A Label-Free Electrochemical Biosensor for Homocysteine Detection Using Molecularly Imprinted Polymer and Nanocomposite-Modified Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Specimens
2.2. Apparatus
2.3. Procedures
2.3.1. Preparation of Molecularly Imprinted Polymers
2.3.2. Preparation of CNT/CS/IL Nanocomposite
2.3.3. Hcy-MIP Electrode Fabrication
2.3.4. Characterization and Electroanalytical Measurements of the Hcy-MIP Electrodes
2.3.5. Determination of Homocysteine
2.3.6. Evaluation of Hcy-MIP Biosensor Performance
3. Results and Discussion
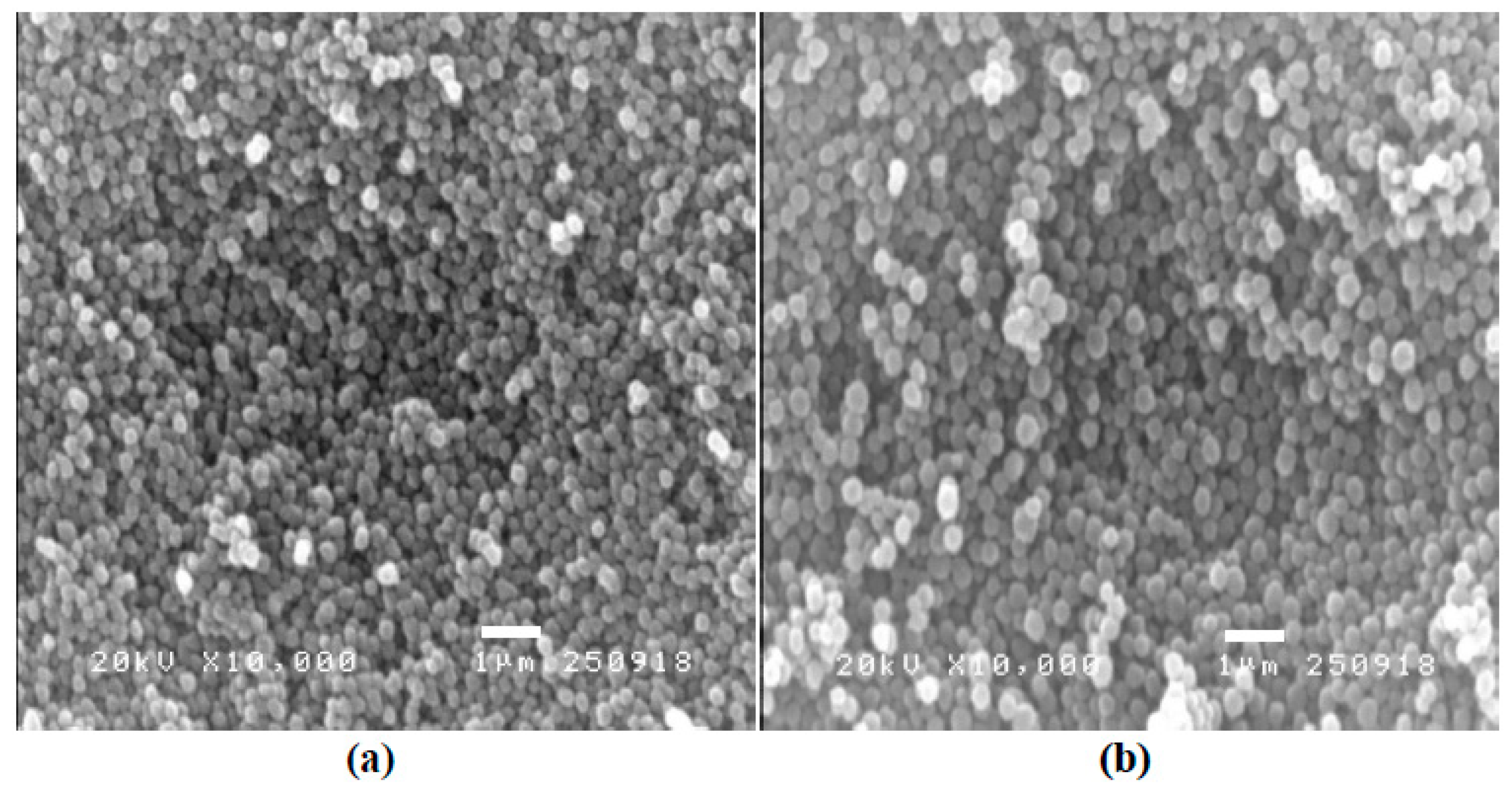
3.1. Characterization of Hcy-MIP
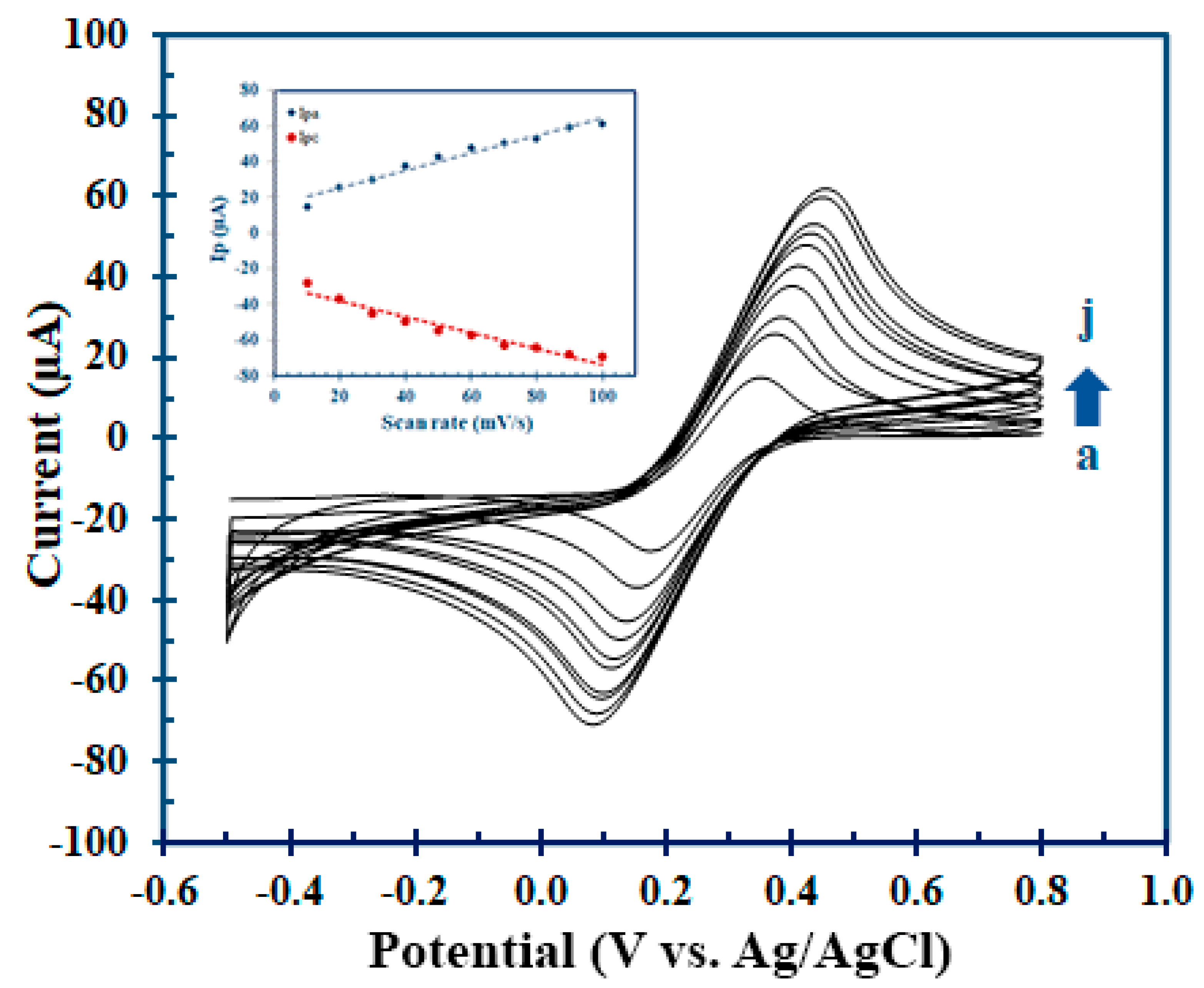
3.2. Electrochemical Characteristics of Hcy-MIP
3.3. Optimization of Hcy-MIP Biosensor
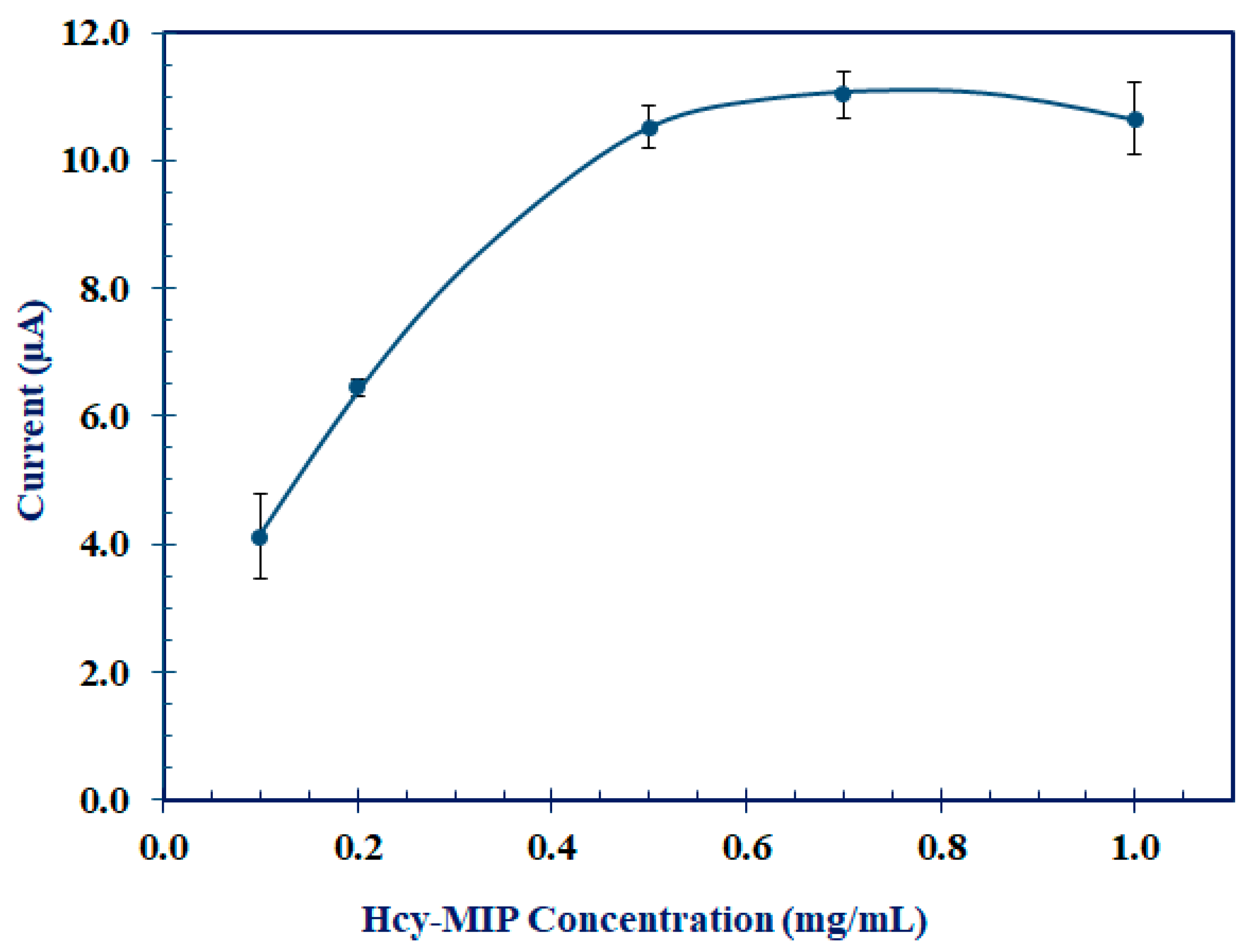
3.3.1. Concentration of MIP in Hcy-MIP Biosensor
3.3.2. Effect of pH
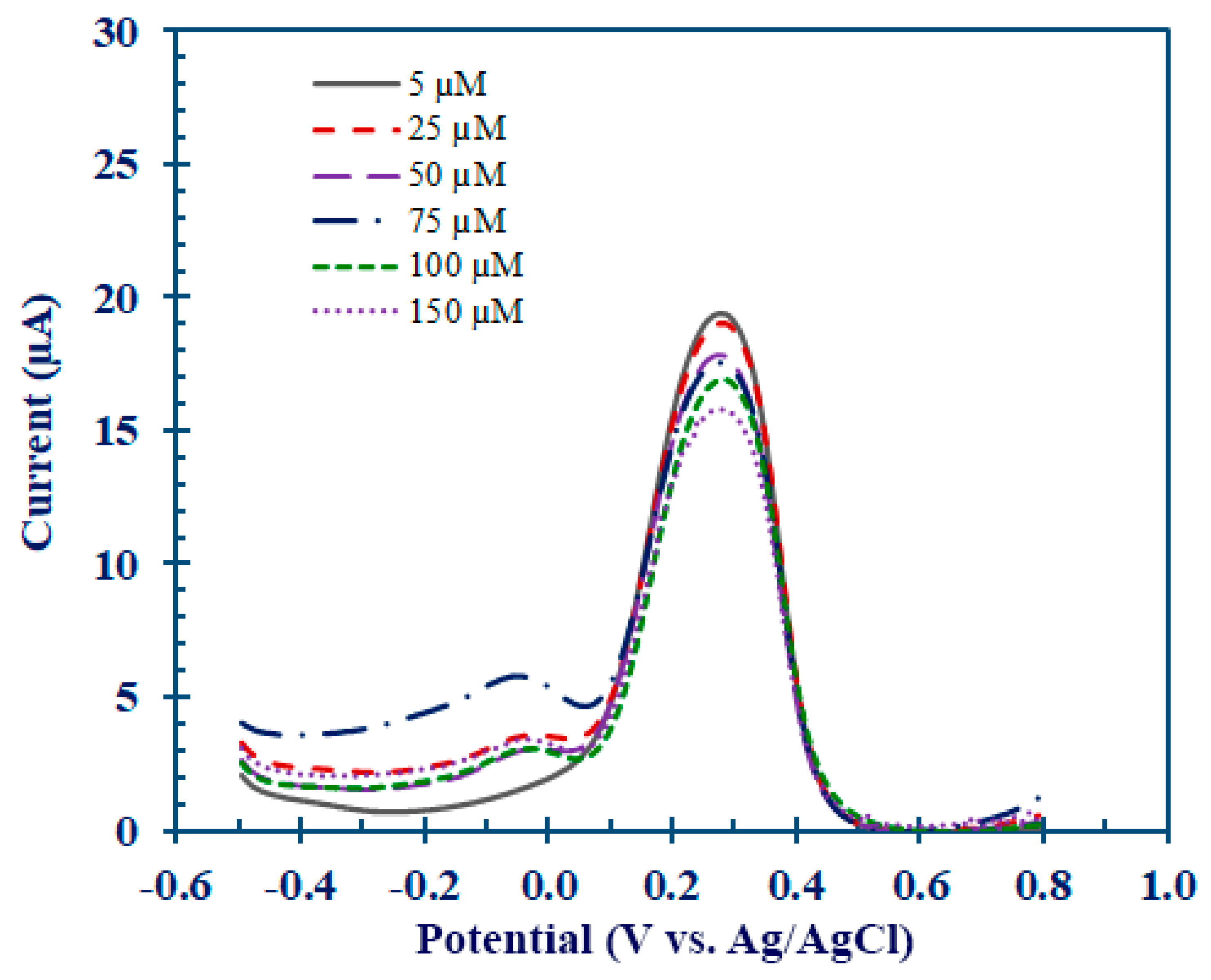
3.4. Determination of Hcy
3.5. Evaluation of Hcy-MIP Biosensor Performance
3.5.1. Dose Response of Hcy-MIP Biosensor
3.5.2. Analytical Accuracy
3.5.3. Analytical Precision
3.5.4. Analytical Specificity
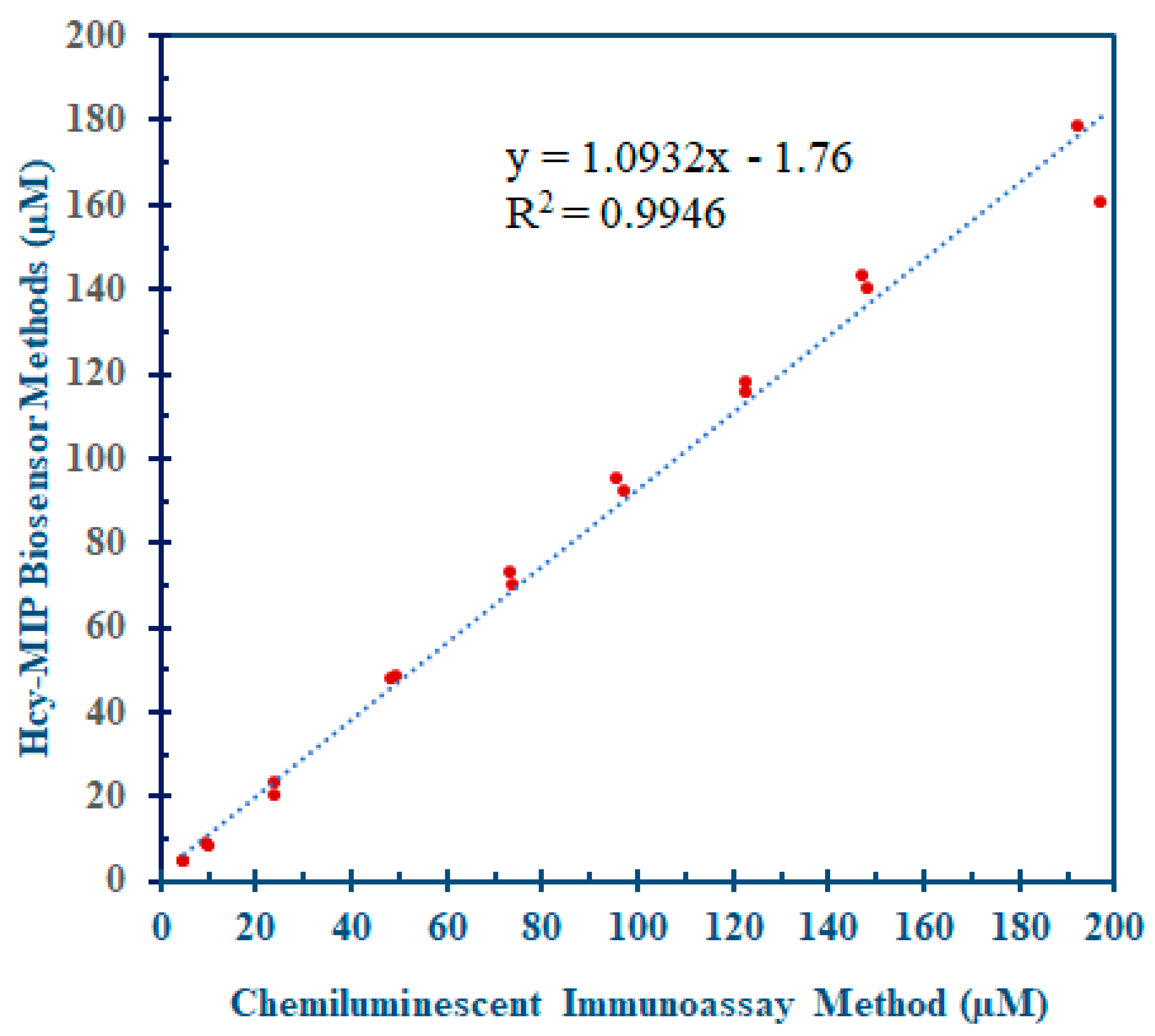
3.5.5. Comparative Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochholzer, W.; Morrow, D.A.; Giugliano, R.P. Novel Biomarkers in Cardiovascular Disease: Update 2010. Am. Heart J. 2010, 160, 583–594. [Google Scholar] [CrossRef]
- Nekrassova, O.; Lawrence, N.S.; Compton, R.G. Analytical Determination of Homocysteine: A Review. Talanta 2003, 60, 1085–1095. [Google Scholar] [CrossRef]
- Splaver, A.; Lamas, G.A.; Hennekens, C.H. Homocysteine and Cardiovascular Disease: Biological Mechanisms, Observational Epidemiology, and the Need for Randomized Trials. Am. Heart J. 2004, 148, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Refsum, H.; Smith, A.D.; Ueland, P.M.; Nexo, E.; Clarke, R.; McPartlin, J.; Johnston, C.; Engbaek, F.; Schneede, J.; McPartlin, C.; et al. Facts and Recommendations about Total Homocysteine Determinations: An Expert Opinion. Clin. Chem. 2004, 50, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Ko, L.E.; Yang, C.S. High Performance Liquid Chromatography with Fluorimetric Detection for the Determination of Total Homocysteine in Human Plasma: Method and Clinical Applications. Anal. Chim. Acta 2001, 429, 331–336. [Google Scholar] [CrossRef]
- Accinni, R.; Bartesaghi, S.; De Leo, G.; Cursano, C.F.; Achilli, G.; Loaldi, A.; Cellerino, C.; Parodi, O. Screening of Homocysteine from Newborn Blood Spots by High-performance Liquid Chromatography with Coulometric Array Detection. J. Chromatogr. A 2000, 896, 183–189. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Zhu, W.; Wang, D.; Ye, J.; Yamamoto, K.; Jin, L. Simultaneous Determination of Total Homocysteine, Cysteine and Methionine in Hypothyroid Patients’ Plasma by Liquid Chromatography Using Platinum/Poly(methyl violet) Modified Electrode. Anal. Chim. Acta 2005, 545, 182–188. [Google Scholar] [CrossRef]
- Amarnath, K.; Amarnath, V.; Amarnath, K.; Valentine, H.; Valentine, W. A Specific HPLC-UV Method for the Determination of Cysteine and Related Aminothiols in Biological Samples. Talanta 2003, 60, 1229–1238. [Google Scholar] [CrossRef]
- Chwatko, G.; Bald, E. Determination of Cysteine in Human Plasma by High-performance Liquid Chromatography and Ultraviolet Detection after Pre-column Derivatization with 2-Chloro-1-Methylpyridinium Iodide. Talanta 2000, 52, 509–515. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Zhang, Z.; Zhang, H. 3-Iodoacetylaminobenzanthrone as a Fluorescent Derivatizing Reagent for Thiols in High-performance Liquid Chromatography. Anal. Chim. Acta 2004, 512, 281–286. [Google Scholar] [CrossRef]
- Guan, X.; Hoffman, B.; Dwivedi, C.; Matthees, D.P. A Simultaneous Liquid Chromatography/Mass Spectrometric Assay of Glutathione, Cysteine, Homocysteine and Their Disulfides in Biological Samples. J. Pharm. Biomed. Anal. 2003, 31, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Götting, C.; Kleesiek, K. Rapid Micro-scale Assay for Homocysteine by Liquid Chromatography-tandem Mass Spectrometry. Clin. Biochem. 2006, 39, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Pasas, S.A.; Lacher, N.A.; Davies, M.I.; Lunte, S.M. Detection of Homocysteine by Conventional and Microchip Capillary Electrophoresis/Electrochemistry. Electrophoresis 2002, 23, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kirchhoff, J.R. Determination of Thiols by Capillary Electrophoresis with Amperometric Detection at a Coenzyme Pyrroloquinoline Quinone Modified Electrode. Anal. Chem. 2002, 74, 1349–1354. [Google Scholar] [CrossRef]
- Zou, Y.; Feng, X.; Zhao, Y.; Wei, Z.; Zhu, W.; Zhao, M.; Wang, T.; Liu, Z.; Tang, S.; Wang, G.; et al. Selective Homocysteine Detection of Nitrogen-doped Graphene Quantum Dots: Synergistic Effect of Surface Catalysis and Photoluminescence Sensing. Synth. Metals 2020, 267, 116432. [Google Scholar] [CrossRef]
- Hock, B. Antibodies for immunosensors a review. Anal. Chim. Acta 1997, 347, 177–186. [Google Scholar] [CrossRef]
- Frantzen, F.; Faaren, A.L.; Alfheim, I.; Nordhei, A.K. Enzyme Conversion Immunoassay for Determining Total Homocysteine in Plasma or Serum. Clin. Chem. 1998, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Su, Y.; Zhu, X.; Liu, G.; Fan, C. Development of Electrochemical Immunosensors Towards Point of Care Diagnostics. Biosens. Bioelectron. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Chen, W.; Ang, C.; Lou, D.; Ji, Y.; Huang, L.; Zhu, Y.; Guo, Z.; Gu, N.; Zhang, Y. Fast Immunofluorescence Lateral Flow Test Strip Approach for Detection of Homocysteine. Micro Nano Lett. 2018, 13, 1719–1723. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, D.; Shuang, S.; Choi, M.M.F. A Homocysteine Biosensor with Eggshell Membrane as an Enzyme Immobilization Platform. Sens. Actuators B 2006, 114, 936–942. [Google Scholar] [CrossRef]
- Alacam, S.; Timur, S.; Telefoncu, A. A Novel Biosensor Based on L-homocysteine Desulfhydrase Enzyme Immobilized in Eggshell Membrane. J. Mol. Catal. B Enzym. 2007, 49, 55–60. [Google Scholar] [CrossRef]
- Ching, C.T.S.; Chang, J.W.; Sun, T.P.; Shieh, H.L.; Tsai, C.L.; Huang, H.W.; Liu, W.H.; Liu, C.M.; Yeh, W.C.; Li, J.H. An Amperometric Biosensor Array for Precise Determination of Homocysteine. Sens. Actuators B 2011, 152, 94–98. [Google Scholar] [CrossRef]
- Madasamy, T.; Santschi, C.; Martin, O.J.F. A Miniaturized Electrochemical Assay for Homocysteine using Screen-printed Electrodes with Cytochrome c Anchored Gold Nanoparticles. Analyst 2015, 140, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.; Mathiyarasua, J. An Electrochemical Sensor for Homocysteine Detection using Gold Nanoparticle Incorporated Reduced Graphene Oxide. Colloids Surf. B 2018, 170, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Agüí, L.; Peña-Farfal, C.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical Determination of Homocysteine at a Gold Nanoparticle-modified Electrode. Talanta 2007, 74, 412–420. [Google Scholar] [CrossRef]
- Gong, K.; Dong, Y.; Xiong, S.; Chen, Y.; Mao, L. Novel Electrochemical Method for Sensitive Determination of Homocysteine with Carbon Nanotube-based Electrodes. Biosens. Bioelectron. 2004, 20, 253–259. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Deo, R.P.; Wang, J. Detection of Homocysteine at Carbon Nanotube Paste Electrodes. Talanta 2004, 63, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Gholami-Orimi, F.; Taleshi, F.; Biparva, P.; Karimi-Maleh, H.; Beitollahi, H.; Ebrahimi, H.R.; Shamshiri, M.; Bagheri, H.; Fouladgar, M.; Taherkhani, A. Voltammetric Determination of Homocysteine using Multiwall Carbon Nanotube Paste Electrode in the Presence of Chlorpromazine as a Mediator. J. Anal. Methods Chem. 2012, 902184. [Google Scholar] [CrossRef]
- Zhao, J.; Du, P.; Sun, X.; Liu, H. Development of Electrochemical Sensor for Homocysteine Determination as a Cerebrovascular Disease Biomarker. Int. J. Electrochem. Sci. 2017, 12, 8642–8650. [Google Scholar] [CrossRef]
- Kannan, P.; Maiyalagan, T.; Sahoo, N.G.; Opallo, M. Nitrogen Doped Graphene Nanosheet Supported Platinum Nanoparticles as High Performance Electrochemical Homocysteine Biosensors. J. Mater. Chem. B 2013, 36, 4655–4666. [Google Scholar] [CrossRef]
- Šimšíkova, M.; Čechal, J.; Zorkovská, A.; Antalík, M.; Šikola, T. Preparation of CuO/ZnO Nanocomposite and Its Application as a Cysteine/Homocysteine Colorimetric and Fluorescence Detector. Colloids Surf. B 2014, 123, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Du, J.; Feng, S.; Li, J.; Yang, R.; Qu, L. Highly Selective and Sensitive Detection of Glutathione over Cysteine and Homocysteine with a Turn-on Fluorescent Biosensor Based on Cysteamine-stabilized CdTe Quantum Dots. Spectrochim. Acta Part A 2022, 267, 120492. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, W.; Chen, J.; Yu, C. A Graphene Quantum Dot-based Method for the Highly Sensitive and Selective Fluorescence Turn on Detection of Biothiols. Talanta 2014, 119, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro Selection of RNA Molecules that Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX-A (R)evolutionary Method to Generate High-affinity Nucleic Acid Ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly Imprinted Polymer Nanoparticles in Chemical Sensing—Synthesis, Characterisation and Application. Sens. Actuators B 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template—“Plastic antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Alcock, S.; Turner, A.P.F. Molecular Imprinting: At the Edge of the Third Millennium. Trends Biotechnol. 2001, 19, 9–12. [Google Scholar] [CrossRef]
- Wen, X.H.; Zhao, X.F.; Peng, B.F.; Yuan, K.P.; Li, X.X.; Zhu, L.Y.; Lu, H.L. Facile Preparation of an Electrochemical Aptasensor Based on Au NPs/Graphene Sponge for Detection of Homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]
- Beitollahi, H.; Zaimbashi, R.; Mahani, M.T.; Tajik, S. A Label-free Aptasensor for Highly Sensitive Detection of Homocysteine Based on Gold Nanoparticles. Bioelectrochemistry 2020, 134, 107497. [Google Scholar] [CrossRef]
- Saeed, J.; Mirzaei, M.; Torkzadeh-Mahani, M. A Selective and Regenerable Voltammetric Aptasensor for Determination of Homocysteine. Microchim. Acta 2016, 183, 2205–2210. [Google Scholar] [CrossRef]
- McKeague, M.; Foster, A.; Miguel, Y.; Giamberardino, A.; Verdin, C.; Chan, J.Y.S.; Derosa, M.C. Development of a DNA Aptamer for Direct and Selective Homocysteine Detection in Human Serum. RSC Adv. 2013, 46, 24415–24422. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, S.; Mergola, L.; Del Sole, R.; Vasapollo, G. Synthesis of Molecularly Imprinted Polymers for Amino Acid Derivates by Using Different Functional Monomers. Int. J. Mol. Sci. 2011, 12, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.F.; Lam, M.H.W.; Leung, M.K.P. Fluorescent Sensing of Homocysteine by Molecular Imprinting. Anal. Chim. Acta 2002, 466, 17–30. [Google Scholar] [CrossRef]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly Imprinted Polymers for the Recognition of Proteins: The State of the Art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Athikomrattanakul, U.; Promptmas, C.; Katterle, M. Synthetic Receptors for Neutral Nitro Derivatives. Tetrahedron Lett. 2009, 50, 359–362. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.-P.; Ragupathy, D. Development of a Stable Cholesterol Biosensor Based on Multi-walled Carbon Nanotubes-Gold Nanoparticles Composite Covered with a Layer of Chitosan-Room-temperature Ionic Liquid Network. Biosens. Bioelectron. 2009, 24, 2211–2217. [Google Scholar] [CrossRef]
- El-Bindary, M.A.; Shahat, A.; El-Deen, I.M.; El-Afify, M.A.M.; Hassan, N. Synthesis of Novel Fluorescent Sensor Based on a Modified Amino Al-MOF for Rapid, Sensitive, and Selective Detection of Arsenic in Aqueous Solution. Appl. Organomet. Chem. 2023, 37, e7029. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, M.; Ignatov, A.V.; Dyakov, S.A.; Wang, J.; Gippius, N.A.; Frueh, J.; Sukhorukov, G.B. Stimuli-Responsive Microarray Films for Real-Time Sensing of Surrounding Media, Temperature, and Solution Properties via Diffraction Patterns. ACS Appl. Mater. Interfaces 2020, 12, 19080–19091. [Google Scholar] [CrossRef]





| Platform | Methods | LOD | Linearity | Ref. |
|---|---|---|---|---|
| Chromatographic Platform | ||||
| Simultaneous liquid chromatography–mass spectrometry | HPLC-MS | 0.75 µM | 1.5–740 µM | [11] |
| Rapid liquid chromatography–tandem mass spectrometry | HPLC-MS/MS | 1.0 µM | 0.0–61.6 µM | [12] |
| HPLC with electrochemical coulometric array detection | HPLC-ED | 0.14 µM | - | [6] |
| HPLC with platinum/poly(methyl violet) (Pt/MV)-modified electrode | HPLC-ED | 0.1 µM | 0.2–100 µM | [7] |
| Thiocarbonyldiimidazole (TCDI) post-column reaction | HPLC-UV | 0.1 µM | 2.5–10 µM | [8] |
| 2-chloro-1-methylpyridinium iodide (CMPI) post-column reaction | HPLC-UV | 0.1 µM | 0.5–50 µM | [9] |
| Methanolic monobromobimane for thiol derivatization | HPLC-FL | 0.12 µM | 3.9–62 µM | [5] |
| Iodoacetylaminobenzanthrone (IAB) post-column reaction | HPLC-FL | 2.3 nM | 0.05–25 µM | [10] |
| Electrophoresis Platform | ||||
| Capillary electrophoresis/electrochemistry | Amperometry | 0.5 µM | 1–100 µM | [13] |
| Capillary electrophoresis with pyrroloquinoline quinone-modified electrode | Amperometry | 0.03 µM | 0.1–5 µM | [14] |
| Immunoassay Platform | ||||
| Lateral flow immunofluorescent | Optical | 0.27 µM | 1.0–50 µM | [16] |
| Enzyme-Based Biosensor Platform | ||||
| Amino acid oxidase immobilized on screen-printed carbon electrode | Amperometry | NA | 6.4–100 µM | [22] |
| Amino acid oxidase immobilized on oxygen electrode | Potentiometry | 2.0 µM | 0.05–1.5 µM | [20] |
| Homocysteine desulfhydrase enzyme electrode | Potentiometry | NA | 0.15–1.8 µM | [21] |
| Nanomaterial-Based Biosensor Platform | ||||
| Cytochrome c-anchored gold nanoparticles on screen-printed electrode | Amperometry | 0.3 µM | 0.4–700 µM | [23] |
| Gold nanoparticle-incorporated reduced graphene oxide electrode | Amperometry | 6.9 µM | 2–14 µM | [24] |
| Reduced graphene oxide–TiO2 (RGO-TiO2) nanocomposite on glassy carbon electrodes | Amperometry | 24 nM | 0.1–80 µM | [29] |
| Carbon nanotube-based electrode | Amperometry | 0.06 µM | 0.1–60 µM | [26] |
| Carbon nanotube-based electrode | Amperometry | 4.6 µM | 5.0–200 µM | [27] |
| Multiwall carbon nanotube paste electrode | Voltammetry | 0.8 µM | 0.1–210 µM | [28] |
| Graphene nanosheet-supported platinum nanoparticle electrode | Voltammetry | 0.2 nM | 0.2–2.4 nM | [30] |
| CuO/ZnO nanocomposite | Optical | 40 µM | 40–96 µM | [31] |
| Aptamer-Based Biosensor Platform | ||||
| Aptamer-modified Au NP/graphene sponge electrode | Voltammetry | 1.0 µM | 1–100 µM | [39] |
| Aptamer-modified gold nanoparticle/carbon electrode | Voltammetry | 0.009 µM | 0.05–20 µM | [40] |
| Aptamer-modified gold electrode | Voltammetry | 10 nM | 0.2–10 µM | [41] |
| Aptamer–gold nanoparticle | Optical | 0.3 µM | 0.5–3.0 µM | [42] |
| Quantum Dot Platform | ||||
| Nitrogen-doped graphene quantum dots | Optical | 0.05 nM | 0.05–50 nM | [15] |
| Cysteamine-stabilized CdTe quantum dots | Optical | 3.3 nM | 6.7–400 nM | [32] |
| Graphene quantum dots | Optical | 5 nM | 0–50 nM | [33] |
| Molecularly Imprinted Polymer-Based Biosensor Platform | ||||
| MIP-based optical sensor | Optical | NA | NA | [45] |
| MIP-modified nanocomposite screen-printed carbon electrode | Voltammetry | 1.2 µM | 5.0–150 µM | This Work |
| Homocysteine Added (µM) | Homocysteine Obtained (µM) | Recovery (%) |
|---|---|---|
| 50 | 46.10 | 91.10 |
| 75 | 72.60 | 96.07 |
| 100 | 94.40 | 93.85 |
| 150 | 144.30 | 95.83 |
| Average % recovery (n = 8) | 94.21 | |
| Homocysteine Concentration (µM) | Intra-Assay (n = 20) | Inter-Assay (n = 20) | ||
|---|---|---|---|---|
| Mean ± SD (µM) | %CV | Mean ± SD (µM) | %CV | |
| 5 | 4.97 ± 0.11 | 2.27 | 4.98 ± 0.17 | 3.42 |
| 150 | 150.35 ± 5.26 | 3.50 | 150.40 ± 6.35 | 4.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongintr, U.; Lertanantawong, B.; Promptmas, C. A Label-Free Electrochemical Biosensor for Homocysteine Detection Using Molecularly Imprinted Polymer and Nanocomposite-Modified Electrodes. Polymers 2023, 15, 2241. https://doi.org/10.3390/polym15102241
Kongintr U, Lertanantawong B, Promptmas C. A Label-Free Electrochemical Biosensor for Homocysteine Detection Using Molecularly Imprinted Polymer and Nanocomposite-Modified Electrodes. Polymers. 2023; 15(10):2241. https://doi.org/10.3390/polym15102241
Chicago/Turabian StyleKongintr, Unchalee, Benchaporn Lertanantawong, and Chamras Promptmas. 2023. "A Label-Free Electrochemical Biosensor for Homocysteine Detection Using Molecularly Imprinted Polymer and Nanocomposite-Modified Electrodes" Polymers 15, no. 10: 2241. https://doi.org/10.3390/polym15102241
APA StyleKongintr, U., Lertanantawong, B., & Promptmas, C. (2023). A Label-Free Electrochemical Biosensor for Homocysteine Detection Using Molecularly Imprinted Polymer and Nanocomposite-Modified Electrodes. Polymers, 15(10), 2241. https://doi.org/10.3390/polym15102241






