1. Introduction
Polyhexene (PH) is a relatively new promising polymer, which may be applied in various fields. The most well-known application is ultrahigh molecular weight polyhexene with the molecular weight higher than 10 × 10
6 g/mol, which is employed as drag reducing additives to reduce hydrodynamic resistance in oil pipelines [
1,
2]. At the same time, PH with different molecular weights necessary for other applications can be obtained by varying the polymerization conditions and the composition of catalysts used for PH production.
Catalysts of different compositions can be employed to produce polyhexene; among them are traditional Ziegler-Natta type catalysts, supported titanium-magnesium catalysts (TMC) [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16], and homogeneous metallocomplex catalysts [
17,
18,
19,
20]. Kinetics of hexene-1 polymerization over Ziegler-Natta catalysts with different composition and data on the effect of catalysts’ composition, and polymerization conditions on the molecular weight and molecular weight distribution of polyhexene are presented and discussed in refs. [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20]. Modern TMC, which are commonly used for stereospecific propylene polymerization, are highly active in hexene-1 polymerization also. It was shown in ref. [
16] that variation of the composition of a catalytic system and polymerization conditions makes it possible to obtain polyhexene with the molecular weight from 7 × 10
4 to 2 × 10
6 g/mol, controllable molecular weight distribution (MWD) (M
w/M
n in the region from 3.7 to 25), and different isotacticity (the content of mmmm pentads from 56 to 96%).
The analysis of literature data shows that polymerization of hexene-1 over titanium-magnesium catalysts strongly differs from the results obtained for propylene. In particular, propylene polymerization over the TMC containing dibutyl phthalate as a stereoregulating component and triethylaluminum (AlEt
3) as a cocatalyst leads to the formation of polypropylene (PP) with quite narrow MWD (M
w/M
n = 4–6). However, polymerization of hexene-1 over a similar TMC with the AlEt
3 cocatalyst yields polyhexene with a broad MWD (M
w/M
n = 15–25) [
12]. When the AlEt
3 cocatalyst is replaced by tri-isobutylaluminium (Al(i-Bu)
3), polyhexene with a narrower MWD (M
w/M
n = 3.7–15) is formed over the same TMC. It should be noted that data concerning the effect of the cocatalyst composition (AlEt
3 or Al(i-Bu)
3) on the polymerization kinetics of propylene and molecular weight characteristics of polypropylene are absent in the literature. We think that possible reasons of differences in the polymerization kinetics of hexene-1 and propylene and in the molecular weight characteristics of the produced polymers could be revealed by a more detailed investigation of the polymerization kinetics of these monomers and molecular weight characteristics of polyhexene and polypropylene obtained on similar samples of titanium-magnesium catalyst.
In this paper, we have presented comparative data on the polymerization kinetics of hexene-1 and propylene over the same supported TMC used for stereospecific polymerization of propylene, as well as the data on molecular weight and MWD of polyhexene and polypropylene obtained under variation of polymerization conditions and cocatalyst composition. The data were used to discuss possible reasons for the great differences in the polymerization kinetics of these monomers and in the MWD of PH and PP samples.
3. Results and Discussion
Our earlier study [
15] revealed that in the case of hexene-1 polymerization over titanium–magnesium catalyst, the composition of cocatalyst (AlEt
3 or Al(i-Bu)
3) substantially affects the MWD of the produced polyhexene. In particular, the use of AlEt
3 as a cocatalyst leads to the formation of a polyhexene with a broader molecular weight distribution in comparison with MWD of polyhexene obtained in the presence of the Al(i-Bu)
3 cocatalyst. More detailed data on MWD of polyhexene produced with the AlEt
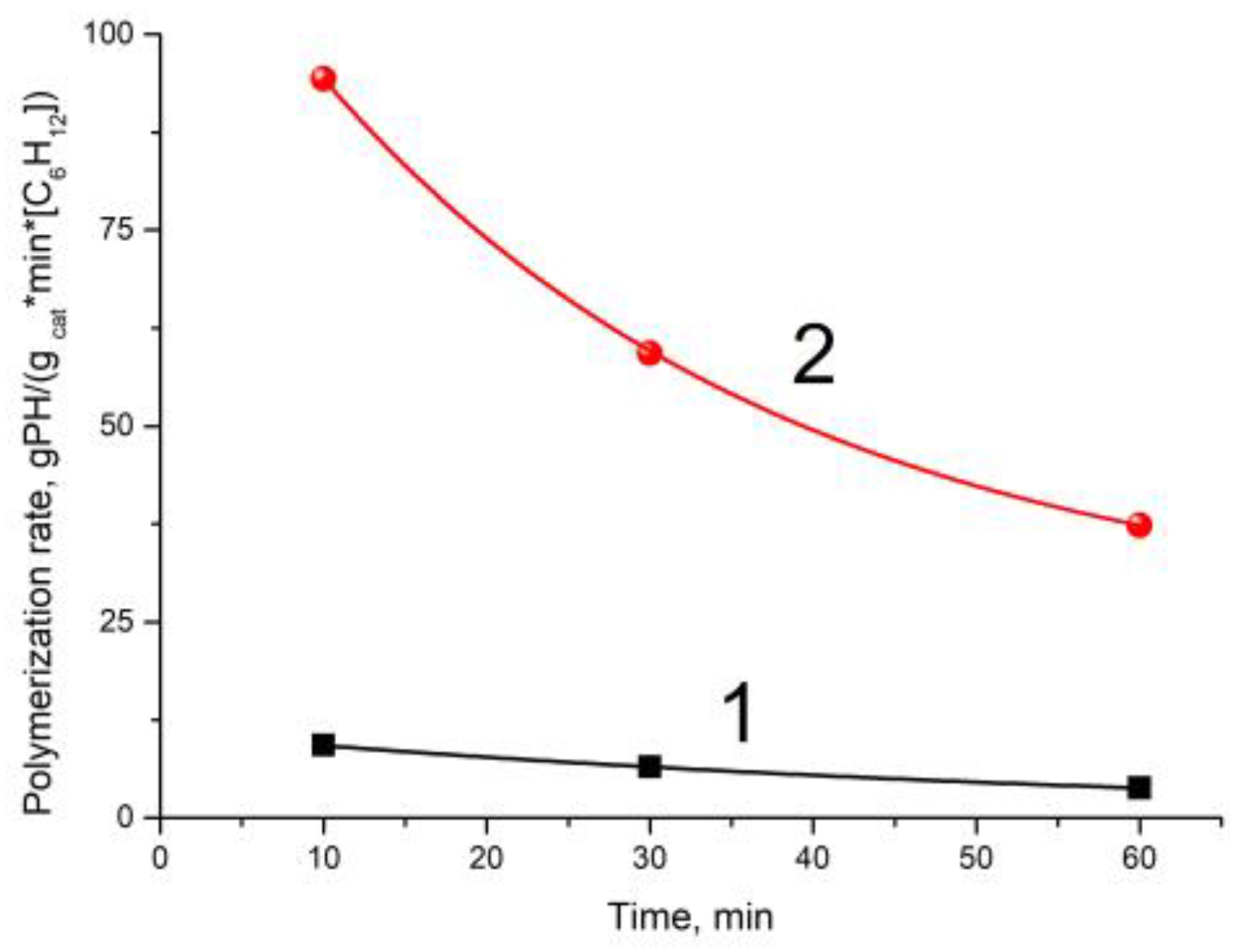
3 cocatalyst under variation of polymerization time and polyhexene yield in the experiments performed in the absence or presence of hydrogen are listed in
Table 1. According to these data, dependences of the polymerization rate vs. polymerization duration were obtained for the experiments carried out in the presence or absence of H
2 (
Figure 1).
It is seen that MWD of polyhexene narrows with an increase in polymerization time (polymer yield); however, the MWD remains quite broad (M
w/M
n = 15 and 10 in Exps. 3 and 6,
Table 1) even at high yields of the polymer. The introduction of hydrogen into polymerization leads to the decrease of the molecular weight and significantly narrows the MWD for both cocatalysts (
Table 1 and Table 3).
It is seen that the rate of hexene-1 polymerization with the AlEt
3 is higher during the initial period of polymerization (10 min) and then decreases with time (
Table 1 and
Figure 1). As was noted earlier [
15], hydrogen introduction into polymerization of hexene-1 leads to a sharp increase in the polymerization rate (ca. 10-fold,
Table 1).
Data on the polymerization rates of propylene and hexene-1 in the case of the same titanium–magnesium catalyst and different cocatalysts (AlEt
3 and Al(i-Bu)
3), as well as the data on MWD and isotacticity of the produced polymers are listed in
Table 2 and
Table 3.
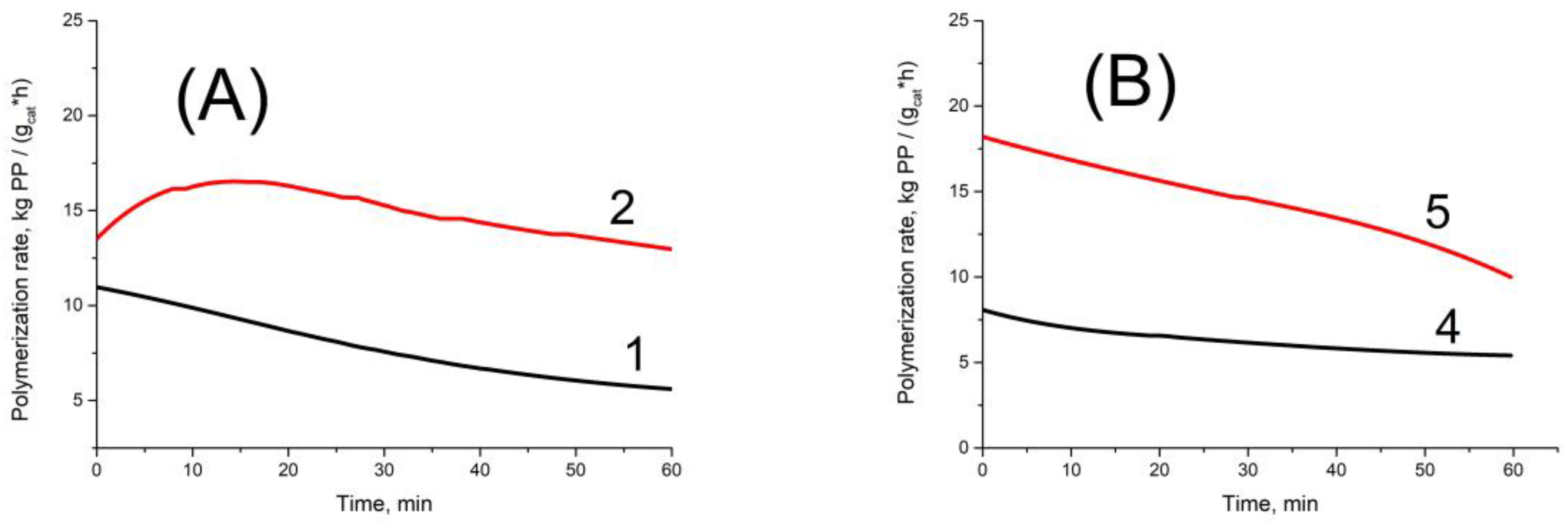
Figure 2 displays typical kinetics curves of propylene polymerization (time dependences of polymerization rate) that were obtained with AlEt
3 and Al(i-Bu)
3 cocatalysts in the presence or absence of H
2. A comparison of the results presented in
Table 2 and
Table 3 reveals the following peculiarities of polymerization of these monomers over titanium–magnesium catalysts.
In polymerization of propylene, the activity of TMC (polymerization rate) is close for AlEt
3 and Al(i-Bu)
3 cocatalysts at polymerization in the absence or presence of H
2 (compare Exps. 1 and 2, 4 and 5 in
Table 2). At the same time, in polymerization of hexene-1 with the AlEt
3 as a cocatalyst, the catalyst activity is much lower compared to the Al(i-Bu)
3 cocatalyst, especially at polymerization in the absence of H
2 (Exps. 1 and 3 in
Table 3). However, in the presence of H
2 the catalyst activity at polymerization of hexene-1 sharply increases by a factor of 12–32 (
Table 3) and approaches the activity of the same catalyst in polymerization of propylene (compare Exps. 2, 3, 5, 6 in
Table 2 with Exps. 2 and 4 in
Table 3). In the case of propene polymerization, activity of the catalyst increases upon hydrogen introduction only by a factor of 1.5–2. Thus, the effect of the catalyst activity growth due to hydrogen introduction at polymerization of hexene-1 is much more pronounced than in the case of propylene polymerization. In refs. [
21] it was shown that the increase of TMC activity during propene polymerization in the presence of H
2 is associated with reactivation of temporarily inactive, so called “dormant” sites, which are formed due to 2,1-addition of propylene to the propagating polymer chain. Our data on a more abrupt increase of the TMC activity after hydrogen introduction at polymerization of hexene-1 testify that the fraction of dormant sites formed during polymerization of hexene-1 in the absence of hydrogen is much greater as compared to polymerization of propylene. In this case, the reactivity of propylene and hexene-1 in the polymer chain propagation reaction should be estimated from the data on the catalyst activity obtained at polymerization in the presence of H
2 (in the absence of dormant sites). Data obtained under the indicated conditions using the Al(i-Bu)
3 cocatalyst (Exps. 5, 6 in
Table 2 and Exp. 4 in
Table 3) demonstrate close reactivities of these monomers in the polymer chain propagation reaction.
In the case of propylene polymerization, the cocatalyst type affects isotacticity of polypropylene; the application of Al(i-Bu)
3 as a cocatalyst significantly decreases isotacticity of polypropylene (
Table 2, Exps. 2 and 5). At the same time, at polymerization of hexene-1, isotacticity of polyhexene-1 does not depend on the composition of cocatalyst (
Table 3, Exps. 2 and 4).
Polypropylene samples produced in the presence of H
2 with AlEt
3 or Al(i-Bu)
3 cocatalysts have close molecular weights (
Table 2, Exps. 2 and 5). These polymers also have close polydispersity values (Mw/Mn = 4.0–5.4). Data on the MWD of polyhexene (
Table 3) greatly differ from the data obtained for polypropylene. Polyhexene produced in the absence of hydrogen with the AlEt
3 cocatalyst has a much lower molecular weight and a very broad MWD (M
w/M
n = 17.4) compared to PH produced with the Al(i-Bu)
3 cocatalyst (
Table 3, Exps. 1 and 3). The introduction of H
2 leads to a sharp decrease in the molecular weight of polyhexene and to some narrowing of MWD in the case of AlEt
3 cocatalyst; nevertheless, the M
w/M
n values for PH produced with the AlEt
3 cocatalyst remain much higher than the values for PH produced with Al(i-Bu)
3 cocatalyst (
Table 3, compare Exps. 1 and 2 with Exps. 3 and 4).
Reactivities of propylene and hexene-1 in the chain transfer reactions can be compared using the polymerization degree (P
n) data for polypropylene and polyhexene obtained under close conditions in the presence of hydrogen. In the case of propylene and hexene polymerization with the AlEt
3 cocatalyst at a low hydrogen content, the polymerization degree of PH is much lower than that of PP (200 and 1900, respectively) (Exp. 2 in
Table 2 and Exp. 2 in
Table 3). However, in polymerization with the Al(i-Bu)
3 cocatalyst, the polymerization degree of PP and PH are close (500 and 440) (Exp. 6 in
Table 2 and Exp. 4 in
Table 3). Presumably, these results may be caused by a great contribution of the chain transfer reaction, with AlEt
3 at a low hydrogen content to polymerization degree of polyhexene in comparison with propylene polymerization. At the same time, during polymerization of propylene and hexene with the Al(i-Bu)
3 cocatalyst at an increased hydrogen content, the contribution of the chain transfer reaction with cocatalyst to the polymerization degree is insignificant. For the indicated polymerization conditions, polymerization degree is determined by the rate constant ratio for chain propagation and polymer chain transfer with H
2. Data on the close polymerization degree of PP and PH obtained for such polymerization conditions indicate that the ratios of these rate constants for polymerization of propylene and hexene-1 over TMC are also close.
Data presented in
Table 2 and
Table 3 show the essential differences in the MWD of polypropylene and polyhexene are observed only at polymerization with cocatalyst AlEt
3. This cocatalyst is the efficient agent of the chain transfer reaction in the case of ethylene, propylene, and hexene-1 polymerization in the absence of hydrogen. In case of ethylene polymerization over TMC it was proposed in [
22] that AlEt
3 is able to form temporarily inactive (“dormant”) sites due to the reversible adsorption on active sites (AS). This reaction proceeds additionally to the decrease of molecular weight of polyhexene and broadening of MWD [
22]. Probably the contribution of this reaction increases at polymerization of hexene-1 compared to ethylene and propylene polymerization due to the elevated concentration of AlEt
3 on the catalyst surface. This phenomenon may be caused by the formation of a homogeneous reaction medium (a polyhexene solution in heptane) in distinction to a heterogeneous medium that appears when solid polypropylene particles are formed as a suspension in a heptane medium.
Earlier in our paper [
15], we have presented data concerning the effect of reaction temperature on the polymerization rate of hexene-1 over TMC with cocatalysts AlEt
3 and Al(i-Bu)
3 in the presence or absence of H
2 at polymerization. We have found in most cases that the polymerization rate decreases when the reaction temperature is increased from 30 °C up to 70 °C. Due to this unusual effect of decreasing polymerization rate with elevation of the reaction temperature, the calculated effective activation energies for polymerization rate (E
eff) have anomalous negative values. The most pronounced effect on the temperature dependence of hexene-1 polymerization rate and the calculated values of E
eff was exerted by the composition of cocatalyst and the presence of H
2 during polymerization (
Table 4).
Data concerning the effect of reaction temperature on the polymerization rate of hexene-1 greatly differ from the results obtained for propylene polymerization over the same titanium–magnesium catalyst with AlEt
3 as a cocatalyst (
Table 5).
One can see that in the case of propylene, the polymerization rate substantially increases with elevation of the reaction temperature. The E
eff values (32–45 kJ/mol) calculated from these data are in the region known for polymerization of propylene over TMC. The indicated values strongly differ from the anomalous negative values of E
eff (−2.2 and −21 kJ/mol) calculated for polymerization of hexene-1 with AlEt
3 or Al(i-Bu)
3 cocatalyst in the absence of H
2 (
Table 4, Exps. 1–3 and Exps. 4–6). The “normal” positive value of E
eff (20 kJ/mol, Exps. 7–8 in
Table 4) was obtained only for polymerization with the Al(i-Bu)
3 cocatalyst in the presence of H
2.
The data on the decreasing of hexene-1 polymerization rate with an increase of polymerization temperature (
Table 4, Exps. 1–3 and 4 and 6) may be related to a decrease in the number of AS that occurs at elevation of polymerization temperature under the indicated conditions. The appearance of this effect is determined by the composition of cocatalyst (AlEt
3) and the absence of hydrogen at polymerization.
We suppose that the decrease of the number of AS and, correspondingly, the decrease of polymerization rate of hexene-1 with elevation of the reaction temperature at polymerization with AlEt3 cocatalyst may be associated with the state of reaction medium. In this case, polymerization proceeds in a homogeneous medium with the formation of a polyhexene solution in heptane. At such state of the reaction medium, the concentration of AlEt3 on the catalyst surface corresponds to its concentration in the polyhexene solution, in distinction to propylene polymerization, when a layer of semicrystalline polymer is formed on the catalyst surface and the concentration of AlEt3 on the catalyst surface is much lower than its concentration in the heptane solution. The high AlEt3 concentration on the catalyst surface at polymerization of hexene-1 may decrease the number of AS at elevation of polymerization temperature due to the reduction of a part of Ti3+ ions in active sites to the inactive Ti2+ sites.
The revealed substantial effect of hydrogen on the dependence of hexene-1 polymerization rate of the reaction temperature (
Table 4, Exps. 4–6 and 7–9) may be related to the known phenomenon consisting in the formation of “dormant” sites at polymerization of α-olefins over TMC in the absence of H
2 and the possibility of their reactivation in the presence of H
2 [
21]. The dormant sites are formed at polymerization of α-olefins in the absence of hydrogen as a result of 2,1-addition of α-olefin to the propagating polymer chain. These sites are reactivated upon interaction with hydrogen, thus enhancing the activity at polymerization of propylene and hexene-1 in the presence of H
2. As was noted above, in the case of hexene-1 polymerization, the fraction of dormant sites formed in the absence of hydrogen and, accordingly, the enhancement of activity after hydrogen introduction are much higher than in the case of propylene polymerization. Presumably, the fraction of dormant sites formed in the absence of hydrogen depends on the polymerization temperature and increases with its elevation. This occurs because the reaction of α-olefin 2,1-addition to the propagating chain has a higher activation energy compared to the normal 1,2-addition; so, the fraction of dormant sites in the absence of H
2 increases with the elevation of polymerization temperature. The effect of hydrogen on the catalyst activity at different temperatures of hexene-1 polymerization and, accordingly, on the estimated E
eff values manifests itself most clearly in experiments with the Al(i-Bu)
3 cocatalyst (
Table 4). It is seen that at polymerization in the absence of hydrogen (Exps. 4–6) the activity weakly decreases when the polymerization temperature is increased from 30 °C to 70 °C (E
eff = −2.2 kJ/mol). At the same time, during polymerization in the presence of hydrogen (Exps. 7–9,
Table 4) the activity noticeably increases with the elevation of polymerization temperature from 30 °C to 70 °C (E
eff = 20 kJ/mol).
Thus, at polymerization of hexene-1 over TMC the composition of cocatalyst and the presence of H
2 strongly affect the dependence of polymerization rate on the reaction temperature and determine the possibility of a substantial decrease in the number of active sites with an increasing polymerization temperature from 30 °C up to 70 °C. This fact leads, firstly, to the appearance of anomalous negative values of the apparent activation energy of polymerization and, secondly, to a pronounced difference in the calculated E
eff values for different compositions of the catalytic system and reaction medium. In particular, according to the data of
Table 4, the calculated values of E
eff vary from −21 to 20 kJ/mol.
4. Conclusions
Data are obtained on the great differences of the kinetics of hexene-1 and propylene polymerization over the TMC as well as the molecular weight and molecular weight distribution of polymers produced. It is found that the composition of cocatalysts (AlEt3 or Al(i-Bu)3) greatly affects the molecular weight and MWD of polyhexene, contrary to polypropylene. Polyhexene produced with AlEt3 cocatalyst has a lower molecular weight and broader MWD (Mw/Mn = 10–22) in comparison with polyhexene produced with Al(i-Bu)3 cocatalyst (Mw/Mn = 4–5). Polypropylene produced with both AlEt3 and Al(i-Bu)3 cocatalysts has a similar MWD (Mw/Mn = 4–5.5). In the case of propylene polymerization, the activity of TMC is similar with AlEt3 and Al(i-Bu)3 cocatalysts, but in the case of hexene-1, the polymerization activity is much higher with Al(i-Bu)3 cocatalyst in comparison with AlEt3 cocatalyst.
The addition of hydrogen at hexene-1 polymerization leads to the great increase of activity (10–32 times), but in the case of propylene polymerization, activity increases only 1.5–2 times. These results show that the fraction of “dormant” sites formed at hexene-1 polymerization in the absence of H2 is much higher in comparison with propylene polymerization. Note that the activity of TMC with Al(i-Bu)3 cocatalyst in the presence of H2 is close to the activity of this catalyst at propylene polymerization in the presence of H2.
In the case of hexene-1 polymerization, we have found the unusual effect of the decrease of polymerization rate at increase of polymerization temperature from 30 °C up to 70 °C. Due to this effect, the activation energies calculated for polymerization rate (Eeff) have anomalous negative values within the range from −2.2 kJ/mol to −21 kJ/mol. These values depend on the composition of the cocatalyst and the presence of H2; the maximal negative value (−21 kJ/mol) is observed for polymerization with AlEt3 cocatalyst in the absence of hydrogen. Note that in the case of propylene polymerization with TEA cocatalyst we have found the usual Eeff values (32–45 kJ/mol). So, two main factors—the composition of the cocatalyst and the presence of H2 leads to differences in the kinetics of hexene-1 and propylene polymerization and molecular mass characteristics of polymers.
The strong effect of cocatalyst AlEt3 on the activity, molecular weight, and MWD of polyhexene may be caused by the formation of a homogeneous reaction medium (solution of polyhexene in heptane), in distinction of a heterogeneous medium when solid polypropylene particles are formed as a slurry in heptane. In this case, the concentration of AlEt3 on the surface of the catalyst is much higher at hexene-1 polymerization in comparison with one at propylene polymerization. High AlEt3 concentration on the surface of a catalyst leads to a decrease in the number of active sites (activity of catalyst), especially at high temperatures (70 °C) and an increase in the rate of chain transfer reaction with AlEt3.
In the case of hexene-1 polymerization in the absence of H2 activity is much lower in comparison with propylene polymerization because a higher fraction of “dormant” sites formed at hexene-1 polymerization in comparison with propylene polymerization. The addition of H2 leads to the reactivation of “dormant” sites and an increase of activity in 10–30 times at hexene-1 polymerization. In this case, activity of TMC with Al(i-Bu)3 cocatalyst in the presence of H2 is close at hexene-1 and propylene polymerization.