PET Waste Recycling into BTX Fraction Using In Situ Obtained Nickel Phosphide
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nickel Phosphide Precursor
2.2. Catalytic Tests
2.3. Characterization
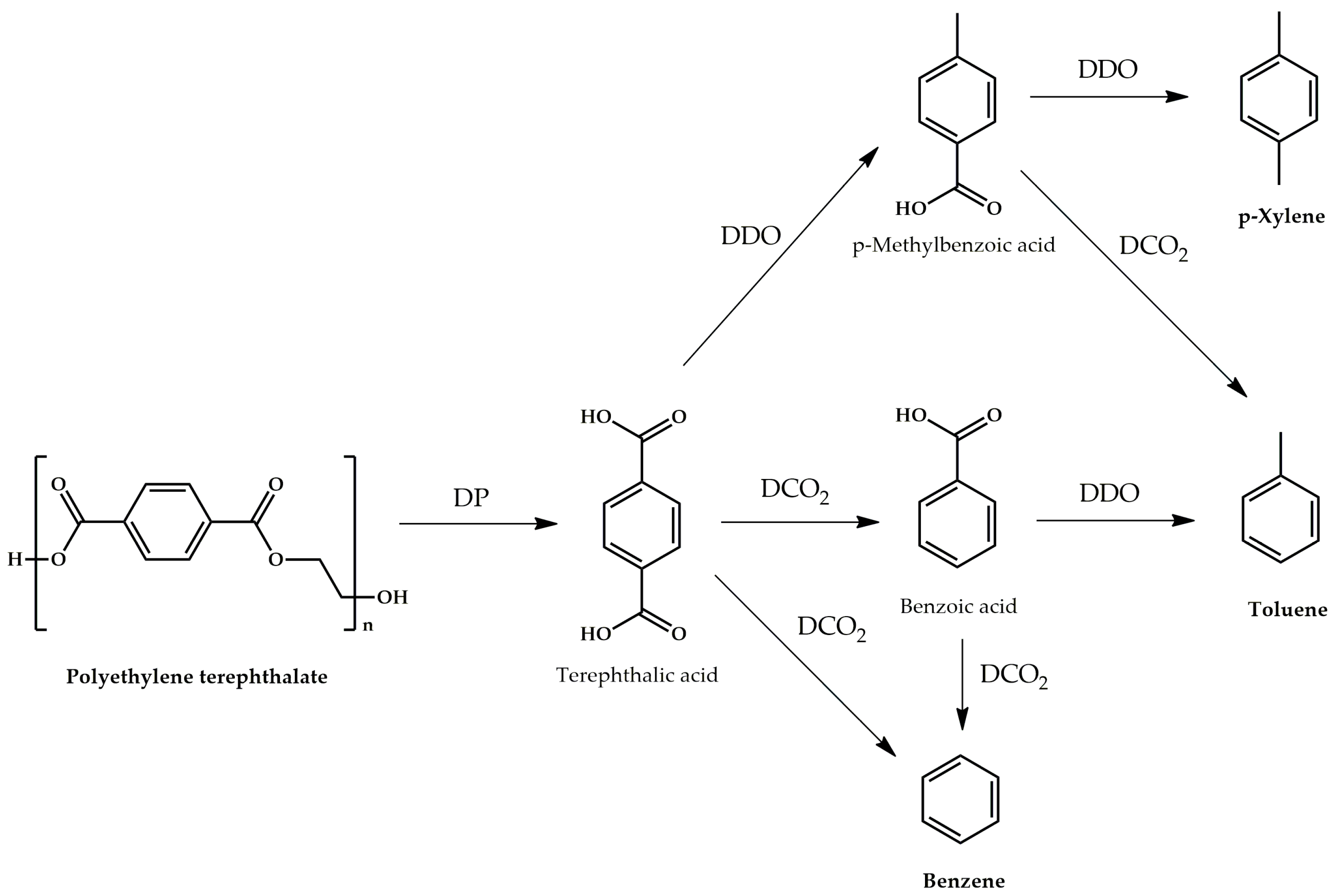
3. Results and Discussion
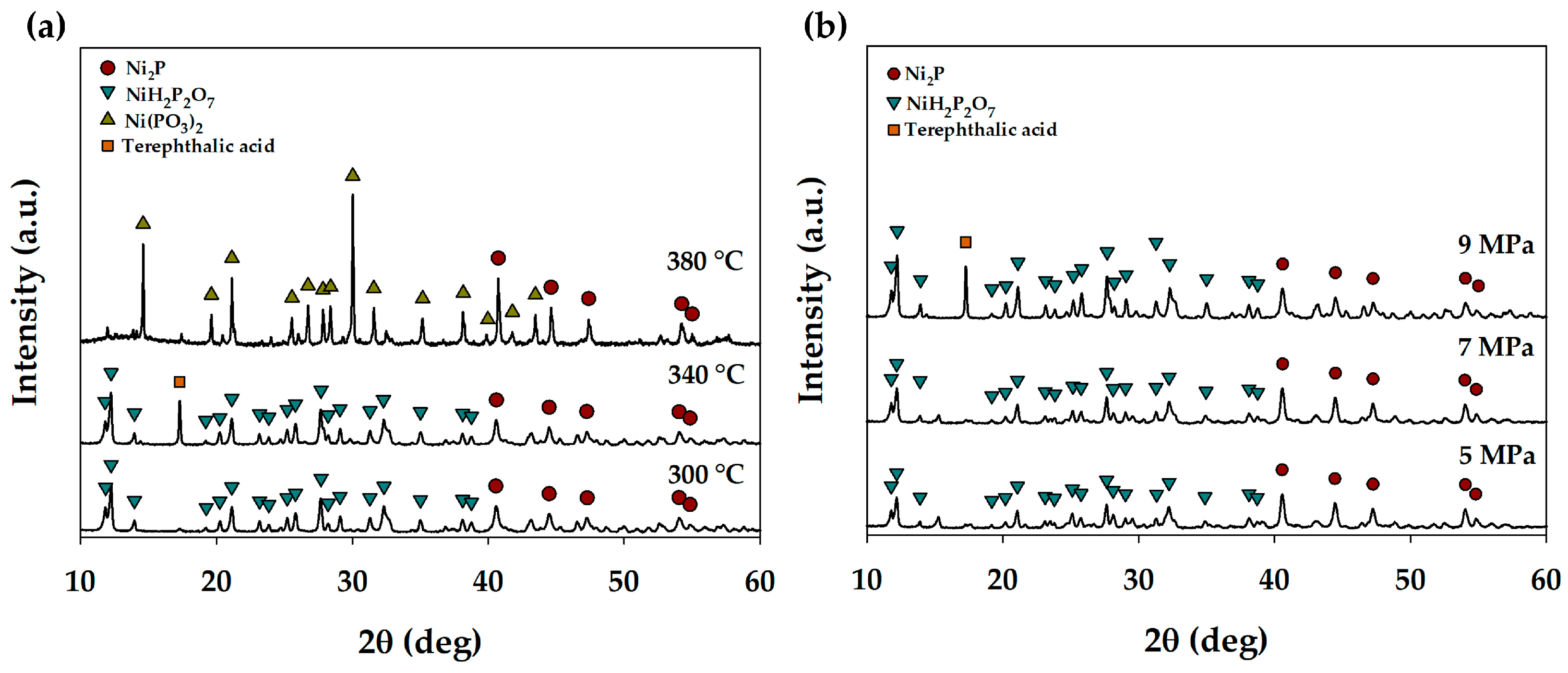
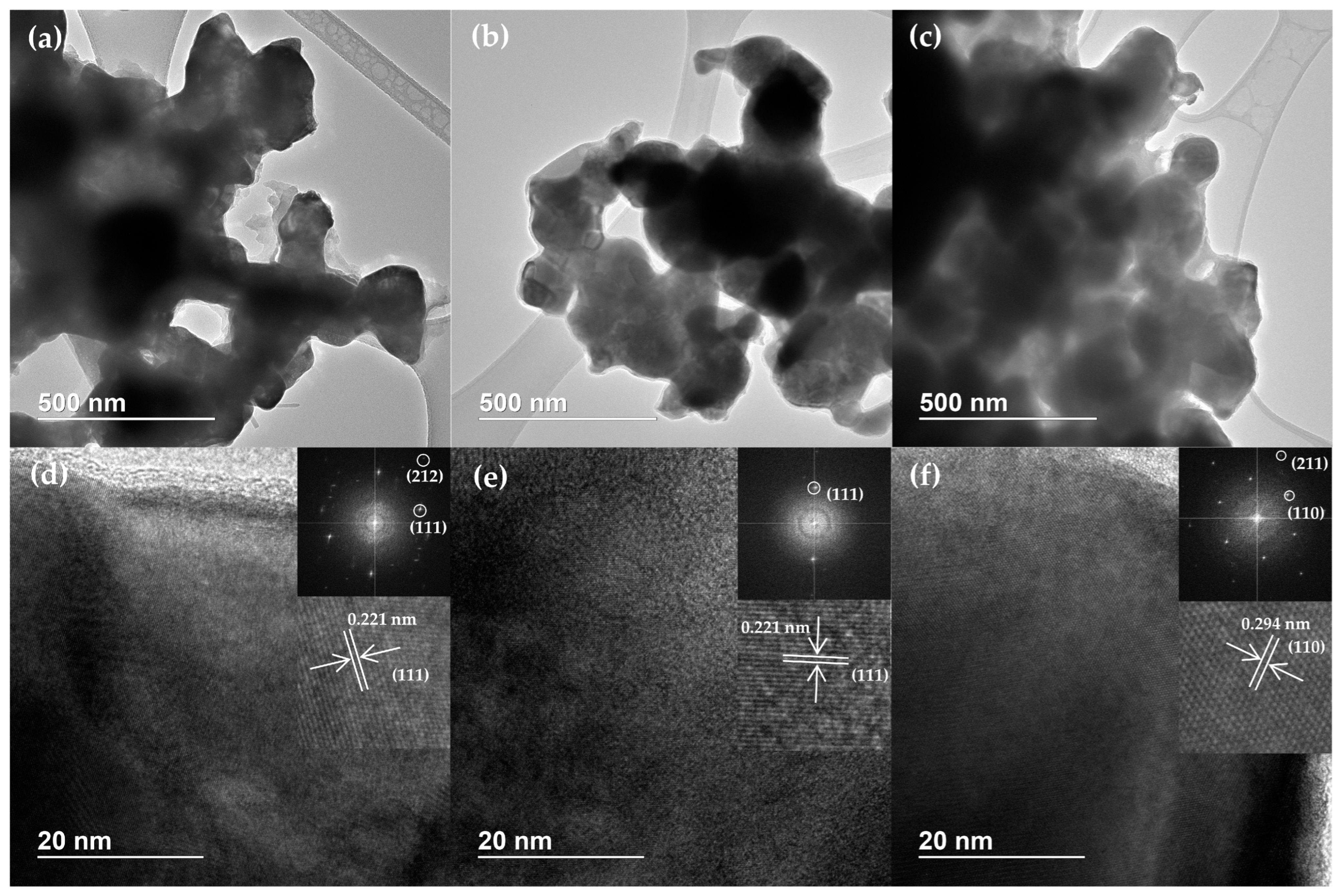
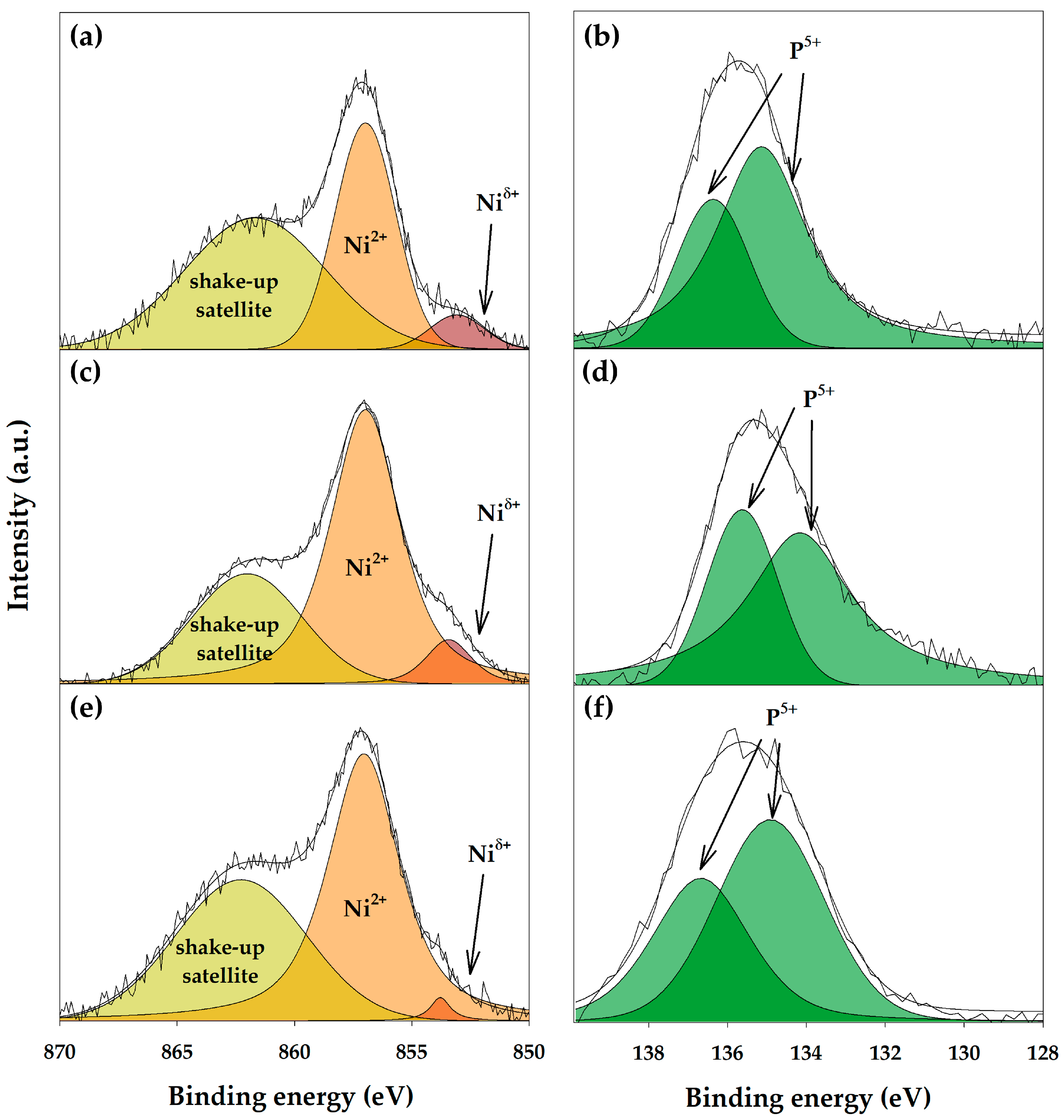
3.1. Nickel Phosphide In Situ Formation
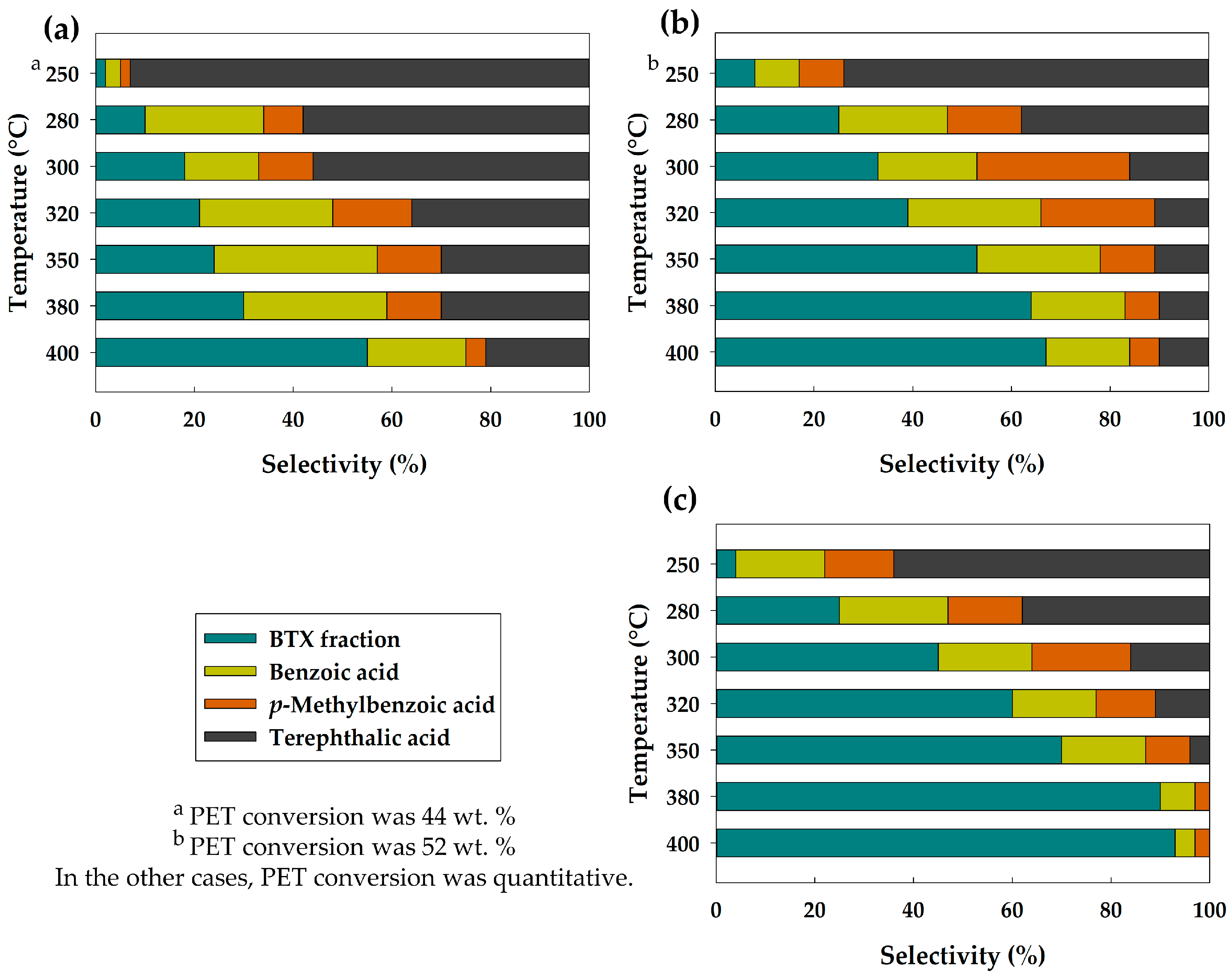
3.2. Catalytic Activity
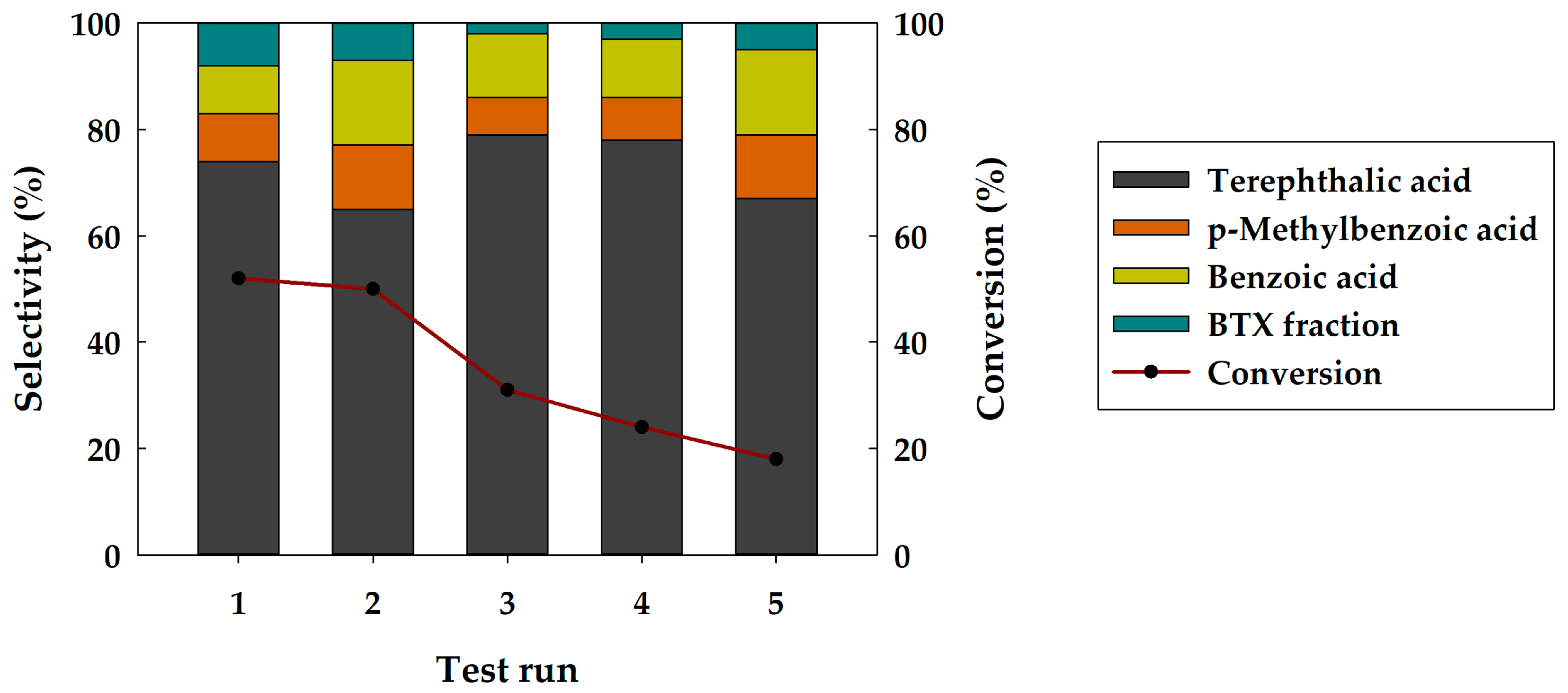
3.3. Recycling Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Plastic Pollution. Our World Data. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 8 May 2023).
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A review. J. Environ. Chem. Eng. 2022, 10, 106867. [Google Scholar] [CrossRef]
- García, J.M. Catalyst: Design challenges for the future of plastics recycling. Chem 2016, 1, 813–815. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Arzhakov, M.S.; Khokhlov, A.R. Disposable polymer packaging: A problem without a solution? Her. Russ. Acad. Sci. 2022, 92, 600–608. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Tomás, R.A.F.; Bordado, J.C.M.; Gomes, J.F.P. p-Xylene oxidation to terephthalic acid: A literature review oriented toward process optimization and development. Chem. Rev. 2013, 113, 7421–7469. [Google Scholar] [CrossRef]
- Tan, T.; Wang, W.; Zhang, K.; Zhan, Z.; Deng, W.; Zhang, Q.; Wang, Y. Upcycling plastic wastes into value-added products by heterogeneous catalysis. ChemSusChem 2022, 15, e202200522. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Furukawa, S.; Xia, J.; Sun, C.; Hülsey, M.J.; Wang, H.; Guo, Y.; Liu, X.; Yan, N. Towards the circular economy: Converting aromatic plastic waste back to arenes over a Ru/Nb2O5 catalyst. Angew. Chem. Int. Ed. 2021, 60, 5527–5535. [Google Scholar] [CrossRef]
- Lu, S.; Jing, Y.; Feng, B.; Guo, Y.; Liu, X.; Wang, Y. H2-free plastic conversion: Converting PET back to BTX by unlocking hidden hydrogen. ChemSusChem 2021, 14, 4242–4250. [Google Scholar] [CrossRef]
- Tang, H.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Xu, G.; Wang, X.; Zhang, T. Synthesis of gasoline and jet fuel range cycloalkanes and aromatics from poly(ethylene terephthalate) waste. Green Chem. 2019, 21, 2709–2719. [Google Scholar] [CrossRef]
- Hongkailers, S.; Jing, Y.; Wang, Y.; Hinchiranan, N.; Yan, N. Recovery of arenes from polyethylene terephthalate (PET) over a Co/TiO2 Catalyst. ChemSusChem 2021, 14, 4330–4339. [Google Scholar] [CrossRef]
- Golubeva, M.A.; Zakharyan, E.M.; Maximov, A.L. Transition metal phosphides (Ni, Co, Mo, W) for hydrodeoxygenation of biorefinery products (a review). Pet. Chem. 2020, 60, 1109–1128. [Google Scholar] [CrossRef]
- Golubeva, M.A.; Maximov, A.L. Hydroprocessing of furfural over in situ generated nickel phosphide based catalysts in different solvents. Appl. Catal. A 2020, 608, 117890. [Google Scholar] [CrossRef]
- Golubeva, M.A.; Maximov, A.L. Catalytic system based on nickel(II) acetate and hypophosphorous acid for the selective hydrodeoxygenation of guaiacol. Mendeleev Commun. 2019, 29, 550–552. [Google Scholar] [CrossRef]
- Golubeva, M.A. In situ generated nickel phosphide based catalysts for hydroprocessing of levulinic acid. Pet. Chem. 2021, 61, 670–675. [Google Scholar] [CrossRef]
- Guan, Q.; Li, W.; Zhang, M.; Tao, K. Alternative synthesis of bulk and supported nickel phosphide from the thermal decomposition of hypophosphites. J. Catal. 2009, 263, 1–3. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Pandey, S.; Rangan, K.K. Convenient route for the synthesis of transition-metal pnictides by direct reduction of phosphate, arsenate, and antimonate precursors. Chem. Mater. 1997, 9, 2113–2116. [Google Scholar] [CrossRef]
- De Andrade, J.B.; Nunes, G.S.; Veiga, M.P.; Costa, A.C.S.; Ferreira, S.L.C.; Amorim, A.M.M.; Reis, S.T. Spectrophotometric and inductively coupled plasma atomic emission spectrometric determination of titanium in ilmenites after rapid dissolution with phosphoric acid. Talanta 1997, 44, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, Version 4.1, National Institute of Standards and Technology, Gaithersburg MD. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 8 May 2023).
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, C.; Kasakov, S.; Foraita, S.; Lercher, J.A. Manipulating catalytic pathways: Deoxygenation of palmitic acid on multifunctional catalysts. Chem. Eur. J. 2013, 19, 4732–4741. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Abdullah, B. The effect of metal loading over Ni/γ-Al2O3 and Mo/γ-Al2O3 catalysts on reaction routes of hydrodeoxygenation of rubber seed oil for green diesel production. Catal. Today 2020, 355, 51–64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubeva, M.; Mukhtarova, M.; Sadovnikov, A.; Maximov, A. PET Waste Recycling into BTX Fraction Using In Situ Obtained Nickel Phosphide. Polymers 2023, 15, 2248. https://doi.org/10.3390/polym15102248
Golubeva M, Mukhtarova M, Sadovnikov A, Maximov A. PET Waste Recycling into BTX Fraction Using In Situ Obtained Nickel Phosphide. Polymers. 2023; 15(10):2248. https://doi.org/10.3390/polym15102248
Chicago/Turabian StyleGolubeva, Maria, Mariyam Mukhtarova, Alexey Sadovnikov, and Anton Maximov. 2023. "PET Waste Recycling into BTX Fraction Using In Situ Obtained Nickel Phosphide" Polymers 15, no. 10: 2248. https://doi.org/10.3390/polym15102248
APA StyleGolubeva, M., Mukhtarova, M., Sadovnikov, A., & Maximov, A. (2023). PET Waste Recycling into BTX Fraction Using In Situ Obtained Nickel Phosphide. Polymers, 15(10), 2248. https://doi.org/10.3390/polym15102248







