Research of Potential Catalysts for Two-Component Silyl-Terminated Prepolymer/Epoxy Resin Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing Methods
2.2.1. Tensile Test
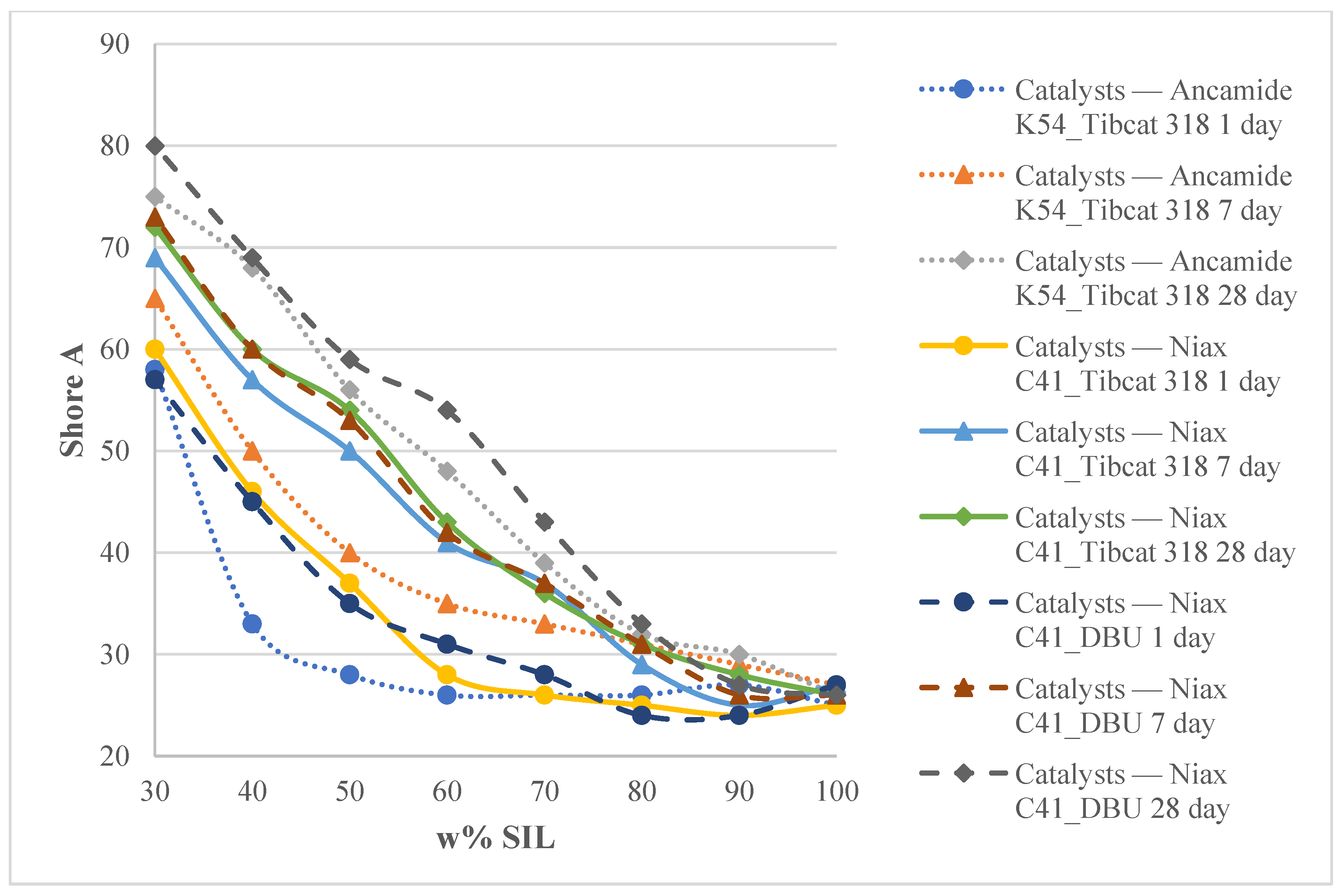
2.2.2. Hardness Test
2.2.3. Rheology Tests
3. Results
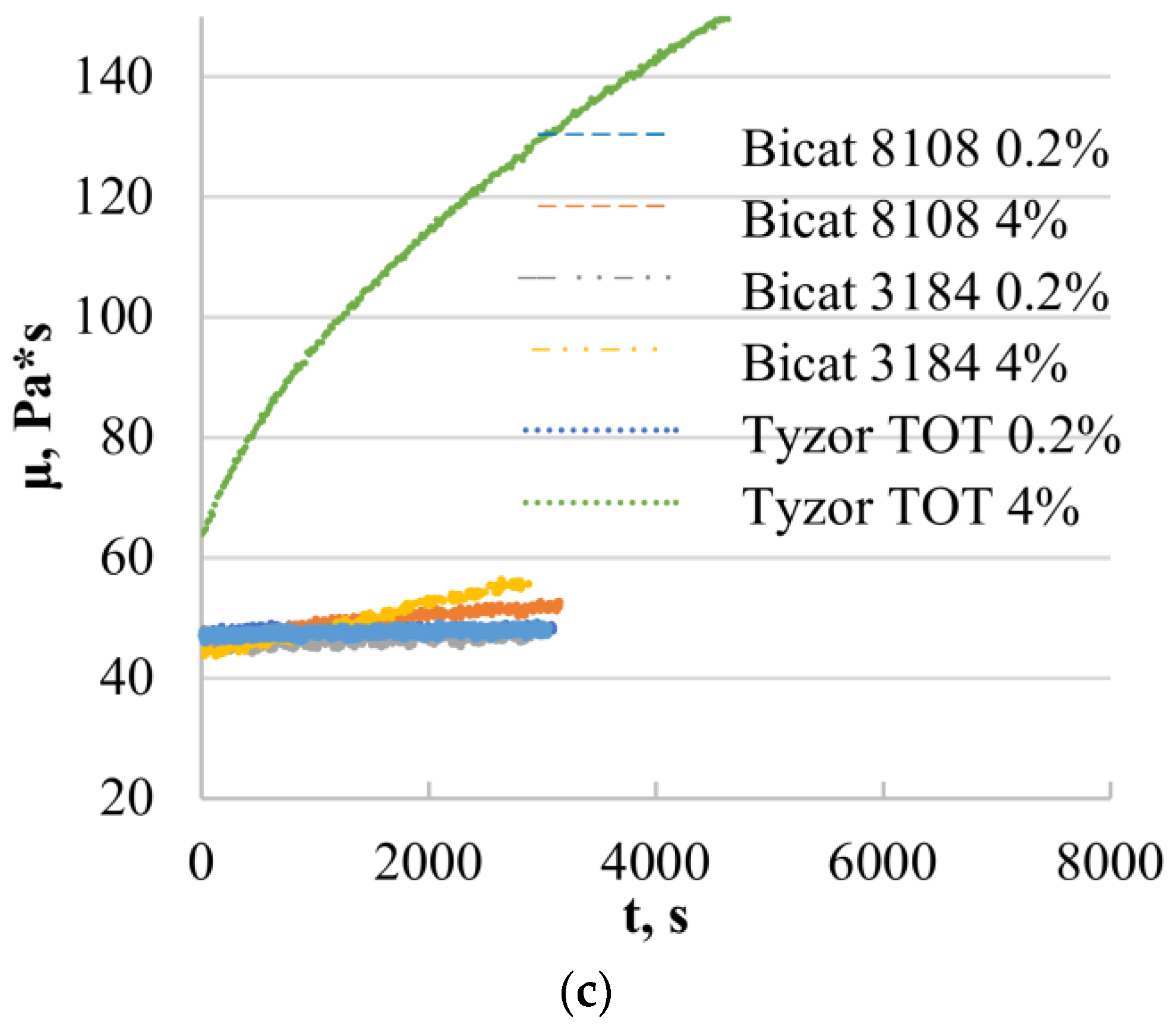
3.1. Rheological Properties of Silyl-Terminated Prepolymer (SAX 520)
3.2. Rheological Properties of Epoxy Resin (D.E.R. 331)
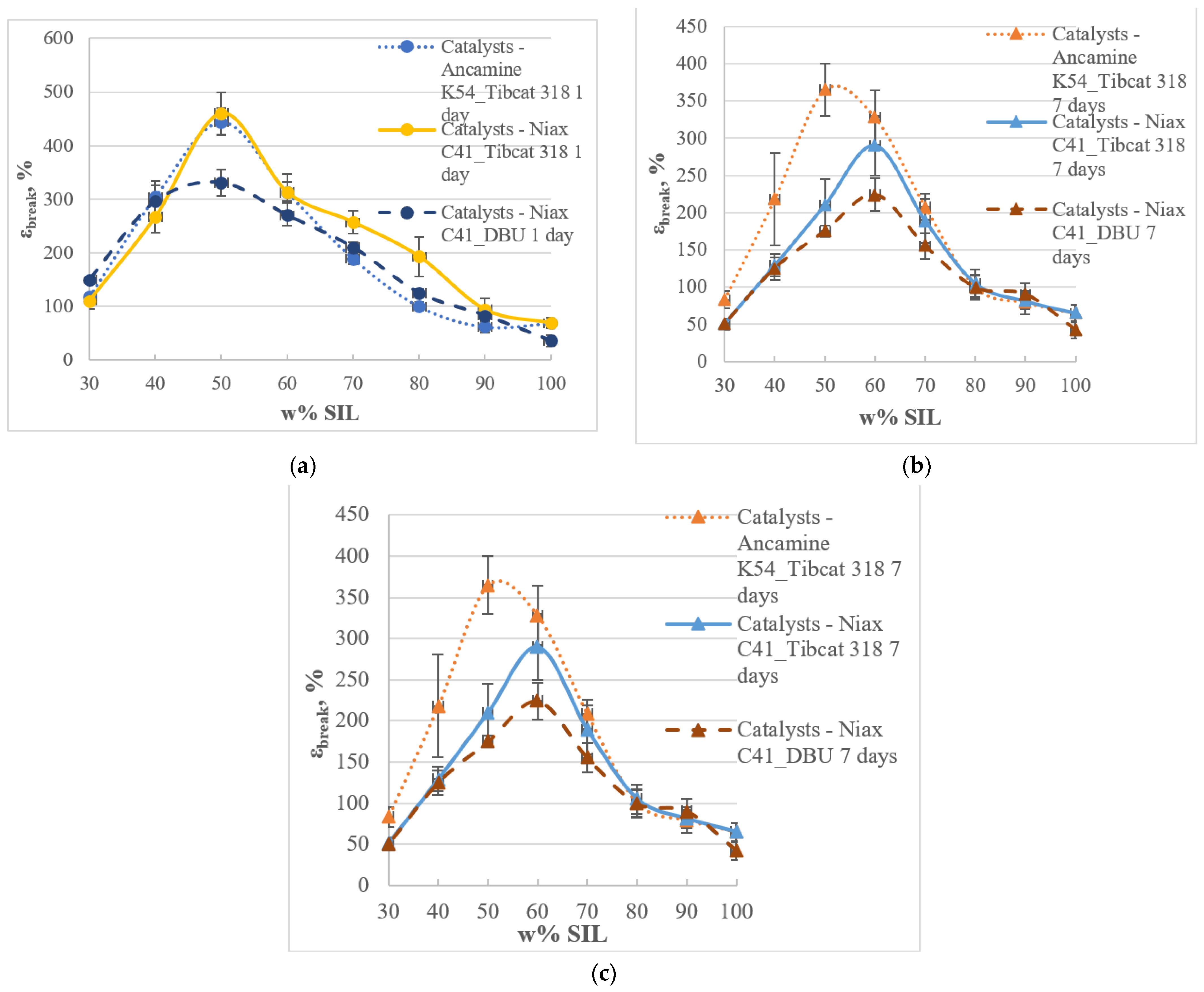
3.3. Mechanical Properties of the Two-Component Model Systems
3.4. Rheological Properties of the Model Systems
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.gminsights.com/industry-analysis/adhesives-and-sealants-market-report (accessed on 15 December 2022).
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and Performance of Polyurethane Elastomers Composed with Different Soft Segments. Materials 2020, 13, 4991. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, R.V.; Gadhave, C.R.; Dhawale, P.V. Silane Terminated Prepolymers: An Alternative to Silicones and Polyurethanes. Open J. Polym. Chem. 2021, 11, 3. [Google Scholar] [CrossRef]
- Sahooa, S.; Mohanty, S.; Nayaka, S.K. Biobased polyurethane adhesive over petroleum-based adhesive. Use Renew. Resour. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 36–48. [Google Scholar] [CrossRef]
- Bockel, S.; Harling, S.; Konnerth, J.; Niemz, P.; Weiland, G.; Hogger, E.; Pichelin, F. Modifying elastic modulus of two-component polyurethane adhesive for structural hardwood bonding. J. Wood Sci. 2020, 66, 69. [Google Scholar] [CrossRef]
- Quinil, J.G.; Marinucci, G. Polyurethane structural adhesives applied in automotive composite joints. Mat. Res. 2012, 15, 3. [Google Scholar]
- Devroey, D.R.E.; Homma, M. Blends of silyl-terminated polyethers and epoxides as elastic adhesives. Int. J. Adhes. Adhes. 2001, 21, 275–280. [Google Scholar] [CrossRef]
- Choffat, F. Silane-Terminated Polyurethane Polymers. U.S. Patent 20110306723A1, 18 February 2010. [Google Scholar]
- Rutz, D.; Oertli, M.; Jucker, B. Silane-Functional Polyesters in Moisture-Curing Compositions Based on Silane-Functional Polymers. U.S. Patent 8,697,815, 15 April 2014. [Google Scholar]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of Polyurethanes Using Organocatalysis: A Perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Belowich, M.E.; Roberts, J.M.; Peterson, T.H.; Bellinger, E.; Syverud, K.; Sidle, T. Lewis Acids As Highly Active Silanol Polycondensation Catalysts Affording Low Levels of Cyclosiloxanes. Macromolecules 2020, 17, 7487–7495. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Proebster, M.; Ge, R.; Etzelstorfer, M.; Manegold, C. Catalysis of Silane-Crosslinkable Polymer Compositions. U.S. Patent 624,653, 22 August 2013. [Google Scholar]
- Zhu, H.D.; Heberer, D.P. Silane Functional Adhesive Composition and Method of Bonding a Window to a Substrate without a Primer. U.S. Patent 6,649,016, 18 November 2003. [Google Scholar]
- Burkett, S.L.; Soukasene, S.; Milton, K.L.; Welch, R.; Little, A.J.; Kasi, R.M.; Coughlin, E.B. Tethered Constrained-Geometry Catalysts in Mesoporous Silica: Probing the Influence of the “Second Sphere” on Polymer Properties. Chem. Mater. 2005, 17, 2716–2723. [Google Scholar] [CrossRef]
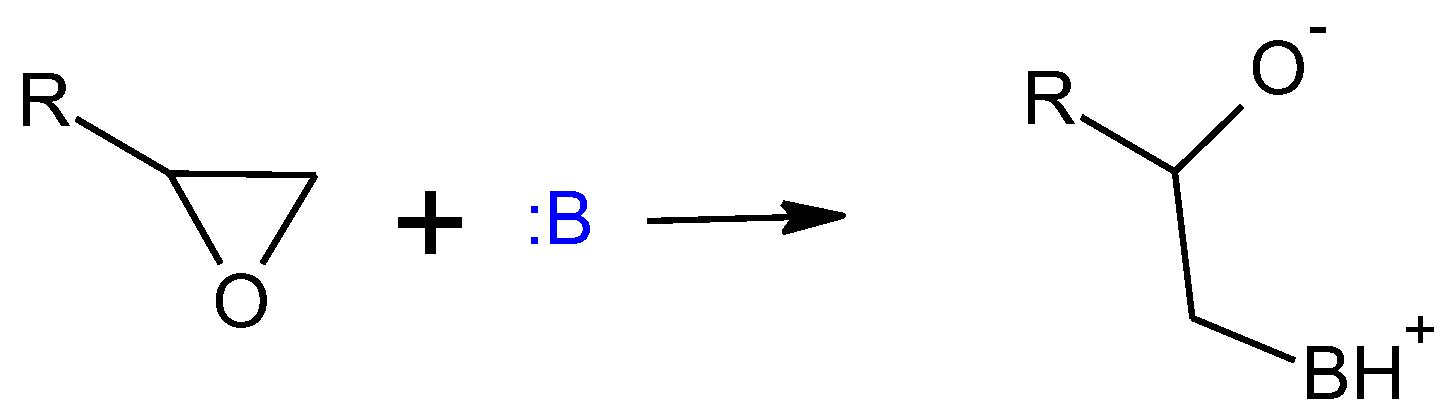
- Konuray, A.O.; Fernández-Francos, X.; Ramis, X. Analysis of the reaction mechanism of the thiol–epoxy addition initiated by nucleophilic tertiary amines. Polym. Chem. 2017, 8, 5934–5947. [Google Scholar] [CrossRef]
- Choi, H.K.; Choi, B.G.; Lee, Y.Y.; Na, J.S. Cationic Catalyst as Latent Curing Agent for Epoxy Resin. Appl. Mech. Mater. 2015, 749, 126–128. [Google Scholar] [CrossRef]
- Berziņš, R.; Merijs-Meri, R.; Zicans, J. Polyether/Epoxy Resin Model and Complete Systems and Evaluation of Their Mechanical, Rheological and Adhesive Properties. Polymers 2022, 14, 2421. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iso.org/standard/50756.html (accessed on 15 December 2022).
- Torry, S.A.; Campbell, A.; Cunliffe, A.V.; Tod, D.A. Kinetic analysis of organosilane hydrolysis and condensation. Inter. J. Adhes. Adhes. 2006, 26, 40–49. [Google Scholar] [CrossRef]
- Bereska, B.; Iłowska, J.; Czaja, K.; Bereska, A. Hardeners for epoxy resins. Przem. Chem. 2014, 93, 443–448. [Google Scholar]






| Raw Material Class | Commercial Name | Raw Material Structure Formula |
|---|---|---|
| Prepolymer | SAX 520 (Kaneka, Belgium, Westerlo, Belgium) |  |
| Prepolymer | D.E.R. 331 (Palmer Holland, Westlake, OH, USA) |  |
| Compatibilizer | Dynasylan 1189 (Evonik Industries, Essen, Germany) | 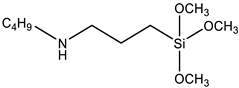 |
| Catalyst | Tibcat 216 (TIB chemicals AG, Mannheim, Germany) |  |
| Catalyst | Tibcat 318 (TIB chemicals AG, Mannheim, Germany) | 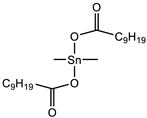 |
| Catalyst | Tibcat 410 (TIB chemicals AG, Mannheim, Germany) | 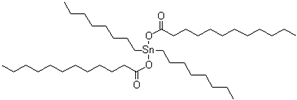 |
| Catalyst | Niax C41 (Air products, Allentown, PA, USA) |  |
| Catalyst | Dabco 33LV (Air products, Allentown, PA, USA) | 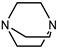 |
| Catalyst | DBU (Air products, Allentown, PA, USA) | 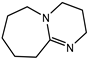 |
| Catalyst | Ancamine K54 (Evonik Industries, Essen, Germany) |  |
| Catalyst | Jeffcat DMDEE (Huntsman Corporation, Conroe, TX, USA) | 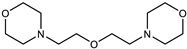 |
| Catalyst | Tyzor TOT (The Shepherd Chemical Company, Cincinnati, OH, USA) |  |
| Catalyst | Bicat 8108M (The Shepherd Chemical Company, Cincinnati, OH, USA) | 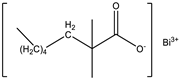 |
| Catalyst | Bicat 3184 (The Shepherd Chemical Company, Cincinnati, OH, USA) | 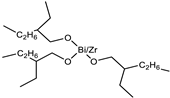 |
| Raw Material Mass (g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| SAX 520/D.E.R. 331 | 100/0 | 90/10 | 80/20 | 70/30 | 60/40 | 50/50 | 40/60 | 30/70 |
| Dynasylan 1189 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Tibcat 318 | 0.2 | 0.18 | 0.16 | 0.14 | 0.12 | 0.1 | 0.08 | 0.06 |
| DBU | 4 | 3.6 | 3.2 | 2.8 | 2.4 | 2 | 1.6 | 1.2 |
| Ancamine K54 | 0 | 0.4 | 0.8 | 1.2 | 1.6 | 2 | 2.4 | 2.8 |
| Niax C41 | 0 | 0.4 | 0.8 | 1.2 | 1.6 | 2 | 2.4 | 2.8 |
| Water | 0.2 | 0.18 | 0.16 | 0.14 | 0.12 | 0.1 | 0.08 | 0.06 |
| SIL/EP | 1–60/40 | 1–50/50 | 2–60/40 | 2–50/50 | 3–60/40 | 3–50/50 |
| Gc, (Pa) | 4705 | 6425 | 5642 | 8647 | 5853 | 9931 |
| tc, (s) | 6395 | 6982 | 5168 | 5852 | 4142 | 6347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berzins, R.; Merijs-Meri, R.; Zicans, J. Research of Potential Catalysts for Two-Component Silyl-Terminated Prepolymer/Epoxy Resin Adhesives. Polymers 2023, 15, 2269. https://doi.org/10.3390/polym15102269
Berzins R, Merijs-Meri R, Zicans J. Research of Potential Catalysts for Two-Component Silyl-Terminated Prepolymer/Epoxy Resin Adhesives. Polymers. 2023; 15(10):2269. https://doi.org/10.3390/polym15102269
Chicago/Turabian StyleBerzins, Ritvars, Remo Merijs-Meri, and Janis Zicans. 2023. "Research of Potential Catalysts for Two-Component Silyl-Terminated Prepolymer/Epoxy Resin Adhesives" Polymers 15, no. 10: 2269. https://doi.org/10.3390/polym15102269
APA StyleBerzins, R., Merijs-Meri, R., & Zicans, J. (2023). Research of Potential Catalysts for Two-Component Silyl-Terminated Prepolymer/Epoxy Resin Adhesives. Polymers, 15(10), 2269. https://doi.org/10.3390/polym15102269







