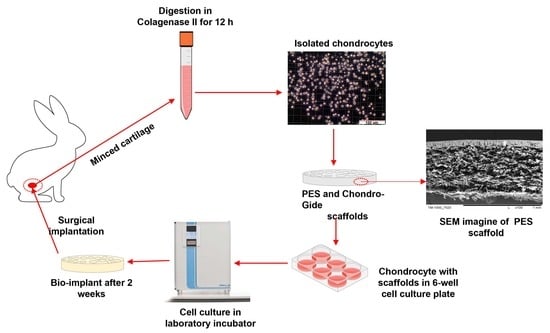
Intraarticular Implantation of Autologous Chondrocytes Placed on Collagen or Polyethersulfone Scaffolds: An Experimental Study in Rabbits
Abstract
1. Introduction
2. Materials
2.1. Membranes
2.2. Rabbits
- Full-thickness defect with implanted chondrocytes placed on a collagen membrane: 28 knees.
- Full-thickness defect and chondrocytes implanted on a PES membrane: 30 knees.
- Full-thickness lesion and implantation of a collagen membrane without cells: 25 knees.
- Full-thickness defect and implantation of a PES membrane without cells: 26 knees.
- Full-thickness defect without any implant, allowing cells from the bone marrow to infiltrate the regenerated tissue: 13 knees.
3. Methods
3.1. Chondrocyte Isolation and Culture Techniques
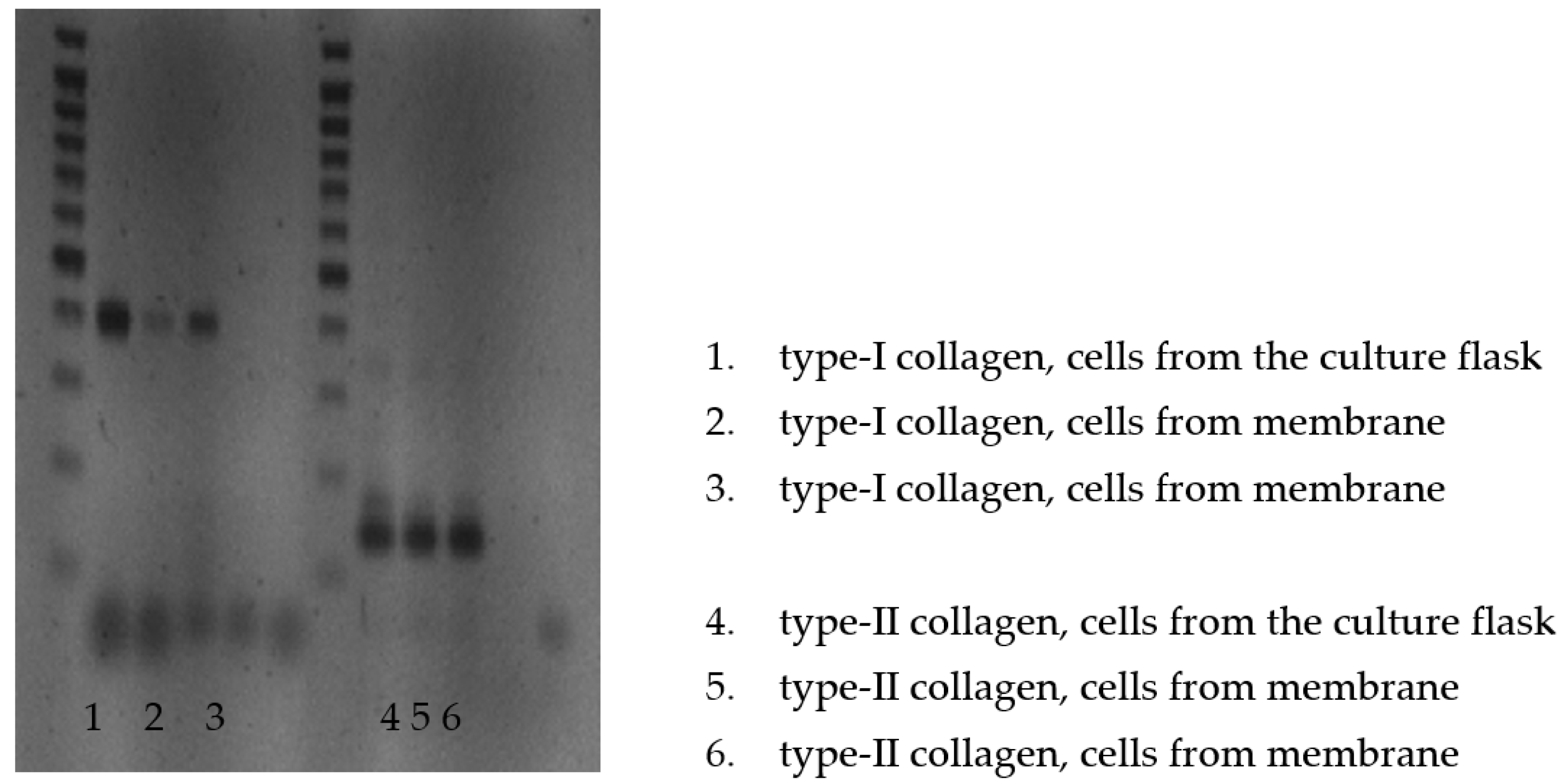
3.2. Identification of Procollagen Type II
- Collagen I (5′ starter—5′-CCAGATTGAGACCCTCCTCA-3′, 3′starter—5′-ATGCAATGCTGTTCTTGCAG-3′)
- Collagen II (5′ starter—5′-GGGGTCCTTTAGGTCCTACG-3′, 3′starter—5′-AGTCGCTGGTGCTGCTGAC-3′)
3.3. Elemental Analysis
3.4. SEM Observation
3.5. Implantation of Grafts
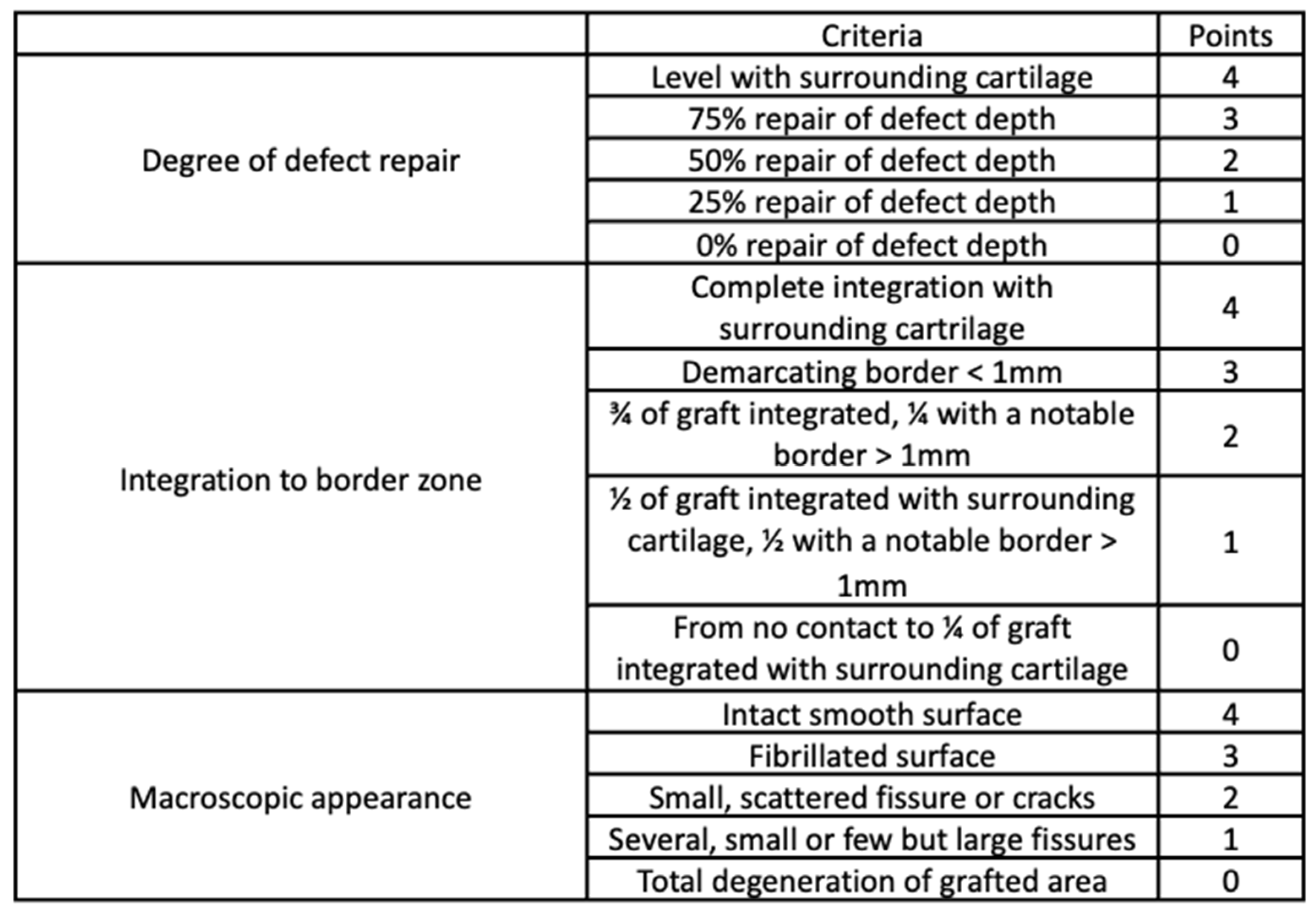
3.6. Macroscopic Evaluation
3.7. Microscopic Analysis
3.8. Statistical Analysis
4. Results
4.1. The Number of Cells
4.2. RT-PCR for mRNA of Type II Collagen
4.3. Elementary Analysis
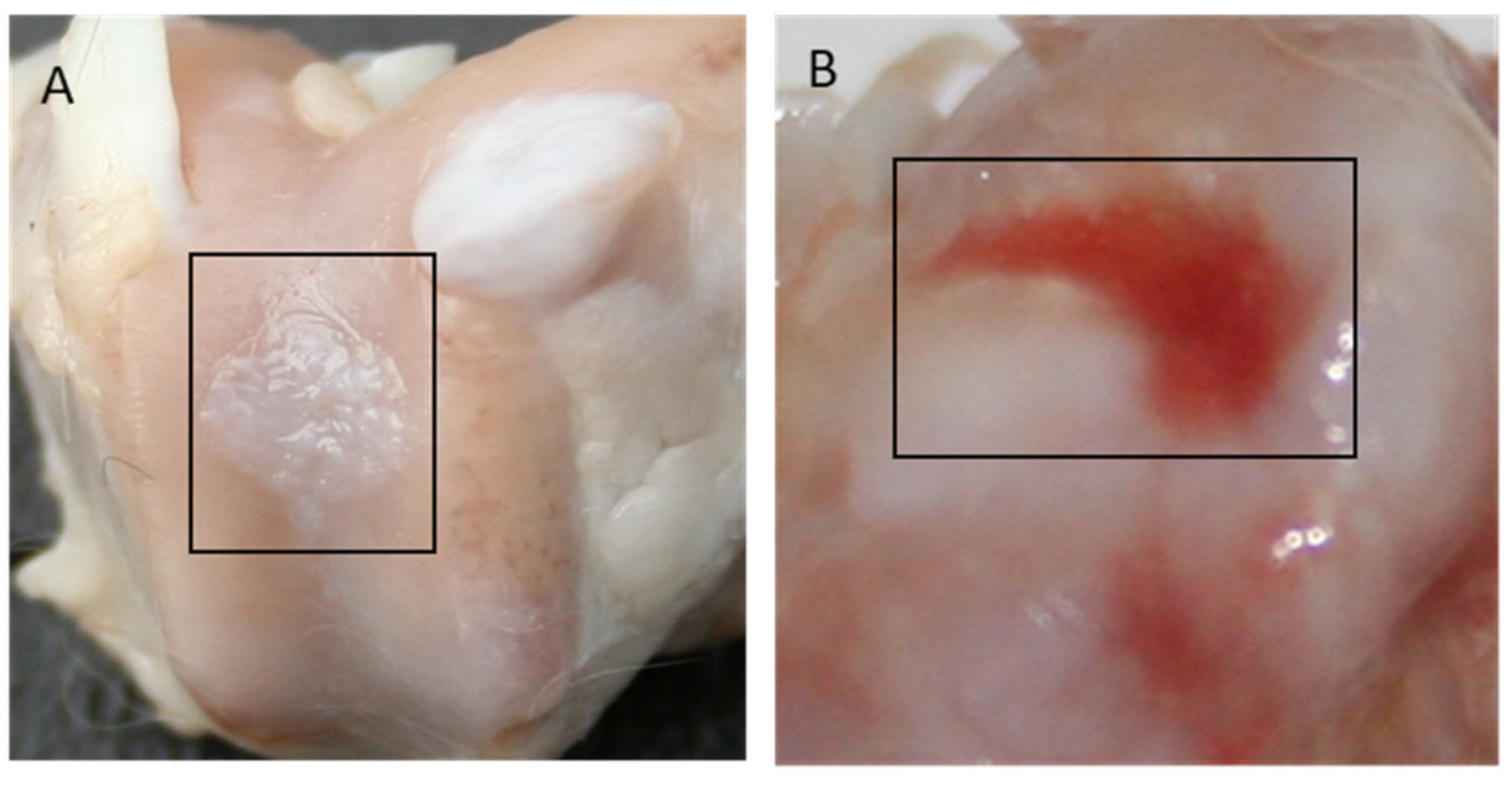
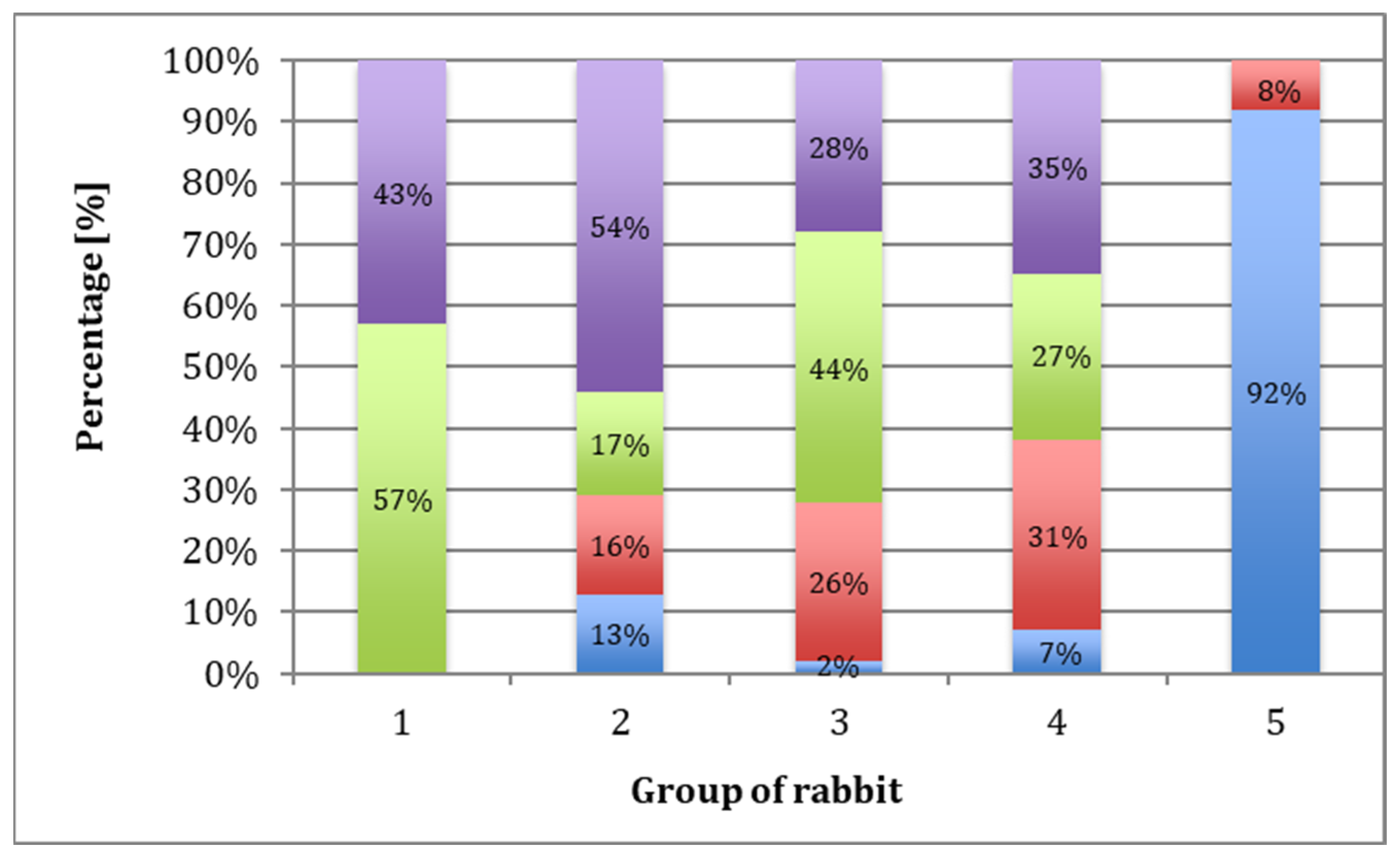
4.4. Macroscopic Evaluation
4.5. Microscopic Evaluation
5. Discussion
- Sterile and non-cytotoxic;
- Preservable without losing its properties;
- Biodegradable after 6 months.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.G.; Mow, V.C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J. Bone Jt. Surg. Ser. A 1982, 64, 88–94. [Google Scholar] [CrossRef]
- Walter, S.G.; Ossendorff, R.; Schildberg, F.A. Articular cartilage regeneration and tissue engineering models: A systematic review. Arch. Orthop. Trauma Surg. 2019, 139, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Vullings, J.; van de Loo, F.A.J. Osteoporosis and osteoarthritis are two sides of the same coin paid for obesity. Nutrition 2020, 70, 110486. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, C.; Chen, F.; Sun, Y.; Xia, Y.; Ji, A.; Wang, D. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol. Biosci. 2019, 20, 1900278. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Homminga, G.N.; Bulstra, S.K.; Kuijer, R.; van der Linden, A.J. Repair of sheep articular cartilage defects with a rabbit costal perichondrial graft. Acta Orthop. 1991, 62, 415–418. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Salter, R.B. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin. Orthop. Relat. Res. 1986, 208, 131–140. [Google Scholar] [CrossRef]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage-Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Mirza, U.; Shubeena, S.; Shah, M.S.; Zaffer, B. Microfracture: A technique for repair of chondral defects. J. Entomol. Zool. Stud. 2018, 6, 1092–1097. [Google Scholar]
- Brittberg, M. Symposium Scaffold based Autologous Chondrocyte Implantation: The Surgical Technique. Asian J Arthrosc. 2019, 4, 23–26. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Tamaddon, M.; Gilja, H.; Wang, L.; Oliveira, J.M.; Sun, X.; Tan, R.; Liu, C. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: From bench to clinic. Biomater. Transl. 2020, 1, 3–17. [Google Scholar]
- Follow-up, F.; Randomized, P.; Brittberg, M.; Recker, D.; Ilgenfritz, J. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture. Am. J. Sport. Med. 2018, 46, 1–9. [Google Scholar] [CrossRef]
- Kon, E.; Gobbi, A.; Filardo, G.; Delcogliano, M.; Zaffagnini, S.; Marcacci, M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: Prospective nonrandomized study at 5 years. Am. J. Sport. Med. 2009, 37, 33–41. [Google Scholar] [CrossRef]
- Baranowski, M.; Wasyłeczko, M.; Kosowska, A.; Plichta, A.; Kowalczyk, S.; Chwojnowski, A.; Bielecki, W.; Czubak, J. Regeneration of Articular Cartilage Using Membranes of Polyester Scaffolds in a Rabbit Model. Pharmaceutics 2022, 14, 1016. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.L.; Reuveny, S.; Oh, S.K.W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Ahmadi, F.; Giti, R.; Mohammadi-Samani, S.; Mohammadi, F. Biodegradable Scaffolds for Cartilage Tissue Engineering GMJ. Gmj 2017, 6, 70–80. [Google Scholar] [CrossRef]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The Use of Scaffolds in Cartilage Regeneration. Eukaryot. Gene Expr. 2018, 28, 343–348. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Jeuken, R.M.; Roth, A.K.; Peters, R.J.R.W.; van Donkelaar, C.C.; Thies, J.C.; van Rhijn, L.W.; Emans, P.J. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-Kafrani, A.; Samadikuchaksaraei, A.; Kaplan, D.L.; Moroni, L.; Joghataei, M.T. Hybrid and composite scaffolds based on extracellular matrices for cartilage tissue engineering. Tissue Eng. Part B Rev. 2019, 25, 202–224. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Geng, Z.; Su, J. The horizon of bone organoid: A perspective on construction and application. Bioact. Mater. 2022, 18, 15–25. [Google Scholar] [CrossRef]
- Sun, C.; Kang, J.; Yang, C.; Zheng, J.; Su, Y.; Dong, E.; Liu, Y.; Yao, S.; Shi, C.; Pang, H.; et al. Additive manufactured polyether-ether-ketone implants for orthopaedic applications: A narrative review. Biomater. Transl. 2022, 3, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Irawan, V.; Akon, T.S.; Toshiyuki, H. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Beck, A.M.; Fritch, M.R.; Tuan, R.S.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Sinha, S.; Singh, B.; Kaur, J.; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Chen, Y.; Mo, X.; Fan, C. Chondroitin sulfate modified 3D porous electrospun nano fi ber scaffolds promote cartilage regeneration. Mater. Sci. Eng. C 2021, 118, 111312. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Krysiak, Z.J.; Łukowska, E.; Gruba, M.; Sikorska, W.; Kruk, A.; Dulnik, J.; Czubak, J.; Chwojnowski, A. Three-dimensional scaffolds for bioengineering of cartilage tissue. Biocybern. Biomed. Eng. 2022, 42, 494–511. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Przytulska, M.; Dulnik, J.; Chwojnowski, A. Polyester membranes as 3D scaffolds for cell culture. Desalination Water Treat. 2021, 214, 181–193. [Google Scholar] [CrossRef]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-roitman, J.; Schroeder, A. Mini Review Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Wen, Y.T.; Dai, N.T.; Hsu, S. Hui Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Mahboudi, H.; Soleimani, M.; Enderami, S.E.; Kehtari, M.; Hanaee-Ahvaz, H.; Ghanbarian, H.; Bandehpour, M.; Nojehdehi, S.; Mirzaei, S.; Kazemi, B. The effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of human induced pluripotent stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, X.; Wang, X.; Su, J. Hydrogels for bone organoid construction: From a materiobiological perspective. J. Mater. Sci. Technol. 2023, 136, 21–31. [Google Scholar] [CrossRef]
- Sikorska, W.; Milner-Krawczyk, M.; Wasyłeczko, M.; Wojciechowski, C.; Chwojnowski, A. Biodegradation process of PSF-PUR blend hollow fiber membranes using escherichia coli Bacteria—Evaluation of changes in properties and porosity. Polymers 2021, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, W.; Wasyłeczko, M.; Przytulska, M.; Wojciechowski, C.; Rokicki, G.; Chwojnowski, A. Chemical degradation of PSF-PUR blend hollow fiber membranes-assessment of changes in properties and morphology after hydrolysis. Membranes 2021, 11, 51. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, L.; Ma, B.; Ding, H.; Tang, C. Effect of component and surface structure on poly (L-lactide-co-ε-caprolactone) (PLCA)-based composite membrane. Compos. Part B 2019, 174, 107031. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Azadbakht, M.; Madaeni, S.S.; Sahebjamee, F. Biocompatibility of polyethersulfone membranes for cell culture systems. Eng. Life Sci. 2011, 11, 629–635. [Google Scholar] [CrossRef]
- Dudziński, K.; Chwojnowski, A.; Gutowska, M.; Płończak, M.; Czubak, J.; Łukowska, E.; Wojciechowski, C. Three dimensional polyethersulphone scaffold for chondrocytes cultivation-The future supportive material for articular cartilage regeneration. Biocybern. Biomed. Eng. 2010, 30, 65–76. [Google Scholar]
- Chwojnowski, A.; Dudziński, K. Method for producing semipermeable polysulfone and polyethersulfone membranes and their applications. Patent: PL 211793 B1, 2012. [Google Scholar]
- Steinwachs, M. Collagen Membrane for Articular Cartilage Repair: Chondro-Gide. Geistlich Company: Freiburg, Germany.
- Harris, J.D.; Flanigan, D.C. Management of knee articular cartilage injuries. In Modern Arthroscopy; Dragoo, J.L., Ed.; InTech: Rijeka, Croatia, 2011; Volume 6, pp. 103–128. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 1993, 15, 532–537. [Google Scholar]
- Cai, H.; Wang, P.; Xu, Y.; Yao, Y.; Liu, J.; Li, T.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. BMSCs-assisted injectable Col i hydrogel-regenerated cartilage defect by reconstructing superficial and calcified cartilage. Regen. Biomater. 2019, 7, 35–45. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Marx, R.G.; Beaton, D.E.; Miura, Y.; Gallay, S.H.; Fitzsimmons, J.S. Validation of a simple histological-histochemical cartilage scoring system. Tissue Eng. 2001, 7, 313–320. [Google Scholar] [CrossRef]
- Metineren, H.; Dülgeroğlu, T.C. Regenerative effect of platelet-rich fibrin on articular cartilage defects in an experimental rat model. Eur. Res. J. 2018, 5, 299–305. [Google Scholar] [CrossRef]
- Speer, D.P.; Chvapil, M.; Volz, R.G.; Holmes, M.D. Enhancement of healing in osteochondral defects by collagen sponge implants. Clin. Orthop. Relat. Res. 1979, 144, 326–335. [Google Scholar] [CrossRef]
- Minns, R.J.; Muckle, D.S. Mechanical and histological response of carbon fibre pads implanted in the rabbit patella. Biomaterials 1989, 10, 273–276. [Google Scholar] [CrossRef] [PubMed]
- AR, C. Arthroplasties using compressed ivalon sponge. South. Med. J. 1960, 53, 957–960. [Google Scholar]
- Burmester, G.R.; Menche, D.; Merryman, P.; Klein, M.; Winchester, R. Application of monoclonal antibodies to the characterization of cells eluted from human articular cartilage. Expression of Ia Antigens in Certain Diseases and Identification of an 85-kD Cell Surface Molecule Accumulated in the Pericellular Matrix. Arthritis Rheum. 1983, 26, 1187–1195. [Google Scholar] [CrossRef]
- Lance, E.M.; Kimura, L.H.; Manibog, C.N. The expression of major histocompatibility antigens on human articular chondrocytes. Clin. Ortopaedics Relat. Res. 1993, 291, 266–282. [Google Scholar] [CrossRef]
- Moskalewski, S.; Kawiak, J. Cartilage Formation after Homotransplatation of Isolated Chondrocytes.pdf. Transplantation 1965, 3, 737–747. [Google Scholar] [CrossRef]
- Moskalewski, S.; Osiecka-Iwan, A.; Hyc, A. Cartilage produced after transplantation of syngeneic chondrocytes is rejected in rats presensitized with allogeneic chondrocytes. Cell Transplant. 2001, 10, 625–632. [Google Scholar] [CrossRef]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)-5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Nilsson, A.; Lindahl, A.; Ohlsson, C.; Peterson, L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin. Orthop. Relat. Res. 1996, 326, 270–283. [Google Scholar] [CrossRef]
- Katsube, K.; Ochi, M.; Uchio, Y.; Maniwa, S.; Matsusaki, M.; Tobita, M.; Iwasa, J. Repair of articular cartilage defects with cultured chondrocytes in Atelocollagen gel. Comparison with cultured chondrocytes in suspension. Arch. Orthop. Trauma Surg. 2000, 120, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R. Yearbook of Cell and Tissue Transplatation; Lanz, R.P., Chick, W.L., Eds.; Kulwer Academic: Hingham, MA, USA, 2021; ISBN 9789401065603. [Google Scholar]
- Matmati, M.; Ng, T.F.; Rosenzweig, D.H.; Quinn, T.M. Protection of bovine chondrocyte phenotype by heat inactivation of allogeneic serum in monolayer expansion cultures. Ann. Biomed. Eng. 2013, 41, 894–903. [Google Scholar] [CrossRef]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Tgfb-, E.; Verhaar, J.A.N. Optimization of chondrocyte expansion in culture. Acta Orthop. Scand. 1999, 70, 55–61. [Google Scholar]
- Barone, L.M. LEESA M. BARONE, Genzyme Tissue Repair. Ph.D. Thesis, Cambridge, MA, USA, 1999. [Google Scholar]
- Im, G.I.; Kim, D.Y.; Shin, J.H.; Hyun, C.W.; Cho, W.H. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J. Bone Jt. Surg. Ser. B 2001, 83, 289–294. [Google Scholar] [CrossRef]
- Von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesized as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- Pulkkinen, H.J.; Tiitu, V.; Valonen, P.; Jurvelin, J.S.; Rieppo, L.; Töyräs, J.; Silvast, T.S.; Lammi, M.J.; Kiviranta, I. Repair of osteochondral defects with recombinant human type II collagen gel and autologous chondrocytes in rabbit. Osteoarthr. Cartil. 2013, 21, 481–490. [Google Scholar] [CrossRef]
- Moskalewski, S.; Osiecka-iwan, A.; Jozwiak, J. Mechanical Barrier as a Protection Against Rejection of Allogeneic Cartilage Formed in Joint Surface Defects in Rats. Cell Transplant. 2000, 9, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, I.; Lips, K.S.; Sommer, U.; Schappat, I.; Martin, A.P.; Szalay, G.; Hartmann, S.; Schnettler, R. Biphasic scaffolds for repair of deep osteochondral defects in a sheep model. J. Surg. Res. 2013, 183, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Odabas, S.; Feichtinger, G.A.; Korkusuz, P.; Inci, I.; Bilgic, E.; Yar, A.S.; Cavusoglu, T.; Menevse, S. Auricular cartilage repair using cryogel scaffolds loaded with BMP-7-expressing primary chondrocytes. J. Tissue Eng. Regen. Med. 2013, 7, 831–840. [Google Scholar] [CrossRef] [PubMed]








| Category | Subcategory | Characteristic | Score |
|---|---|---|---|
| 1. Nature of predominant tissue | Cellular morphology | Hyaline articular cartilage | 4 |
| Young hyaline cartilage | 3 | ||
| Incompletely differentiated mesenchyme | 2 | ||
| Fibrous cartilage | 1 | ||
| Fibrous tissue or bone | 0 | ||
| 2. Structural characteristics | Surface regularity | Smooth and intact | 3 |
| Superficial horizontal lamination | 2 | ||
| Fissures 25–100% of the thickness | 1 | ||
| Severe disruption including fibrillation | 0 | ||
| Structural integrity | Normal | 2 | |
| Slight disruption including cysts | 1 | ||
| Severe disintegration | 0 | ||
| Thickness | 100% of normal adjacent cartilage | 2 | |
| 50–99% of normal cartilage | 1 | ||
| 0–50% of normal cartilage | 0 | ||
| Bonding to the adjacent cartilage | Bonded at both ends of graft | 2 | |
| Bonded at one end or partially at both ends | 1 | ||
| Not bonded | 0 | ||
| 3. Freedom from cellular changes of degeneration | Hypocellularity | Normal cellularity | 3 |
| Slight hypocellularity | 2 | ||
| Moderate hypocellularity | 1 | ||
| Severe hypocellularity | 0 | ||
| Degenerative changes | None | 2 | |
| Moderate | 1 | ||
| Significant changes | 0 | ||
| 4. Subchondral bone reconstruction | 100% | 2 | |
| 50–99% | 1 | ||
| <50% | 0 | ||
| Number of PES Scaffold | Tissue Mass Content [mg] |
|---|---|
| 1 | 0.28 |
| 2 | 0.19 |
| 3 | 0.25 |
| 4 | 0.21 |
| 12 Weeks | 25 Weeks | 52 Weeks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | I | II | III | IV | V | I | II | III | IV | V | I | II | III | IV | V |
| mean | 8.3 | 8.6 | 6.8 | 8 | 1.7 | 8.2 | 7.3 | 7 | 7.2 | 1.8 | 9.4 | 8.5 | 7 | 6.8 | 1.5 |
| SD | 1.5 | 2.8 | 1.3 | 2.4 | 1 | 1.7 | 2.6 | 1.3 | 2 | 1.3 | 1.5 | 2.5 | 2.1 | 2.1 | 1.1 |
| median value | 8 | 10 | 7 | 9 | 2 | 8 | 9 | 7 | 7 | 2 | 9 | 8.5 | 7 | 7 | 1.5 |
| Groups | Level of Statistical Significance of the Differences between Groups. Highlighted Fields Indicate Statistically Significant Differences (p ≤ 0.05) | ||
|---|---|---|---|
| 12 Weeks | 25 Weeks | 52 Weeks | |
| I/II | 0.369 | 0.462 | 0.473 |
| I/III | 0.083 | 0.178 | 0.030 |
| I/IV | 0.930 | 0.354 | 0.029 |
| II/III | 0.083 | 0.534 | 0.178 |
| II/IV | 0.514 | 0.806 | 0.131 |
| III/IV | 0.312 | 0.962 | 0.962 |
| Groups | Level of Statistical Significance of the Difference between Observation Times Highlighted Fields Indicate Statistically Significant Differences (p ≤ 0.05) | ||
|---|---|---|---|
| 12 Weeks ↔ 25 Weeks | 12 Weeks ↔ 52 Weeks | 25 Weeks ↔ 52 Weeks | |
| I | 0.895 | 0.153 | 0.178 |
| II | 0.174 | 0.910 | 0.385 |
| III | 0.834 | 0.962 | 0.885 |
| IV | 0.596 | 0.470 | 0.700 |
| 12 Weeks | 25 Weeks | 52 Weeks | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | I | II | III | IV | V | I | II | III | IV | V | I | II | III | IV | V |
| mean | 14 | 14 | 13 | 14 | 4 | 15 | 13 | 13 | 12 | 4 | 16 | 14 | 13 | 13 | 4 |
| SD | 1.5 | 6 | 3 | 4.4 | 1.2 | 1.7 | 4.7 | 2.9 | 3.4 | 2.2 | 2.1 | 4.3 | 4.1 | 3.2 | 1.1 |
| median value | 14 | 16 | 13.5 | 15 | 3 | 15 | 14.5 | 14.5 | 12 | 3 | 16.5 | 14.5 | 13 | 13.5 | 4.5 |
| Groups | The Level of Statistical Significance of Differences Between Groups. Highlighted Fields Indicate Statistically Significant Differences (p ≤ 0.05) | ||
|---|---|---|---|
| 12 Weeks | 25 Weeks | 52 Weeks | |
| I/II | 0.221 | 0.462 | 0.326 |
| I/III | 0.441 | 0.441 | 0.066 |
| I/IV | 0.895 | 0.064 | 0.046 |
| II/III | 0.198 | 0.756 | 0.391 |
| II/IV | 0.596 | 0.744 | 0.424 |
| III/IV | 0.564 | 0.501 | 0.885 |
| Groups | Level of Statistical Significance of the Difference between the Observation Times Highlighted Fields Indicate Statistically Significant Differences (p ≤ 0.05) | ||
|---|---|---|---|
| 12 Weeks ↔ 25 Weeks | 12 Weeks ↔ 52 Weeks | 25 Weeks ↔ 52 Weeks | |
| I | 0.233 | 0.018 | 0.121 |
| II | 0.307 | 0.650 | 0.406 |
| III | 0.431 | 0.847 | 0.847 |
| IV | 0.536 | 0.847 | 0.700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płończak, M.; Wasyłeczko, M.; Jakutowicz, T.; Chwojnowski, A.; Czubak, J. Intraarticular Implantation of Autologous Chondrocytes Placed on Collagen or Polyethersulfone Scaffolds: An Experimental Study in Rabbits. Polymers 2023, 15, 2360. https://doi.org/10.3390/polym15102360
Płończak M, Wasyłeczko M, Jakutowicz T, Chwojnowski A, Czubak J. Intraarticular Implantation of Autologous Chondrocytes Placed on Collagen or Polyethersulfone Scaffolds: An Experimental Study in Rabbits. Polymers. 2023; 15(10):2360. https://doi.org/10.3390/polym15102360
Chicago/Turabian StylePłończak, Maciej, Monika Wasyłeczko, Tomasz Jakutowicz, Andrzej Chwojnowski, and Jarosław Czubak. 2023. "Intraarticular Implantation of Autologous Chondrocytes Placed on Collagen or Polyethersulfone Scaffolds: An Experimental Study in Rabbits" Polymers 15, no. 10: 2360. https://doi.org/10.3390/polym15102360
APA StylePłończak, M., Wasyłeczko, M., Jakutowicz, T., Chwojnowski, A., & Czubak, J. (2023). Intraarticular Implantation of Autologous Chondrocytes Placed on Collagen or Polyethersulfone Scaffolds: An Experimental Study in Rabbits. Polymers, 15(10), 2360. https://doi.org/10.3390/polym15102360