Polylactic Acid/Polyaniline Nanofibers Subjected to Pre- and Post-Electrospinning Plasma Treatments for Refined Scaffold-Based Nerve Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Polymer Solutions
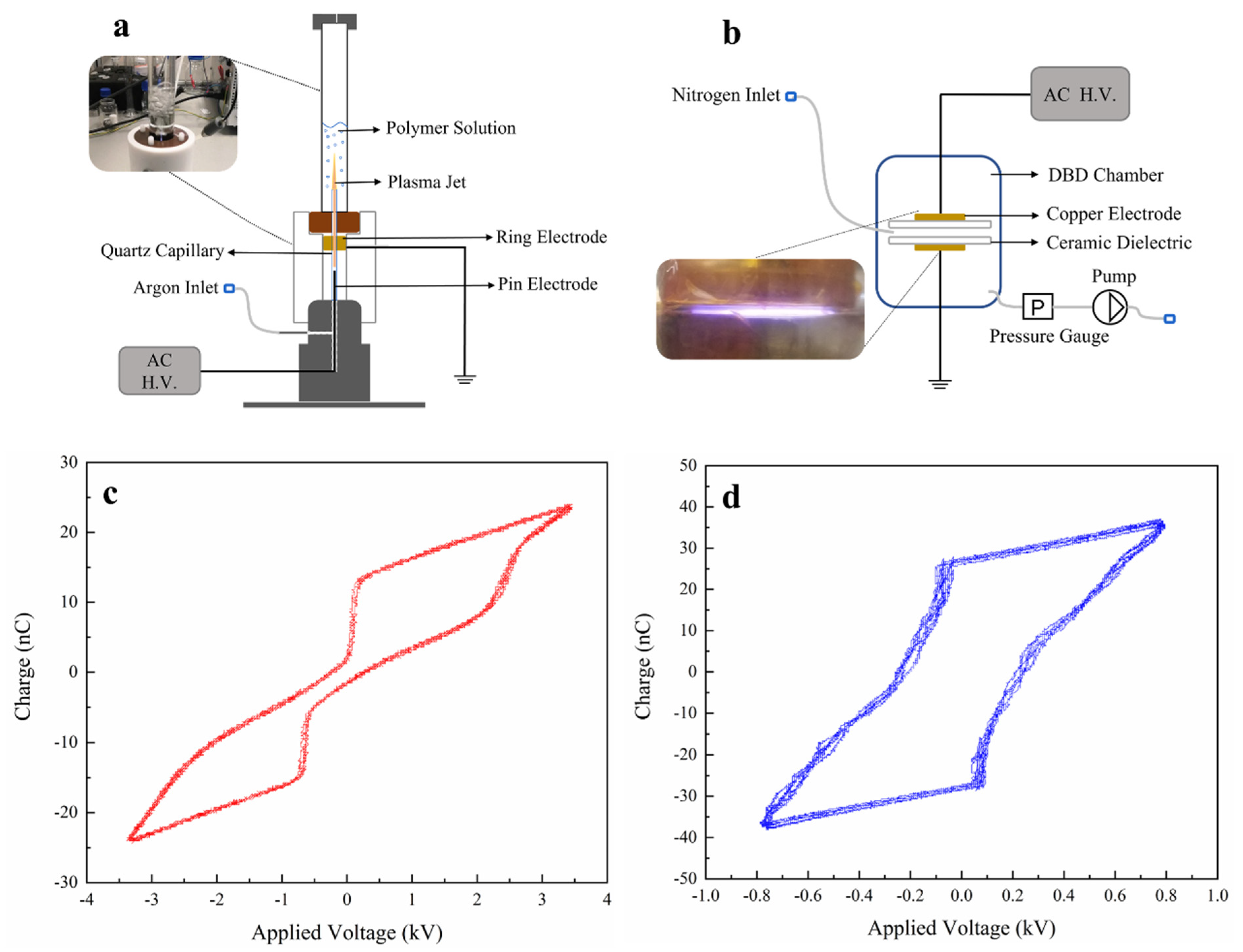
2.2. APPJ Treatment of Polymer Solutions
2.3. Liquid Properties Characterization of Polymeric Solutions
2.4. Fabrication of Nanofibrous Scaffolds
2.5. DBD Treatment of the Nanofibrous Scaffolds
2.6. Characterization of the Nanofibrous Scaffolds
2.7. PC-12 Cell Culture Tests on the Nanofibrous Scaffolds
3. Results and Discussion
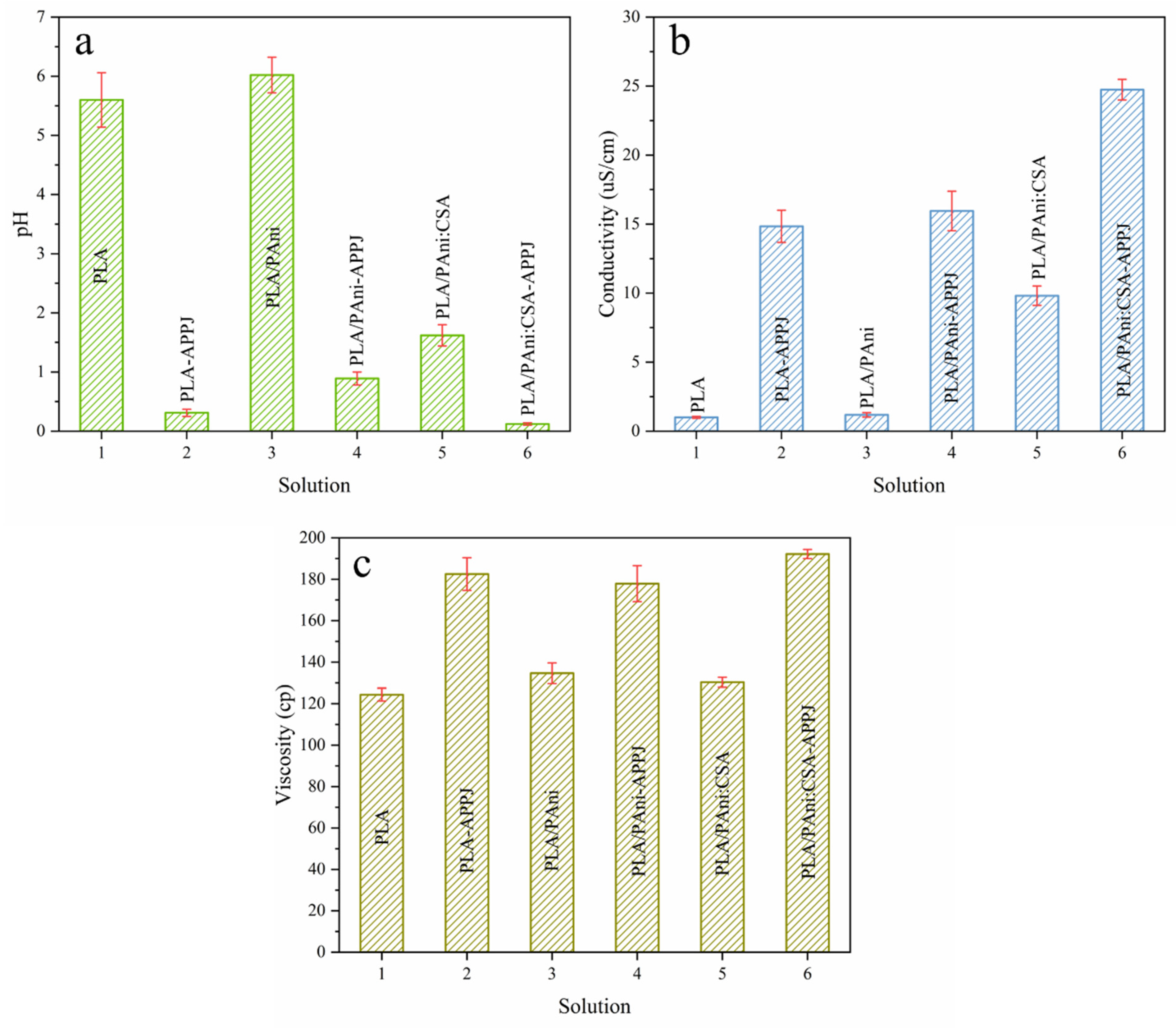
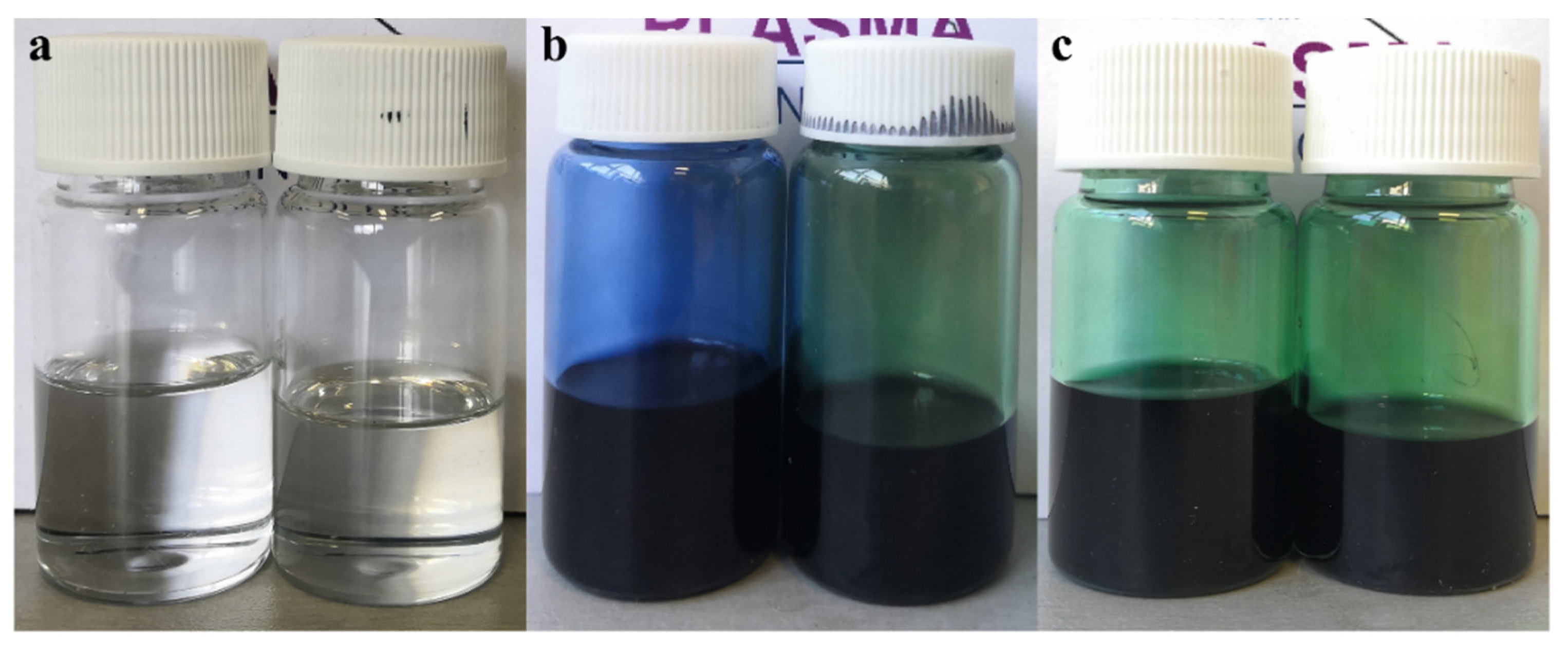
3.1. Physical Characterization of the Polymer Solutions
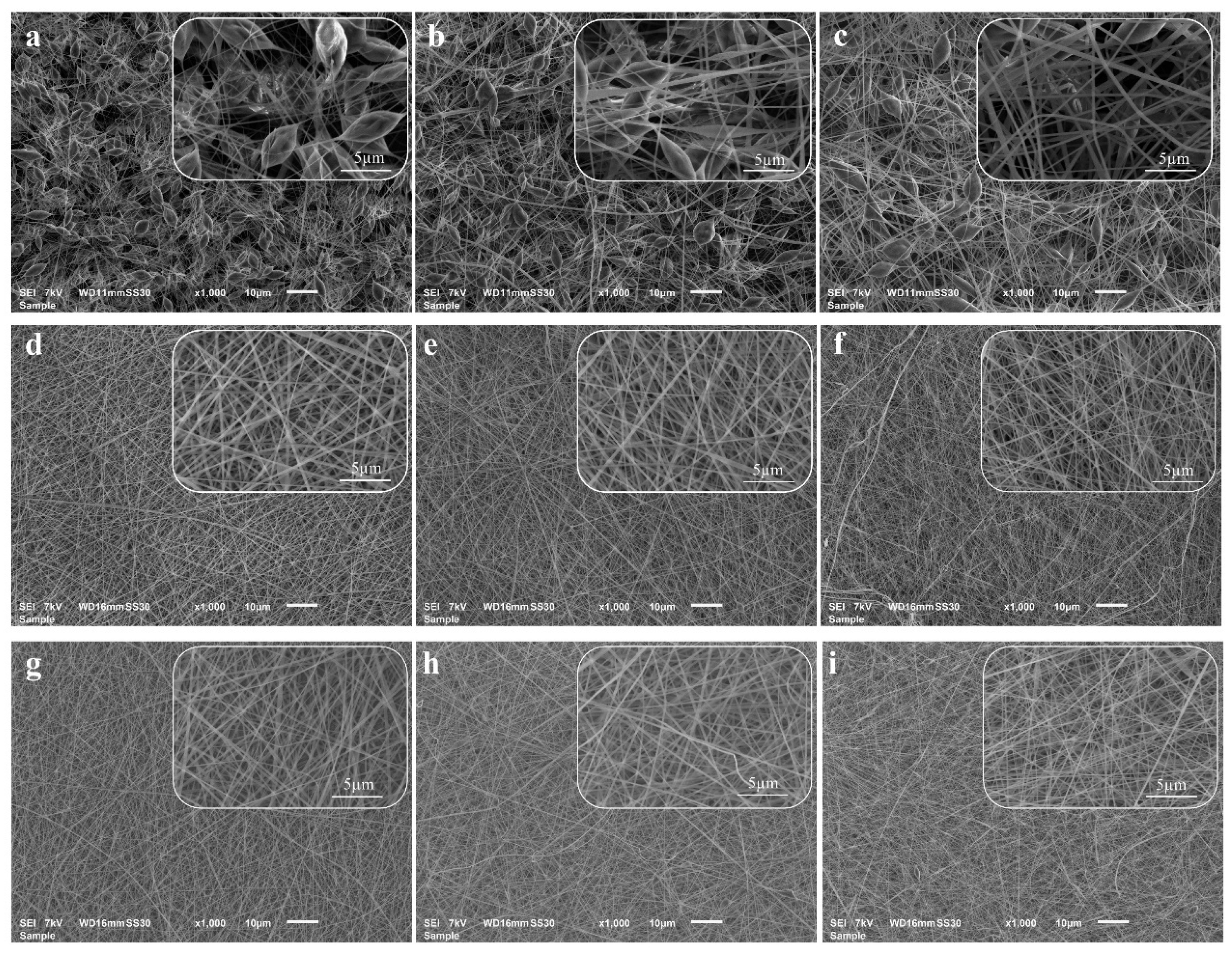
3.2. Morphology of the Electrospun Nanofibrous Scaffolds
3.3. Wettability of Nanofiber Scaffolds
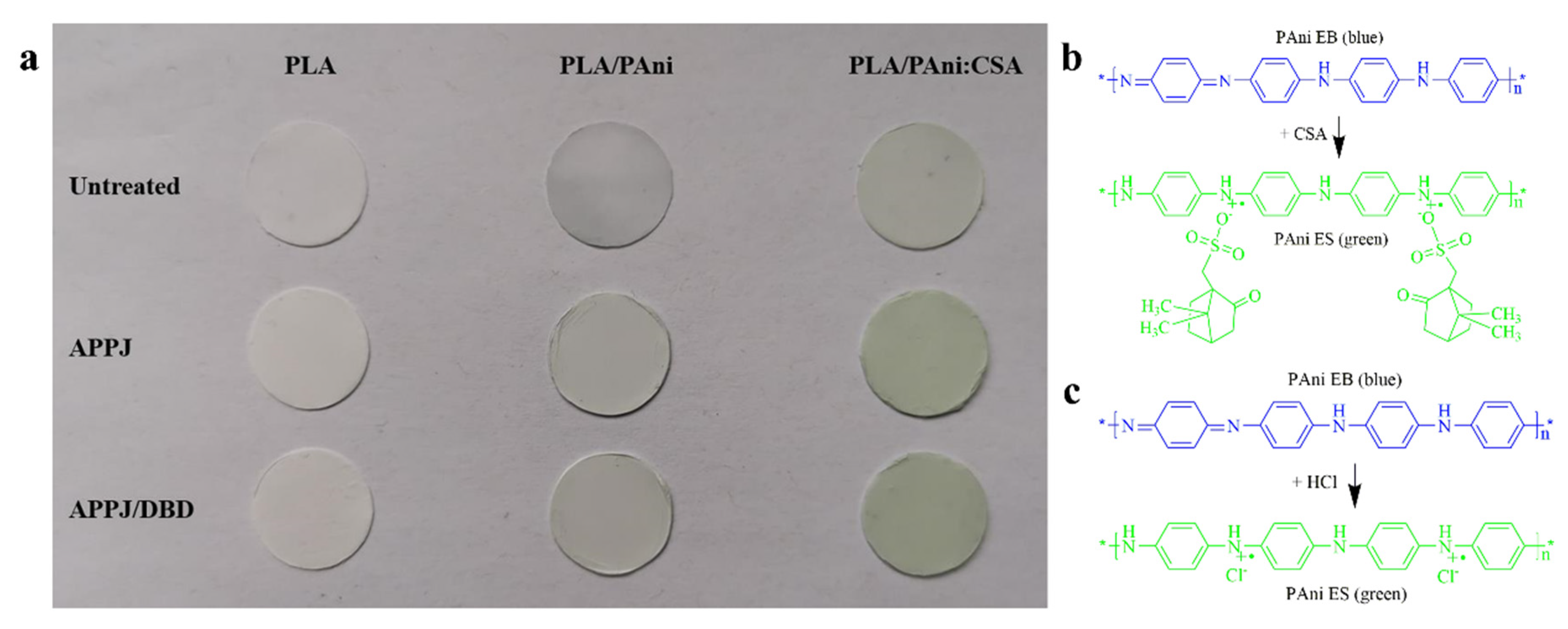
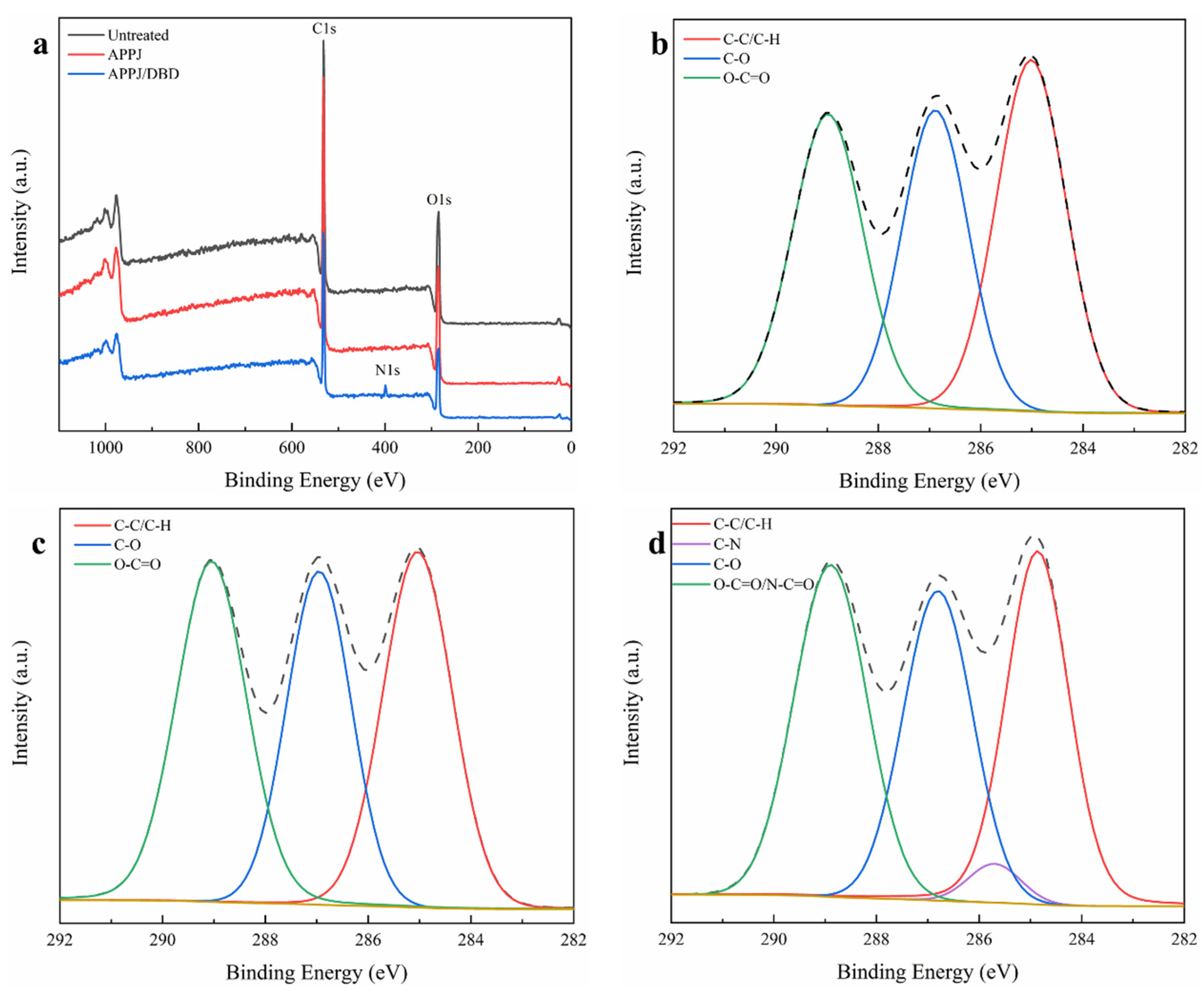
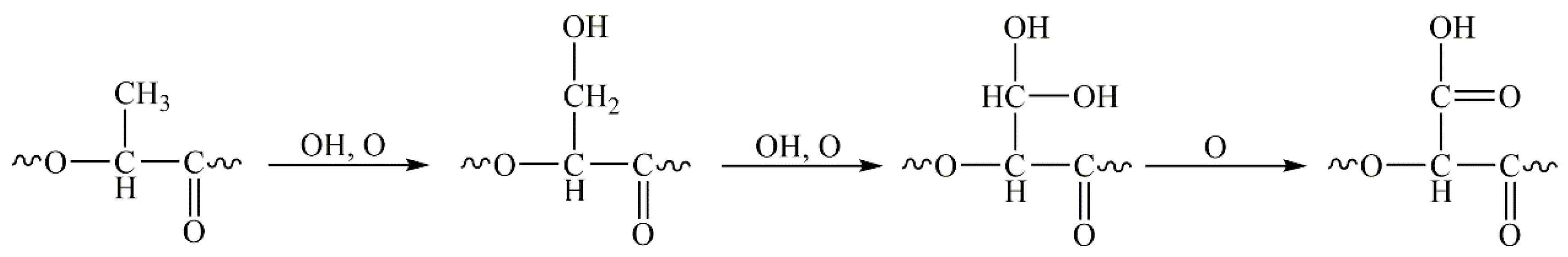
3.4. Surface Chemical Characterization of the Nanofibers
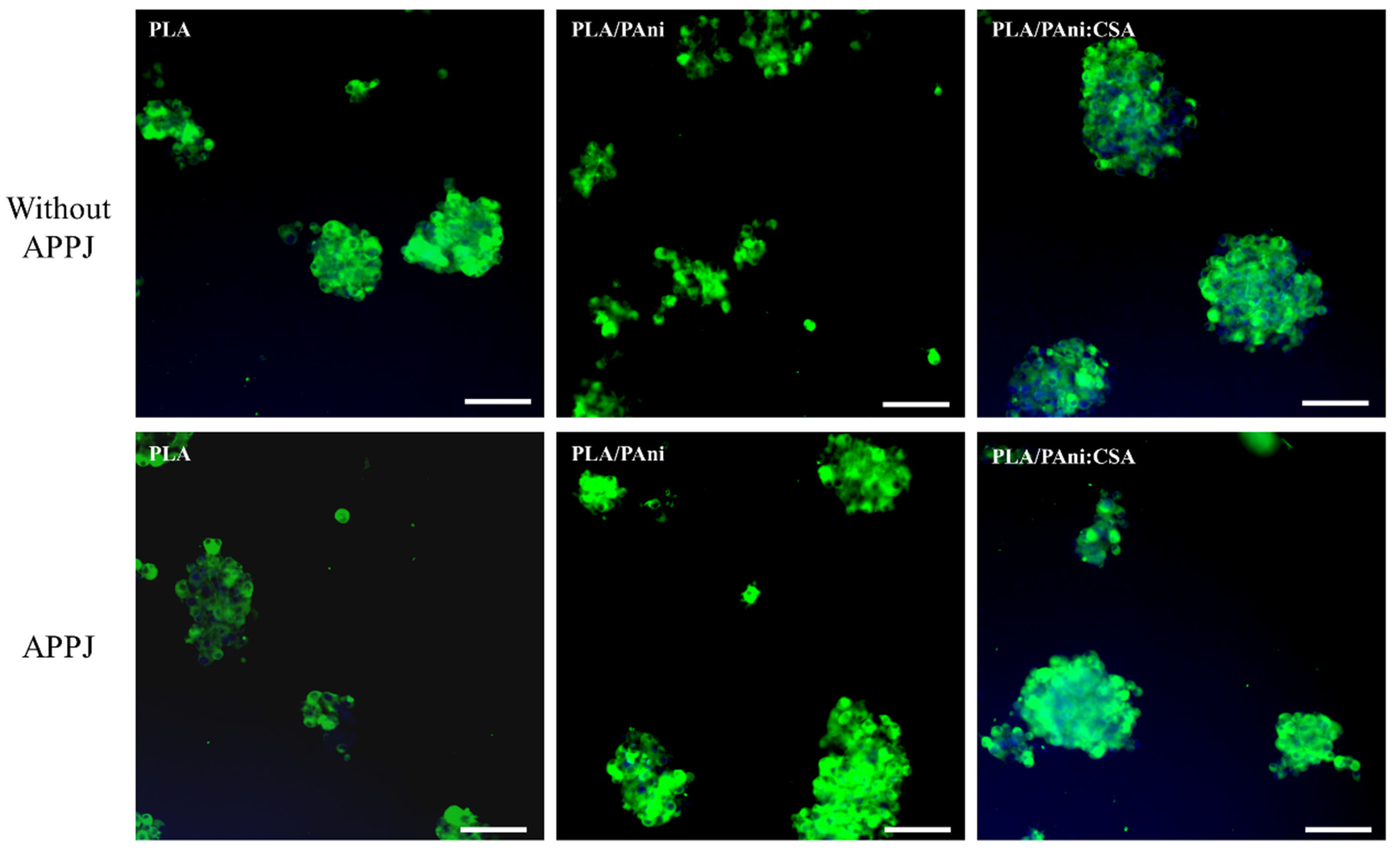
3.5. Cell-Scaffold Interactions: In Vitro PC-12 Cell Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.H.; Rutledge, G.C. 50th Anniversary Perspective: Advanced Polymer Fibers: High Performance and Ultrafine. Macromolecules 2017, 50, 5627–5642. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-Dimensional Composite Nanomaterials: Synthesis by Electrospinning and Their Applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; Recum, H.V. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Nanotechnology and Tissue Engineering: The Scaffold Based Approach. Nanotechnologies Life Sci. 2007, 1, 4–23. [Google Scholar] [CrossRef]
- Ghobeira, R.; de Geyter, N.; Morent, R. Plasma surface functionalization of biodegradable electrospun scaffolds for tissue en-gineering applications. In Biodegradable Polymers: Recent Developments and New Perspectives; IAPC Publishing(Zagreb): Zagreb, Croatia, 2017; pp. 191–236. [Google Scholar]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunc-tional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Vogt, L.; Ruther, F.; Roether, J.A.; Boccaccini, A.R.; Engel, F.B. Nanofibrous Composite with Tailorable Electrical and Mechanical Properties for Cardiac Tissue Engineering. Adv. Funct. Mater. 2019, 30, 1908612. [Google Scholar] [CrossRef]
- Xu, H.; Holzwarth, J.M.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P.X. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials 2014, 35, 225–235. [Google Scholar] [CrossRef]
- Guex, A.G.; Puetzer, J.L.; Armgarth, A.; Littmann, E.; Stavrinidou, E.; Giannelis, E.P.; Malliaras, G.G.; Stevens, M.M. Highly porous scaffolds of PEDOT:PSS for bone tissue engineering. Acta Biomater. 2017, 62, 91–101. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wang, C.; Li, F.; Qiao, Z.; Zeng, L.; Wang, Z.; Liu, H.; Ding, J.; Yang, H. Conductive Composite Fiber with Optimized Alignment Guides Neural Regeneration under Electrical Stimulation. Adv. Health Mater. 2020, 10, e2000604. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Mo, X.; Wang, H. Reduced Graphene Oxide-Encapsulated Microfiber Patterns Enable Controllable Formation of Neuronal-Like Networks. Adv. Mater. 2020, 32, e2004555. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011, 112, 501–507. [Google Scholar] [CrossRef]
- Xu, D.; Fan, L.; Gao, L.; Xiong, Y.; Wang, Y.; Ye, Q.; Yu, A.; Dai, H.; Yin, Y.; Cai, J.; et al. Micro-Nanostructured Polyaniline Assembled in Cellulose Matrix via Interfacial Polymerization for Applications in Nerve Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 17090–17097. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jeong, S.I.; Lee, T.J.; Jun, I.; Lee, Y.B.; Kim, B.S.; Shin, H. Electroactive electrospun polyani-line/poly[(L-lactide)-co-(epsilon-caprolactone)] fibers for control of neural cell function. Macromol. Biosci. 2012, 12, 402–411. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef]
- Li, Y.; Dong, T.; Li, Z.; Ni, S.; Zhou, F.; Alimi, O.A.; Chen, S.; Duan, B.; Kuss, M.; Wu, S. Review of advances in electrospinning-based strategies for spinal cord regeneration. Mater. Today Chem. 2022, 24, 100944. [Google Scholar] [CrossRef]
- Wu, S.; Qi, Y.; Shi, W.; Kuss, M.; Chen, S.; Duan, B. Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction. Acta Biomater. 2020, 139, 91–104. [Google Scholar] [CrossRef]
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-art review of advanced electrospun nanofiber yarn-based textiles for biomedical applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [CrossRef]
- Baker, C.O.; Huang, X.; Nelson, W.; Kaner, R.B. Polyaniline nanofibers: Broadening applications for conducting polymers. Chem. Soc. Rev. 2017, 46, 1510–1525. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Boccaccini, A.R. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials 2014, 35, 9068–9086. [Google Scholar] [CrossRef] [PubMed]
- Kamalesh, S.; Tan, P.; Wang, J.; Lee, T.; Kang, E.T.; Wang, C.H. Biocompatibility of electroactive polymers in tissues. J. Biomed. Mater. Res. 2000, 52, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Mattioli-Belmonte, M.; Giavaresi, G.; Biagini, G.; Virgili, L.; Giacomini, M.; Fini, M.; Giantomassi, F.; Natali, D.; Torricelli, P.; Giardino, R. Tailoring Biomaterial Compatibility: In Vivo Tissue Response versus in Vitro Cell Behavior. Int. J. Artif. Organs 2003, 26, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Guo, B.; Ma, P.X.; Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 2018, 514, 517–527. [Google Scholar] [CrossRef]
- Bertuoli, P.T.; Ordono, J.; Armelin, E.; Perez-Amodio, S.; Baldissera, A.F.; Ferreira, C.A.; Puiggali, J.; Engel, E.; del Valle, L.J.; Aleman, C. Electrospun Conducting and Biocompatible Uniaxial and Core-Shell Fibers Having Poly(lactic acid), Poly(ethylene glycol), and Polyaniline for Cardiac Tissue Engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar] [CrossRef]
- Ghobeira, R.; Philips, C.; Liefooghe, L.; Verdonck, M.; Asadian, M.; Cools, P.; Declercq, H.; De Vos, W.H.; De Geyter, N.; Morent, R. Synergetic effect of electrospun PCL fiber size, orientation and plasma-modified surface chemistry on stem cell behavior. Appl. Surf. Sci. 2019, 485, 204–221. [Google Scholar] [CrossRef]
- Cheng, L.; Ghobeira, R.; Cools, P.; Liu, Z.; Yan, K.; De Geyter, N.; Morent, R. Comparative study of different nitrogen-containing plasma modifications applied on 3D porous PCL scaffolds and 2D PCL films. Appl. Surf. Sci. 2020, 516, 146067. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Desmet, T.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Plasma modification of polylactic acid in a medium pressure DBD. Surf. Coat. Technol. 2010, 204, 3272–3279. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, N.; Ramakrishna, S.; Tian, L.; Mo, X. The Effect of Plasma Treated PLGA/MWCNTs-COOH Composite Nanofibers on Nerve Cell Behavior. Polymers 2017, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Xu, H.; Ren, Y.; Qiu, Y. Influence of atmospheric pressure plasma treatment time on penetration depth of surface modification into fabric. Appl. Surf. Sci. 2007, 254, 2499–2505. [Google Scholar] [CrossRef]
- Mao, T.; Kusefoglu, D.; Hooks, B.M.; Huber, D.; Petreanu, L.; Svoboda, K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 2011, 72, 111–123. [Google Scholar] [CrossRef]
- Foley, J.D.; Grunwald, E.W.; Nealey, P.F.; Murphy, C.J. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials 2005, 26, 3639–3644. [Google Scholar] [CrossRef]
- Olăreț, E.; Drăgușin, D.-M.; Serafim, A.; Lungu, A.; Șelaru, A.; Dobranici, A.; Dinescu, S.; Costache, M.; Boerașu, I.; Vasile, B.; et al. Electrospinning Fabrication and Cytocompatibility Investigation of Nanodiamond Particles-Gelatin Fibrous Tubular Scaffolds for Nerve Regeneration. Polymers 2021, 13, 407. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded nanofibers formed during electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Hsu, C.M.; Shivkumar, S. N,N-dimethylformamide additions to the solution for the electrospinning of poly(epsilon-caprolactone) nanofibers. Macromol. Mater. Eng. 2004, 289, 334–340. [Google Scholar] [CrossRef]
- Picciani, P.H.S.; Medeiros, E.S.; Pan, Z.; Wood, D.F.; Orts, W.J.; Mattoso, L.H.C.; Soares, B.G. Structural, Electrical, Mechanical, and Thermal Properties of Electrospun Poly(lactic acid)/Polyaniline Blend Fibers. Macromol. Mater. Eng. 2010, 295, 618–627. [Google Scholar] [CrossRef]
- Picciani, P.H.; Medeiros, E.S.; Pan, Z.; Orts, W.J.; Mattoso, L.H.; Soares, B.G. Development of conducting polyani-line/poly (lactic acid) nanofibers by electrospinning. J. Appl. Polym. Sci. 2009, 112, 744–753. [Google Scholar] [CrossRef]
- McKeon, K.D.; Lewis, A.; Freeman, J.W. Electrospun poly(D,L-lactide) and polyaniline scaffold characterization. J. Appl. Polym. Sci. 2009, 115, 1566–1572. [Google Scholar] [CrossRef]
- Brunetti, V.; Maiorano, G.; Rizzello, L.; Sorce, B.; Sabella, S.; Cingolani, R.; Pompa, P.P. Neurons sense nanoscale roughness with nanometer sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Gorbanev, Y.; Chys, M.; Nikiforov, A.; Van Hulle, S.W.H.; Cos, P.; Bogaerts, A.; De Geyter, N. Investigation of plasma-induced chemistry in organic solutions for enhanced electrospun PLA nanofibers. Plasma Process. Polym. 2018, 15, 1700226. [Google Scholar] [CrossRef]
- Grande, S.; Tampieri, F.; Nikiforov, A.; Giardina, A.; Barbon, A.; Cools, P.; Morent, R.; Paradisi, C.; Marotta, E.; de Geyter, N. Rad-icals and Ions Formed in Plasma-Treated Organic Solvents: A Mechanistic Investigation to Rationalize the Enhancement of Electrospinnability of Polycaprolactone. Front Chem. 2019, 7, 344. [Google Scholar] [CrossRef]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Lin, Z.; Guo, B.; Mccord, M.; Bourham, M.; Zhang, X. Atmospheric plasma treatment of pre-electrospinning polymer solution: A feasible method to improve electrospinnability. J. Polym. Sci. Part B: Polym. Phys. 2010, 49, 115–122. [Google Scholar] [CrossRef]
- Colombo, V.; Fabiani, D.; Focarete, M.L.; Gherardi, M.; Gualandi, C.; Laurita, R.; Zaccaria, M. Atmospheric Pressure Non- Equi-librium Plasma Treatment to Improve the Electrospinnability of Poly(L- Lactic Acid) Polymeric Solution. Plasma Process Polym. 2014, 11, 247–255. [Google Scholar] [CrossRef]
- Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Plasma Modification of Poly Lactic Acid Solutions to Generate High Quality Electrospun PLA Nanofibers. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Grande, S.; van Guyse, J.; Nikiforov, A.Y.; Onyshchenko, I.; Asadian, M.; Morent, R.; Hoogenboom, R.; Geyter, N. Atmospheric Pressure Plasma Jet Treatment of Poly-epsilon-caprolactone Polymer Solutions to Improve Electrospinning. ACS Appl. Mater. Inter. 2017, 9, 33080–33090. [Google Scholar] [CrossRef]
- Wagner, H.-E.; Brandenburg, R.; Kozlov, K.; Sonnenfeld, A.; Michel, P.; Behnke, J. The barrier discharge: Basic properties and applications to surface treatment. Vacuum 2003, 71, 417–436. [Google Scholar] [CrossRef]
- Ghobeira, R.; Philips, C.; De Naeyer, V.; Declercq, H.; Cools, P.; De Geyter, N.; Cornelissen, R.; Morent, R. Comparative Study of the Surface Properties and Cytocompatibility of Plasma-Treated Poly-ε-Caprolactone Nanofibers Subjected to Different Sterilization Methods. J. Biomed. Nanotechnol. 2017, 13, 699–716. [Google Scholar] [CrossRef]
- Rezaei, F.; Planckaert, T.; Vercruysse, C.; Verjans, J.; van der Voort, P.; Declercq, H.; Hoogenboom, R.; Morent, R.; de Geyter, N. The Influence of Pre-Electrospinning Plasma Treatment on Physicochemical Characteristics of PLA Nanofibers. Macromol. Mater. Eng. 2019, 304. [Google Scholar]
- Guo, Y.; Ghobeira, R.; Sun, Z.; Shali, P.; Morent, R.; de Geyter, N. Atmospheric pressure plasma jet treatment of PLA/PAni solu-tions: Enhanced morphology, improved yield of electrospun nanofibers and concomitant doping behaviour. Polymer 2022, 262, 125502. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Mor-phology. IEEE T. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Venkatraman, S. Importance of viscosity parameters in electrospinning: Of monolithic and core–shell fibers. Mater. Sci. Eng. C 2012, 32, 1037–1042. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Verschuren, J.; De Clerck, K.; Kiekens, P.; Leys, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Asadian, M.; Grande, S.; Onyshchenko, I.; Morent, R.; Declercq, H.; de Geyter, N. A comparative study on pre- and post-production plasma treatments of PCL films and nanofibers for improved cell-material interactions. Appl. Surf. Sci. 2019, 481, 1554–1565. [Google Scholar] [CrossRef]
- Grande, S.; Cools, P.; Asadian, M.; van Guyse, J.; Onyshchenko, I.; Declercq, H.; Morent, R.; Hoogenboom, R.; de Geyter, N. Fab-rication of PEOT/PBT Nanofibers by Atmospheric Pressure Plasma Jet Treatment of Electrospinning Solutions for Tissue Engineering. Macromol. Biosci. 2018, 18, 1800309. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Gengembre, L.; Leys, C.; Payen, E.; Van Vlierberghe, S.; Schacht, E. Surface treatment of a polypropylene film with a nitrogen DBD at medium pressure. Eur. Phys. J. Appl. Phys. 2008, 43, 289–294. [Google Scholar] [CrossRef]
- Cools, P.; Asadian, M.; Nicolaus, W.; Declercq, H.; Morent, R.; de Geyter, N. Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure. Materials 2018, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Tabaei, P.S.E.; Ghobeira, R.; Cools, P.; Rezaei, F.; Nikiforov, A.; Morent, R.; De Geyter, N. Comparative study between in-plasma and post-plasma chemical processes occurring at the surface of UHMWPE subjected to medium pressure Ar and N2 plasma activation. Polymer 2020, 193, 122383. [Google Scholar] [CrossRef]
- Gonzalez, E., 2nd; Hicks, R.F. Surface analysis of polymers treated by remote atmospheric pressure plasma. Langmuir 2010, 26, 3710–3719. [Google Scholar] [CrossRef]
- Li, H.; Hall, M.B. Mechanism of the Formation of Carboxylate from Alcohols and Water Catalyzed by a Bipyridine-Based Ruthenium Complex: A Computational Study. J. Am. Chem. Soc. 2013, 136, 383–395. [Google Scholar] [CrossRef]
- Massines, F.; Gouda, G.; Gherardi, N.; Duran, M.; Croquesel, E. The Role of Dielectric Barrier Discharge Atmosphere and Physics on Polypropylene Surface Treatment. Plasmas Polym. 2001, 6, 35–49. [Google Scholar] [CrossRef]
- Ghobeira, R.; Tabaei, P.S.E.; Morent, R.; De Geyter, N. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Malagelada, C.; Greene, L.A. PC12 Cells as a model for parkinson’s disease research. In Parkinson's Disease; Academic Press: Cambridge, MA, USA, 2008; pp. 375–387. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef]
- Chua, J.S.; Chng, C.P.; Moe, A.A.; Tann, J.Y.; Goh, E.L.; Chiam, K.H.; Yim, E.K. Extending neurites sense the depth of the under-lying topography during neuronal differentiation and contact guidance. Biomaterials 2014, 35, 7750–7761. [Google Scholar] [CrossRef]
- Mukhatyar, V.J.; Salmeron-Sanchez, M.; Rudra, S.; Mukhopadaya, S.; Barker, T.H.; Garcia, A.J.; Bellamkonda, R.V. Role of fi-bronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials 2011, 32, 3958–3968. [Google Scholar] [CrossRef]


| Condition | PLA | PLA/PAni | PLA/PAni:CSA |
|---|---|---|---|
| APPJ | 231.5 ± 48.4 | 217.7 ± 51.2 | 170.0 ± 26.9 |
| APPJ/DBD | 217.5 ± 36.9 | 217.9 ± 40.3 | 175.3 ± 40.1 |
| Condition | PLA | PLA/PAni | PLA/PAni:CSA |
|---|---|---|---|
| Untreated | 141.9 ± 2.8 | 137.1 ± 1.0 | 138.6 ± 0.7 |
| APPJ | 129.5 ± 1.0 | 128.5 ± 1.4 | 127.2 ± 1.2 |
| Condition | C (at%) | O (at%) | N (at%) | O/C | (O+N)/C |
|---|---|---|---|---|---|
| Untreated | 61.2 ± 0.5 | 38.8 ± 0.5 | 0 | 0.634 | 0.634 |
| APPJ | 60.4 ± 0.4 | 39.6 ± 0.4 | 0 | 0.656 | 0.656 |
| APPJ/DBD | 58.3 ± 0.4 | 38.4 ± 0.6 | 3.3 ± 0.6 | 0.659 | 0.715 |
| Condition | C–C/C–H (285.0 eV) | C–N (285.7 eV) | C–O (286.8 eV) | O–C=O/N–C=O (289.0 eV) |
|---|---|---|---|---|
| Untreated | 37.6 ± 0.6 | 0 | 30.0 ± 0.6 | 32.4 ± 0.4 |
| APPJ | 35.2 ± 0.6 | 0 | 30.2 ± 0.5 | 34.6 ± 0.4 |
| APPJ/DBD | 33.1 ± 0.5 | 3.0 ± 1.4 | 30.2 ± 1.1 | 33.7 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Ghobeira, R.; Aliakbarshirazi, S.; Morent, R.; De Geyter, N. Polylactic Acid/Polyaniline Nanofibers Subjected to Pre- and Post-Electrospinning Plasma Treatments for Refined Scaffold-Based Nerve Tissue Engineering Applications. Polymers 2023, 15, 72. https://doi.org/10.3390/polym15010072
Guo Y, Ghobeira R, Aliakbarshirazi S, Morent R, De Geyter N. Polylactic Acid/Polyaniline Nanofibers Subjected to Pre- and Post-Electrospinning Plasma Treatments for Refined Scaffold-Based Nerve Tissue Engineering Applications. Polymers. 2023; 15(1):72. https://doi.org/10.3390/polym15010072
Chicago/Turabian StyleGuo, Yongjian, Rouba Ghobeira, Sheida Aliakbarshirazi, Rino Morent, and Nathalie De Geyter. 2023. "Polylactic Acid/Polyaniline Nanofibers Subjected to Pre- and Post-Electrospinning Plasma Treatments for Refined Scaffold-Based Nerve Tissue Engineering Applications" Polymers 15, no. 1: 72. https://doi.org/10.3390/polym15010072
APA StyleGuo, Y., Ghobeira, R., Aliakbarshirazi, S., Morent, R., & De Geyter, N. (2023). Polylactic Acid/Polyaniline Nanofibers Subjected to Pre- and Post-Electrospinning Plasma Treatments for Refined Scaffold-Based Nerve Tissue Engineering Applications. Polymers, 15(1), 72. https://doi.org/10.3390/polym15010072









