Encapsulation of Thymol and Eugenol Essential Oils Using Unmodified Cellulose: Preparation and Characterization
Abstract
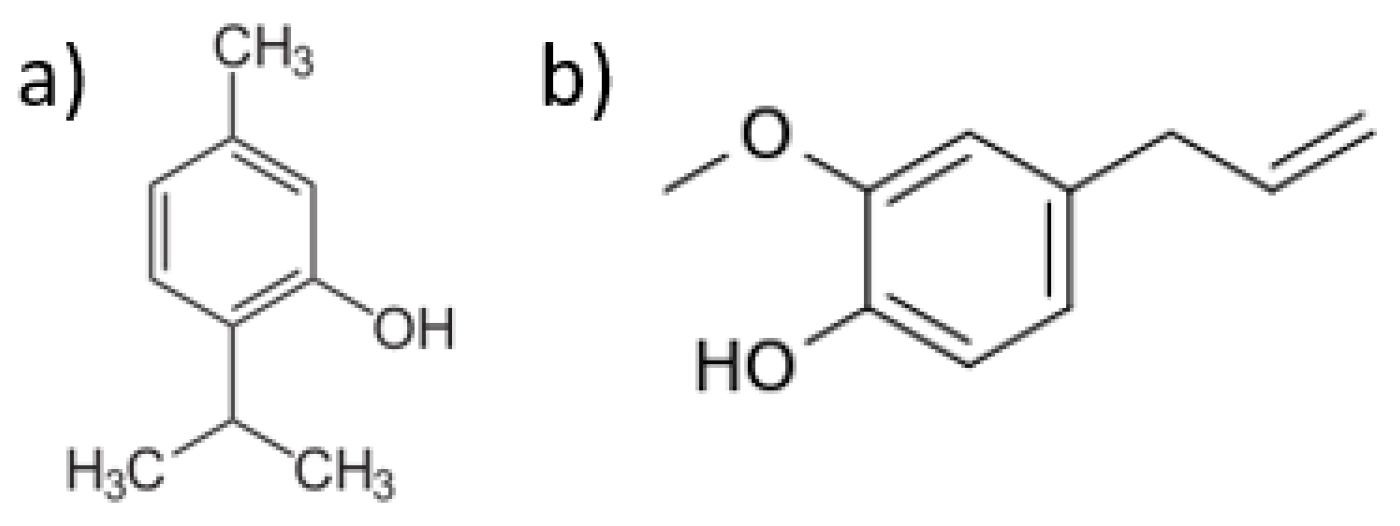
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication of Encapsulated Essential Oil Emulsions
2.2.2. Characterization
Scanning Electron Microscopy
Light Microscopy
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
Emulsion Stability and Particle Size Distribution
Emulsion Anti-Mold Activity
3. Results and Discussion
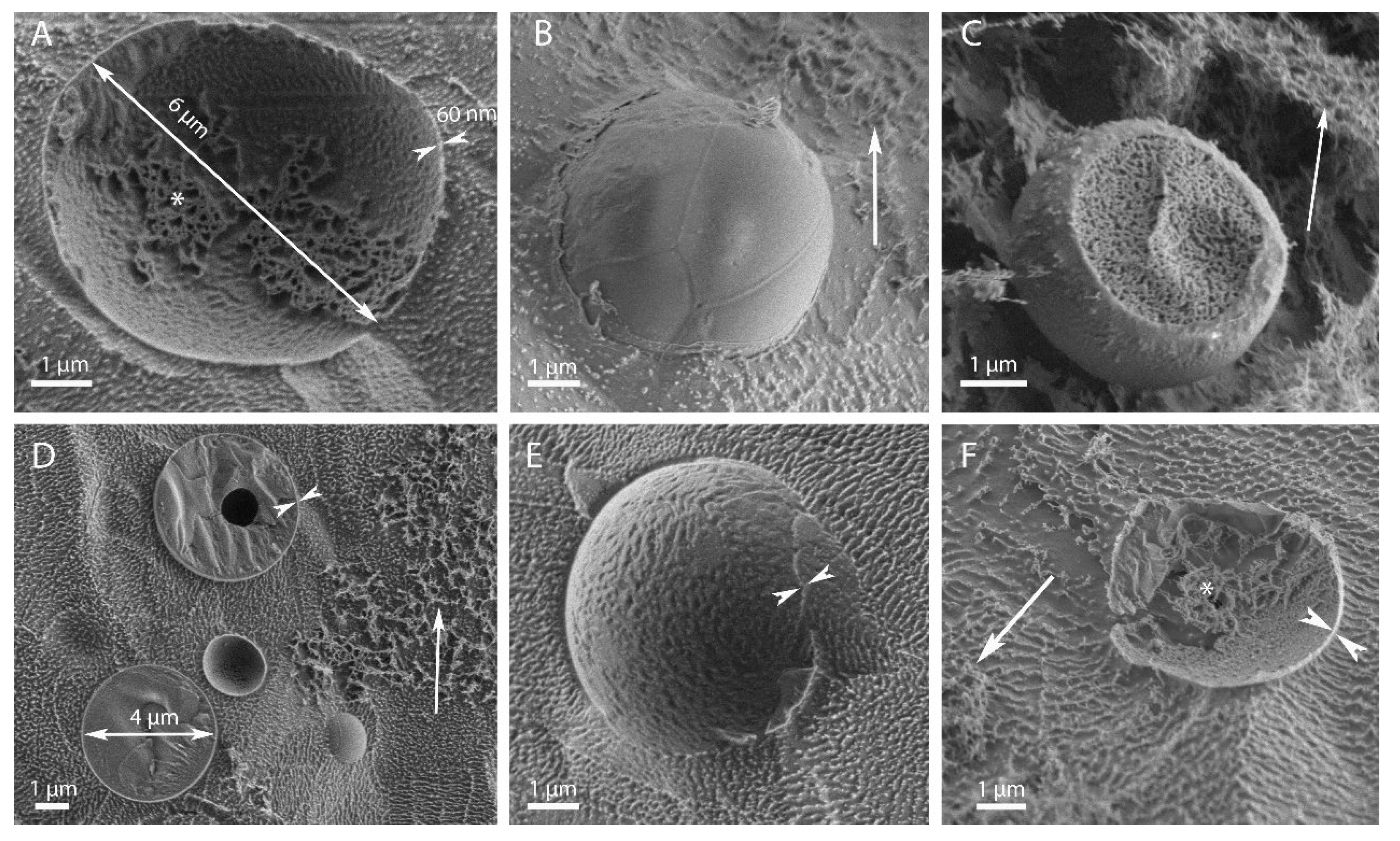
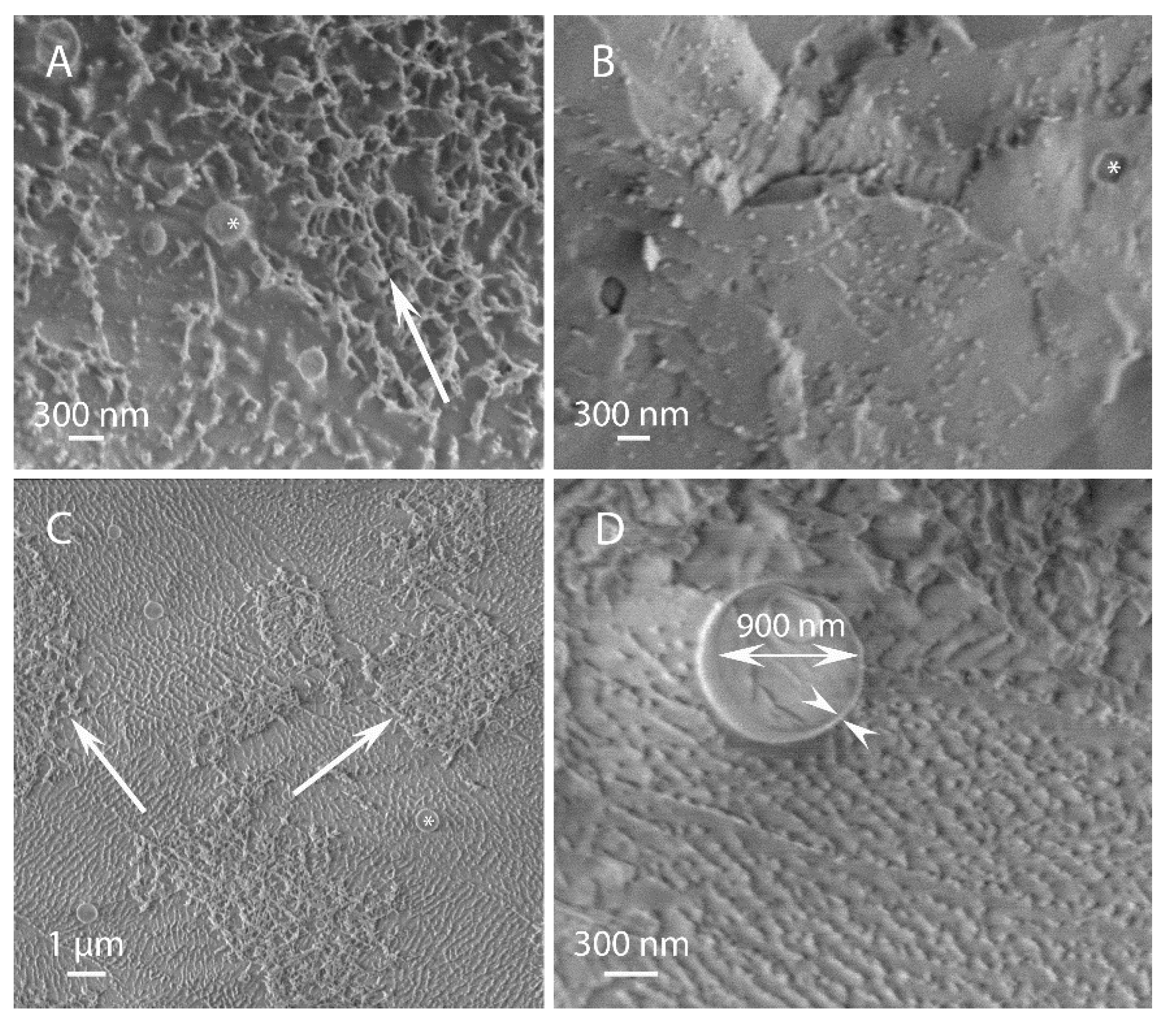
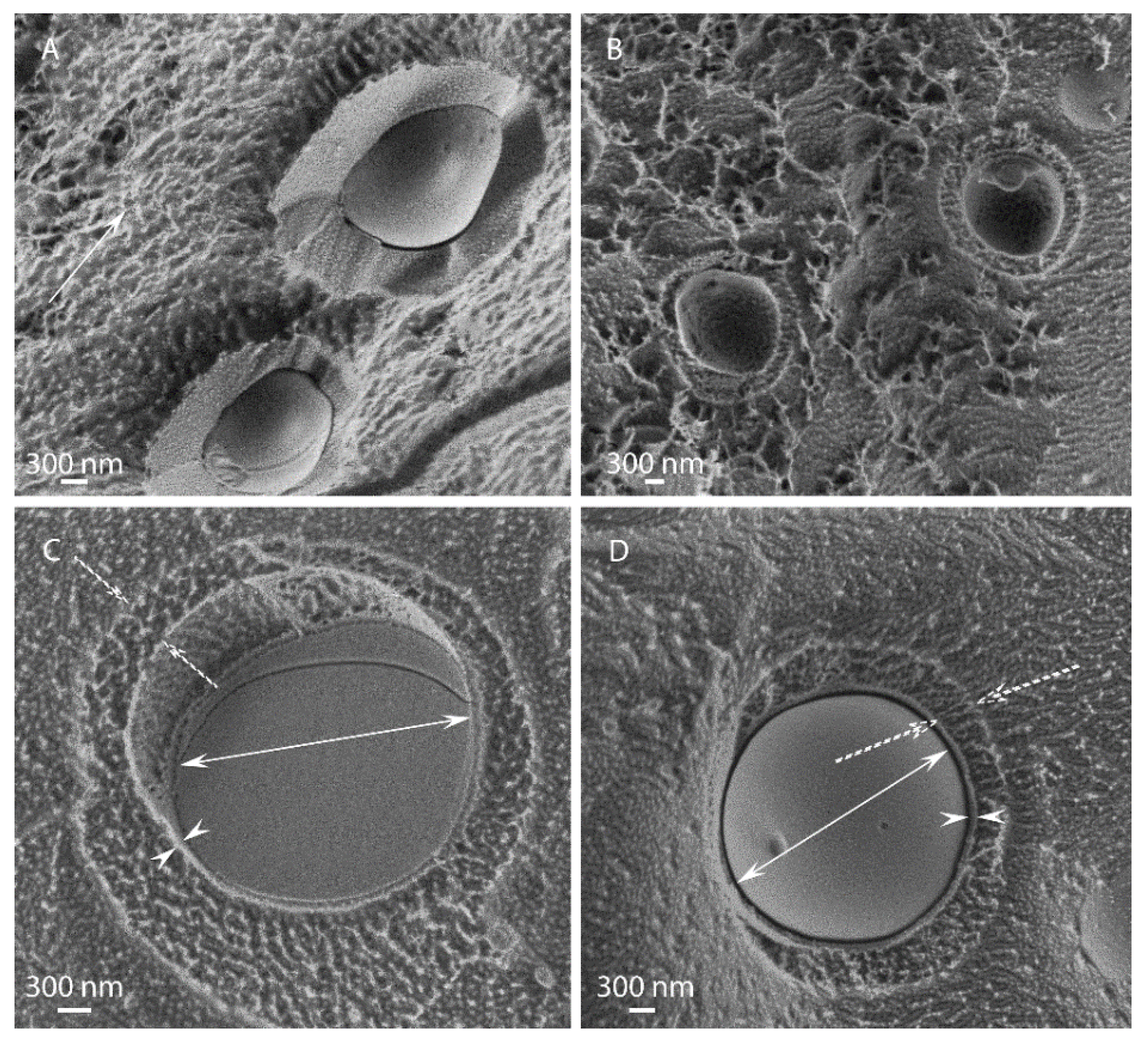
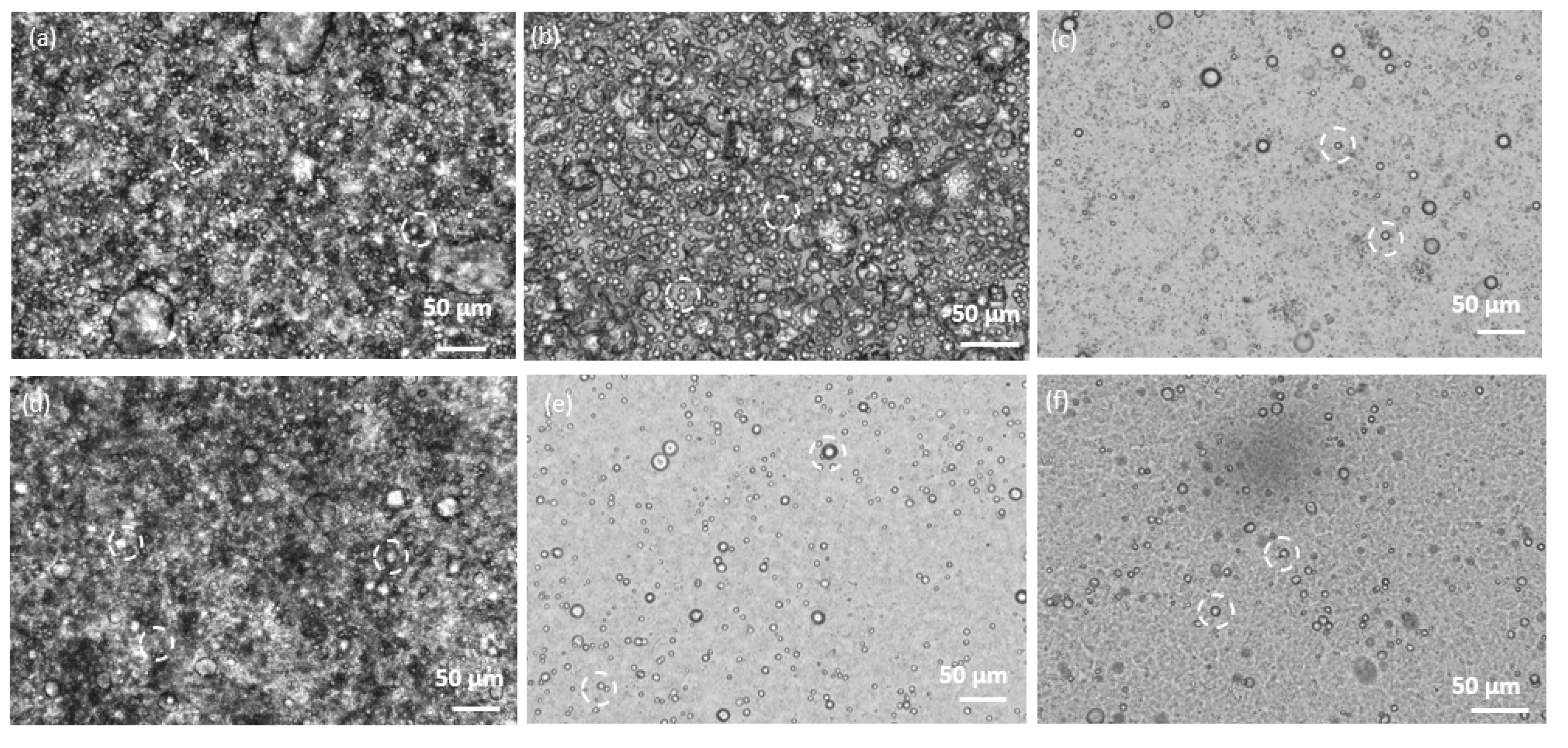
3.1. Morphological Observations by Scanning-Electron and Light Microscopies
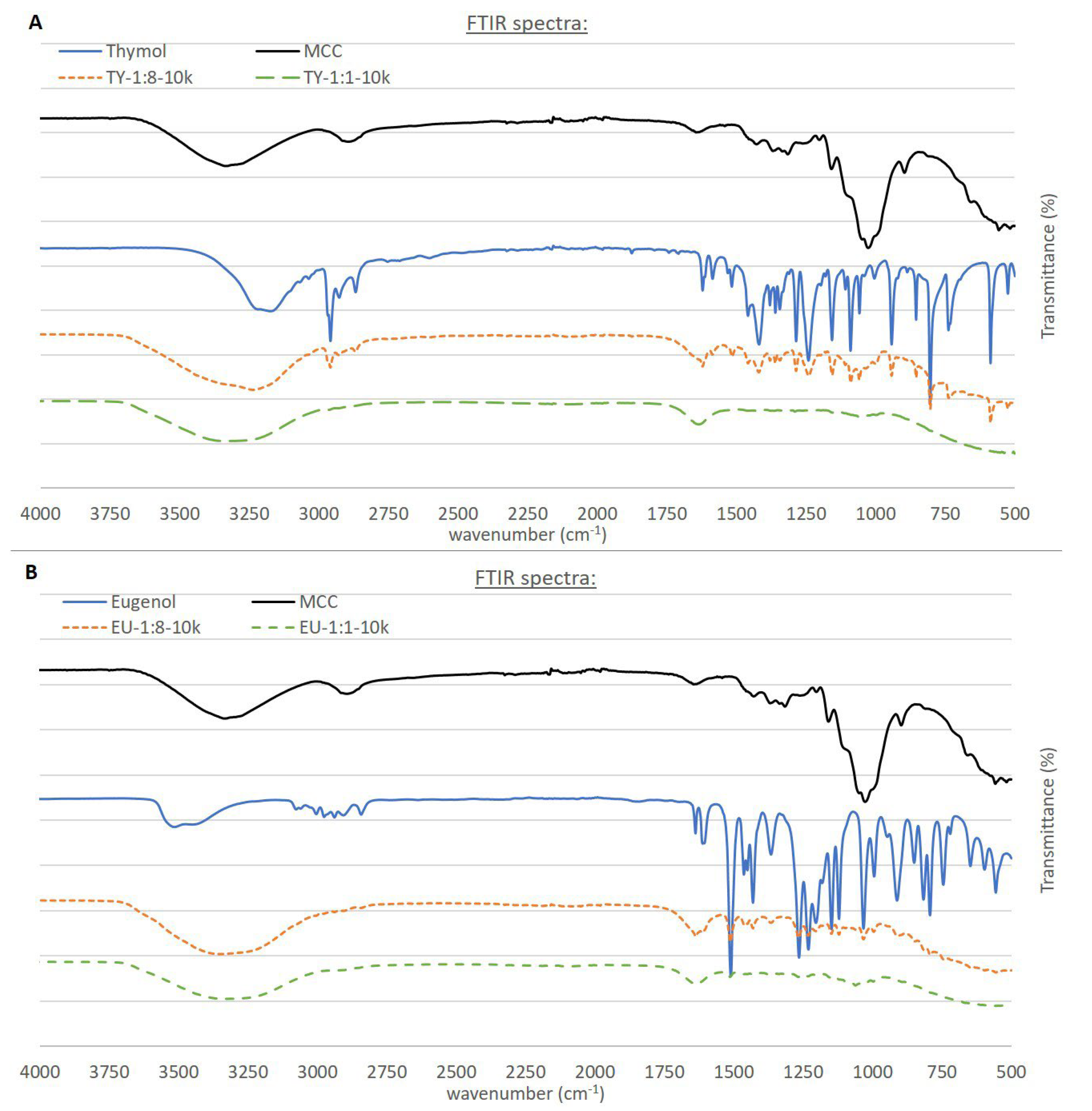
3.2. Attenuated Total Reflectance (ATR) Fourier Transform Infrared (FTIR) Spectroscopy
3.3. Emulsion Stability and Particle Size Distribution
3.4. Emulsion Anti-Mold Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Villanueva Bermejo, D.; Angelov, I.; Vicente, G.; Stateva, R.P.; Rodriguez García-Risco, M.; Reglero, G.; Ibañez, E.; Fornari, T. Extraction of thymol from different varieties of thyme plants using green solvents. J. Sci. Food Agric. 2015, 95, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre-Urizar, J.M.; Quindós, G.; Eraso, E. In vitro activities of carvacrol, cinnamaldehyde and thymol against Candida biofilms. Biomed. Pharmacother. 2021, 143, 112218. [Google Scholar] [CrossRef]
- Abd-Alla, M.; Abd-El-Kader, M.M.; Abd-El-Kareem, F.; El-Mohamedy, R.S.R. Evaluation of lemongrass, thyme and peracetic acid against gray mold of strawberry fruits. J. Agric. Technol. 2011, 7, 1775–1787. [Google Scholar]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Mittal, V.; Rao, R. Encapsulation of babchi essential oil into microsponges: Physicochemical properties, cytotoxic evaluation and anti-microbial activity. J. Food Drug Anal. 2019, 27, 60–70. [Google Scholar] [CrossRef]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose acetate—essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Hamzah, H.; Tunjung Pratiwi, S.U.; Hertiani, T. Efficacy of Thymol and Eugenol Against Polymicrobial Biofilm. Indones. J. Pharm. 2018, 29, 214. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Julaeha, E.; Nugeraha, R.; Nurzaman, M.; Kurnia, D.; Wahyudi, T.; Rosandi, Y. Characterization of ethyl cellulose (EC) microcapsules for lime oil encapsulation. J. Phys. Conf. Ser. 2018, 1080, 012038. [Google Scholar] [CrossRef]
- Enciso-Sáenz, S.; Borrás-Enriquez, A.J.; Ventura-Canseco, L.M.C.; Gutiérrez-Miceli, F.; Dendooven, L.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Ruíz-Valdiviezo, V.; Archila, M.A. Lemongrass (Cymbopogon citratus (DC) Stapf) essential oil encapsulation by freeze-drying. Rev. Mex. Ing. Quim. 2018, 17, 407–420. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion formation and stabilization by biomolecules: The leading role of cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Deeming, L.; Edler, K.J. Recent progress in Pickering emulsions stabilised by bioderived particles. RSC Adv. 2021, 11, 39027–39044. [Google Scholar] [CrossRef]
- De Souza, A.G.; Ferreira, R.R.; Aguilar, E.S.F.; Zanata, L.; Rosa, D.d.S. Cinnamon Essential Oil Nanocellulose-Based Pickering Emulsions: Processing Parameters Effect on Their Formation, Stabilization, and Antimicrobial Activity. Polysaccharides 2021, 2, 608–625. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef] [PubMed]
- Aksoylu Özbek, Z.; Günç Ergönül, P. A Review on Encapsulation of Oils. Celal Bayar Univ. J. Sci. 2017, 13, 293–309. [Google Scholar] [CrossRef]
- Yang, M.; Liang, Z.; Wang, L.; Qi, M.; Luo, Z.; Li, L. Microencapsulation Delivery System in Food Industry—Challenge and the Way Forward. Adv. Polym. Technol. 2020, 2020, 7531810. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–52. ISBN 978-3-319-26015-0. [Google Scholar]
- Costa, C.; Medronho, B.; Lindman, B.; Edlund, H.; Norgren, M. Cellulose as a Natural Emulsifier: From Nanocelluloses to Macromolecules. In Cellulose Science and Derivatives; Sand, A., Banga, S., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Rein, D.M.; Khalfin, R.; Cohen, Y. Cellulose as a novel amphiphilic coating for oil-in-water and water-in-oil dispersions. J. Colloid Interface Sci. 2012, 386, 456–463. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Napso, S.; Rein, D.M.; Fu, Z.; Radulescu, A.; Cohen, Y. Structural Analysis of Cellulose-Coated Oil-in-Water Emulsions Fabricated from Molecular Solution. Langmuir 2018, 34, 8857–8865. [Google Scholar] [CrossRef]
- Alfassi, G.; Rein, D.M.; Cohen, Y. Cellulose emulsions and their hydrolysis. J. Chem. Technol. Biotechnol. 2019, 94, 178–184. [Google Scholar] [CrossRef]
- Hamal, E.K.; Alfassi, G.; Khalfin, R.; Rein, D.M.; Cohen, Y. Structural Insights into Cellulose-Coated Oil in Water Emulsions. Langmuir 2022, 38, 11171–11179. [Google Scholar] [CrossRef]
- Meir, I.; Alfassi, G.; Arazi, Y.; Rein, D.M.; Fishman, A.; Cohen, Y. Lipase Catalyzed Transesterification of Model Long-Chain Molecules in Double-Shell Cellulose-Coated Oil-in-Water Emulsion Particles as Microbioreactors. Int. J. Mol. Sci. 2022, 23, 12122. [Google Scholar] [CrossRef]
- Zamani, Z.; Alipour, D.; Moghimi, H.R.; Mortazavi, S.A.R.; Saffaryc, M. Development and evaluation of thymol microparticles using cellulose derivatives as controlled release dosage form. Iran. J. Pharm. Res. 2015, 14, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.N.; Mishra, A.; Dutta, A.K. Preface. In Proceedings of the International Conference on Recent Trends in Physics (ICRTP 2016), Indore, India, 13–14 February 2016. J. Phys. Conf. Ser. 2016, 755, 011001. [Google Scholar] [CrossRef]
- Doncea, S.M.; Ion, R.M.; Fierascui, R.C.; Bacalum, E.; Bunaciu, A.A.; Aboul-Enein, H.Y. Spectral methods for historical paper analysis: Composition and age approximation. Instrum. Sci. Technol. 2010, 38, 96–106. [Google Scholar] [CrossRef]
- Chaib, S.; Benali, N.; Arhab, R.; Sadraoui Ajmi, I.; Bendaoued, H.; Romdhane, M. Preparation of Thymus vulgaris Essential Oil Microcapsules by Complex Coacervation and Direct Emulsion: Synthesis, Characterization and Controlled Release Properties. Arab. J. Sci. Eng. 2021, 46, 5429–5446. [Google Scholar] [CrossRef]
- Rodríguez, J.D.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef] [PubMed]
- Taraj, K.; Andoni, A.; Ylli, F.; Ylli, A.; Hoxha, R.; Llupa, J.; Malollari, I. Spectroscopic Investigation of Syzygium aromaticum L. Oil by Water Distillation Extraction. J. Int. Environ. Appl. Sci. 2020, 15, 122–126. [Google Scholar] [CrossRef]
- Zhu, Z.; Min, T.; Zhang, X.; Wen, Y. Microencapsulation of thymol in poly(lactide-co-glycolide) (PLGA): Physical and antibacterial properties. Materials 2019, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Lerche, D.; Sobisch, T. Direct and Accelerated Characterization of Formulation Stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- Dewitte, K.; Landschoot, S.; Carrette, J.; Audenaert, K.; Haesaert, G. Exploration of essential oils as alternatives to conventional fungicides in lupin cultivation. Org. Agric. 2019, 9, 107–116. [Google Scholar] [CrossRef]

| Sample ID | EO 1 Type | Cellulose: EO Ratio wt.:wt. | HPH 2 Pressure (psi) |
|---|---|---|---|
| MCC | None | Neat MCC | 5000 |
| EU-1:8-5k | EU | 1:8 | 5000 |
| TY-1:8-5k | TY | 1:8 | 5000 |
| TY-1:1-10k | TY | 1:1 | 10,000 |
| EU-1:1-10k | EU | 1:1 | 10,000 |
| TY-1:8-10k | TY | 1:8 | 10,000 |
| EU-1:8-10k | EU | 1:8 | 10,000 |
| TY-1:8-20k | TY | 1:8 | 20,000 |
| EU-1:8-20k | EU | 1:8 | 20,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shlosman, K.; Rein, D.M.; Shemesh, R.; Koifman, N.; Caspi, A.; Cohen, Y. Encapsulation of Thymol and Eugenol Essential Oils Using Unmodified Cellulose: Preparation and Characterization. Polymers 2023, 15, 95. https://doi.org/10.3390/polym15010095
Shlosman K, Rein DM, Shemesh R, Koifman N, Caspi A, Cohen Y. Encapsulation of Thymol and Eugenol Essential Oils Using Unmodified Cellulose: Preparation and Characterization. Polymers. 2023; 15(1):95. https://doi.org/10.3390/polym15010095
Chicago/Turabian StyleShlosman, Koranit, Dmitry M. Rein, Rotem Shemesh, Na’ama Koifman, Ayelet Caspi, and Yachin Cohen. 2023. "Encapsulation of Thymol and Eugenol Essential Oils Using Unmodified Cellulose: Preparation and Characterization" Polymers 15, no. 1: 95. https://doi.org/10.3390/polym15010095
APA StyleShlosman, K., Rein, D. M., Shemesh, R., Koifman, N., Caspi, A., & Cohen, Y. (2023). Encapsulation of Thymol and Eugenol Essential Oils Using Unmodified Cellulose: Preparation and Characterization. Polymers, 15(1), 95. https://doi.org/10.3390/polym15010095







