Exploration of Breakdown Strength Decrease and Mitigation of Ultrathin Polypropylene
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
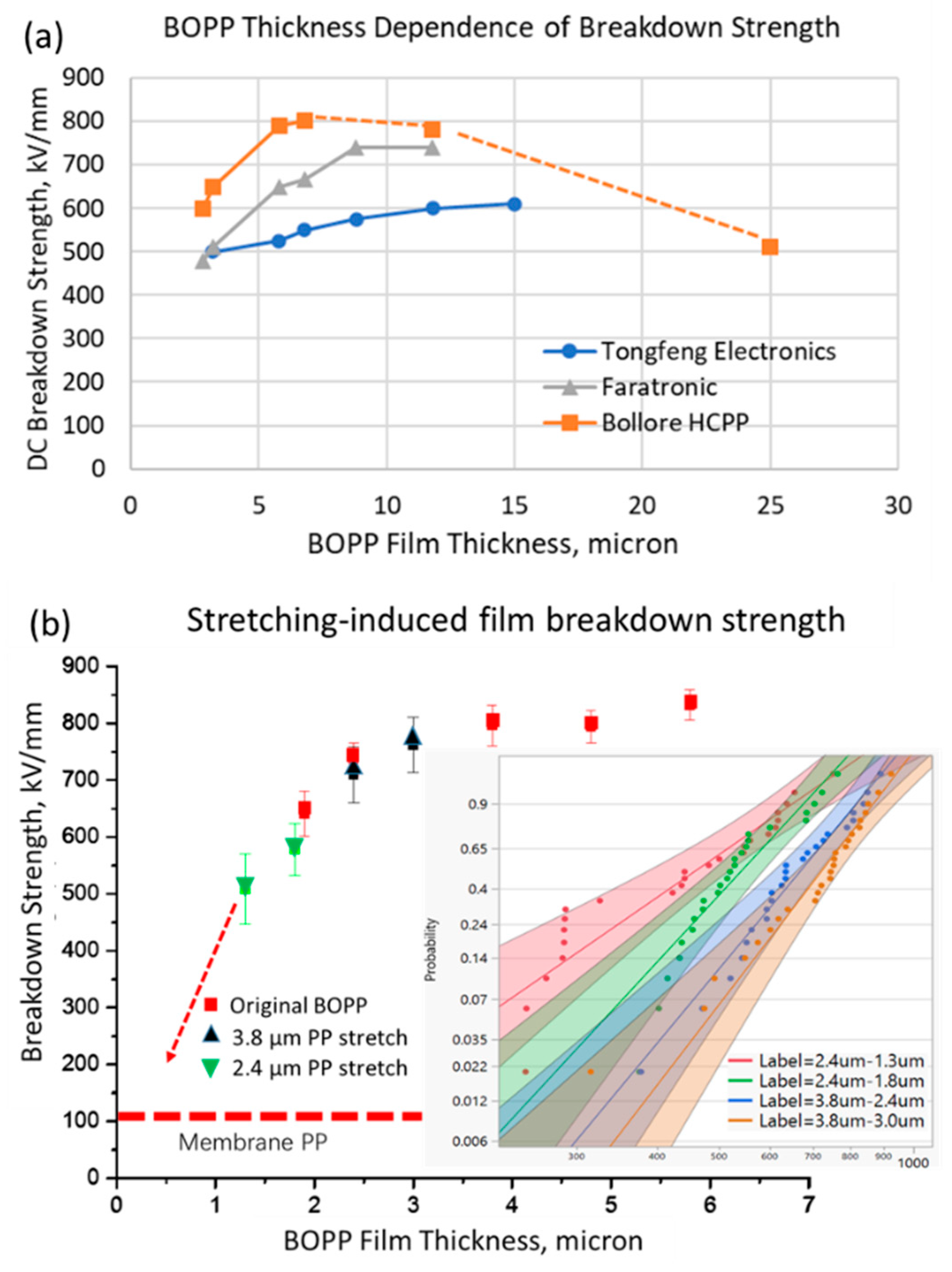
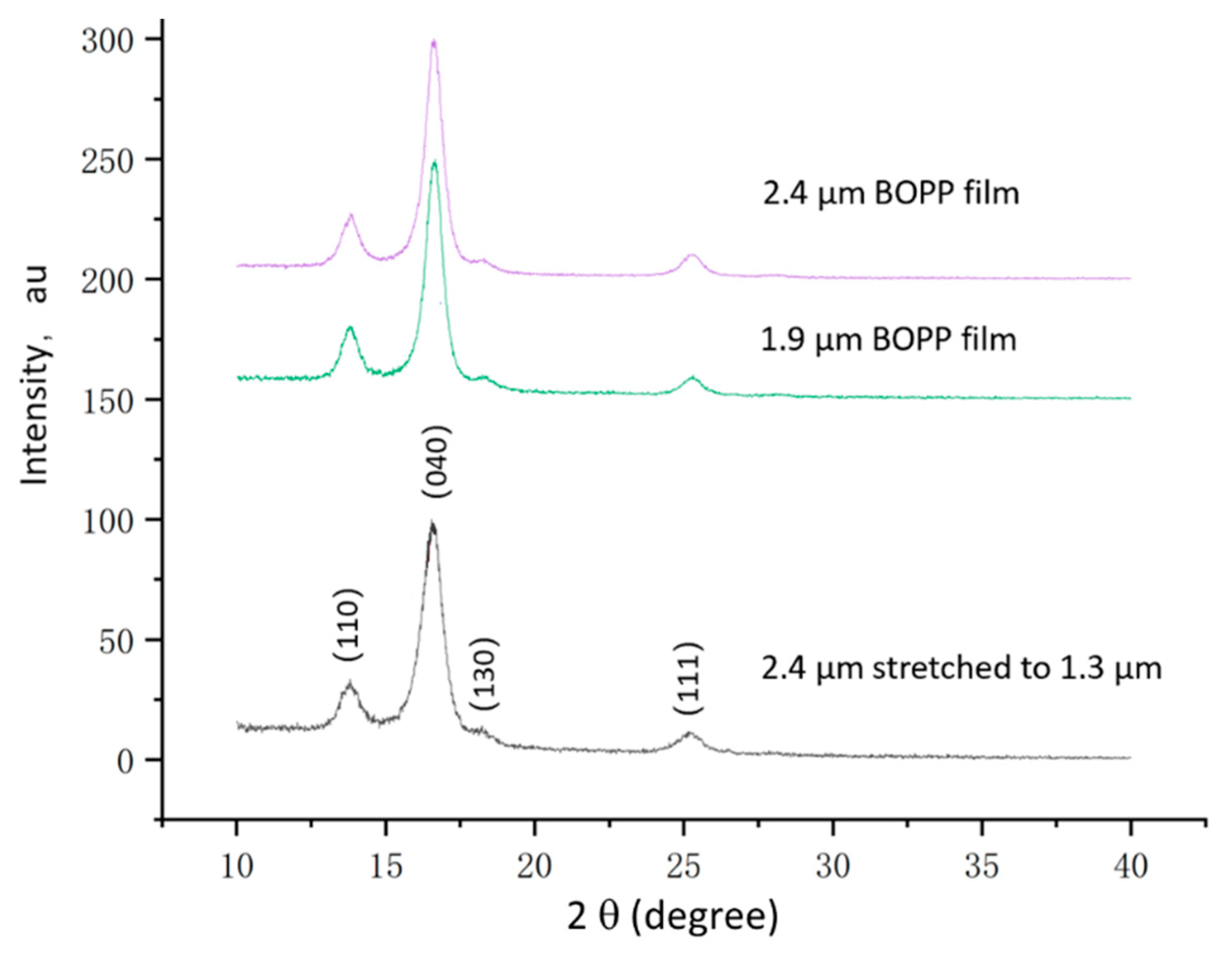
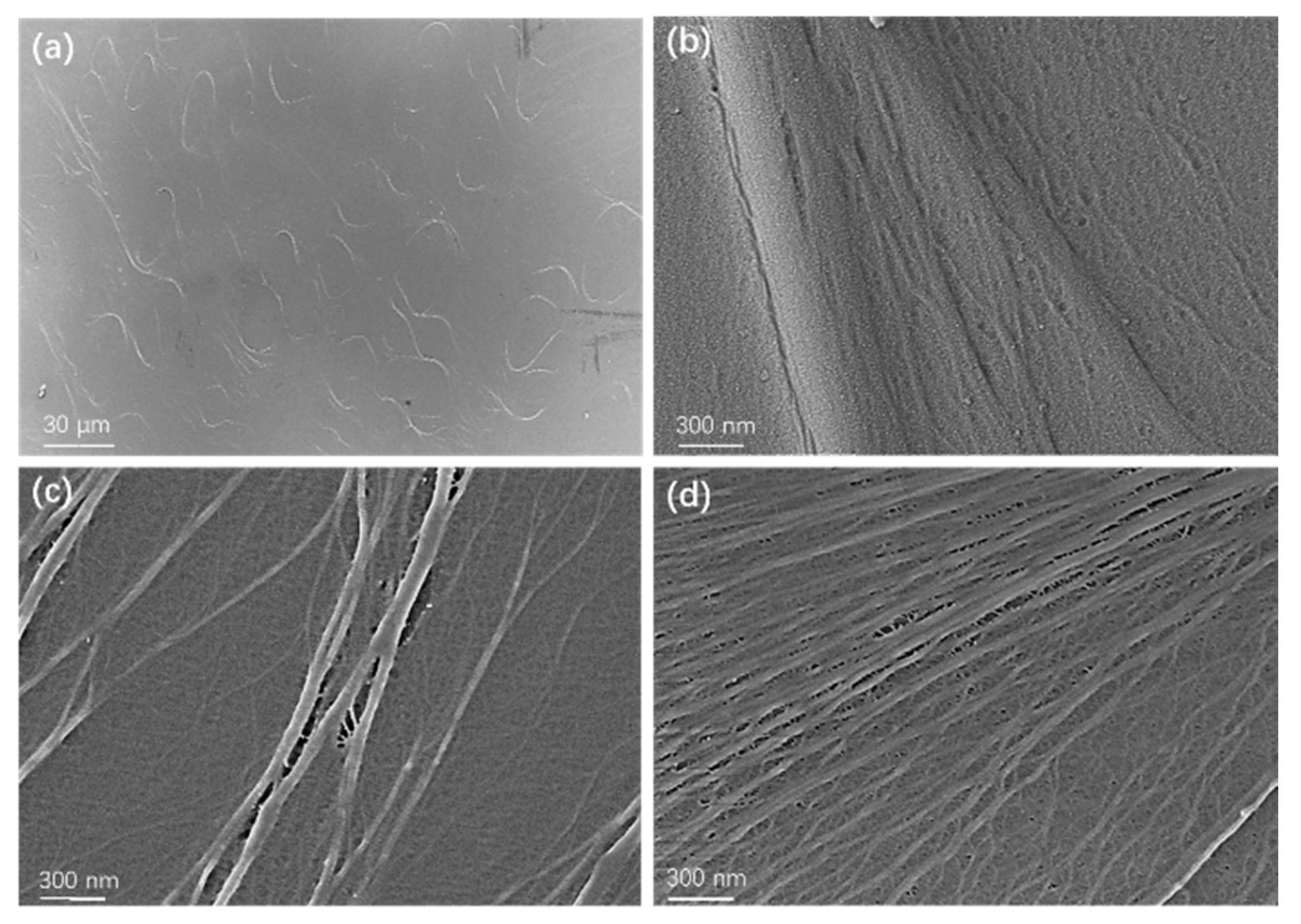
3.1. Electrical Breakdown Behavior of Ultra-Thin BOPP
3.2. Mitigation Scheme of Breakdown Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Boggs, S.A.; Ho, J.; Jow, T.R. Overview of laminar dielectric capacitors. IEEE Electr. Insul. Mag. 2010, 26, 7–13. [Google Scholar] [CrossRef]
- Nash, J.L. Biaxially oriented polypropylene film in power capacitors. Polym. Eng. Sci. 1988, 28, 862–870. [Google Scholar] [CrossRef]
- Cygan, S.; Laghari, J.R. Dependence of the Electric Strength on Thickness Area and Volume of Polypropylene. IEEE Trans. El Ectrical Insul. 1987, EIT-22, 737. [Google Scholar] [CrossRef]
- Lu, J.Y.; Zhu, B.F.; Zhang, X.; Wang, X. Dielectric Strength Structure-Activity Relationship of BOPP Film for High Energy Density Pulse Capacitor. IEEE Trans. Plasma Sci. 2019, 47, 4342. [Google Scholar] [CrossRef]
- Laihonen, S.J.; Gäfvert, U. DC Breakdown Strength of Polypropylene Films: Area Dependence and Statistical Behavior. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 275. [Google Scholar] [CrossRef]
- Tan, D.Q. Review of polymer-based nanodielectric exploration and film scale-up for advanced capacitors. Adv. Funct. Mater. 2019, 1808567, 1–23. [Google Scholar] [CrossRef]
- Baer, E.; Zhu, L. 50th anniversary perspective: Dielectric phenomena in polymers and multilayered dielectric films. Macromolecules 2017, 50, 2239. [Google Scholar] [CrossRef]
- Tan, Q.; Irwin, P.; Cao, Y. Advanced dielectrics for capacitors. IEEE Trans. 2006, 126, 1209–1215. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, J.W.; Li, S.T.; Zhong, L.S. Origin of superb electrical insulating capability of cellulose-liquid biphasic dielectrics by interfacial charge behaviors. Appl. Phys. Lett. 2012, 100, 222904. [Google Scholar] [CrossRef]
- Neusel, C.; Schneider, G.A. Size-dependence of the dielectric breakdown strength from nano to millimeter scale. J. Mech. Phys. Solids 2014, 63, 201–213. [Google Scholar] [CrossRef]
- Tan, D.Q. The search for enhanced dielectric strength of polymer-based dielectrics: A focused review on polymer nanocomposites. J. Appl. Polym. Sci. 2020, 37, 49379. [Google Scholar] [CrossRef]
- Wu, X.D.; Chen, X.; Zhang, Q.M.; Tan, D.Q. Advanced Dielectric Polymer Films for Energy Storage. Energy Storage Mater. 2021, 44, 29–47. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Thakur, Y.; Lu, B.; Zhang, Q.; Runt, J.; Zhang, Q.M. A highly scalable dielectric metamaterial with superior capacitor performance over a broad temperature. Sci. Adv. 2020, 6, eaax6622. [Google Scholar] [CrossRef]
- Zheng, W.W.; Li, Z.H.; Chen, K.J.; Liu, S.W.; Chi, Z.G.; Xu, J.R.; Zhang, Y. Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials. Molecules 2022, 27, 6337. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, B.S.; Kim, H.; Xie, Z.; Lainé, A.; Ma, L.; Xu, T.; Yang, C.; Kwon, J.; Shelton, S.W.; et al. High-performing polysulfate dielectrics for electrostatic energy storage under harsh conditions. Joule 2023, 7, 95–111. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Huang, X.; Yu, C.; Han, D.; Wang, A.; Zhu, Y.; Shi, K.; Kang, Q.; Li, P.; et al. Ladderphane copolymers for high-temperature capacitive energy storage. Nature 2023, 615, 62–66. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Fu, J.; Yang, M.; Ran, Z.; Li, J.; Li, M.; Hu, J.; He, J.; Li, Q. Designing tailored combinations of structural units in polymer dielectrics for high-temperature capacitive energy storage. Nat. Commun. 2023, 14, 2406. [Google Scholar] [CrossRef]
- Xie, Z.L.; Liu, D.Y.; Tang, X.H.; Fu, Z.Z.; Wang, K.; Zhang, Q.; Wang, J.; Fu, Q. Largely improved dielectric energy performances and safety of BOPP film via surface engineering. Compos. Sci. Technol. 2023, 232, 109856. [Google Scholar] [CrossRef]
- Zhang, T.D.; Yu, H.N.; Jung, Y.H.; Zhang, C.H.; Feng, Y.; Chen, Q.G.; Lee, K.J.; Chi, Q.G. Significantly Improved High-Temperature Energy Storage Performance of BOPP Films by Coating Nanoscale Inorganic Layer. Energy Environ. Mater. 2022, e12594. [Google Scholar] [CrossRef]
- Ding, L.P.; Zhang, X.Y.; Wang, Y.Q. Study on the Behavior of BOPP Film Treated by Corona Discharge. Coatings 2020, 10, 1195. [Google Scholar] [CrossRef]
- SteinerFilm Dielectrics for Film Capacitors. Available online: https://www.steinerfilm.de/en/our-products/steinerfilmr-p/ (accessed on 30 April 2023).
- Xiamen Faratronic Co., Ltd. Available online: http://www.faratronic.com/cn/ (accessed on 30 April 2023).
- Tongfeng Electronics. Available online: http://www.tong-feng.com/uploads/file/2021/08/25611bea35c37ba37.pdf (accessed on 30 April 2023).
- Ho, J.; Jow, T.R. High Field Conduction in Biaxially Oriented Polypropylene at Elevated Temperature. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 990. [Google Scholar] [CrossRef]
- Rytöluoto, I.; Gitsas, A.; Pasanen, S.; Lahti, K. Effect of film structure and morphology on the dielectric breakdown characteristics of cast and biaxially oriented polypropylene films. Eur. Polym. J. 2017, 95, 606–624. [Google Scholar] [CrossRef]
- Tan, D.Q. Differentiation of Roughness and Surface Defect Impact on Dielectric Strength of Polymeric Thin Films. IET Nanodielectrics 2020, 3, 28–31. [Google Scholar] [CrossRef]
- Song, G.H.; Tan, D.Q. Atomic Layer Deposition for Polypropylene Film Engineering—A Review. Macromol. Mater. Eng. 2020, 305, 2000127. [Google Scholar] [CrossRef]
- Dai, X.; Xing, Z.; Yang, W.; Zhang, C.; Li, F.; Chen, X.; Li, C.; Zhou, J.; Li, L. The Effect of Annealing on the Structure and Electric Performance of Polypropylene Films. Hindawi Int. J. Polym. Sci. 2022, 2022, 5970484. [Google Scholar]
- Sainan, S.; Xia, W.; Ruijuan, M.; Zhaoliang, X.; Xiying, D. Performance Evaluation of Polypropylene Resin for Capacitor Film. J. Phys. Conf. Ser. 2021, 2009, 012003. [Google Scholar] [CrossRef]
- Wu, X.D.; Tang, S.K.; Song, G.H.; Zhang, Z.H.; Tan, D.Q. High-temperature resistant polypropylene films enhanced by atomic layer deposition. Nanoexpress 2021, 2, 010025. [Google Scholar] [CrossRef]
- Wu, X.D.; Liu, Y.C.; Lin, X.T.; Huang, E.L.; Song, G.H.; Tan, D.Q. Atomic Layer Deposition Coated Polymer Films with Enhanced High-Temperature Dielectric Strength Suitable for Film Capacitors. Surf. Interfaces 2022, 28, 101686. [Google Scholar] [CrossRef]
- Ho, J.; Ramprasad, R.; Boggs, S. Effect of Alteration of Antioxidant by UV Treatment on the Dielectric Strength of BOPP Capacitor Film. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1295. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.Q.; Liu, Y.; Lin, X.; Huang, E.; Lin, X.; Wu, X.; Lin, J.; Luo, R.; Wang, T. Exploration of Breakdown Strength Decrease and Mitigation of Ultrathin Polypropylene. Polymers 2023, 15, 2257. https://doi.org/10.3390/polym15102257
Tan DQ, Liu Y, Lin X, Huang E, Lin X, Wu X, Lin J, Luo R, Wang T. Exploration of Breakdown Strength Decrease and Mitigation of Ultrathin Polypropylene. Polymers. 2023; 15(10):2257. https://doi.org/10.3390/polym15102257
Chicago/Turabian StyleTan, Daniel Q., Yichen Liu, Xiaotian Lin, Enling Huang, Xi Lin, Xudong Wu, Jintao Lin, Ronghai Luo, and Tianxiang Wang. 2023. "Exploration of Breakdown Strength Decrease and Mitigation of Ultrathin Polypropylene" Polymers 15, no. 10: 2257. https://doi.org/10.3390/polym15102257
APA StyleTan, D. Q., Liu, Y., Lin, X., Huang, E., Lin, X., Wu, X., Lin, J., Luo, R., & Wang, T. (2023). Exploration of Breakdown Strength Decrease and Mitigation of Ultrathin Polypropylene. Polymers, 15(10), 2257. https://doi.org/10.3390/polym15102257






