Nanomaterials 2022, 12, 2451. [Google Scholar] [CrossRef] [PubMed]
- Miletic, V. Development of dental composites. In Dental Composite Materials for Direct Restorations; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–9. [Google Scholar]
- Lutz, F.; Phillips, R.W. A classification and evaluation of composite resin systems. J. Prosthet. Dent. 1983, 50, 480–488. [Google Scholar] [CrossRef]
- Raptis, C.N.; Fan, P.L.; Powers, J.M. Properties of microfilled and visible light-cured composite resins. J. Am. Dent. Assoc. (1939) 1979, 99, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Topouzi, M.; Kontonasaki, E.; Bikiaris, D.; Papadopoulou, L.; Paraskevopoulos, K.M.; Koidis, P. Reinforcement of a PMMA resin for interim fixed prostheses with silica nanoparticles. J. Mech. Behav. Biomed. Mater. 2017, 69, 213–222. [Google Scholar] [CrossRef]
- Ai, M.; Du, Z.; Zhu, S.; Geng, H.; Zhang, X.; Cai, Q.; Yang, X. Composite resin reinforced with silver nanoparticles–laden hydroxyapatite nanowires for dental application. Dent. Mater. 2017, 33, 12–22. [Google Scholar] [CrossRef]
- de Castro, D.T.; Valente, M.L.d.C.; Aires, C.P.; Alves, O.L.; Dos Reis, A.C. Elemental ion release and cytotoxicity of antimicrobial acrylic resins incorporated with nanomaterial. Gerodontology 2017, 34, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Rahoma, A.; Abualsaud, R.; Al-Harbi, F.A.; Akhtar, S. Reinforcement of PMMA denture base material with a mixture of ZrO2 nanoparticles and glass fibers. Int. J. Dent. 2019, 2019, 2489393. [Google Scholar] [CrossRef]
- Alhareb, A.O.; Ahmad, Z.A. Effect of Al2O3/ZrO2 reinforcement on the mechanical properties of PMMA denture base. J. Reinf. Plast. Compos. 2011, 30, 86–93. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, X.Y.; Zhu, B.S.; Qian, C. Effect of nano ZrO2 on flexural strength and surface hardness of polymethylmethacrylate. Shanghai J. Stomatol. 2011, 20, 358–363. [Google Scholar]
- Persson, C.; Unosson, E.; Ajaxon, I.; Engstrand, J.; Engqvist, H.; Xia, W. Nano grain sized zirconia–silica glass ceramics for dental applications. J. Eur. Ceram. Soc. 2012, 32, 4105–4110. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Kumar, R.; Kumar, R.; Pruncu, C.I. Fabrication and characterization of ZrO2 incorporated SiO2–CaO–P2O5 bioactive glass scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 109, 103854. [Google Scholar] [CrossRef]
- El-Tamimi, K.M.; Bayoumi, D.A.; Ahmed, M.M.Z.; Albaijan, I.; El-Sayed, M.E. The effect of salinized nano ZrO2 particles on the microstructure, hardness, and wear behavior of acrylic denture tooth nanocomposite. Polymers 2022, 14, 302. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A.; Patnaik, A. Biomaterials for dental composite applications: A comprehensive review of physical, chemical, mechanical, thermal, tribological, and biological properties. Polym. Adv. Technol. 2022, 33, 1762–1781. [Google Scholar] [CrossRef]
- de Castro, D.T.; Valente, M.L.C.; Agnelli, J.A.M.; da Silva, C.H.L.; Watanabe, E.; Siqueira, R.L.; Alves, O.L.; Holtz, R.D.; dos Reis, A.C. In Vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. J. Prosthet. Dent. 2016, 115, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, S.; Dhamodharan, D.; Kale, M.; Divakaran, N.; Senthil, T.; Wu, L.; Wang, J. A novel approach to enhance mechanical and thermal properties of SLA 3D printed structure by incorporation of metal–metal oxide nanoparticles. Nanomaterials 2020, 10, 217. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, S.; Wang, Z.; Miao, J.-T.; Zheng, L.; Wu, L.; Weng, Z. UV-curable, low-viscosity resin with a high silica filler content for preparing ultrastiff, 3D-printed molds. ACS Appl. Polym. Mater. 2022, 4, 2636–2647. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 100, 1444–1450. [Google Scholar] [CrossRef]
- Kim, G.-T.; Go, H.-B.; Yu, J.-H.; Yang, S.-Y.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Cytotoxicity, Colour Stability and Dimensional Accuracy of 3D Printing Resin with Three Different Photoinitiators. Polymers 2022, 14, 979. [Google Scholar] [CrossRef] [PubMed]
- Baldion, P.A.; Velandia-Romero, M.L.; Castellanos, J.E. Dental resin monomers induce early and potent oxidative damage on human odontoblast-like cells. Chem. Biol. Interact. 2021, 333, 109336. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Reichl, F.-X.; Shi, J.; He, X.; Hickel, R.; Högg, C. Cytotoxicity and DNA double-strand breaks in human gingival fibroblasts exposed to eluates of dental composites. Dent. Mater. 2018, 34, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dizon, J.R.C.; Gache, C.C.L.; Cascolan, H.M.S.; Cancino, L.T.; Advincula, R.C. Post-processing of 3D-printed polymers. Technologies 2021, 9, 61. [Google Scholar] [CrossRef]
- Hardiman, K. Post-Processing considerations for biomedical 3D printing of polymers. In Polymer-Based Additive Manufacturing: Biomedical Applications; Springer: Cham, Switzerland, 2019; pp. 219–241. [Google Scholar]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef]
- Hwangbo, N.-K.; Nam, N.-E.; Choi, J.-H.; Kim, J.-E. Effects of the washing time and washing solution on the biocompatibility and mechanical properties of 3D printed dental resin materials. Polymers 2021, 13, 4410. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Gad, M.M. Effects of SiO2 incorporation on the flexural properties of a denture base resin: An in vitro study. Eur. J. Dent. 2022, 16, 188–194. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.-B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef]
- de Almeida Rossato, T.C.; Gallas, J.A.; da Rosa, W.L.O.; da Silva, A.F.; Piva, E.; Peralta, S.L.; Lund, R.G. Experimental sealers containing metal methacrylates: Physical and biological properties. J. Endod. 2017, 43, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lee, S.-Y.; Lin, Y.-M. Synthesis and formulation of PCL-based urethane acrylates for DLP 3D printers. Polymers 2020, 12, 1500. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef]
- Abduo, J.; Lyons, K.; Bennamoun, M. Trends in computer-aided manufacturing in prosthodontics: A review of the available streams. Int. J. Dent. 2014, 2014, 783948. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- dos Santos, R.L.; de Sampaio, G.A.; de Carvalho, F.G.; Pithon, M.M.; Guênes, G.M.; Alves, P.M. Influence of degree of conversion on the biocompatibility of different composites in vivo. J. Adhes. Dent. 2014, 16, 15–20. [Google Scholar]
- Alshamrani, A.A.; Raju, R.; Ellakwa, A. Effect of Printing Layer Thickness and Postprinting Conditions on the Flexural Strength and Hardness of a 3D-Printed Resin. BioMed Res. Int. 2022, 2022, 8353137. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.; Wismeijer, D. Effects of build direction on the mechanical properties of 3D-printed complete coverage interim dental restorations. J. Prosthet. Dent. 2016, 115, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L. Evaluation of the mechanical properties and degree of conversion of 3D printed splint material. J. Mech. Behav. Biomed. Mater. 2021, 115, 104254. [Google Scholar] [CrossRef] [PubMed]
- Haselton, D.R.; Diaz-Arnold, A.M.; Vargas, M.A. Flexural strength of provisional crown and fixed partial denture resins. J. Prosthet. Dent. 2002, 87, 225–228. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Investigations on mechanical behaviour of dental composites. Clin. Oral. Investig. 2009, 13, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.R.; Palin, W.M.; Fleming, G.J.P.; Shortall, A.C.C.; Marquis, P.M. The mechanical properties of nanofilled resin-based composites: Characterizing discrete filler particles and agglomerates using a micromanipulation technique. Dent. Mater. 2009, 25, 180–187. [Google Scholar] [CrossRef]
- Digholkar, S.; Madhav, V.N.V.; Palaskar, J. Evaluation of the flexural strength and microhardness of provisional crown and bridge materials fabricated by different methods. J. Indian Prosthodont. Soc. 2016, 16, 328. [Google Scholar] [PubMed]
- Balkenhol, M.; Mautner, M.C.; Ferger, P.; Wöstmann, B. Mechanical properties of provisional crown and bridge materials: Chemical-Curing versus dual-curing systems. J. Dent. 2008, 36, 15–20. [Google Scholar] [CrossRef]
- Astudillo-Rubio, D.; Delgado-Gaete, A.; Bellot-Arcís, C.; Montiel-Company, J.M.; Pascual-Moscardó, A.; Almerich-Silla, J.M. Mechanical properties of provisional dental materials: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0193162. [Google Scholar]
- Nakamura, T.; Ohyama, T.; Waki, T.; Kinuta, S.; Wakabayashi, K.; Takano, N.; Yatani, H. Finite element analysis of fiber-reinforced fixed partial dentures. Dent. Mater. J. 2005, 24, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Bijelic-Donova, J.; Garoushi, S.; Lassila, L.V.J.; Keulemans, F.; Vallittu, P.K. Mechanical and structural characterization of discontinuous fiber-reinforced dental resin composite. J. Dent. 2016, 52, 70–78. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Nejatian, T.; Nathwani, N.; Taylor, L.; Sefat, F. Denture base composites: Effect of surface modified nano-and micro-particulates on mechanical properties of polymethyl methacrylate. Materials 2020, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Ergun, G.; Sahin, Z.; Ataol, A.S. The effects of adding various ratios of zirconium oxide nanoparticles to poly (methyl methacrylate) on physical and mechanical properties. J. Oral. Sci. 2018, 60, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Karci, M.; Demir, N.; Yazman, S. Evaluation of flexural strength of different denture base materials reinforced with different nanoparticles. J. Prosthodont. 2019, 28, 572–579. [Google Scholar] [CrossRef]
- Hakim, M.L.N.; Faza, Y.; Hasratiningsih, Z.; Djustiana, N.; Sunendar, B. Hardness evaluation of dental composites fabricated from the uniform size and well-distributed zirconia-alumina-silica fillers with sol-gel technique. Padjadjaran J. Dent. 2018, 30, 78–83. [Google Scholar] [CrossRef]
- Mustafa, B.S.; Jamal, G.M.; Abdullah, O.G. Improving the tensile, toughness, and flexural properties of epoxy resin based nanocomposites filled with ZrO2 and Y2O3 nanoparticles. Results Phys. 2022, 38, 105662. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural strength and hardness of filler-reinforced PMMA targeted for denture base application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Taghavimehr, M.; Famili, M.H.N.; Shirsavar, M.A. Effect of nanoparticle network formation on electromagnetic properties and cell morphology of microcellular polymer nanocomposite foams. Polym. Test. 2020, 86, 106469. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Assessing fracture toughness and impact strength of PMMA reinforced with nano-particles and fibre as advanced denture base materials. Materials 2021, 14, 4127. [Google Scholar] [CrossRef]
- Kj, A. Phillips’ science of dental materials. St. Louis WB Saunders 2003, 596, 41–43. [Google Scholar]
- Sun, L.; Gibson, R.F.; Gordaninejad, F.; Suhr, J. Energy absorption capability of nanocomposites: A review. Compos. Sci. Technol. 2009, 69, 2392–2409. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Cellulose Fibers: Bio-and Nano-Polymer Composites: Green Chemistry and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Adnan, S.; Khan, F.R. Comparison of micro-leakage around temporary restorative materials placed in complex endodontic access cavities: An in-vitro study. J. Coll. Physicians Surg. Pak. 2016, 26, 182. [Google Scholar] [PubMed]
- Alifui-Segbaya, F.; Varma, S.; Lieschke, G.J.; George, R. Biocompatibility of photopolymers in 3D printing. 3d Print. Addit. Manuf. 2017, 4, 185–191. [Google Scholar] [CrossRef]
- Frasheri, I.; Aumer, K.; Keßler, A.; Miosge, N.; Folwaczny, M. Effects of resin materials dedicated for additive manufacturing of temporary dental restorations on human gingival keratinocytes. J. Esthet. Restor. Dent. 2022, 34, 1105–1112. [Google Scholar] [CrossRef]
- Bieger, V.; Thieringer, F.M.; Fischer, J.; Rohr, N. Fibroblast behavior on conventionally processed, milled, and printed occlusal device materials with different surface treatments. J. Prosthet. Dent. 2021, 28, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Atria, P.J.; Bordin, D.; Marti, F.; Nayak, V.V.; Conejo, J.; Benalcázar Jalkh, E.; Witek, L.; Sampaio, C.S. 3D-printed resins for provisional dental restorations: Comparison of mechanical and biological properties. J. Esthet. Restor. Dent. 2022, 34, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, B.; Chen, R.; Huang, Z.; Zhu, C.; Zhang, X. Effect of silver-supported materials on the mechanical and antibacterial properties of reinforced acrylic resin composites. Materials Design (1980–2015) 2015, 65, 1245–1252. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, J.-K.; Kim, D.-A.; Patel, K.D.; Kim, H.-W.; Lee, H.-H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Hamid, S.K.; Alghamdi, L.A.; Alshahrani, F.A.; Khan, S.Q.; Matin, A.; Gad, M.M. In Vitro assessment of artificial aging on the antifungal activity of PMMA denture base material modified with ZrO2 nanoparticles. Int. J. Dent. 2021, 2021, 5560443. [Google Scholar] [CrossRef] [PubMed]

| Material | Compositions (Wight%) | Manufacturer |
|---|---|---|
| Everes Temporary (dental resin) | Aliphaticdifunctional methacrylate < 50% 2.2 ethylenedioxydiethyl dimethacrylate < 40% Aliphatic unethane acrylate < 20% Phosphine oxide < 2.5% Ethylenedioxydiethyl dimethacrylate < 40% Aliphatic unethane acrylate < 20% | Sisma, Italy |
| Glass fillers (ultrafine GM35429) | SiO2 < 30%, CaO < 10%, Al2O3 < 30%, F < 15%, P2O < 10%, Na2O < 10% | Shofu Inc., Ratingen, Germany |
| Zirconia glass (ultrafine GM018-307) | Al2O3 < 5.0%, B2O3 < 15%, K2O < 5%, SiO2< 65%, ZrO2 < 5.0% | Shofu Inc., Ratingen, Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers 2023, 15, 2523. https://doi.org/10.3390/polym15112523
Alshamrani A, Alhotan A, Kelly E, Ellakwa A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers. 2023; 15(11):2523. https://doi.org/10.3390/polym15112523
Chicago/Turabian StyleAlshamrani, Abdullah, Abdulaziz Alhotan, Elizabeth Kelly, and Ayman Ellakwa. 2023. "Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study" Polymers 15, no. 11: 2523. https://doi.org/10.3390/polym15112523
APA StyleAlshamrani, A., Alhotan, A., Kelly, E., & Ellakwa, A. (2023). Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers, 15(11), 2523. https://doi.org/10.3390/polym15112523






