Analysis of the Influencing Factors of the Efficient Degradation of Waste Polyurethane and Its Scheme Optimization
Abstract
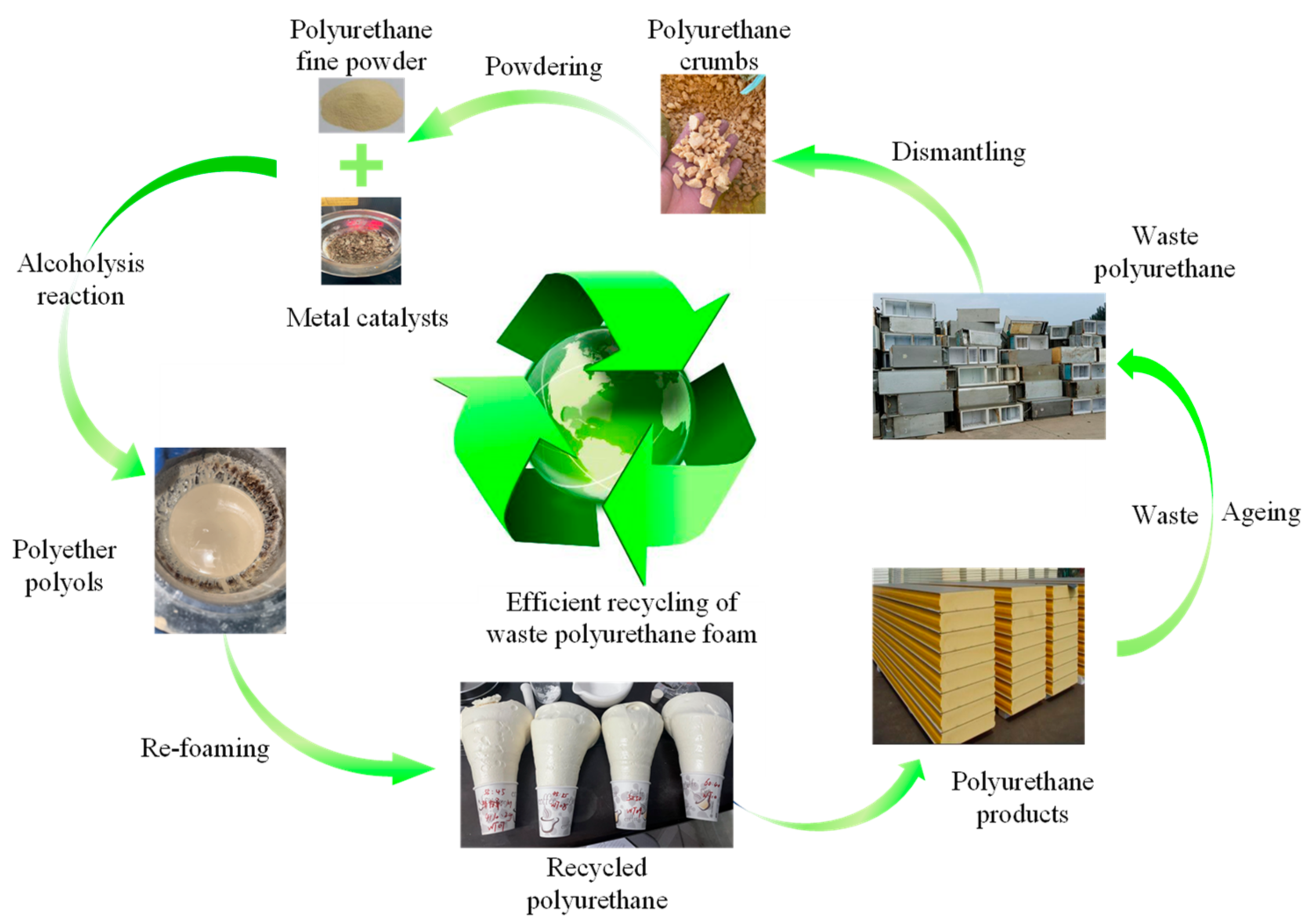
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Analytical Instruments and Methods
2.2.1. Fourier-Transform Infrared Spectrometer (FTIR)
2.2.2. Scanning Electron Microscope (SEM)
2.2.3. Thermal Gravimetric Analyzer (TG)
2.2.4. Other Analytical Instruments
2.3. Analytical Instruments and Methods
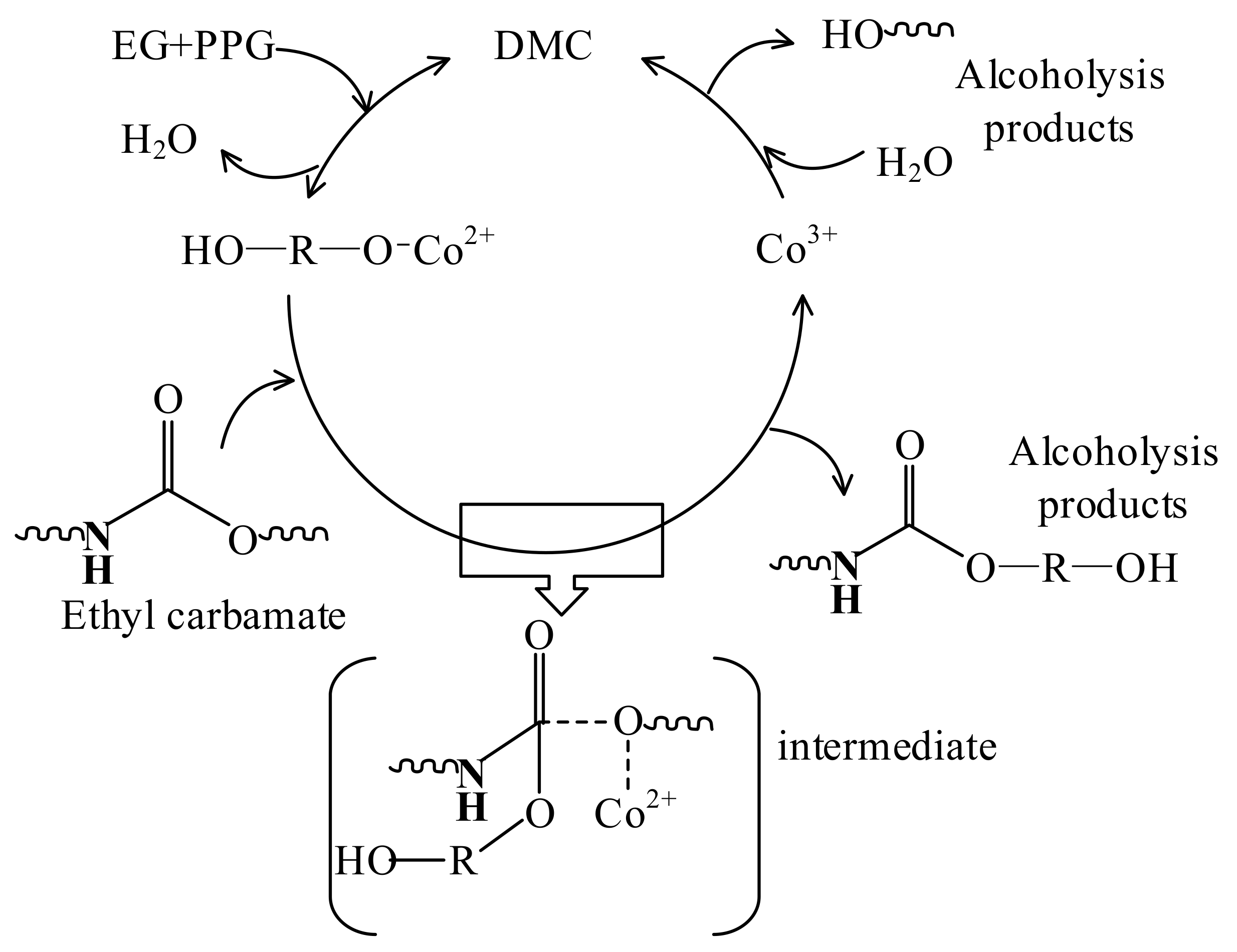
2.3.1. Analysis of the Mechanism of Waste Polyurethane Catalytic Degradation
2.3.2. Preparation of Recycled Polyurethane Foam
2.3.3. Preparation and Catalytic Mechanism of DMC
3. Results and Discussion
3.1. Analysis of Waste Polyurethane Foam Raw Materials
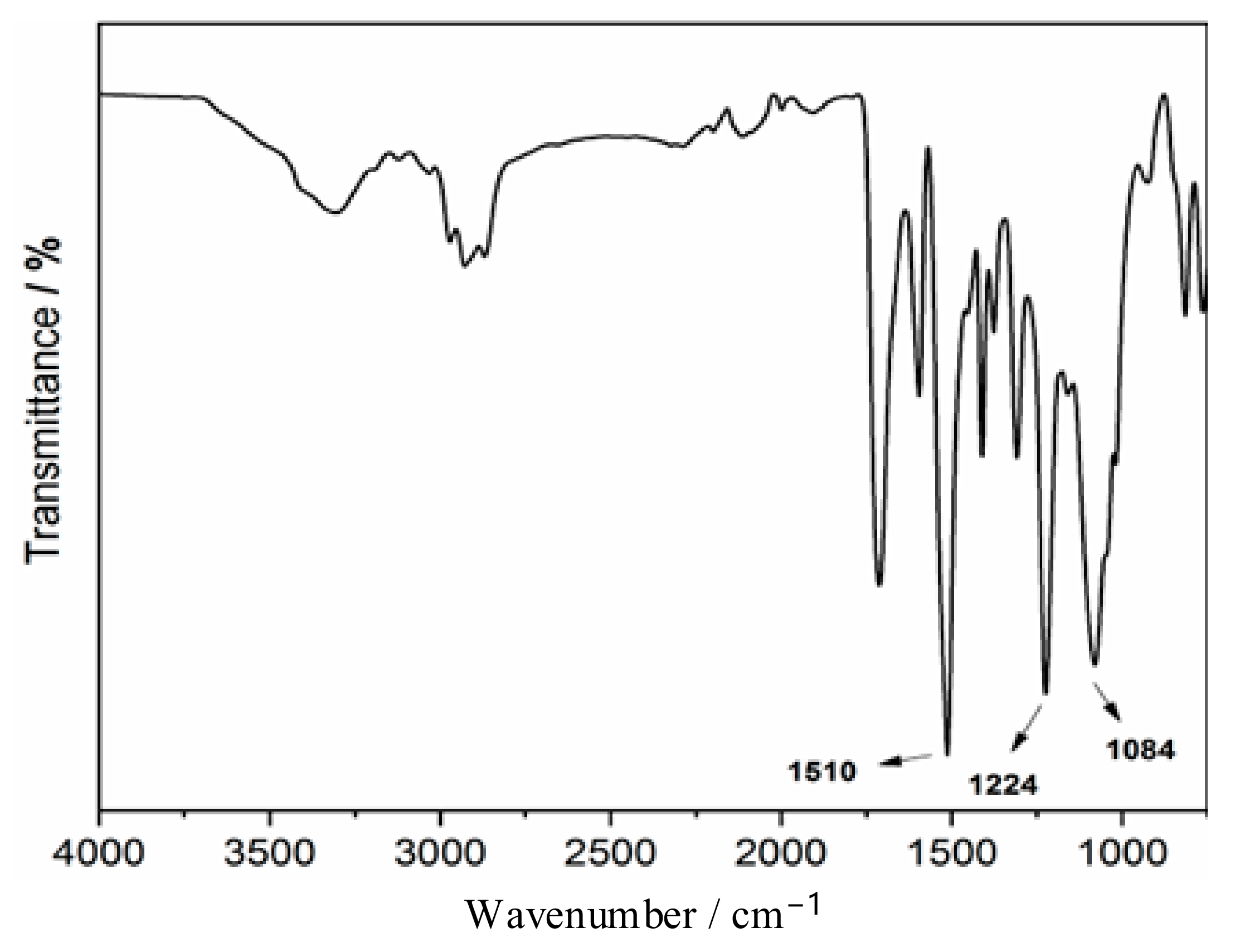
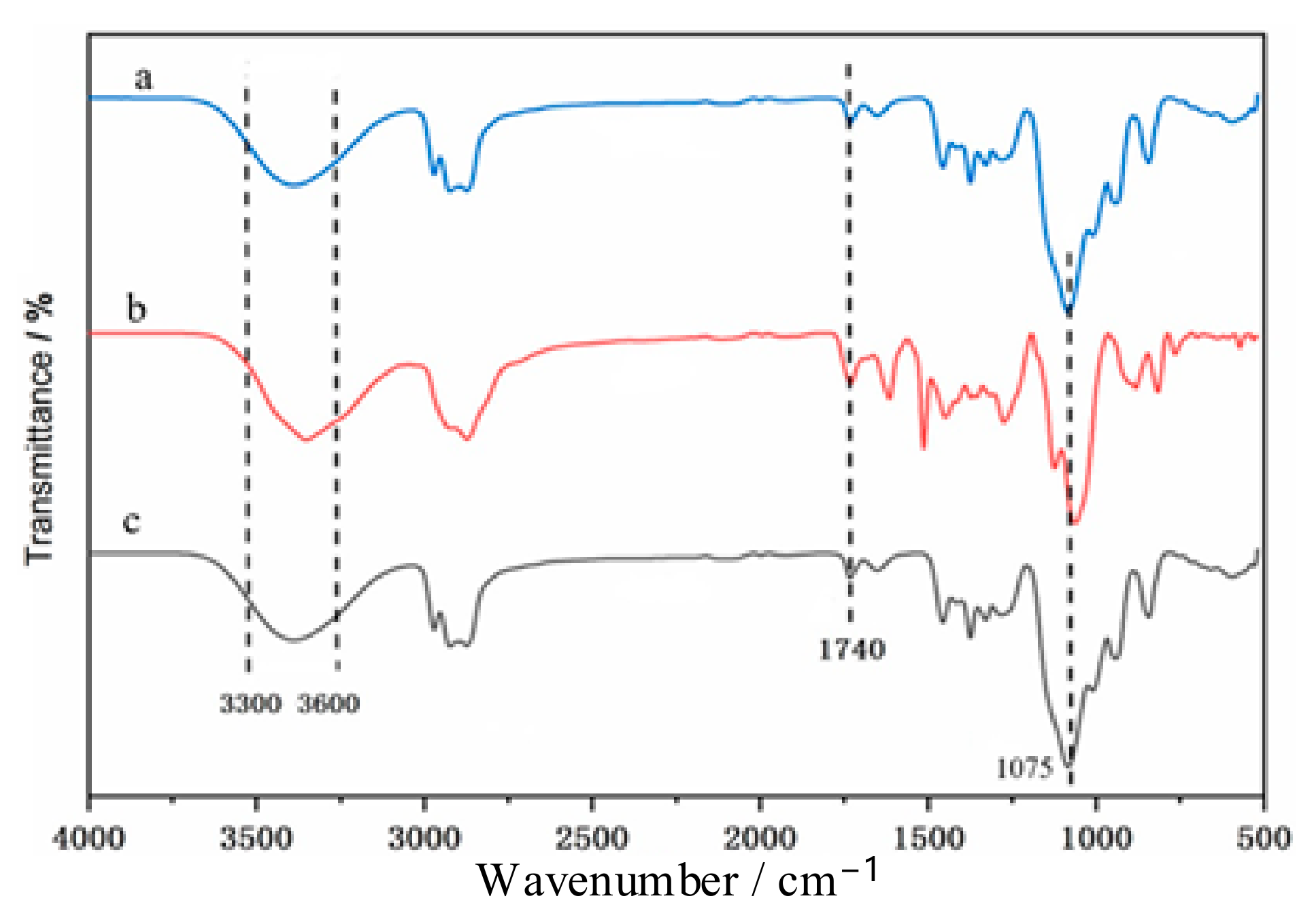
3.1.1. Infrared Analysis
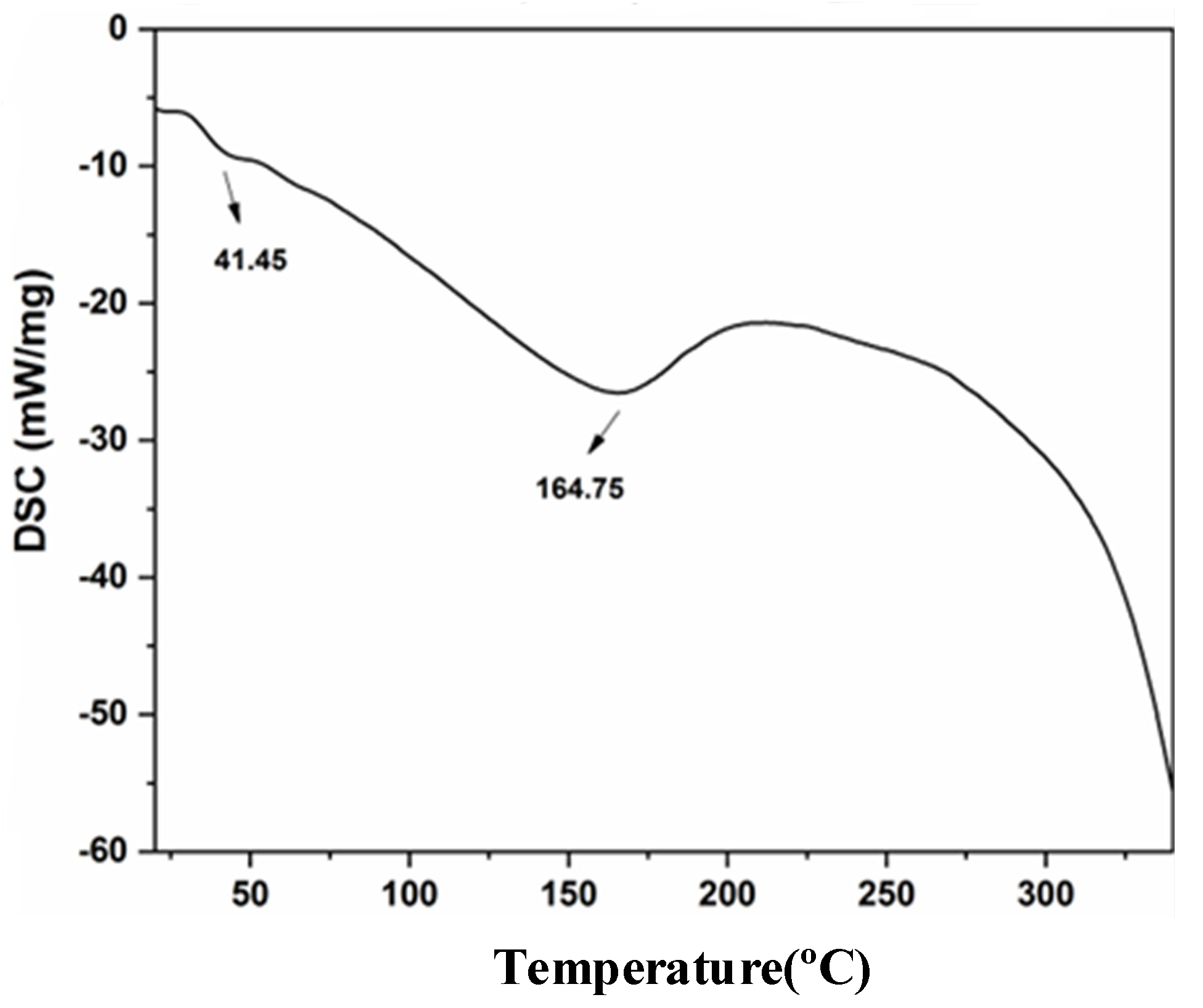
3.1.2. Analysis of Differential Scanning Calorimetry
3.2. Waste Polyurethane Degradation Material Situation
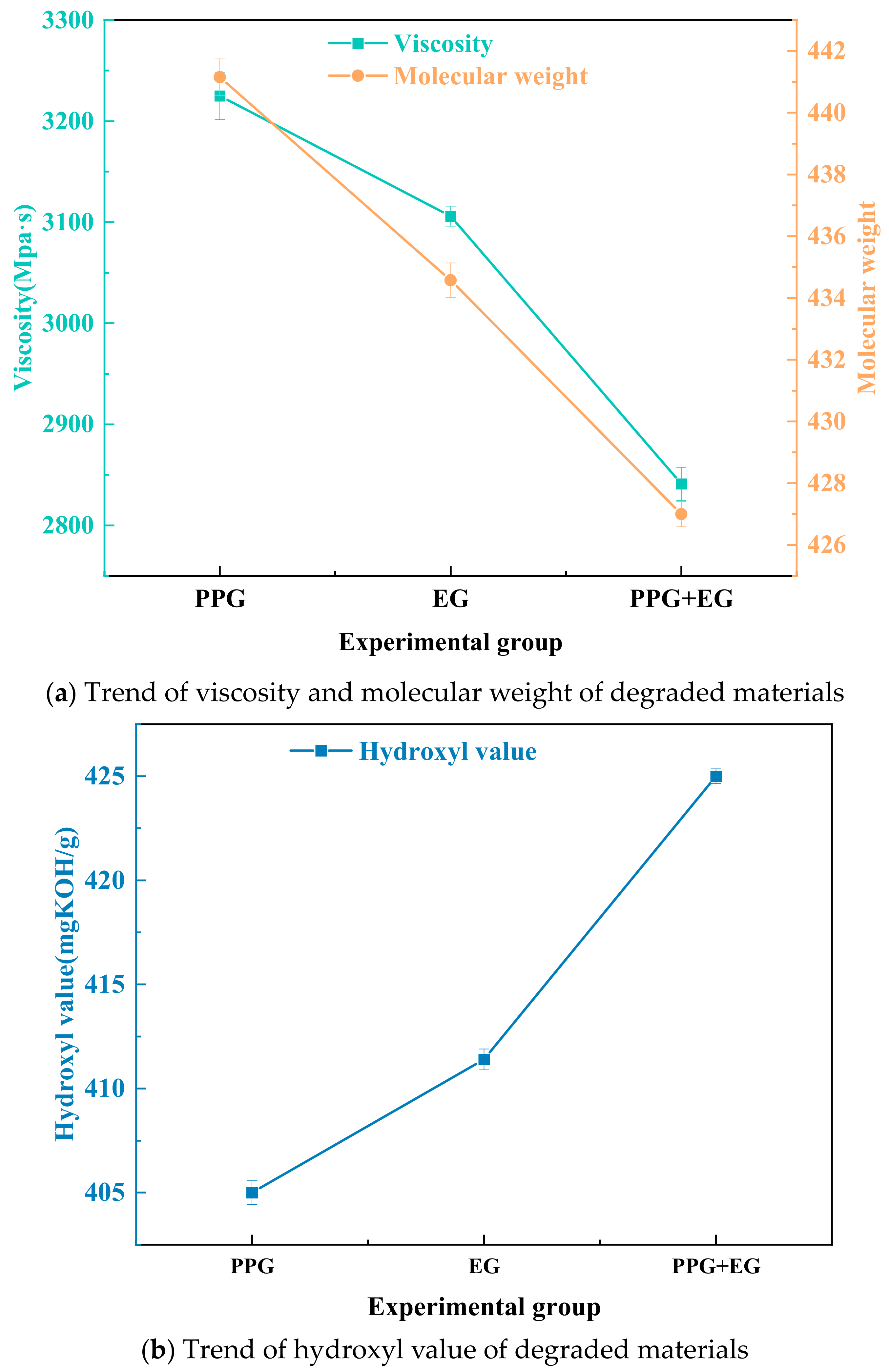
3.3. Effect of Two-Component Alcoholic Solvents on Degradation Materials and Analysis of Degradation Product Properties
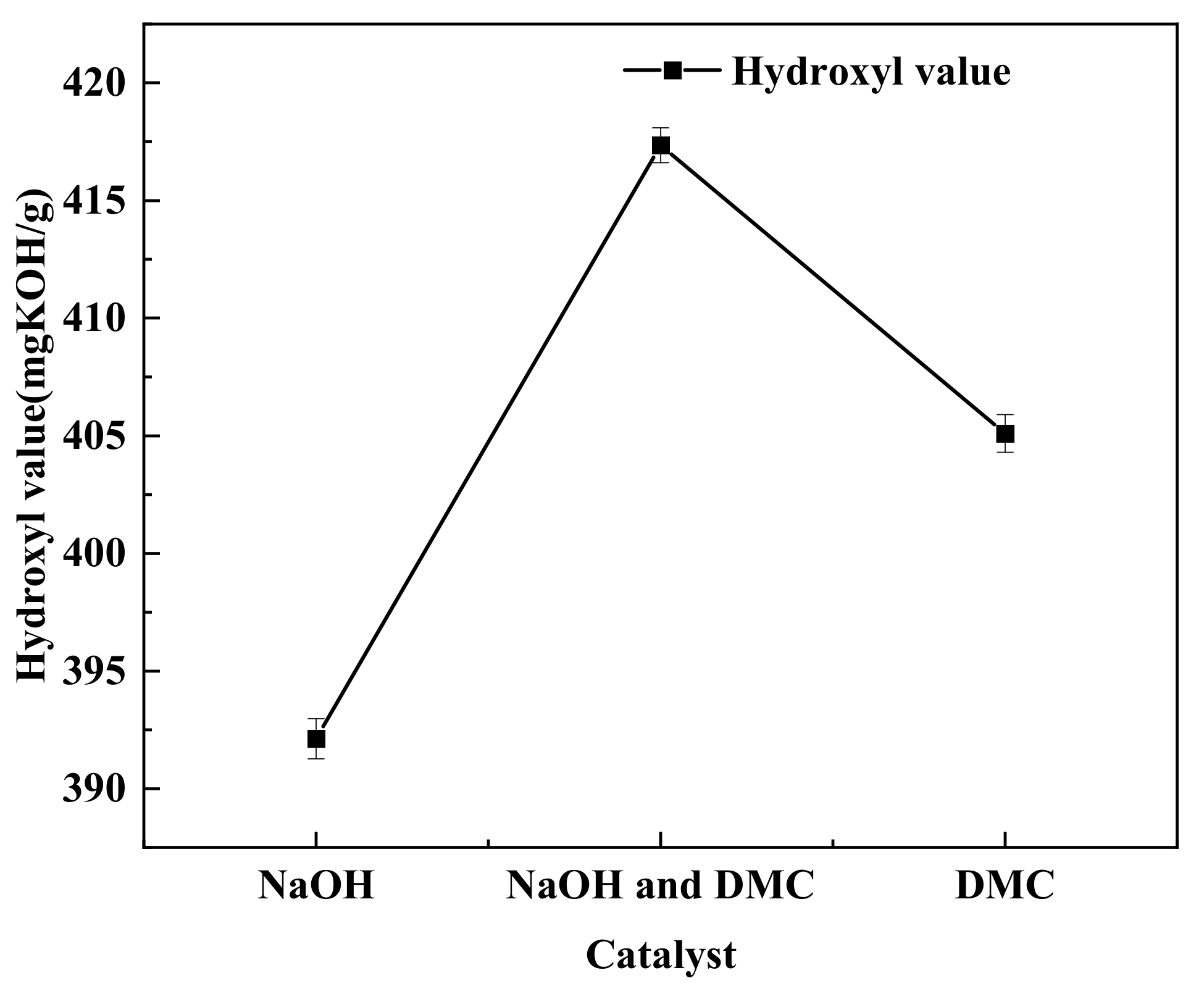
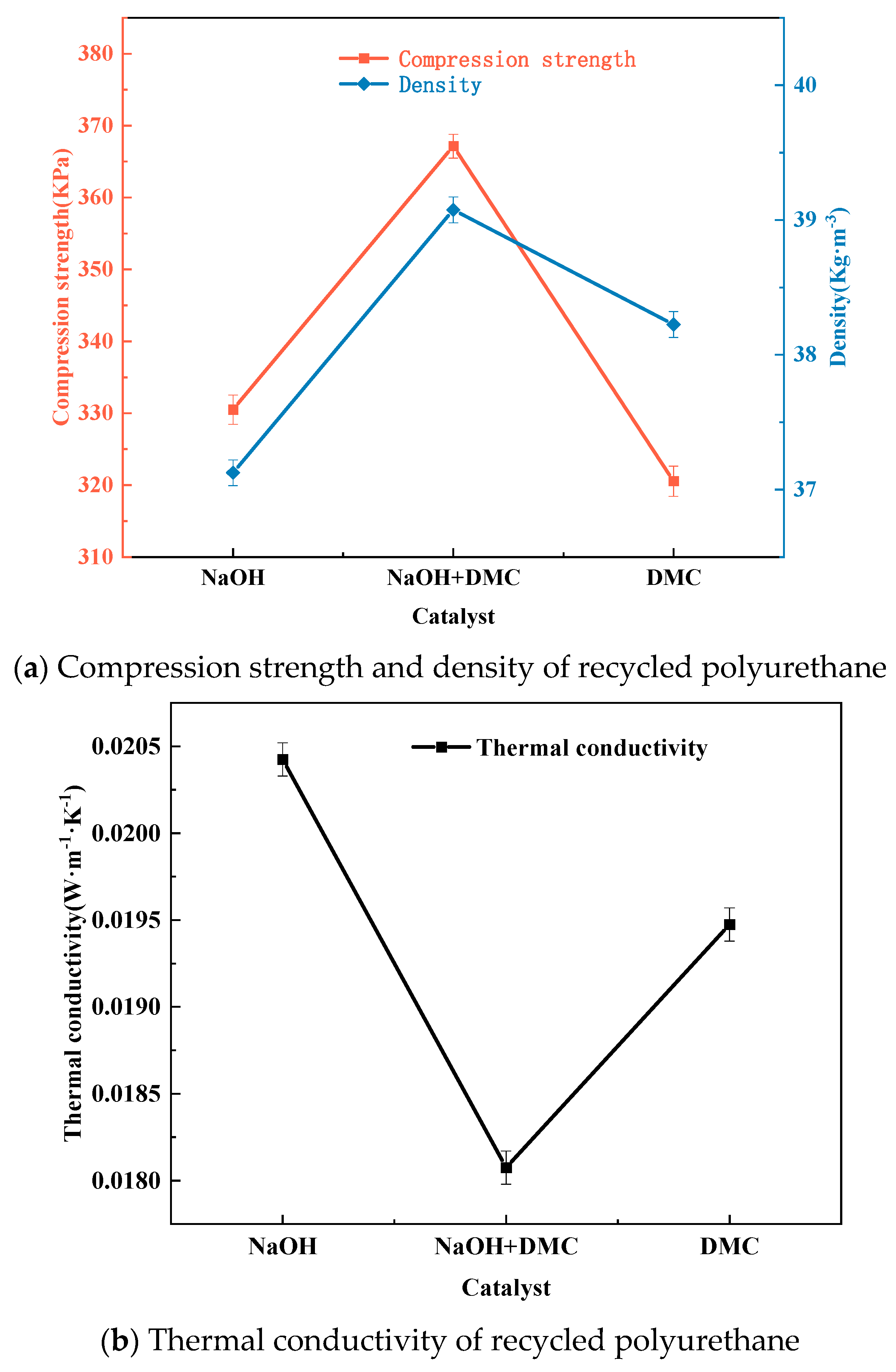
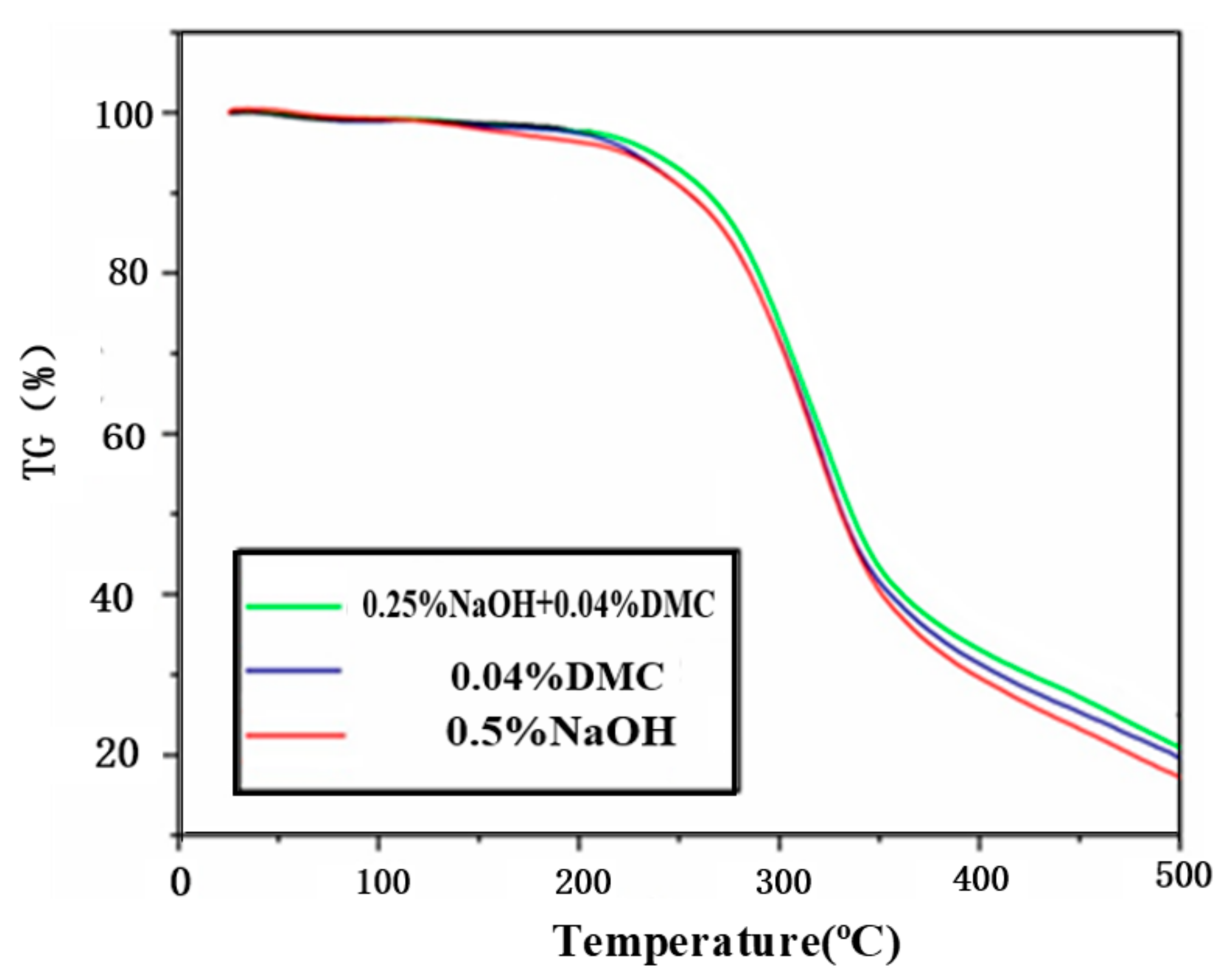
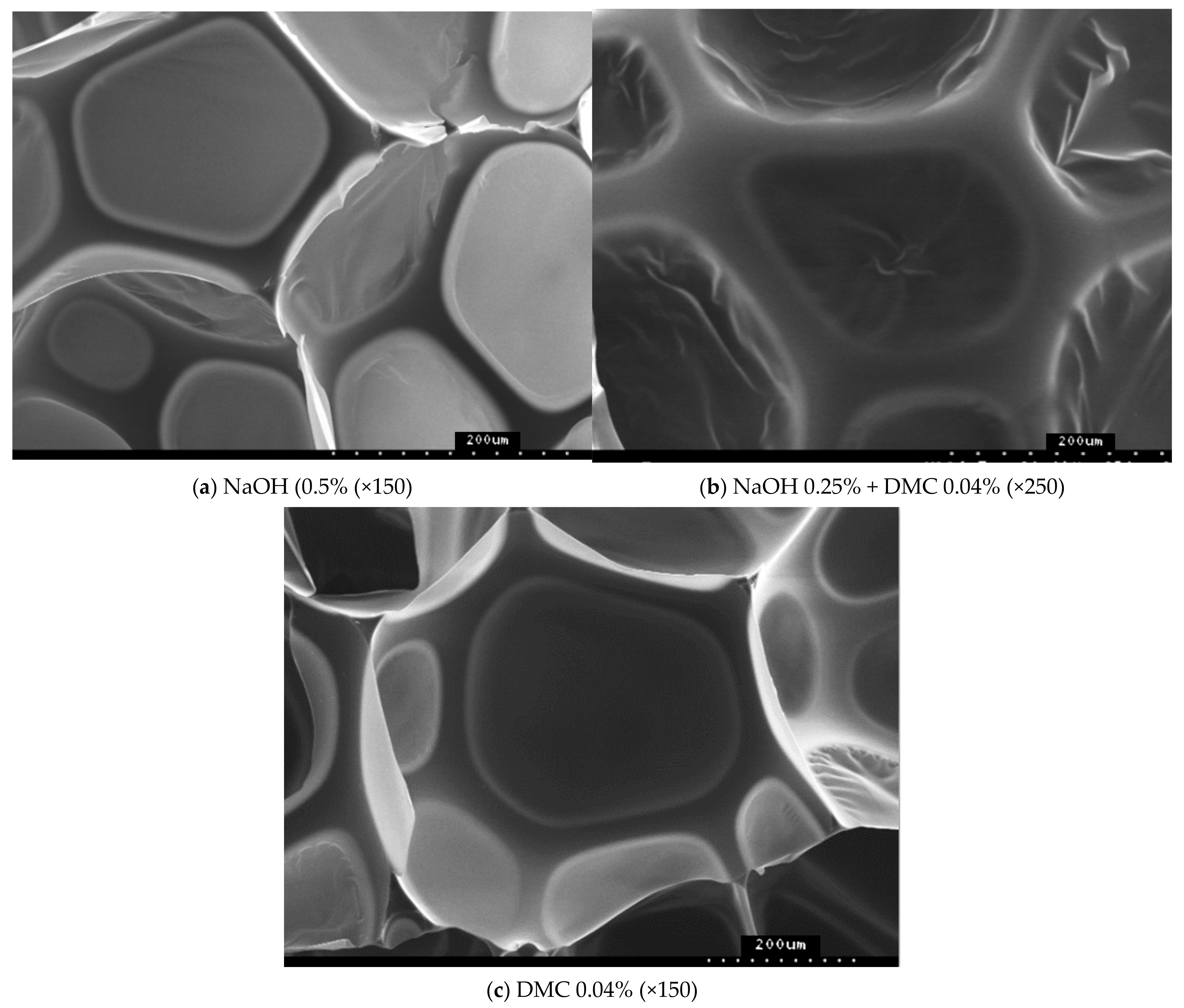
3.4. Effect of Catalysts on the Degradation of Waste Polyurethane
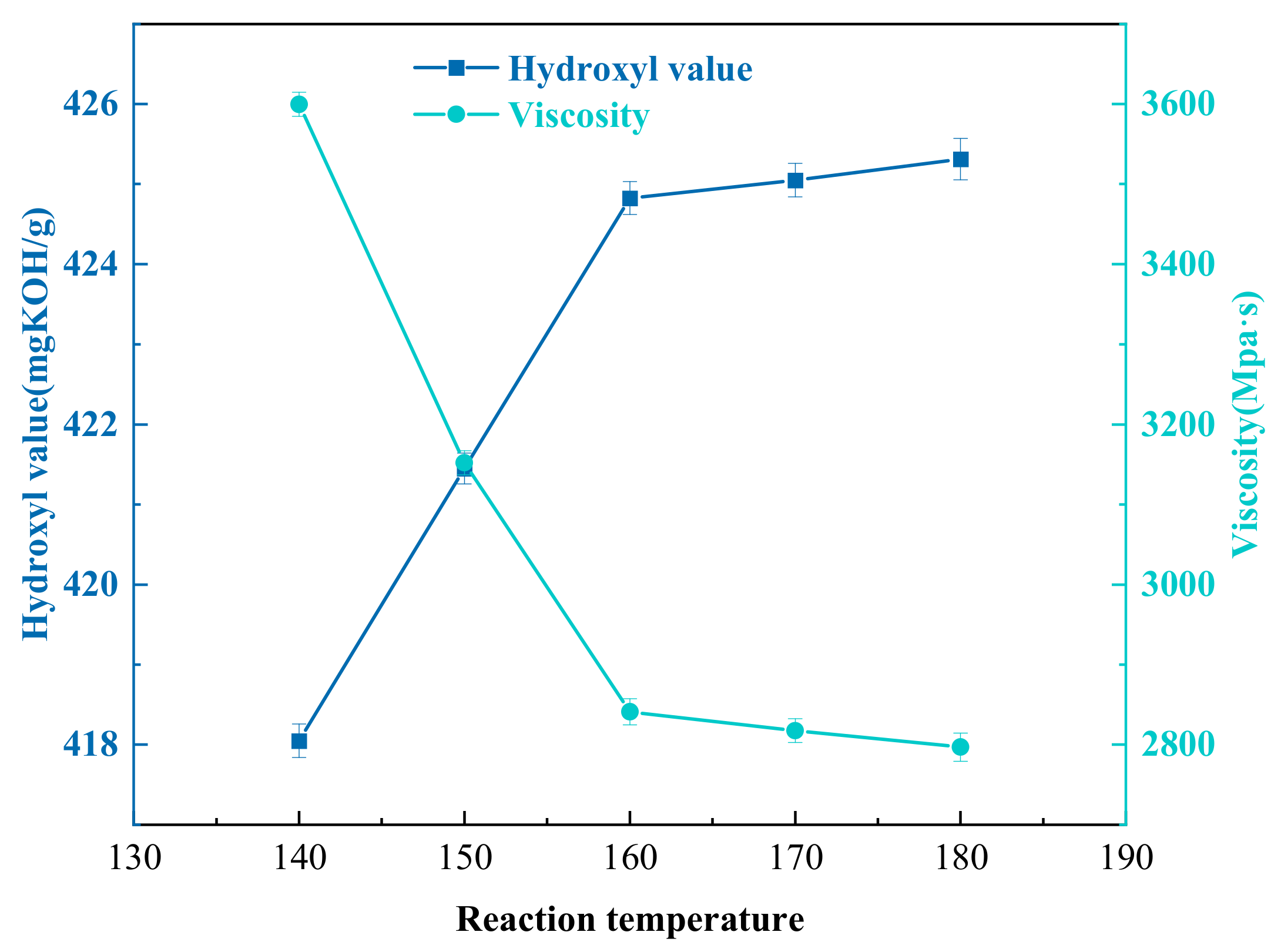
3.5. Effect of Reaction Temperature on the Degradation of Waste Polyurethane
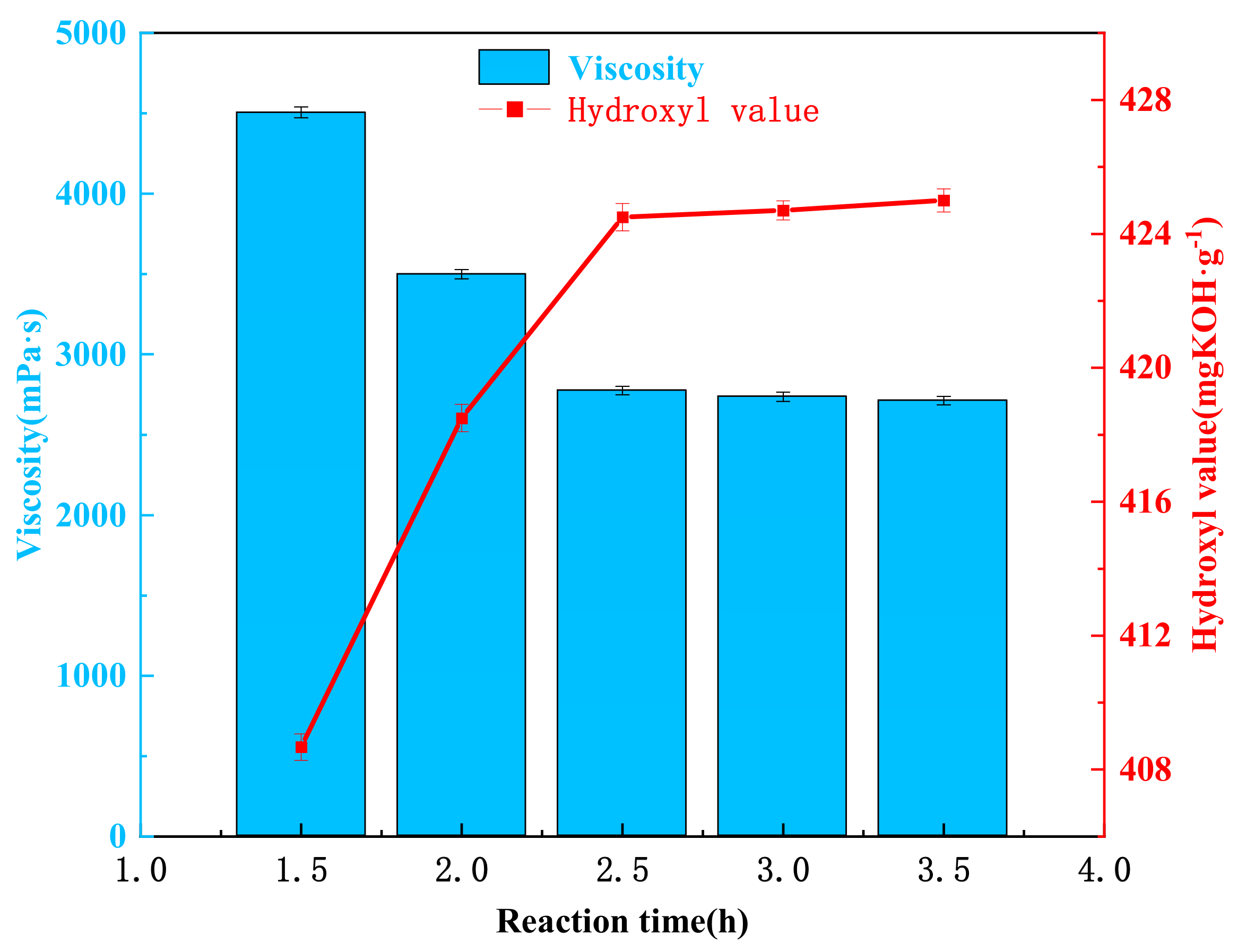
3.6. Effect of Reaction Time on the Degradation of Waste Polyurethane
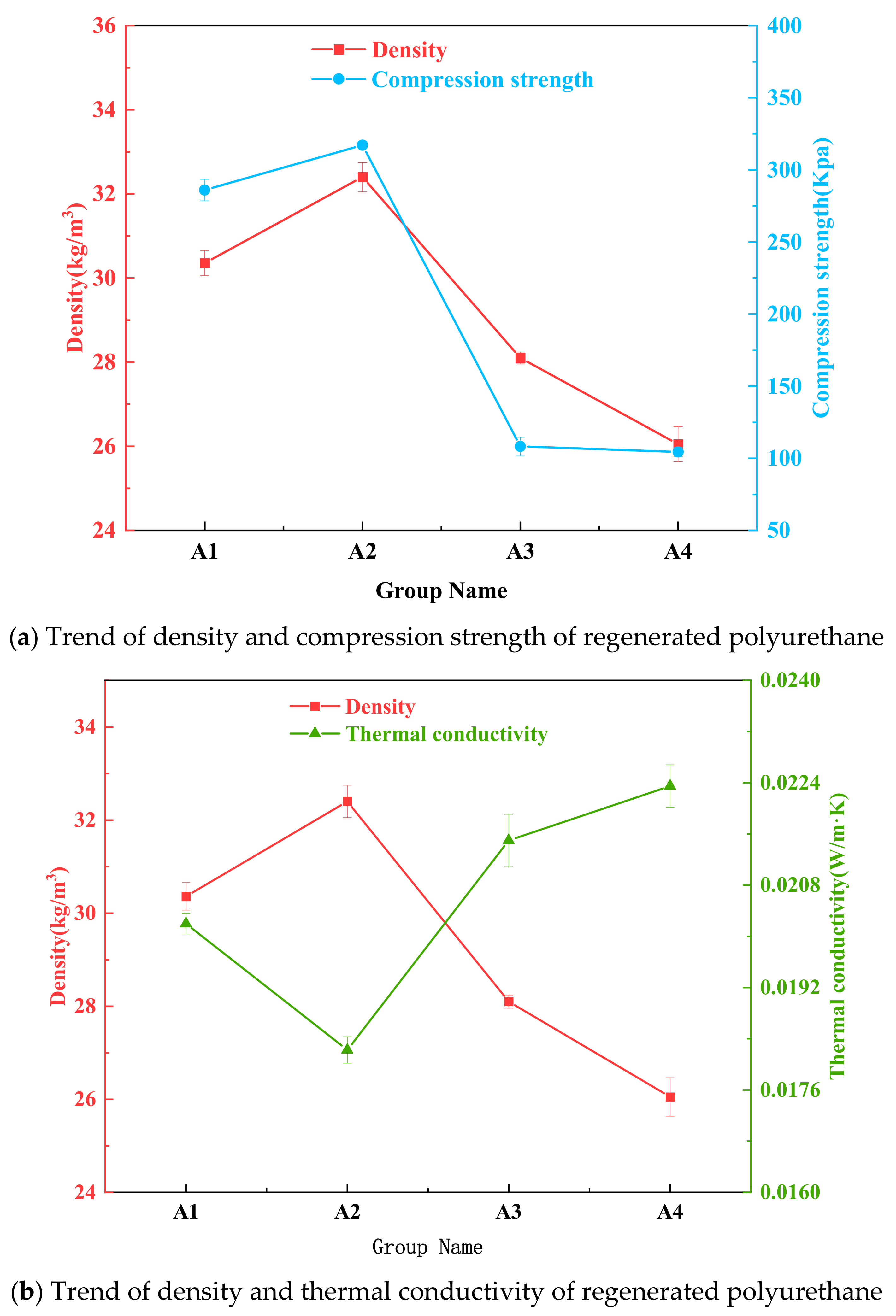
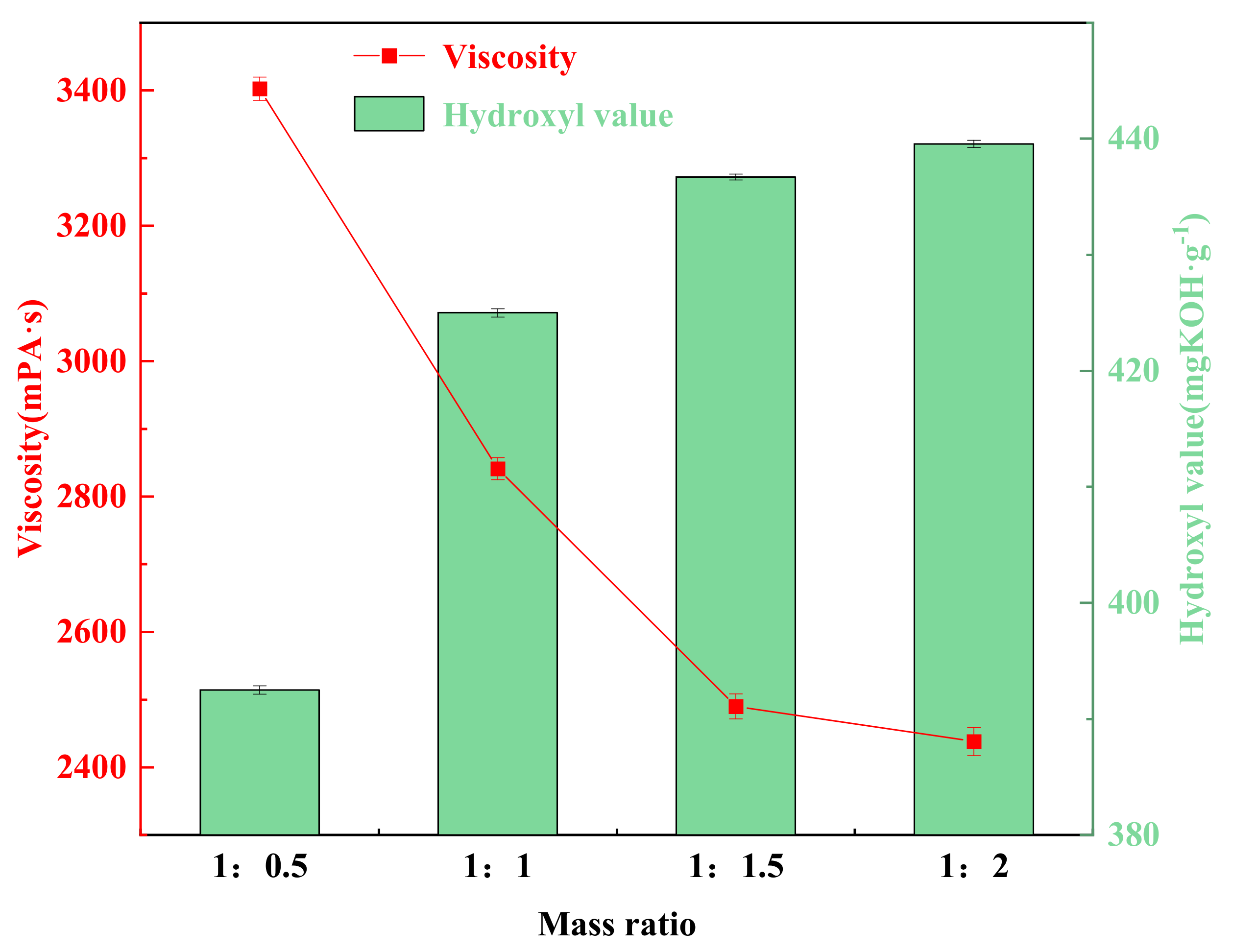
3.7. Effect of Mass Ratio of Waste Polyurethane Foam to Two-Component Alcoholic Solvents on the Degradation of Waste Polyurethane
3.8. Infrared Analysis of Waste Polyurethane Foam Degradation Material under Optimized Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simón, D.; Borreguero, A.; Lucas, A.; Rodríguez, J. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Somarathna, H.; Raman, S.; Mohotti, D.; Mutalib, A.; Badri, K. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Abhijit, D.; Prakash, M. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, J.; Zhang, X.; Peng, H. Polyurethane foam based composite phase change microcapsules with reinforced thermal conductivity for cold energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129875. [Google Scholar] [CrossRef]
- Behrooz, M.; Mohsen, R.; Mohammad, Y. Thermoeconomic analysis for determining optimal insulation thickness for new composite prefabricated wall block as an external wall member in buildings. J. Build. Eng. 2020, 31, 101354. [Google Scholar] [CrossRef]
- Antonio, S.; Carmen, D.; Konstantin, V.; Ángela, B. Providing a feasible energy retrofitting technique based on polyurethane foam injection to improve windows performance in the building stock. Energy Build. 2023, 278, 112595. [Google Scholar] [CrossRef]
- El-Sayed, A.; Abdou, A.; Rashad, E. On the cathodic protection of thermally insulated pipelines. Eng. Fail. Anal. 2009, 16, 2047–2053. [Google Scholar] [CrossRef]
- Zhu, H.; Dai, S.; Cao, J.; Bai, H.; Zhong, Y.; Zhang, Z.; Cheng, G.; Yuan, N.; Ding, J. A high-performance textile pressure sensor based on carbon black/carbon nanotube-polyurethane coated fabrics with porous structure for monitoring human motion. Mater. Today Commun. 2022, 33, 104541. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Wang, L.; Wang, W.; Liu, S.; Wang, C.; Xu, Z.; Liu, L.; Qian, X. Current advances of Polyurethane/Graphene composites and its prospects in synthetic leather: A review. Eur. Polym. J. 2021, 161, 110837. [Google Scholar] [CrossRef]
- Saad, R.; Ehsan, B.; Reza, J.; Gelareh, M. Superhydrophobic and icephobic polyurethane coatings: Fundamentals, progress, challenges and opportunities. Prog. Org. Coat. 2022, 165, 106715. [Google Scholar] [CrossRef]
- Huang, G.; Yang, T.; He, Z.; Yu, L.; Xiao, H. Polyurethane as a modifier for road asphalt: A literature review. Constr. Build. Mater. 2022, 356, 129058. [Google Scholar] [CrossRef]
- Irini, G.; Margherita, T.; Federico, M.; Camilla, R.; Cristina, M.; Luisa, O.; Mariacaterina, T.; Scott, A.; Alessandro, T.; Cristina, L. Exploring the potential of polyurethane-based soft foam as cell-free scaffold for soft tissue regeneration. Acta Biomater. 2018, 73, 141–153. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, J.; Yuan, J.; Jiang, F.; Yu, X.; Xiao, F. Recent applications and developments of Polyurethane materials in pavement engineering. Constr. Build. Mater. 2021, 304, 124639. [Google Scholar] [CrossRef]
- AgileIntel Research. Global Polyurethane Market—Trends, COVID-19 Impact and Growth Forecasts to 2029. Chemintel360, AICIBUPO1119. 2022. Available online: https://www.chemintel360.com/reportdetails/Global-Polyurethane-Market/25 (accessed on 24 March 2023).
- Suhailuddin, S.; Aprajith, K.; Sanjay, B.; Shabeeruddin, S.; Begum, S. Development and characterization of flame retardant property in flexible polyurethane foam. Mater. Today Proc. 2022, 59, 819–826. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, W.; Liu, X.; Wang, Y.; Han, D.; Zhang, Q. Effect of bio-based polyols and chain extender on the microphase separation structure, mechanical properties and morphology of rigid polyurethane foams. Eur. Polym. J. 2022, 179, 111572. [Google Scholar] [CrossRef]
- Lu, J.; Concellón, A.; Wang, P.; Swager, T.; Hsieh, A. Supramolecular hierarchical polyurethane elastomers for thermal and mechanical property optimization. Polymer 2022, 260, 125363. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of different types of polyurethane foam wastes via acidolysis to produce polyurethane coatings. Sustain. Mater. Technol. 2021, 29, e00330. [Google Scholar] [CrossRef]
- Du, L.; Liu, Z.; Ye, Z.; Hao, X.; Ou, R.; Liu, T.; Wang, Q. Dynamic cross-linked polyurethane hot-melt adhesive with high biomass content and high adhesive strength simultaneously. Eur. Polym. J. 2023, 182, 111732. [Google Scholar] [CrossRef]
- Mehmet Emin Çetin. Investigation of carbon nanotube reinforcement to polyurethane adhesive for improving impact performance of carbon fiber composite sandwich panels. Int. J. Adhes. Adhes. 2022, 112, 103002. [Google Scholar] [CrossRef]
- BCC Research. Polyurethane Global Market Report 2022. BCC Research, TBR691C. 2022. Available online: https://www.bccresearch.com (accessed on 24 March 2023).
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef]
- Cregut, M.; Bedas, M.; Durand, M.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2013, 13, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, X.; Chen, R.; Mao, X.; Qi, X. The United States and China on the paths and policies to carbon neutrality. J. Environ. Manag. 2022, 320, 115785. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, K.; Kang, J.; Chen, W.; Wang, X.; Zhang, X. Policy and Management of Carbon Peaking and Carbon Neutrality: A Literature Review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Hu, P.; Kumar, A.; Gharibi, R.; Agarwal, S. Tailor-made compostable polyurethanes. Polym. Chem. 2022, 13, 622–630. [Google Scholar] [CrossRef]
- Garrido, M.; Font, R.; Conesa, J. Pollutant emissions during the pyrolysis and combustion of flexible polyurethane foam. Waste Manag. 2016, 52, 138–146. [Google Scholar] [CrossRef]
- Ren, M.; Wu, W.; Shi, Q.; Wu, L.; Zhang, C. Study on the surface properties of the regenerated polyurethane foam micropowder via cryogenic pulverization and its application. J. Mater. Res. Technol. 2023, 23, 808–818. [Google Scholar] [CrossRef]
- Oenema, J.; Liu, H.; Coensel, N.; Eschenbacher, A.; Vijver, R.; Weng, J.; Li, L.; Wang, C.; Geem, K. Review on the pyrolysis products and thermal decomposition mechanisms of polyurethanes. J. Anal. Appl. Pyrolysis 2022, 168, 105723. [Google Scholar] [CrossRef]
- Njuguna, J.; Muchiri, P.; Mwema, F.; Karuri, N.; Herzog, M.; Dimitrov, K. Determination of thermo-mechanical properties of recycled polyurethane from glycolysis polyol. Sci. Afr. 2021, 12, e00755. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef]
- Valencia, L.; Rogaume, T.; Guillaume, E.; Rein, G.; Torero, J. Analysis of principal gas products during combustion of polyether polyurethane foam at different irradiance levels. Fire Saf. J. 2009, 44, 933–940. [Google Scholar] [CrossRef]
- Howard, G. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Cuenca-Romero, L.; Arroyo, R.; Alonso, Á.; Gutiérrez-González, S.; Calderón, V. Characterization properties and fire behaviour of cement blocks with recycled polyurethane roof wastes. J. Build. Eng. 2022, 50, 104075. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Caballero, J.; Candela, J.; García, A. Pyrolysis study of polyurethane. J. Anal. Appl. Pyrolysis 2001, 58, 63–77. [Google Scholar] [CrossRef]
- Cárdenas, E.; Colina, B.; Tabea, S.; Katharina, A.; Uwe, K.; Dirk, T.; Dietmar, H.; Hermann, J.; Christian, E. Toward Biorecycling: Isolation of a Soil Bacterium That Grows on a Polyurethane Oligomer and Monomer. Front. Microbiol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Xue, R.; Xu, B.; Qian, X.; Xin, F.; Blank, L.; Zhou, J.; Wei, R.; Dong, W.; et al. Biodegradation and up-cycling of polyurethanes: Progress, challenges, and prospects. Biotechnol. Adv. 2021, 48, 107730. [Google Scholar] [CrossRef]
- Gunawan, N.; Tessman, M.; Schreiman, A.; Simkovsky, R.; Samoylov, A.; Neelakantan, N.; Bemis, T.; Burkart, M.; Pomeroy, R.; Mayfield, S. Rapid biodegradation of renewable polyurethane foams with identification of associated microorganisms and decomposition products. Bioresour. Technol. Rep. 2020, 11, 100513. [Google Scholar] [CrossRef]
- Wang, G.; Santerre, J.; Labow, R. High-performance liquid chromatographic separation and tandem mass spectrometric identification of breakdown products associated with the biological hydrolysis of a biomedical polyurethane. J. Chromatogr. B Biomed. Sci. Appl. 1997, 698, 69–80. [Google Scholar] [CrossRef]
- Wang, W.; Hao, K.; Guo, X.; Liu, F.; Xu, Y.; Guo, S.; Bai, L.; Liu, G.; Qu, L.; Liu, M.; et al. Mechano-chemical rubber reclamation using aminolysis products of waste flexible polyurethane foams as the devulcanizing agent. J. Clean. Prod. 2023, 384, 135421. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.; Lucas, A.; Rodríguez, J. Glycolysis of flexible polyurethane wastes containing polymeric polyols. Polym. Degrad. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane by acidolysis: The effect of reaction conditions on the properties of the recovered polyol. Polymer 2021, 219, 123561. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R.; Tsekova, A. A novel approach to recycling of polyurethanes: Chemical degradation of flexible polyurethane foams by triethyl phosphate. Polymer 2000, 41, 7017–7022. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, W.; Wang, L.; Hao, J. Comparative study of nitrogen migration among the products from catalytic pyrolysis and gasification of waste rigid polyurethane foam. J. Anal. Appl. Pyrolysis 2016, 120, 144–153. [Google Scholar] [CrossRef]
- Jung, J.; Lee, T.; Jung, S.; Tsang, Y.; Bhatnagar, A.; Lee, S.; Song, H.; Park, W.; Kwon, E. Control of the fate of toxic pollutants from catalytic pyrolysis of polyurethane by oxidation using CO2. Chem. Eng. J. 2022, 442, 136358. [Google Scholar] [CrossRef]
- Bozi, J.; Mihályi, M.; Blazsó, M. Study on temperature dependence of catalytic thermal decomposition of polyamides and polyurethanes mixed with acidic zeolites. J. Anal. Appl. Pyrolysis 2013, 101, 103–110. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, Y.; Liu, S.; Zhu, S.; Liu, Y. Preparation of Mullite/PU Nanocomposites by Double Waste Co-Recycling. Sustainability 2022, 14, 14310. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X.; Guo, X.; Liu, S.; Li, Q.; Liu, Y. Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane. Polymers 2023, 15, 1445. [Google Scholar] [CrossRef]
- Gu, X.; Chen, P.; Wang, T.; Liu, S.; Zhu, S.; Zhu, Y.; Liu, Y. Analysis of Influencing Factors in the Preparation of Mullite Whiskers from Recovering Silicon-Rich Waste under Low-Temperature Conditions. Nanomaterials 2023, 13, 1143. [Google Scholar] [CrossRef]
- He, D.; Song, X.; Li, W.; Tang, C.; Liu, J.; Ke, Z.; Jiang, C.; Xiao, X. Active Electron Density Modulation of Co3O4-Based Catalysts Enhances their Oxygen Evolution Performance. Angew. Chem. Int. Ed. 2020, 59, 6929–6935. [Google Scholar] [CrossRef]
| Group Name | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| Proportion of EG and PPG | 70:30 | 60:40 | 50:50 | 40:60 |
| Alcoholic Solvents | Color | Status | Mobility |
|---|---|---|---|
| Ethylene glycol | Sepia | Silty | Rather poor |
| Propylene glycol | Sepia | Silty | General |
| Group Name | Mass Ratio of EG and PPG | Reaction Temperature/°C | Viscosity/Mpa·s | Compression Strength/Kpa | Molecular Weight | Density/kg·m−3 | Thermal Conductivity/W·m−1·K−1 |
|---|---|---|---|---|---|---|---|
| A1 | 7:3 | 160 | 3909.6 | 286.05 | 469 | 30.36 | 0.0202 |
| A2 | 6:4 | 160 | 2842.2 | 317.2 | 427 | 32.4 | 0.01823 |
| A3 | 5:5 | 160 | 1655.2 | 108.2 | 392 | 28.1 | 0.0215 |
| A4 | 4:6 | 160 | 2984.1 | 104.4 | 431 | 26.075 | 0.02215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Zhu, S.; Liu, S.; Liu, Y. Analysis of the Influencing Factors of the Efficient Degradation of Waste Polyurethane and Its Scheme Optimization. Polymers 2023, 15, 2337. https://doi.org/10.3390/polym15102337
Gu X, Zhu S, Liu S, Liu Y. Analysis of the Influencing Factors of the Efficient Degradation of Waste Polyurethane and Its Scheme Optimization. Polymers. 2023; 15(10):2337. https://doi.org/10.3390/polym15102337
Chicago/Turabian StyleGu, Xiaohua, Shangwen Zhu, Siwen Liu, and Yan Liu. 2023. "Analysis of the Influencing Factors of the Efficient Degradation of Waste Polyurethane and Its Scheme Optimization" Polymers 15, no. 10: 2337. https://doi.org/10.3390/polym15102337
APA StyleGu, X., Zhu, S., Liu, S., & Liu, Y. (2023). Analysis of the Influencing Factors of the Efficient Degradation of Waste Polyurethane and Its Scheme Optimization. Polymers, 15(10), 2337. https://doi.org/10.3390/polym15102337








