Excellent Energy Storage Performance in Epoxy Resin Dielectric Polymer Films by a Facile Hot−Pressing Method
Abstract
1. Introduction
2. Materials and Methods
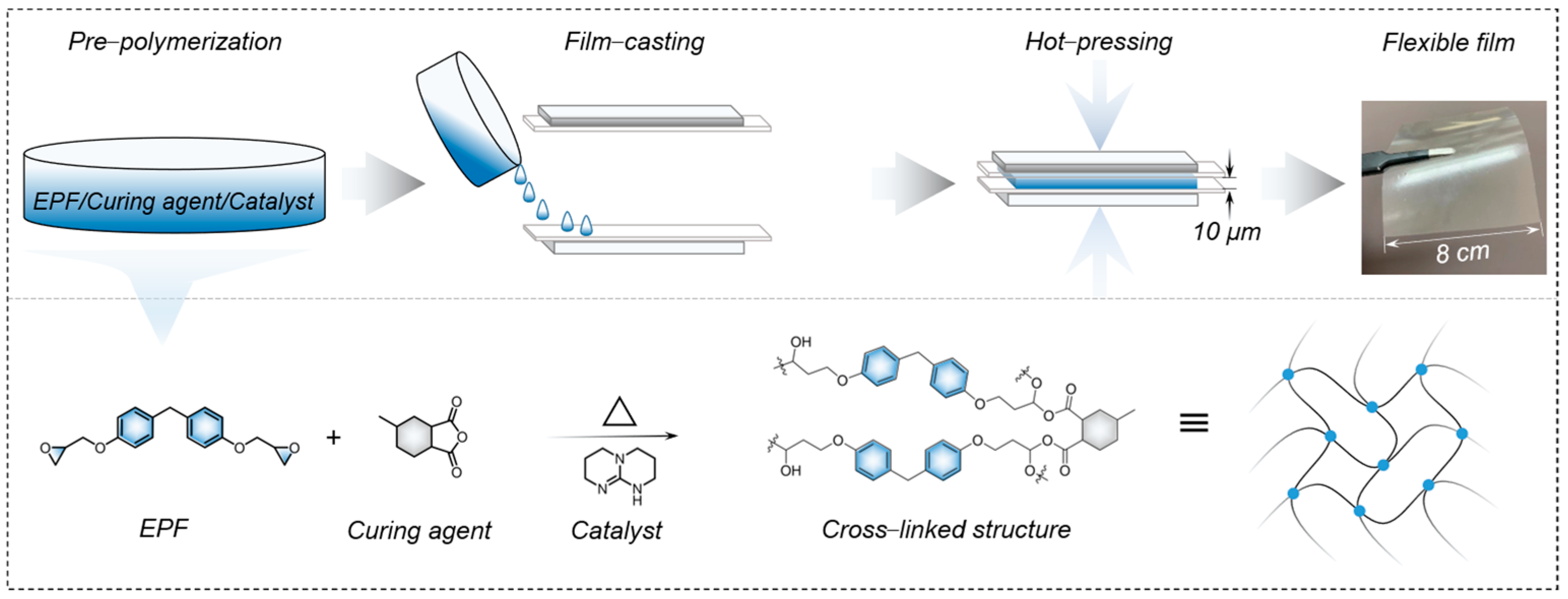
2.1. Preparation of EPF Film
2.2. Characterization
3. Results
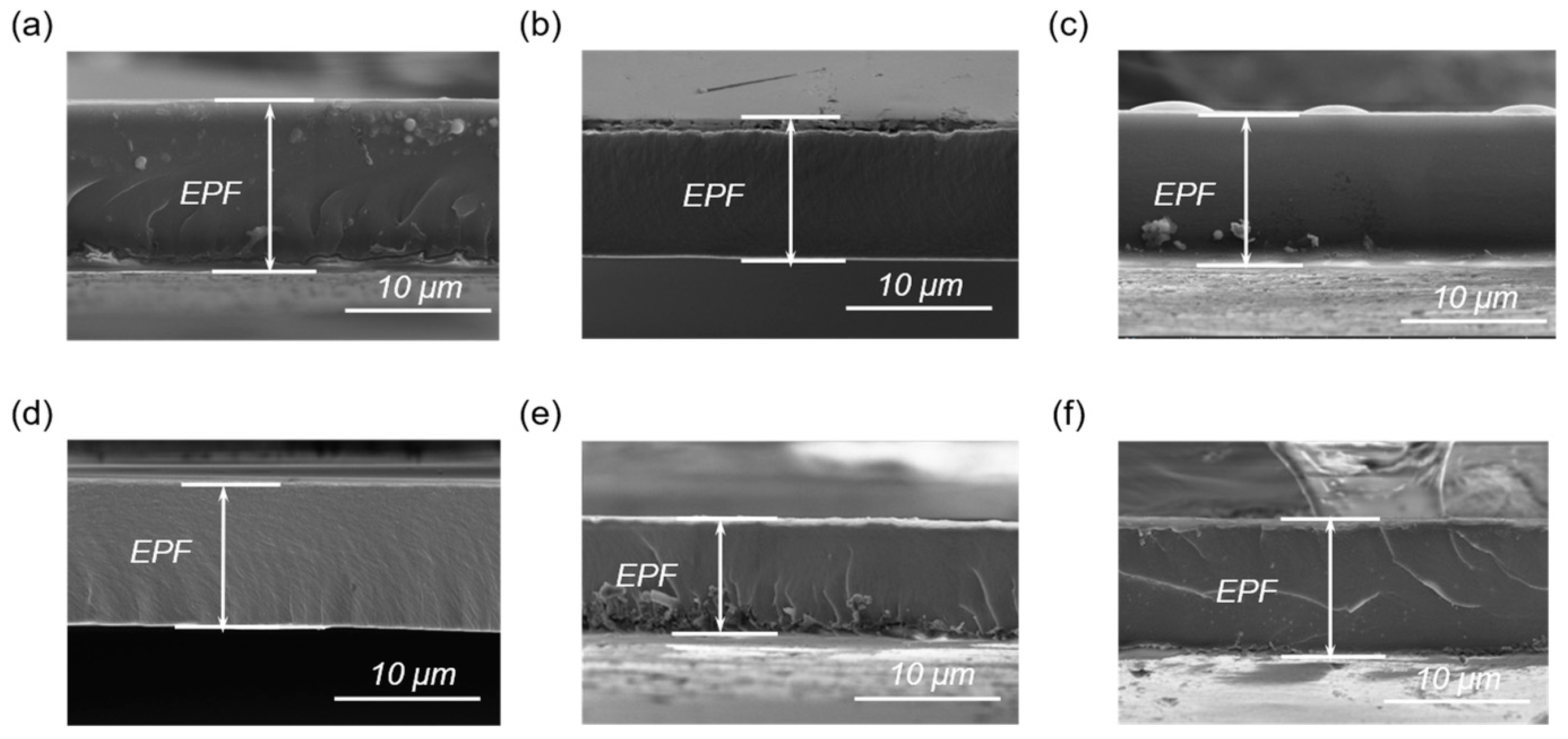
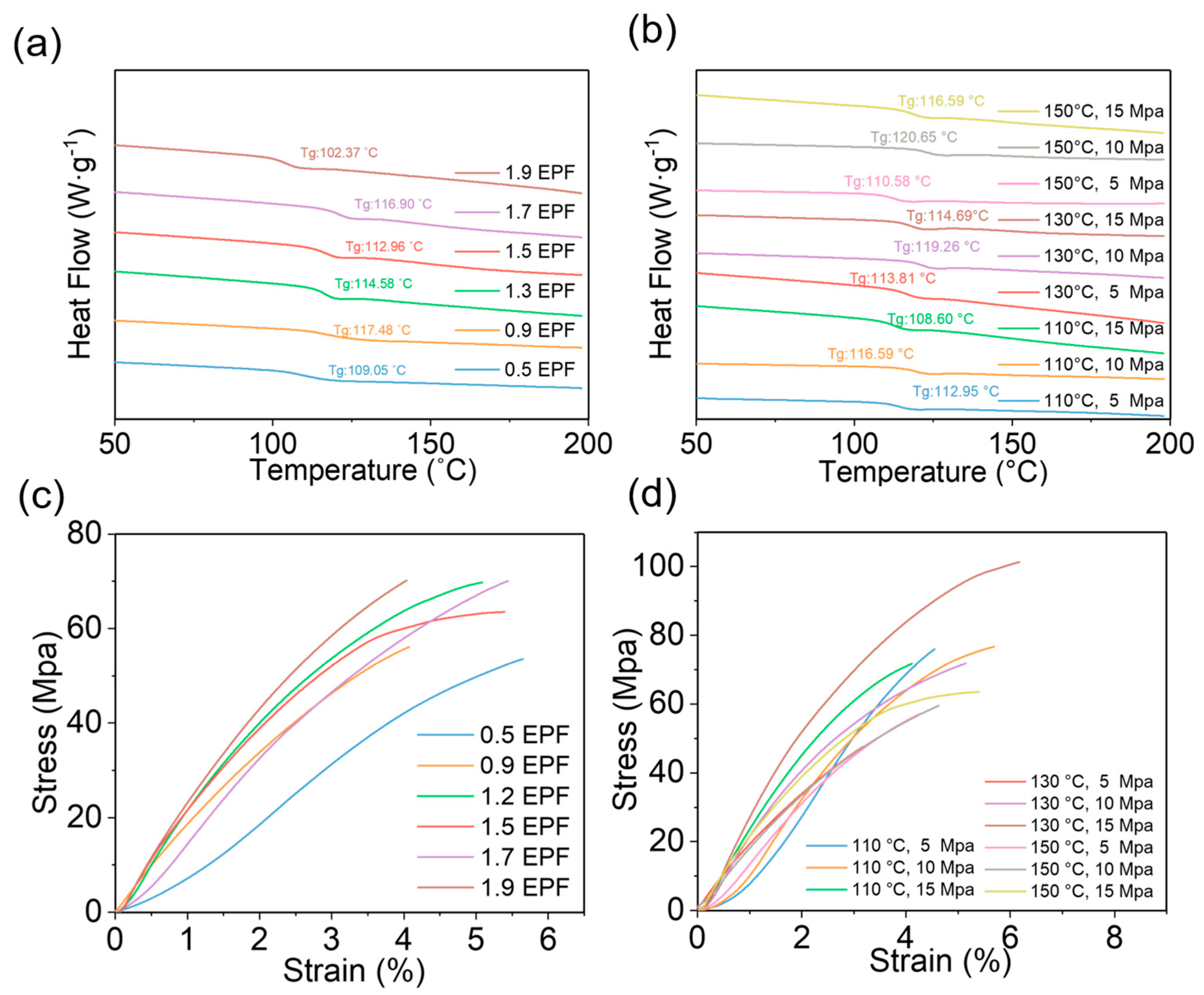
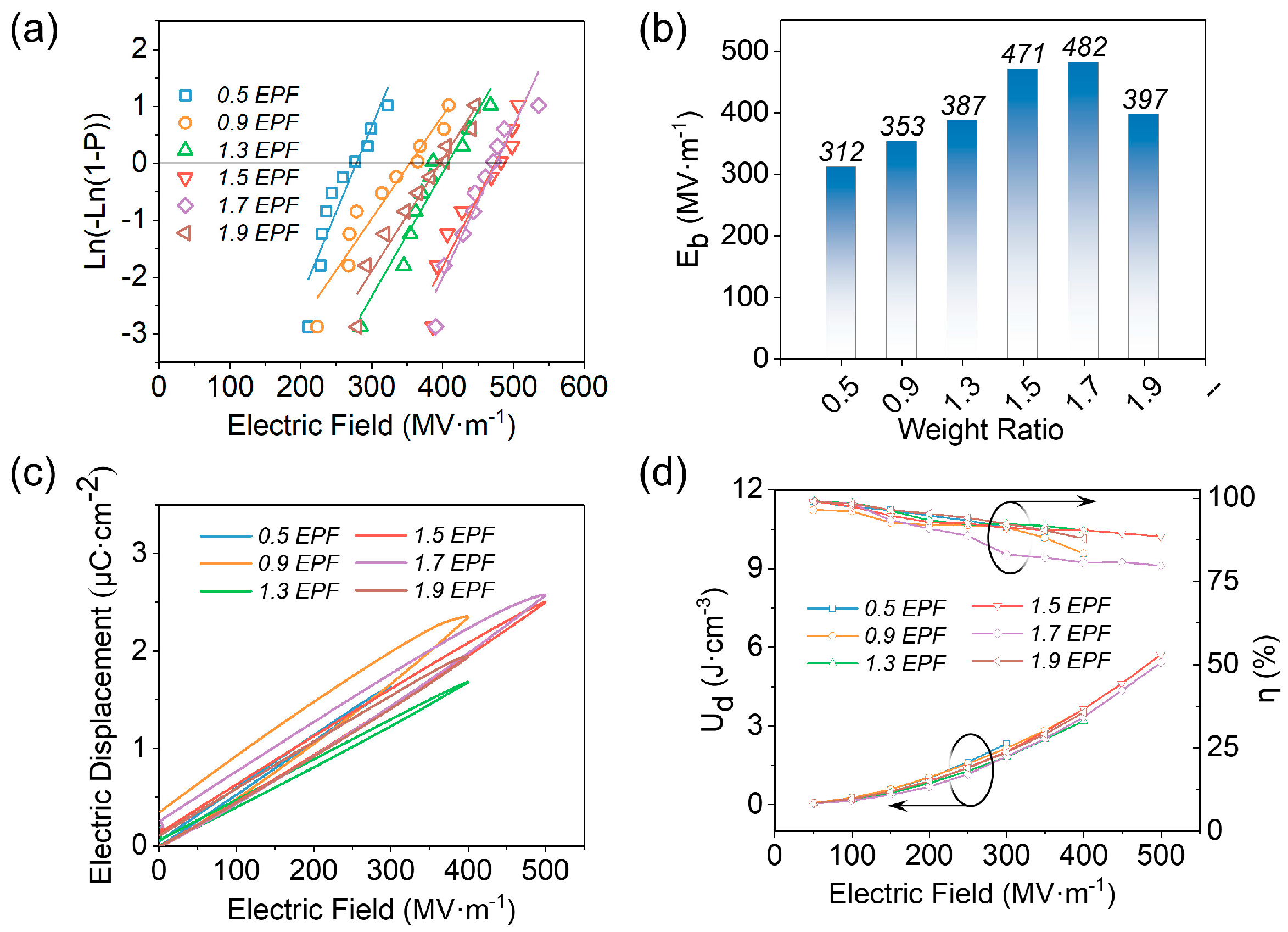
3.1. Different Ratios of Curing Agent to EPF
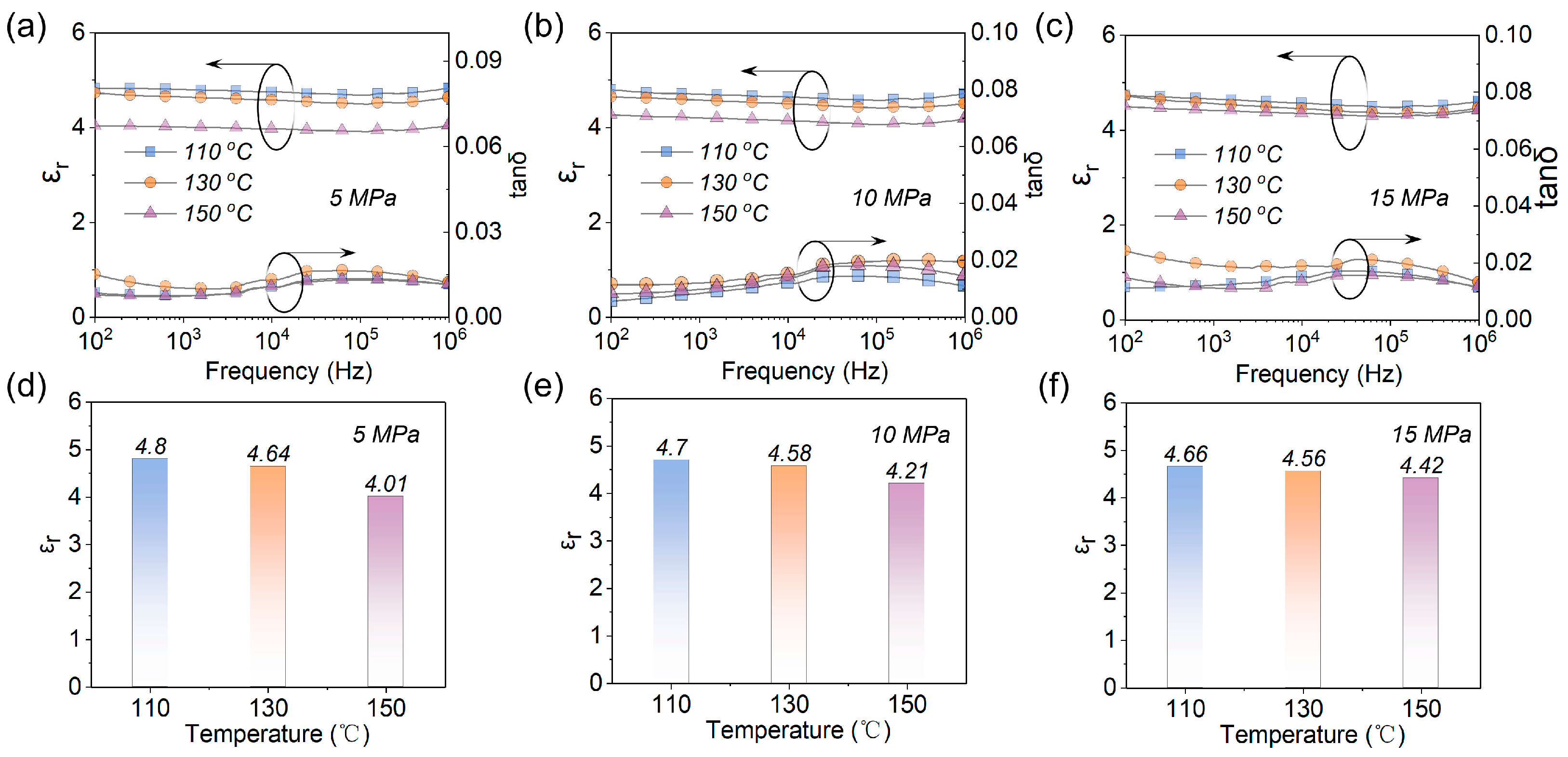
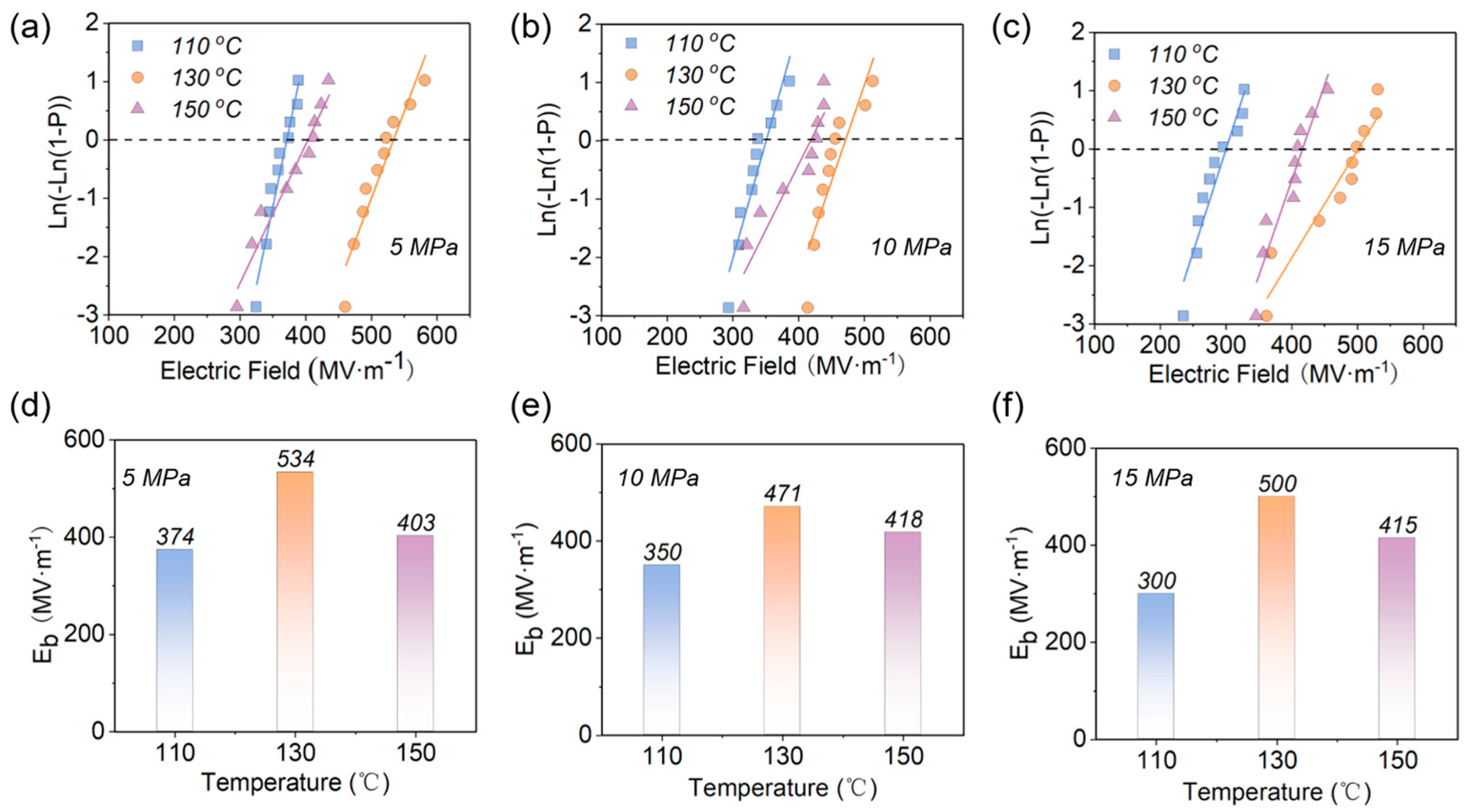
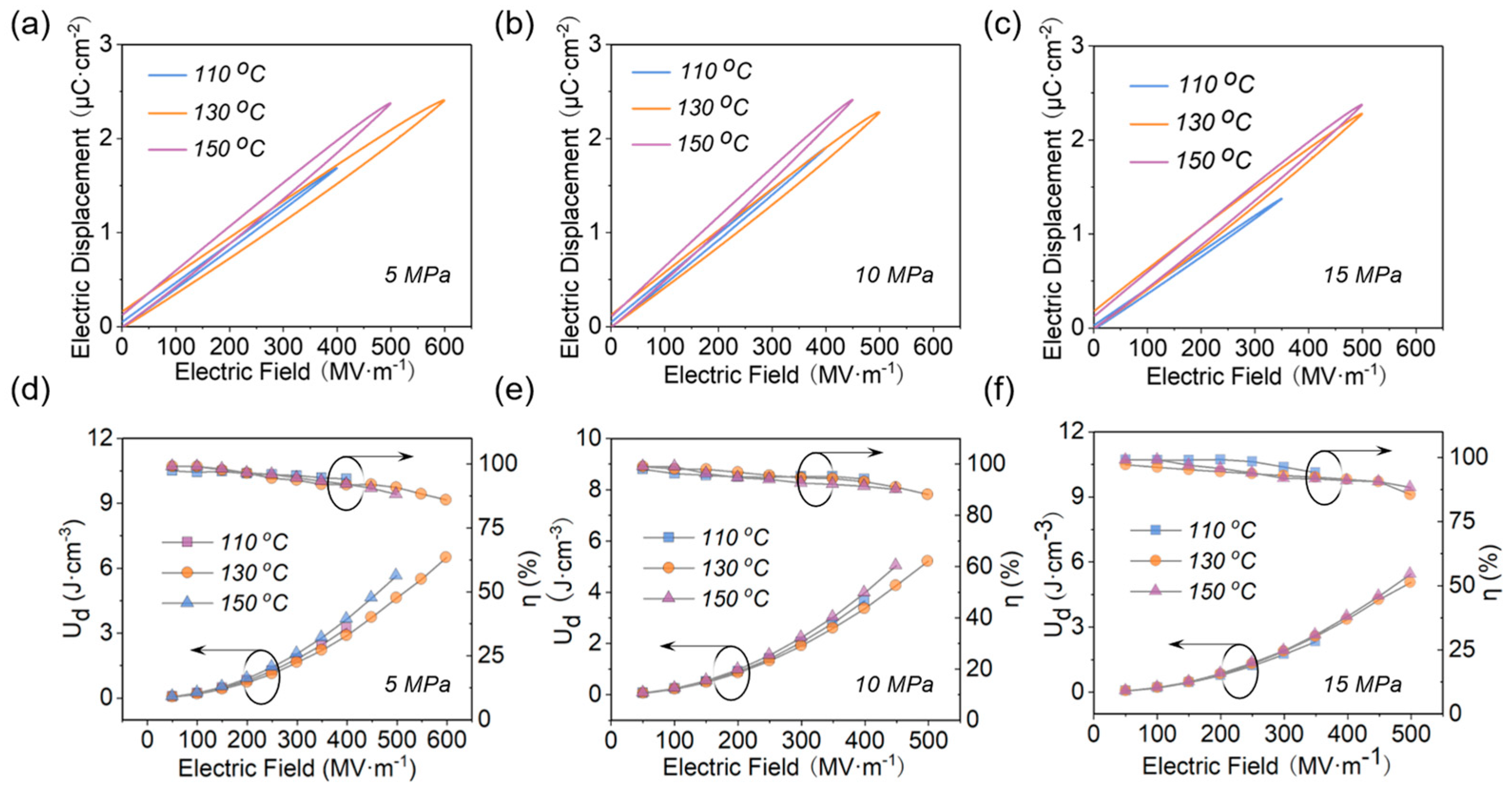
3.2. Different Hot−Pressing Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Fan, B.; Liu, F.; Yang, G.; Li, H.; Zhang, G.; Jiang, S.; Wang, Q. Dielectric materials for high-temperature capacitors. IET Nanodielectr. 2018, 1, 32–40. [Google Scholar] [CrossRef]
- Li, D.; Zeng, X.; Li, Z.; Shen, Z.-Y.; Hao, H.; Luo, W.; Wang, X.; Song, F.; Wang, Z.; Li, Y. Progress and perspectives in dielectric energy storage ceramics. J. Adv. Ceram. 2021, 10, 675–703. [Google Scholar] [CrossRef]
- Wang, C.; He, G.; Chen, S.; Zhai, D.; Luo, H.; Zhang, D. Enhanced performance of all-organic sandwich structured dielectrics with linear dielectric and ferroelectric polymers. J. Mater. Chem. A 2021, 9, 8674–8684. [Google Scholar] [CrossRef]
- Zhao, P.; Cai, Z.; Wu, L.; Zhu, C.; Li, L.; Wang, X. Perspectives and challenges for lead-free energy-storage multilayer ceramic capacitors. J. Adv. Ceram. 2021, 10, 1153–1193. [Google Scholar] [CrossRef]
- Feng, M.; Feng, Y.; Zhang, T.; Li, J.; Chen, Q.; Chi, Q.; Lei, Q. Recent Advances in Multilayer-Structure Dielectrics for Energy Storage Application. Adv. Sci. 2021, 8, 2102221. [Google Scholar] [CrossRef]
- Sun, B.; Hu, P.; Ji, X.; Fan, M.; Zhou, L.; Guo, M.; He, S.; Shen, Y. Excellent Stability in Polyetherimide/SiO2 Nanocomposites with Ultrahigh Energy Density and Discharge Efficiency at High Temperature. Small 2022, 18, 2202421. [Google Scholar] [CrossRef]
- Venkatesan, T.R.; Gulyakova, A.A.; Gerhard, R. Influence of film stretching on crystalline phases and dielectric properties of a 70/30 mol% poly(vinylidenefluoride-tetrafluoroethylene) copolymer. J. Adv. Dielectr. 2020, 10, 2050023. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Liu, F.; Han, K.; Gadinski, M.R.; Xiong, C.; Wang, Q. Solution-processed ferroelectric terpolymer nanocomposites with high breakdown strength and energy density utilizing boron nitride nanosheets. Energy Environ. Sci. 2015, 8, 922–931. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Z.; Xu, W.; Zhang, Y.; Qian, X.; Jiang, Z.; Zhang, Y. Interface-strengthened polymer nanocomposites with reduced dielectric relaxation exhibit high energy density at elevated temperatures utilizing a facile dual crosslinked network. Small 2020, 16, 2000714. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.-m.; Wu, W.-w.; Khesro, A.; Liu, P.; Mao, M.; Song, K.; Sun, R.; Wang, D. Outstanding discharge energy density and efficiency of the bilayer nanocomposite films with BaTiO3-dispersed PVDF polymer and polyetherimide layer. Chem. Eng. J. 2022, 446, 136926. [Google Scholar] [CrossRef]
- Liu, X.; Luo, H.; Yan, C.; Liu, Y.; Luo, H.; Zhang, D.; Chen, S. Achieving synergistic improvement in dielectric constant and energy storage properties of all-organic liquid crystal molecule/PVDF composites. J. Mater. Chem. C 2022, 10, 17757–17767. [Google Scholar] [CrossRef]
- Prateek; Bhunia, R.; Garg, A.; Gupta, R.K. Poly(vinylpyrrolidone)/Poly(vinylidene fluoride) as Guest/Host Polymer Blends: Understanding the Role of Compositional Transformation on Nanoscale Dielectric Behavior through a Simple Solution–Process Route. ACS Appl. Energy Mater. 2019, 2, 6146–6152. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.; Liu, H.; Li, J.-F.; Zhang, S. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sci. 2019, 102, 72–108. [Google Scholar] [CrossRef]
- Yu, S.; Ding, C.; Liu, Y.; Liu, Y.; Zhang, Y.; Luo, H.; Zhang, D.; Chen, S. Enhanced breakdown strength and energy density over a broad temperature range in polyimide dielectrics using oxidized MXenes filler. J. Power Sources 2022, 535, 231415. [Google Scholar] [CrossRef]
- Bai, H.; Zhu, K.; Wang, Z.; Shen, B.; Zhai, J. 2D Fillers Highly Boost the Discharge Energy Density of Polymer-Based Nanocomposites with Trilayered Architecture. Adv. Funct. Mater. 2021, 31, 2102646. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.m.; Pan, Z.; Liu, P.; Mao, M.; Song, K.; Mao, Z.; Sun, R.; Wang, D.; Zhang, S. Superior High-Temperature Energy Density in Molecular Semiconductor/Polymer All-Organic Composites. Adv. Funct. Mater. 2023, 33, 2210050. [Google Scholar] [CrossRef]
- Riggs, B.C.; Adireddy, S.; Rehm, C.H.; Puli, V.S.; Elupula, R.; Chrisey, D.B. Polymer nanocomposites for energy storage applications. Mater. Today Proc. 2015, 2, 3853–3863. [Google Scholar] [CrossRef]
- Zhu, X.; Ng, L.W.; Hu, G.; Wu, T.C.; Um, D.S.; Macadam, N.; Hasan, T. Hexagonal boron nitride–enhanced optically transparent polymer dielectric inks for printable electronics. Adv. Funct. Mater. 2020, 30, 2002339. [Google Scholar] [CrossRef]
- Li, H.; Ren, L.; Ai, D.; Han, Z.; Liu, Y.; Yao, B.; Wang, Q. Ternary polymer nanocomposites with concurrently enhanced dielectric constant and breakdown strength for high-temperature electrostatic capacitors. InfoMat 2020, 2, 389–400. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Z.; Li, Y.; Li, L.; Ji, H.; Feteira, A.; Zhou, D.; Wang, D.; Zhang, S.; Reaney, I.M. Electroceramics for high-energy density capacitors: Current status and future perspectives. Chem. Rev. 2021, 121, 6124–6172. [Google Scholar] [CrossRef]
- Yang, M.; Ren, W.; Guo, M.; Shen, Y. High-Energy-Density and High Efficiency Polymer Dielectrics for High Temperature Electrostatic Energy Storage: A Review. Small 2022, 18, 2205247. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, G.; Chen, S.; Luo, H.; Yang, Y.; Zhang, D. Achieving high breakdown strength and energy density in all-organic sandwich-structured dielectrics by introducing polyacrylate elastomers. J. Mater. Chem. A 2022, 10, 9103–9113. [Google Scholar] [CrossRef]
- Huan, T.D.; Boggs, S.; Teyssedre, G.; Laurent, C.; Cakmak, M.; Kumar, S.; Ramprasad, R. Advanced polymeric dielectrics for high energy density applications. Prog. Mater. Sci. 2016, 83, 236–269. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Utracki, L.; Utracki, L. Polymer industry. In Commercial Polymer Blends; Springer: Boston, MA, USA, 1998; pp. 8–52. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Zhang, X.; Sun, R.; Tian, X.; Zeng, Y.; Xu, J.-B.; Wong, C.-P. Silver nanoparticle-modified alumina microsphere hybrid composites for enhanced energy density and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2019, 119, 299–309. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, X.; Yao, Y.; Sun, R.; Meng, F.; Xu, J.; Wong, C. A novel h-BN–RGO hybrids for epoxy resin composites achieving enhanced high thermal conductivity and energy density. RSC Adv. 2017, 7, 23355–23362. [Google Scholar] [CrossRef]
- Zhao, D.; Yuan, L.; Liang, G.; Gu, A. Orientating carbon nanotube bundles and barium titanate nanofibers in tri-layer structure to develop high energy density epoxy resin composites with greatly improved dielectric constant and breakdown strength. Compos. Part B Eng. 2019, 173, 107030. [Google Scholar] [CrossRef]
- Tomer, V.; Polizos, G.; Manias, E.; Randall, C.A. Epoxy-based nanocomposites for electrical energy storage. I: Effects of montmorillonite and barium titanate nanofillers. J. Appl. Phys. 2010, 108, 074116. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, L.; Sun, W.; Mao, J.; Zheng, Y.; Wang, S.; Zhang, Z.; Chen, Y.; Cheng, Y. Influence of imidazole derivatives on the dielectric and energy storage performance of epoxy. High Volt. 2022, 7, 782–791. [Google Scholar] [CrossRef]
- Wang, P.-J.; Zhou, D.; Guo, H.-H.; Liu, W.-F.; Su, J.-Z.; Fu, M.-S.; Singh, C.; Trukhanov, S.; Trukhanov, A. Ultrahigh enhancement rate of the energy density of flexible polymer nanocomposites using core–shell BaTiO3@MgO structures as the filler. J. Mater. Chem. A 2020, 8, 11124–11132. [Google Scholar] [CrossRef]
- Xie, D.; Min, D.; Yang, L.; Li, S. Temperature- and thickness-dependent electrical breakdown modulated by a coupling model of charge transport and molecular chain dynamics. J. Phys. D Appl. Phys. 2019, 52, 365305. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Pham, V.H.; Ha, Y.W.; Kim, S.H.; Jeong, H.T.; Jung, M.Y.; Ko, B.S.; Hwang, Y.J.; Chung, J.S. Synthesis of epoxy encapsulated organoclay nanocomposite latex via phase inversion emulsification and its gas barrier property. J. Ind. Eng. Chem. 2014, 20, 108–112. [Google Scholar] [CrossRef]
- Xiang, Q.; Xiao, F. Applications of epoxy materials in pavement engineering. Constr. Build. Mater. 2020, 235, 117529. [Google Scholar] [CrossRef]
- Ghaemy, M.; Barghamadi, M.; Behmadi, H. Cure kinetics of epoxy resin and aromatic diamines. J. Appl. Polym. Sci. 2004, 94, 1049–1056. [Google Scholar] [CrossRef]
- Jilani, W.; Mzabi, N.; Fourati, N.; Zerrouki, C.; Gallot-Lavallée, O.; Zerrouki, R.; Guermazi, H. Effects of curing agent on conductivity, structural and dielectric properties of an epoxy polymer. Polymer 2015, 79, 73–81. [Google Scholar] [CrossRef]
- Ramesh, P.; Ravikumar, L.; Burkanudeen, A. Curing properties of novel curing agent based on phenyl bisthiourea for an epoxy resin system. J. Therm. Anal. Calorim. 2011, 115, 713–722. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. Toughening of epoxy resins. Des. Monomers Polym. 2012, 9, 129–152. [Google Scholar] [CrossRef]
- Wan, J.; Bu, Z.-Y.; Xu, C.-J.; Li, B.-G.; Fan, H. Preparation, curing kinetics, and properties of a novel low-volatile starlike aliphatic-polyamine curing agent for epoxy resins. Chem. Eng. J. 2011, 171, 357–367. [Google Scholar] [CrossRef]
- Zhang, C.; Mi, X.; Tian, J.; Zhang, J.; Xu, T. Supported Ionic Liquid Silica as Curing Agent for Epoxy Composites with Improved Mechanical and Thermal Properties. Polymers 2017, 9, 478. [Google Scholar] [CrossRef]
- Businova, P.; Chomoucka, J.; Prasek, J.; Hrdy, R.; Drbohlavova, J.; Sedlacek, P.; Hubalek, J. Polymer coated iron oxide magnetic nanoparticles: Preparation and characterization. Nanocon 2011, 9, 21–23. [Google Scholar]
- Guo, Y.; Bao, C.; Song, L.; Yuan, B.; Hu, Y. In Situ Polymerization of Graphene, Graphite Oxide, and Functionalized Graphite Oxide into Epoxy Resin and Comparison Study of On-the-Flame Behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Käppler, A.; Windrich, F.; Löder, M.G.; Malanin, M.; Fischer, D.; Labrenz, M.; Eichhorn, K.-J.; Voit, B. Identification of microplastics by FTIR and Raman microscopy: A novel silicon filter substrate opens the important spectral range below 1300 cm− 1 for FTIR transmission measurements. Anal. Bioanal. Chem. 2015, 407, 6791–6801. [Google Scholar] [CrossRef] [PubMed]
- Aridoss, G.; Kim, M.S.; Son, S.M.; Kim, J.T.; Jeong, Y.T. Synthesis of Poly (P-phenylenediamine-co-o-aminophenol)/multi-walled Carbon Nanotube Composites by Emulsion Polymerization. Polym. Adv. Technol. 2010, 21, 881–887. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, F.; Yang, Y.; Chang, Z.; Wu, Y. An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. RSC Adv. 2014, 4, 76–81. [Google Scholar] [CrossRef]
- Chen, S.; Meng, G.; Kong, B.; Xiao, B.; Wang, Z.; Jing, Z.; Gao, Y.; Wu, G.; Wang, H.; Cheng, Y. Asymmetric alicyclic amine-polyether amine molecular chain structure for improved energy storage density of high-temperature crosslinked polymer capacitor. Chem. Eng. J. 2020, 387, 123662. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, S.; Chang, Y.; Wang, D.; Zhang, X.; Huang, H.; He, Y.; Zhang, Q. Ultra-superior high-temperature energy storage properties in polymer nanocomposites via rational design of core–shell structured inorganic antiferroelectric fillers. J. Mater. Chem. A 2023, 11, 7227–7238. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Yan, S.; Shen, Z.; Dong, L.; Zhang, S.; Zhang, X.; Nan, C.W. Ultrahigh Energy Density in Continuously Gradient-Structured All-Organic Dielectric Polymer Films. Adv. Funct. Mater. 2022, 32, 2200848. [Google Scholar] [CrossRef]
- Wang, P.; Pan, Z.; Wang, W.; Hu, J.; Liu, J.; Yu, J.; Zhai, J.; Chi, Q.; Shen, Z. Ultrahigh energy storage performance of a polymer-based nanocomposite via interface engineering. J. Mater. Chem. A 2021, 9, 3530–3539. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Mao, M.; Zhang, B.; Li, Z.; Song, K.; Li, H.-F.; Mao, Z.; Wang, D. Excellent Energy Storage Performance in Epoxy Resin Dielectric Polymer Films by a Facile Hot−Pressing Method. Polymers 2023, 15, 2315. https://doi.org/10.3390/polym15102315
Pan Z, Mao M, Zhang B, Li Z, Song K, Li H-F, Mao Z, Wang D. Excellent Energy Storage Performance in Epoxy Resin Dielectric Polymer Films by a Facile Hot−Pressing Method. Polymers. 2023; 15(10):2315. https://doi.org/10.3390/polym15102315
Chicago/Turabian StylePan, Zhe, Minmin Mao, Bin Zhang, Zhongyu Li, Kaixin Song, Hai-Feng Li, Zhu Mao, and Dawei Wang. 2023. "Excellent Energy Storage Performance in Epoxy Resin Dielectric Polymer Films by a Facile Hot−Pressing Method" Polymers 15, no. 10: 2315. https://doi.org/10.3390/polym15102315
APA StylePan, Z., Mao, M., Zhang, B., Li, Z., Song, K., Li, H.-F., Mao, Z., & Wang, D. (2023). Excellent Energy Storage Performance in Epoxy Resin Dielectric Polymer Films by a Facile Hot−Pressing Method. Polymers, 15(10), 2315. https://doi.org/10.3390/polym15102315










