Spin Trapping Analysis of Radical Intermediates on the Thermo-Oxidative Degradation of Polypropylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oxidized PP
2.3. Preparation of ESR Samples
2.4. Characterizations
2.4.1. DSC Measurements
2.4.2. FTIR Measurements
2.4.3. TGA Measurements
2.4.4. 1H-NMR Measurements
2.4.5. ESR Measurements
2.4.6. CL Measurements
3. Results and Discussion
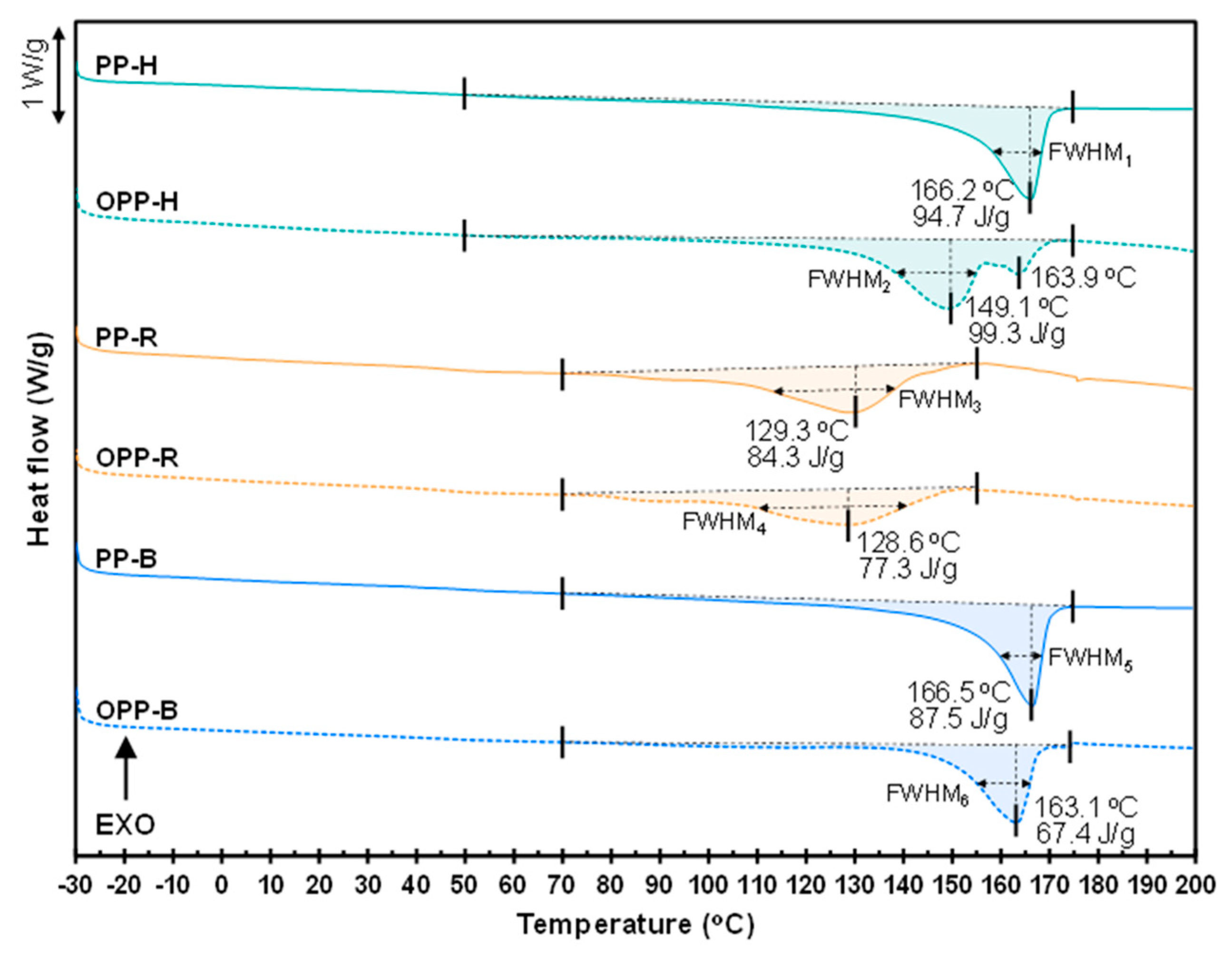
3.1. Thermophysical Properties of PP
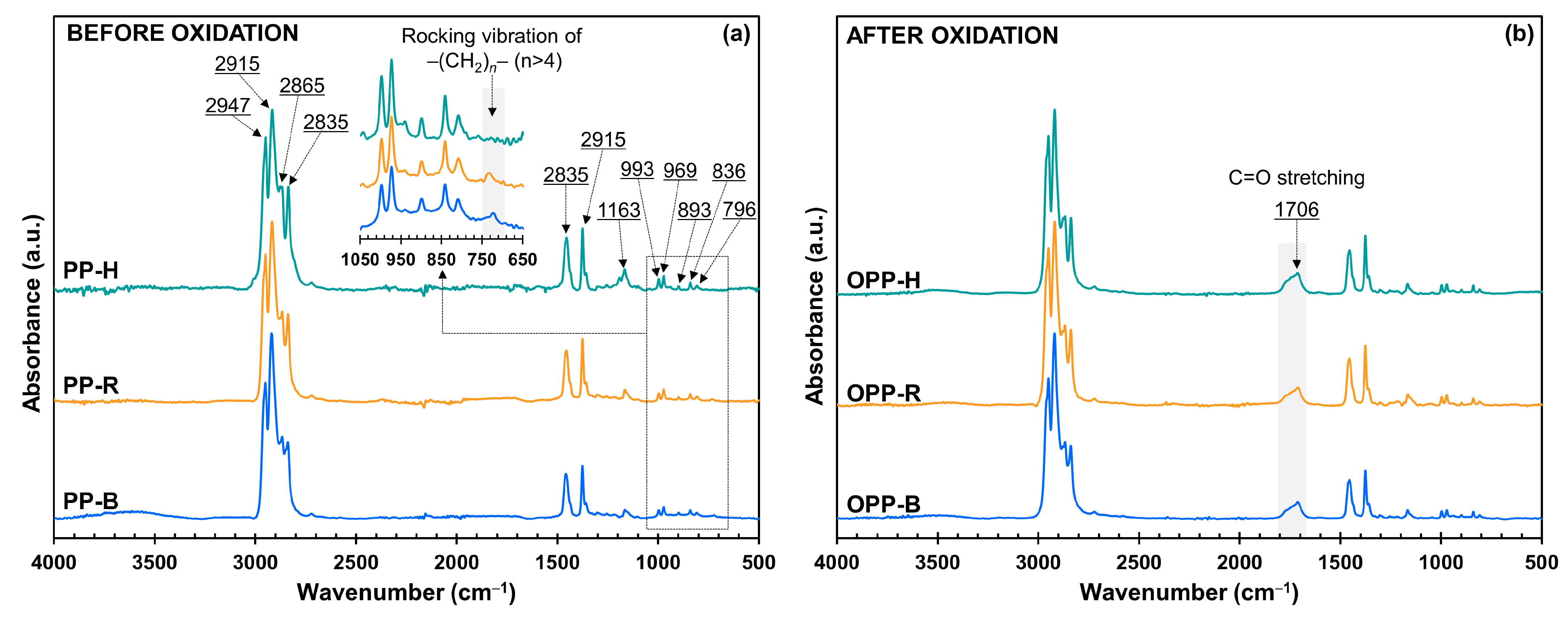
3.2. Chemical Analysis by FTIR Measurements
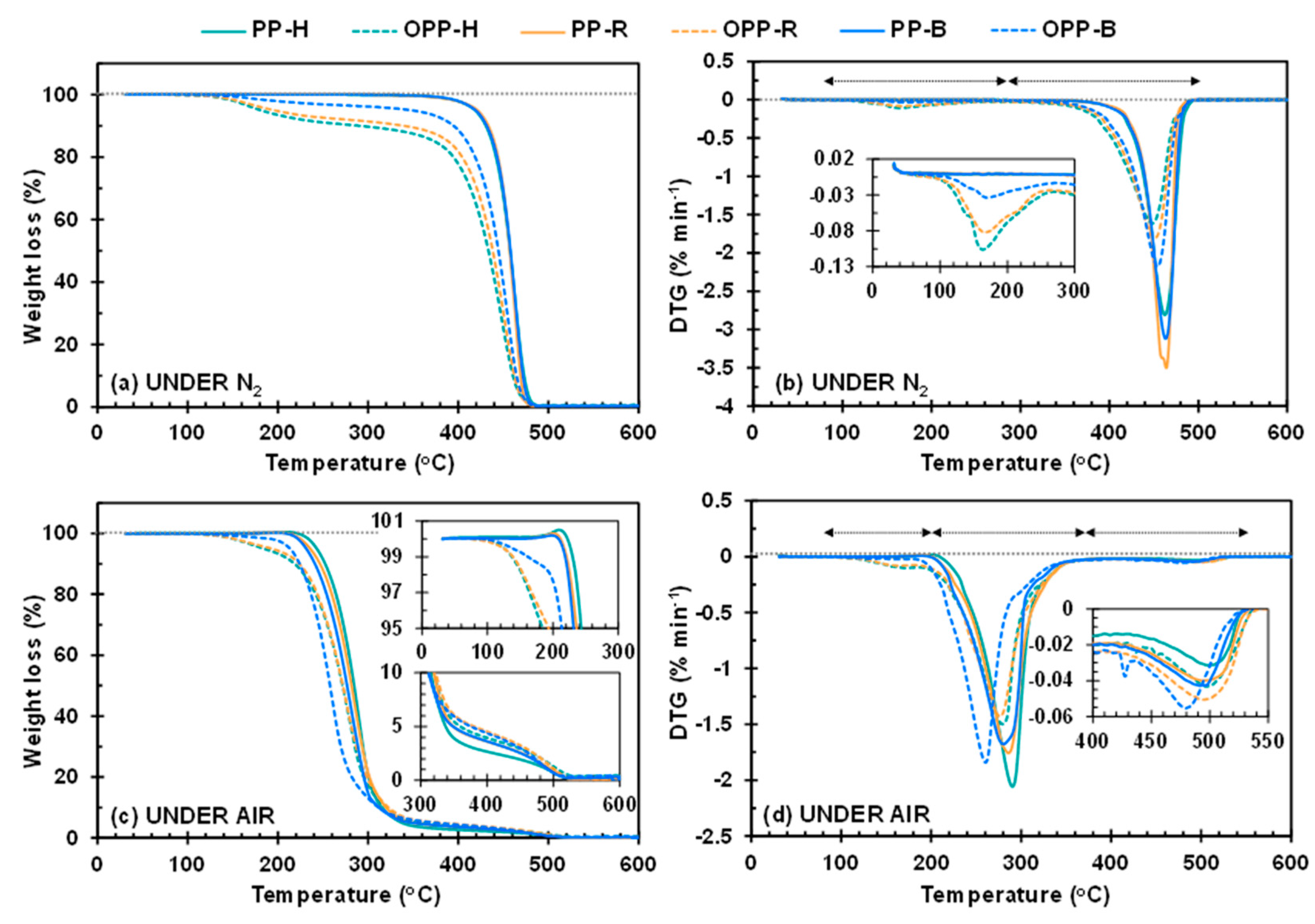
3.3. Thermal Decomposition Analysis by TGA
3.4. Evaluation by 1H-NMR of the Effect of scCO2 Treatment
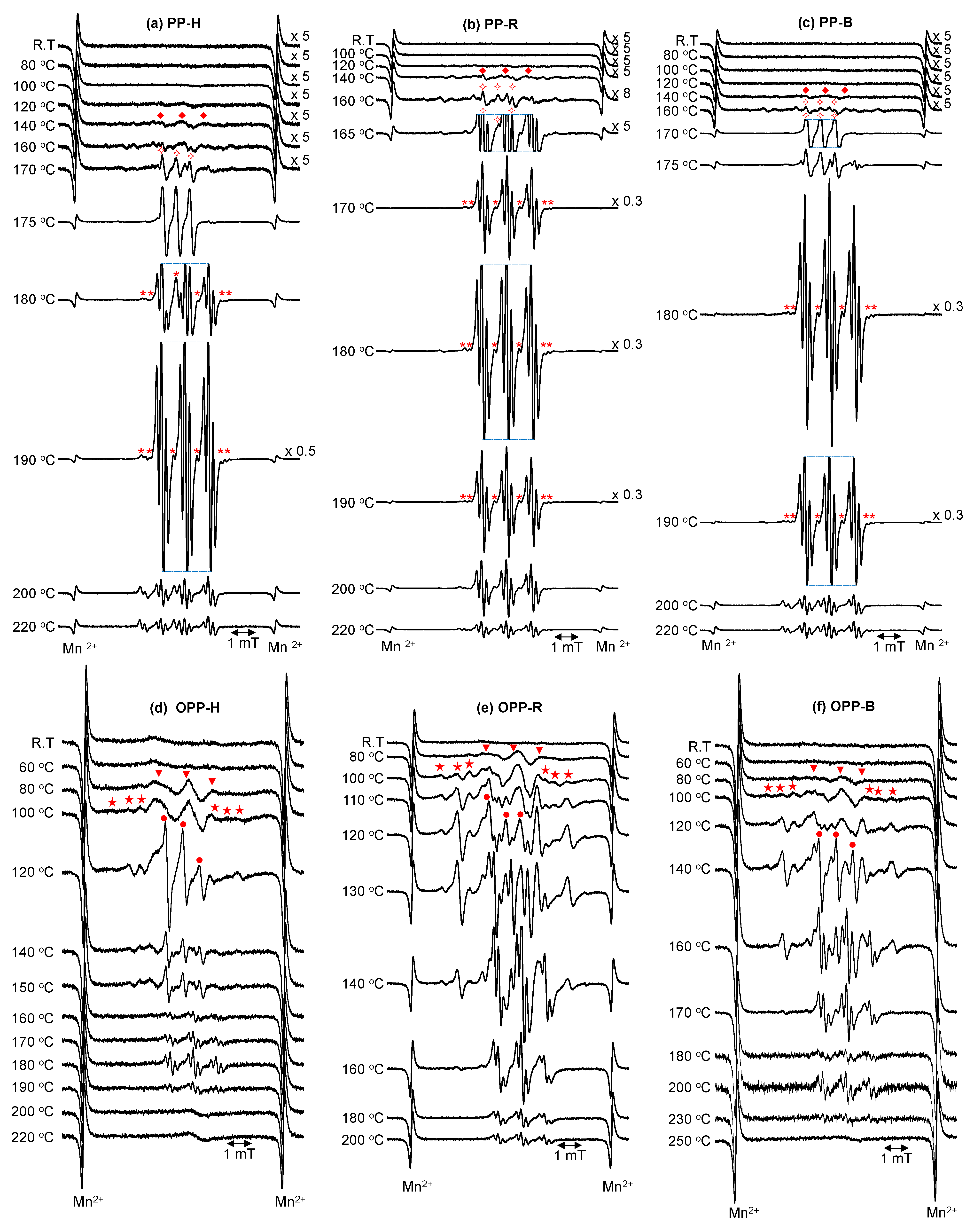
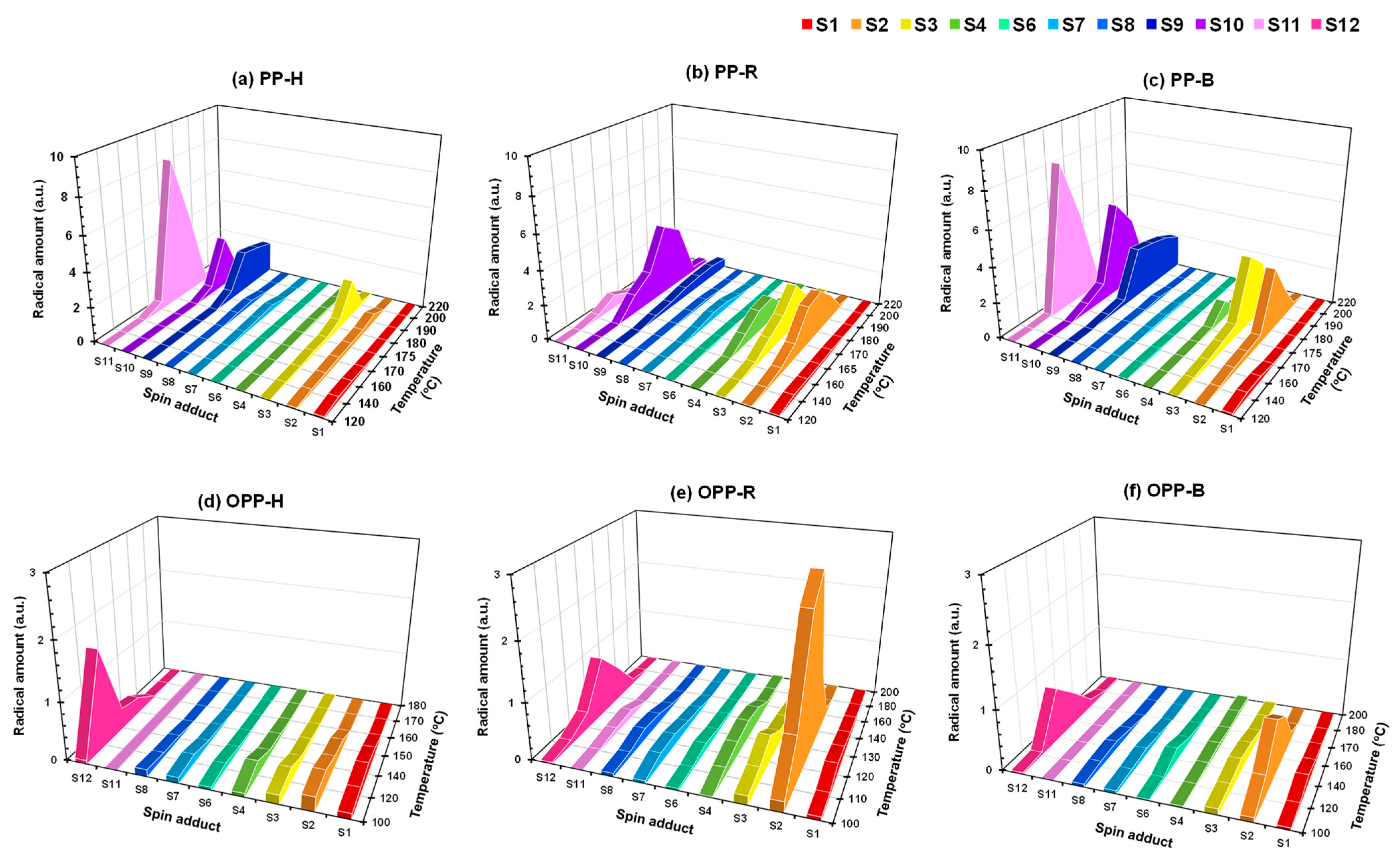
3.5. Analysis of Radicals by Spin Trapping ESR
3.5.1. ESR Measurements
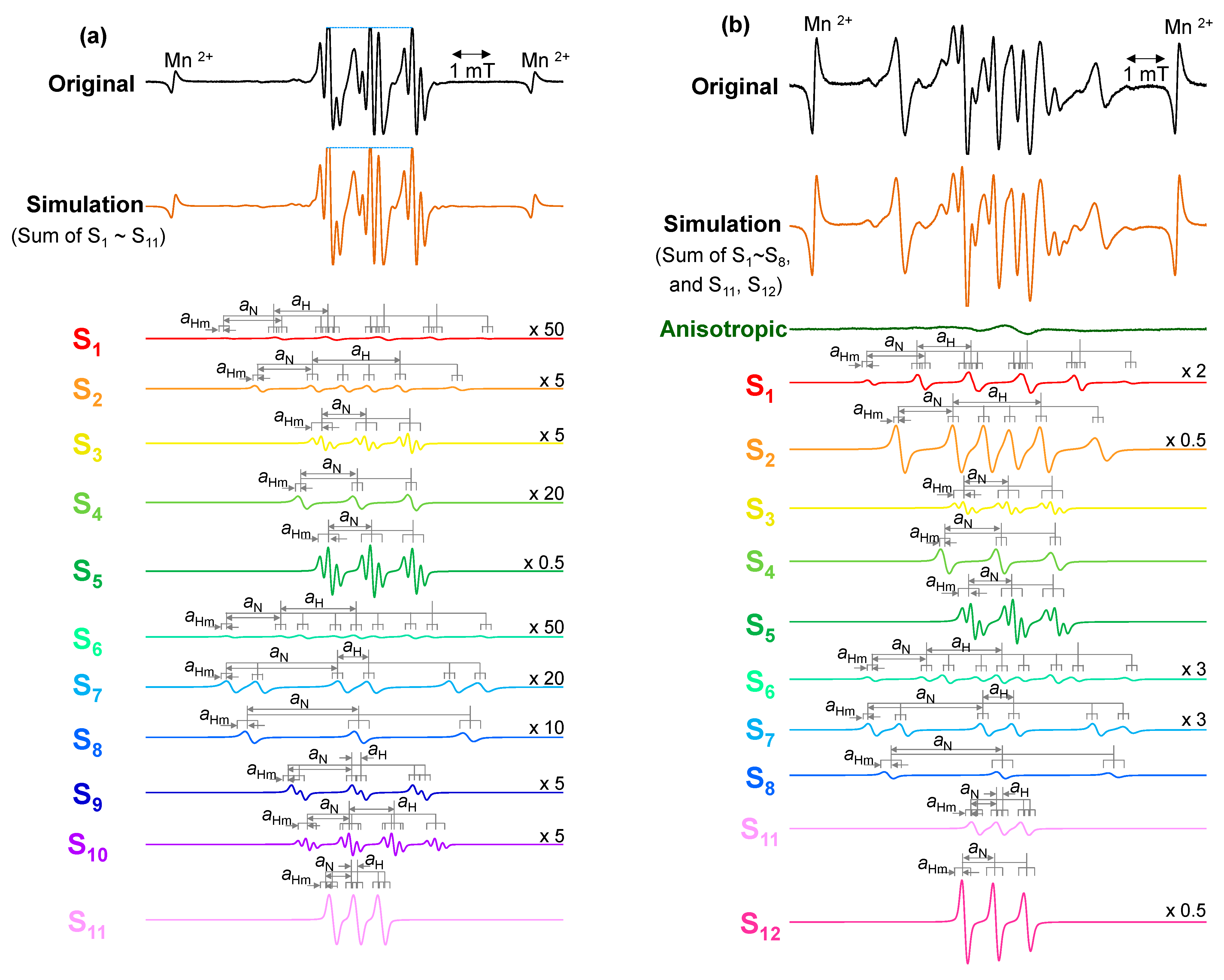
3.5.2. Assignments of Spin Adducts
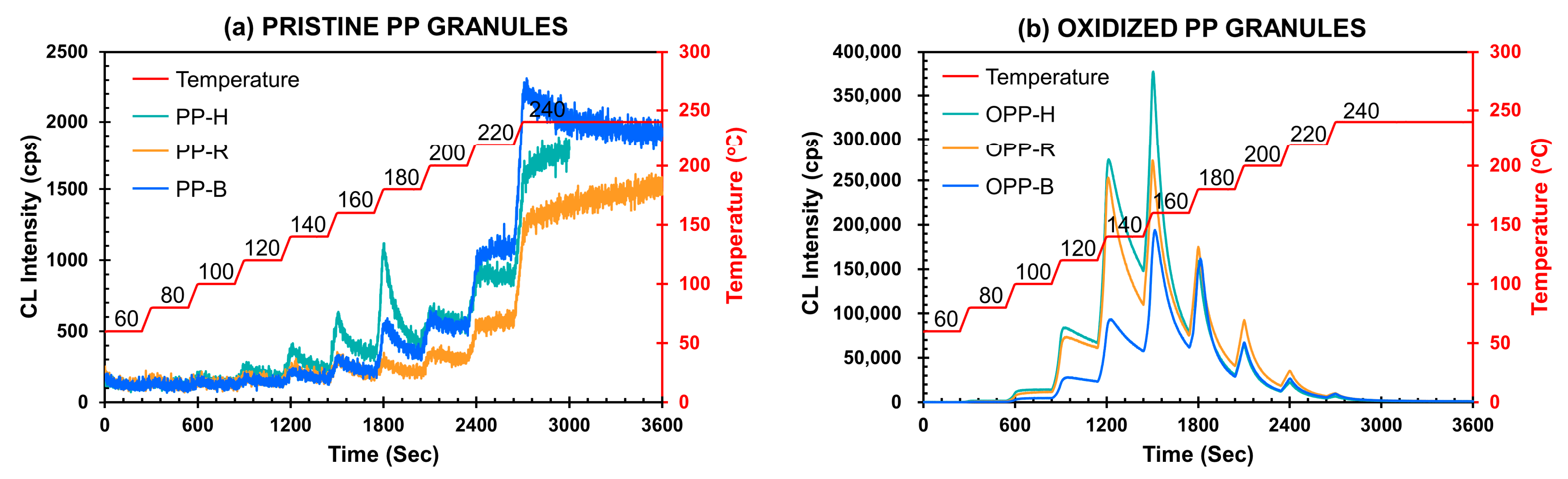
3.6. Chemiluminescence from PP Oxidation
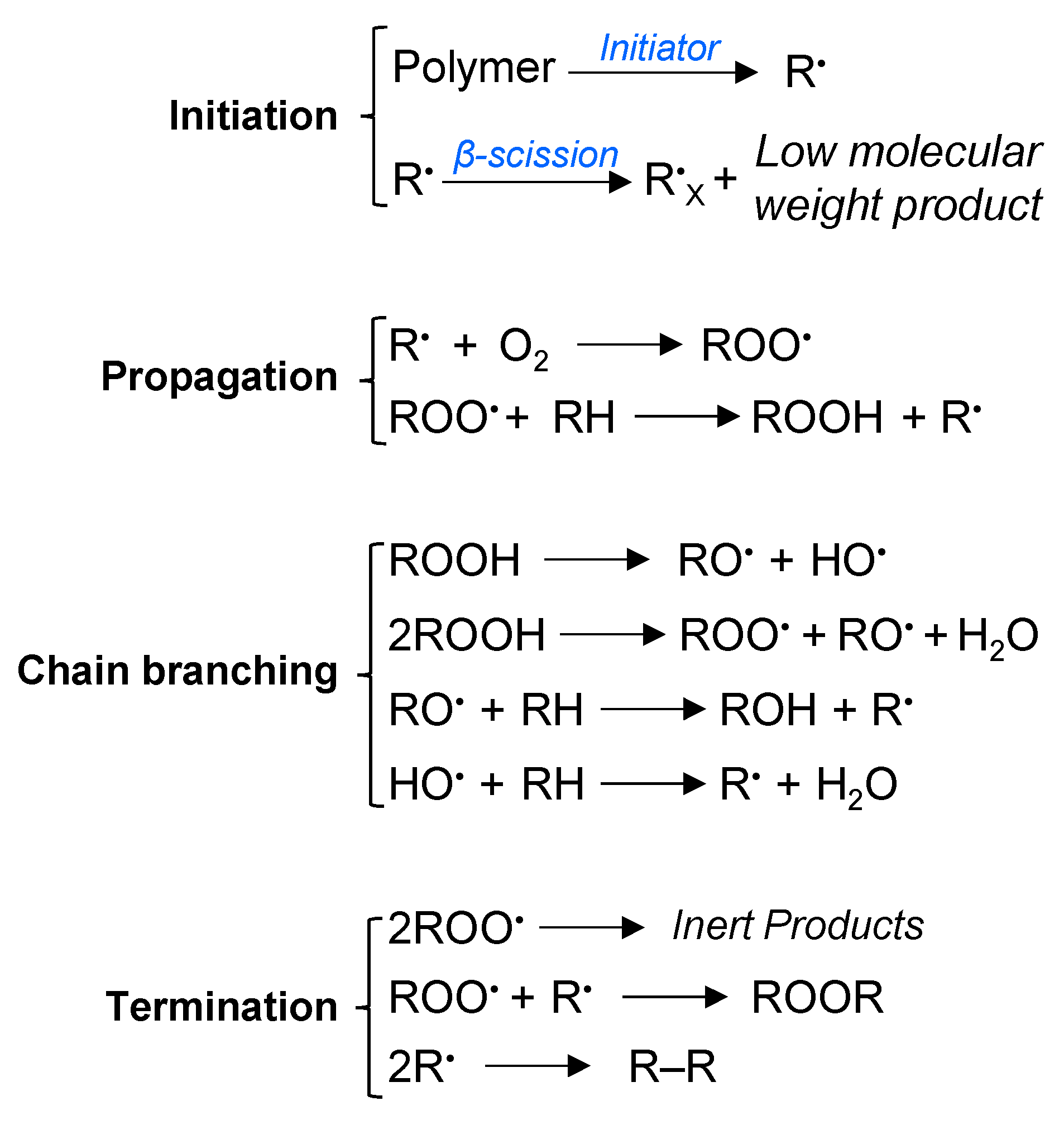
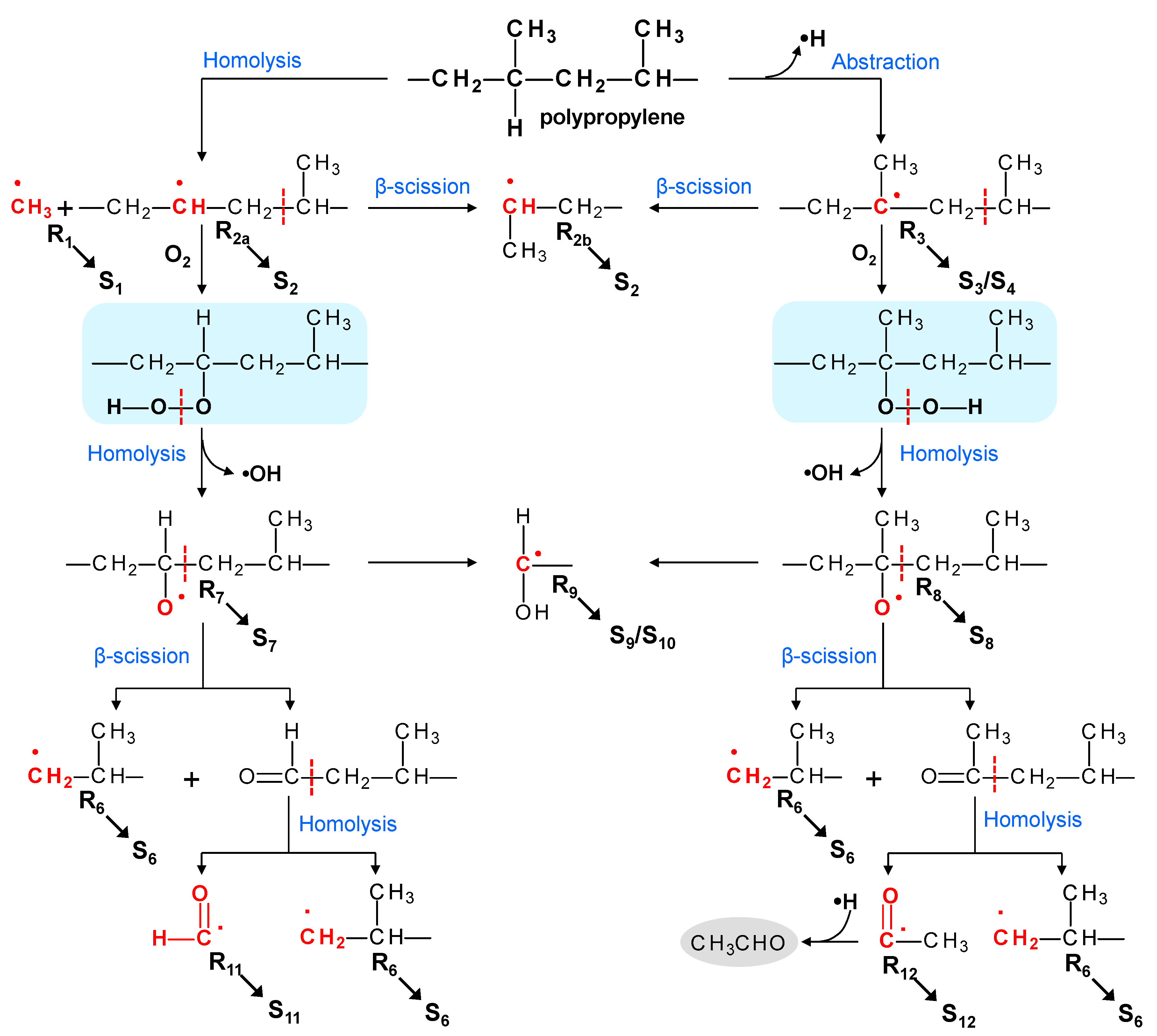
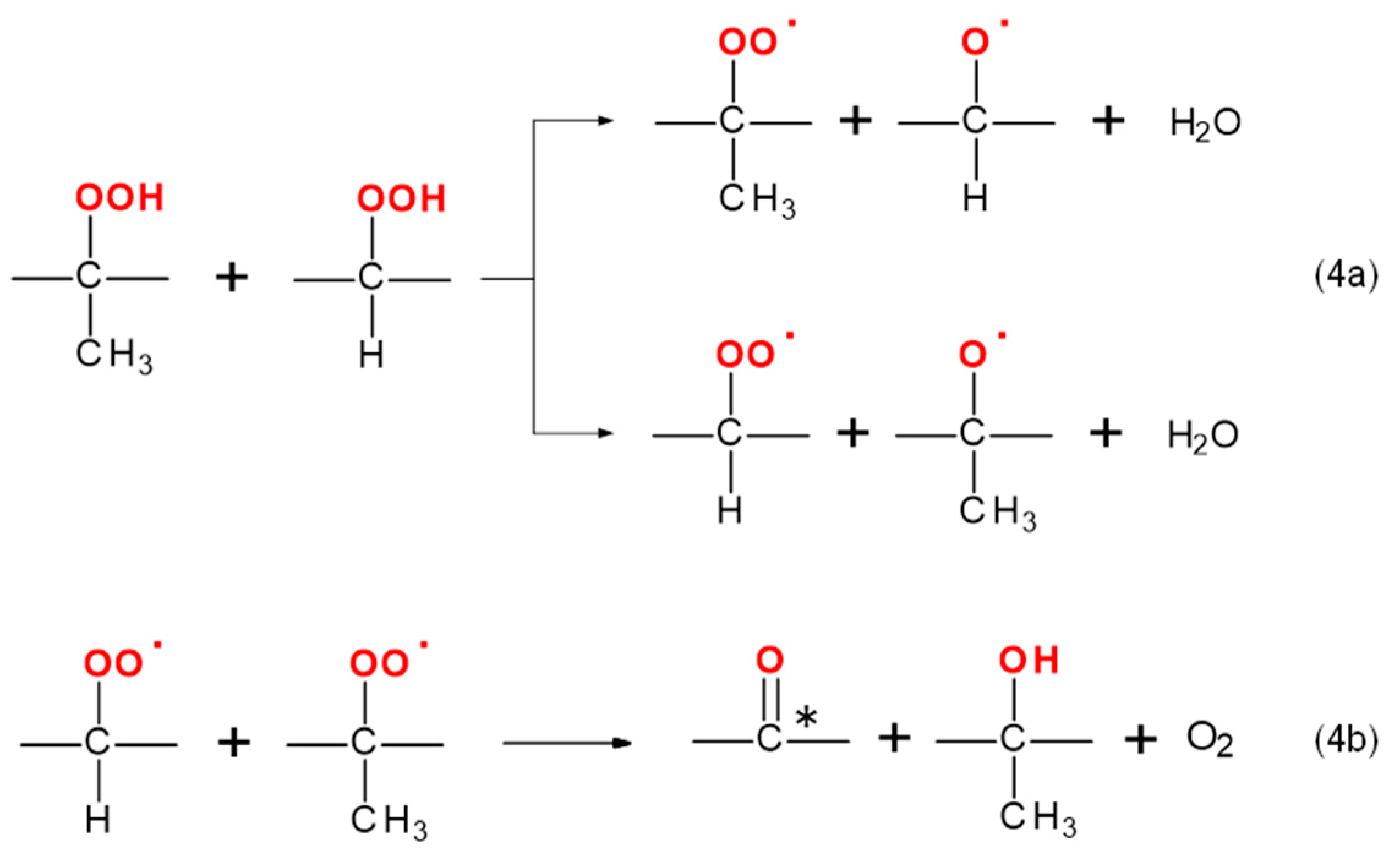
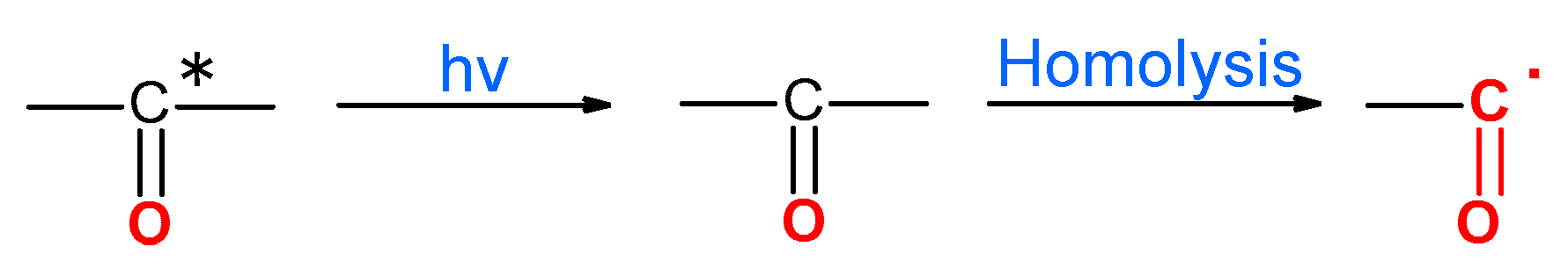
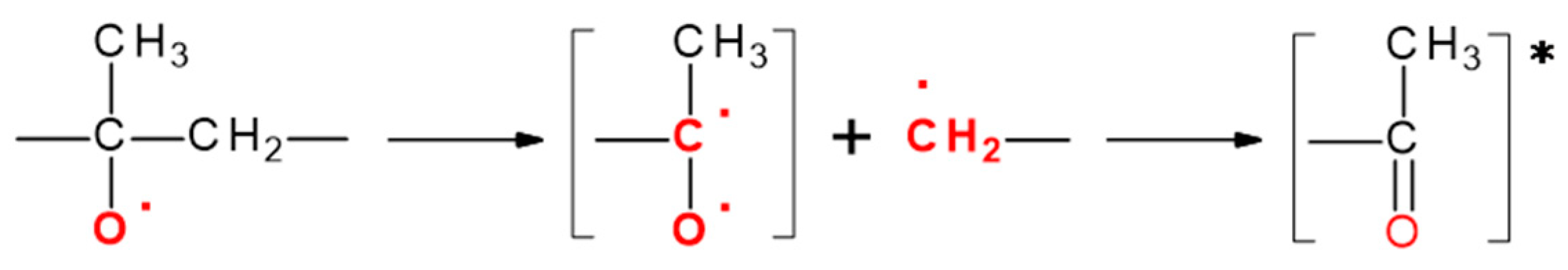
3.7. Reaction Mechanism of PP Thermo-Oxidative Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.M.; Aitken, H.M.; Coote, M.L. The fate of the peroxyl radical in autoxidation: How does polymer degradation really occur? Acc. Chem. Res. 2018, 51, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Bolland, J.L.; Gee, G. Kinetic studies in the chemistry of rubber and related materials. II. The kinetics of oxidation of unconjugated olefins. Rubber Chem. Technol. 1947, 20, 609–617. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Org. Biomol. Chem. 2011, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.P. Polypropylene Handbook: Polymerization, Characterization, Properties, Processing, Applications; Hanser-Gardner: Cincinnati, OH, USA, 1996. [Google Scholar]
- Gijsman, P.; Hennekens, J.; Vincent, J. The mechanism of the low-temperature oxidation of polypropylene. Polym. Degrad. Stab. 1993, 42, 95–105. [Google Scholar] [CrossRef]
- Gardette, J.L.; Lemaire, J. Advantages and limits of hydroperoxide titration methods in solid polymers. Polym. Photochem. 1986, 7, 409–416. [Google Scholar] [CrossRef]
- Qian, S.; Igarashi, T.; Nitta, K. Thermal degradation behavior of polypropylene in the melt state: Molecular weight distribution changes and chain scission mechanism. Polym. Bull. 2011, 67, 1661–1670. [Google Scholar] [CrossRef]
- Vaillant, D.; Lacoste, J.; Dauphin, G. The oxidation mechanism of polypropylene: Contribution of 13C-NMR spectroscopy. Polym. Degrad. Stab. 1994, 45, 355–360. [Google Scholar] [CrossRef]
- Corvaja, C. Introduction to Electron Paramagnetic Resonance. In Electron Paramagnetic Resonance: A Practitioner’s Toolkit; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–36. [Google Scholar]
- Rånby, R.; Rabek, J.F. ESR Spectroscopy in Polymer Research; Springer-Verlag: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods. 2016, 109, 21–30. [Google Scholar] [CrossRef]
- Bunce, N.J. Introduction to the interpretation of electron spin resonance spectra of organic radicals. J. Chem. Educ. 1987, 64, 907–914. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S.; Barr, D.P.; Weber, R.T. Quantitative EPR; Springer: Wein, NY, USA, 2010. [Google Scholar]
- Janzen, E.G. Spin Trapping. Acc. Chem. Res. 1971, 4, 31–40. [Google Scholar] [CrossRef]
- Janzen, E.G.; Stronks, H.J.; Dubose, C.M.; Poyer, J.L.; McCay, P.B. Chemistry and biology of spin-trapping radicals associated with halocarbon metabolism in vitro and in vivo. Environ. Health Perspect. 1985, 64, 151–170. [Google Scholar] [CrossRef]
- Hayashi, T.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Fujii, A.; Inada, S.; Yamamoto, H. Spin-trapping analysis for thermal degradation of poly(vinyl alcohol). Polymer 2021, 217, 123416. [Google Scholar] [CrossRef]
- Sono, M.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Spin-trapping analysis of thermal degradation reaction of poly(butylene terephthalate). Macromolecules 2017, 50, 254–263. [Google Scholar] [CrossRef]
- Sono, M.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Spin-trapping analysis and characterization of thermal degradation of thermoplastic poly(ether–ester) elastomer. Macromolecules 2018, 51, 1088–1099. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Ichise, S.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Okubayashi, S. Spin trapping analysis of the thermal degradation of polypropylene. Polym. Degrad. Stab. 2022, 197, 109871. [Google Scholar] [CrossRef]
- Ashby, G.E. Oxyluminescence from polypropylene. J. Polym. Sci. 1961, 50, 99–106. [Google Scholar] [CrossRef]
- Osawa, Z.; Someya, M.; Fu, Y.S.; Konoma, F. Studies of chemiluminescence observed in photo-irradiated and weathered polypropylene in the early stages. Polym. Degrad. Stab. 1994, 43, 461–470. [Google Scholar] [CrossRef]
- Osawa, Z.; Wu, S.; Konoma, F. Properties and chemiluminescence of polypropylene stored for a long period. Polym. Degrad. Stab. 1988, 22, 97–107. [Google Scholar] [CrossRef]
- Kihara, H.; Hosoda, S. Chemiluminescence parameter for the degradation of polyolefins. Polym. J. 1990, 22, 763–770. [Google Scholar] [CrossRef]
- Billingham, N.C.; Then, E.T.H.; Gijsman, P.J. Chemiluminescence from peroxides in polypropylene. Part I: Relation of luminescence to peroxide content. Polym. Degrad. Stab. 1991, 34, 263–277. [Google Scholar] [CrossRef]
- Kron, A.; Stenberg, B.; Reitberger, T. Characterisation of polypropylene peroxides; their thermo-oxidative stability and reactivity towards dimethylsulfide. Polym. Degrad. Stab. 1996, 54, 89–98. [Google Scholar] [CrossRef]
- Kron, A.; Stenberg, B.; Reitberger, T.; Billingham, N.C. Chemiluminescence from oxidation of polypropylene: Correlation with peroxide concentration. Polym. Degrad. Stab. 1996, 53, 119–127. [Google Scholar] [CrossRef]
- Yano, A.; Akai, N.; Ishii, H.; Satoh, C.; Hironiwa, T.; Millington, K.R.; Nakata, M. Thermal oxidative degradation of additive-free polypropylene pellets investigated by multichannel Fourier-transform chemiluminescence spectroscopy. Polym. Degrad. Stab. 2013, 98, 2680–2686. [Google Scholar] [CrossRef]
- Hosoda, S.; Sato, T.; Takahashi, M.; Tanuma, I.; Yamada, R. Chemiluminescence studies on the photooxidation of isotactic polypropylene. Polym. Degrad. Stab. 2021, 188, 109575. [Google Scholar] [CrossRef]
- Akovantseva, A.A.; Bagratashvili, V.N.; Chumakova, N.A.; Golubeva, E.N.; Gromov, O.I.; Kuzin, S.V.; Mrlnikov, M.Y.; Timashev, P.S. Impregnation of Polycarbonate by Paramagnetic Probe 2,2,6,6-tetramethyl-4-hydroxy-piperidine-1-oxyl (TEMPOL) in supercritical CO2. Appl. Magn. Reson. 2018, 49, 403–413. [Google Scholar] [CrossRef]
- Gromov, O.I.; Kostenko, M.O.; Petrunin, A.V.; Popova, A.A.; Parenago, O.O.; Minaev, N.V.; Golubeva, E.N.; Melnikov, M.Y. Solute Diffusion into Polymer Swollen by Supercritical CO2 by and Chromatography. Polymers 2021, 13, 3059. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics, 3rd ed.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Bucci, G.; Simonazzi, T. Contribution to the study of ethylene-propylene copolymers by infrared spectroscopy. Distribution of monomeric units. J. Polym. Sci. Part C Polym. Symp. 1964, 7, 203–212. [Google Scholar] [CrossRef]
- Coates, J.; Meyers, R.A. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Heory and Instrumentation; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–23. [Google Scholar]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless melt-electrospinning of polypropylene nanofibers. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Mouaci, S.; Saidi, M.; Saidi-Amroun, N. Oxidative degradation and morphological properties of gamma-irradiated isotactic polypropylene films. Micro Nano Lett. 2017, 12, 478–481. [Google Scholar] [CrossRef]
- van Schooten, J.; Duck, E.W.; Berkenbosch, R. The constitution of ethylene-propylene copolymers. Polymer 1961, 2, 357–363. [Google Scholar] [CrossRef]
- Longo, C.; Savaris, M.; Zeni, M.; Brandalise, R.N.; Grisa, A.M.C. Degradation study of polypropylene (PP) and bioriented polypropylene (BOPP) in the environment. Mater. Res. 2011, 14, 442–448. [Google Scholar] [CrossRef]
- Wang, K.; Addiego, F.; Bahlouli, N.; Ahzi, S.; Rémond, Y.; Toniazzo, V.; Muller, R. Analysis of thermomechanical reprocessing effects on polypropylene/ethylene octene copolymer blends. Polym. Degrad. Stab. 2012, 97, 1475–1484. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T.H. Degradation behavior of polypropylene during reprocessing and its biocomposites: Thermal and oxidative degradation kinetics. Polymers 2020, 12, 1627. [Google Scholar] [CrossRef]
- Ng, H.M.; Saidi, N.M.; Omar, F.S.; Ramesh, K.; Ramesh, S.; Bashir, S. Thermogravimetric Analysis of Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–29. [Google Scholar]
- Alam, M.S.; Razzaque, S.M.A.; Khan, G.M.A.; Chakraborty, A.K.; Moniruzzaman, M. Study of the thermo-oxidative degradation behavior of isotactic polypropylene with the varying ethylene content. Indian J. Chem. Sect. B 2007, 46B, 1198–1202. [Google Scholar]
- Purohit, V.; Orzel, R.A. Polypropylene: A literature review of the thermal decomposition products and toxicity. J. Am. Coll. Toxicol. 1988, 7, 221–242. [Google Scholar] [CrossRef]
- Qu, B.; Xu, Y.; Shi, W.; Rånby, B. Photoinitiated crosslinking of low-density polyethylene. 7. Initial radical reactions with model compounds studied by spin-trapping ESR spectroscopy. Macromolecules 1992, 25, 5220–5224. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin Trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Terabe, S.; Konaka, R. Spin trapping of short-lived free radicals by use of 2,4,6-tri-tert-butylnitrosobenzene. J. Am. Chem. Soc. 1971, 93, 4306–4307. [Google Scholar]
- Tabata, M.; Shoma, J.; Yamaoka, H.; Matsuyama, T. A spin-trapping study on crosslinks induced by γ- and neutron irradiations of n-eicosane. Int. J. Radiat. Appl. Istrum. C Radiat. Phys. Chem. 1986, 27, 35–39. [Google Scholar] [CrossRef]
- Qu, B.; Xu, Y.; Shi, W.; Rånby, B. Photoinitiated cross-linking of low-density polyethylene. 6. Spin-trapping ESR studies on radical intermediates. Macromolecules 1992, 25, 5215–5219. [Google Scholar] [CrossRef]
- Tabata, M.; Sohma, J.; Shiotani, M.; Heusinger, H. Spin trapping of carboxylic esters γ-irradiated in solid and liquid phases. Radiat. Phys. Chem. 1980, 16, 369–378. [Google Scholar] [CrossRef]
- Niki, E.; Ohto, N.; Kanauchi, T.; Kamiya, Y. Hydrogen atom abstraction from polypropylene and polystyrene by t-butoxy radical site of radical attack studied by spin trapping. Eur. Polym. J. 1980, 16, 559–563. [Google Scholar] [CrossRef]
- Rosenthal, I.; Mossoba, M.M.; Riesz, P. Photoinduced reactions of dibenzoyl peroxide as studied by EPR and spin-trapping. J. Magn. Reson. 1982, 47, 200–208. [Google Scholar] [CrossRef]
- Hartgerink, J.W.; Engberts, J.B.F.N.; de Boer, T.J. Photolysis of polyhalomethanes in the presence of nitrosoalkanes formation of acyl alkyl nitroxides. Tetrahedron Lett. 1971, 12, 2709–2712. [Google Scholar] [CrossRef]
- Kapustina, N.I.; Popkov, A.Y.; Gasanov, R.G.; Nikishin, G.I. Oxidation of secondary cyclic alcohols by Pb(OAc)4 catalyzed by Cu(II) compounds. Bull. Acad. Sci. USSR Div. Chem. Sci. 1988, 37, 2095–2099. [Google Scholar] [CrossRef]
- Luo, Y.-R. Handbook of Bond Dissociation Energies in Organic Compounds; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Iring, M.; Tudos, F. Thermal oxidation of polyethylene and polypropylene: Effects of chemical structure and reaction conditions on the oxidation process. Prog. Polym. Sci. 1990, 15, 217–262. [Google Scholar] [CrossRef]
- Zlatkevich, L. Luminescence Techniques in Solid State Polymer Research; Marcel Dekker, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Deblieck, R.; Remerie, K.; den Fonteyne, W.V.; Boerakker, M. A morphology-based model to describe the low-temperature impact behaviour of rubber-toughened polypropylene. Polymers 2021, 13, 2218. [Google Scholar] [CrossRef]


| Type | Sample Code | Polymer Structure | Oxidation Status |
|---|---|---|---|
| Homopolymer | PP-H | No ethylene units | None |
| OPP-H | Oxidized by oxygen flow at 90 °C | ||
| Random copolymer | PP-R | Contained ~5 wt% of ethylene units | None |
| OPP-R | Oxidized by oxygen flow at 90 °C | ||
| Block copolymer | PP-B | Contained ~14 wt% of ethylene units | None |
| OPP-B | Oxidized by oxygen flow at 90 °C |
| Sample | Tm (°C) | ΔHm (J/g) | χc (%) | FWHM (°C) | Remarks |
|---|---|---|---|---|---|
| PP-H | 166.2 | 94.7 | 51.2 | 9.0 ± 0.1 | No ethylene units |
| OPP-H | 149.1 | 99.3 | 53.7 | 16.7 ± 0.1 | |
| 163.9 | |||||
| PP-R | 129.3 | 84.3 | 45.6 | 24.8 ± 0.1 | 5 wt% of ethylene units |
| OPP-R | 128.6 | 77.3 | 41.8 | 30.3 ± 0.1 | |
| PP-B | 166.5 | 87.5 | 47.3 | 8.8 ± 0.1 | 14 wt% of ethylene units |
| OPP-B | 163.1 | 67.4 | 36.4 | 11.9 ± 0.1 |
| Abs. Band (cm−1) | Vibration Type | Assignment | Refs. |
|---|---|---|---|
| 2947/2865 | Asym./sym. stretching | CH3 | [33,34] |
| 2915/2835 | Asym./sym. stretching | CH2 | [33,34] |
| 1452/1347 | Asym./sym. bending | CH3 | [33,34,35] |
| 1163 | Wagging/rocking | C–H/CH3 | [33,34] |
| 993/969 | Rocking/stretching | CH3/C–C | [33,34,35] |
| 893/796 | Stretching | C–C | [33] |
| 836 | Rocking | C–H | [33,34] |
| 722 | Rocking | –(CH2)n– | [32,36] |
| 1650–1790 | Stretching | C=O | [33,37] |
| Sample | Peak Integral (a.u.) | TTBNB Concentration (%) |
|---|---|---|
| at 1.21 ppm | ||
| PP-H | 12.13 | 0.60 |
| OPP-H | 12.89 | 0.63 |
| PP-R | 23.68 | 1.18 |
| OPP-R | 23.24 | 1.21 |
| PP-B | 13.02 | 0.65 |
| OPP-B | 13.23 | 0.68 |
| Spin Adduct | Trapped Radical | hfcc (mT) | g-Value | Investigated Materials in Reference | Ref | ||
|---|---|---|---|---|---|---|---|
| aH | aN | aHm | |||||
| S1 | •CH3 | 1.20 | 1.30 | 0.08 | 2.0059 | ||
| •CH3 (ref) | 1.23 | 1.30 | 0.081 | 2.0060 | di-tert-butyl-peroxide/ TTBNB/benzene | [45] | |
| S2 | •CH(CH3)− or −CH2−•CH−CH2− | 2.10 | 1.36 | 0.08 | 2.0058 | ||
| −CH2−•CH−CH2− (ref) | 2.16 | 1.33 | N/A | 2.0061 | paraffin n-C12C26/TTBNB | [43] | |
| S3 | −CH2−•C(CH3)−CH2− | - | 1.05 | 0.20 | 2.0046 | ||
| −CH2−•C(CH3)−CH2− (ref) | - | 1.05 | 0.193 | 2.0046 | n-eicosane/TTBNB | [46] | |
| S4 | −CH2−•C(CH3)−CH2− | - | 1.33 | 0.08 | 2.0059 | ||
| −CH2−•C(CH2−)−CH2− (ref) | - | 1.37 | N/A | 2.0060 | LDPE/nitrosodurene/ benzophenone | [47] | |
| S5 | •C(CH3)3 | - | 1.02 | 0.20 | 2.0038 | ||
| −CH2−•C(CH2−)−CH2− (ref) | - | 1.03 | 0.19 | 2.0039 | paraffin n-C24C50/TTBNB | [43] | |
| S6 | •CH2−CH(CH3)− | 1.80 | 1.30 | 0.08 | 2.0058 | ||
| •CH2−CH3 (ref) | 1.799 | 1.346 | 0.083 | 2.0060 | (n-Bu)3Sn-Br(or I)/ TTBNB/benzene | [45] | |
| S7 | −CH2−CH(O•)−CH2− | 0.75 | 2.70 | 0.080 | 2.0059 | ||
| •O−CH(CH3)2 (ref) | 0.183 | 2.50 | 0.085 | 2.0057 | CH3COOCH(CH3)2/TTBNB | [48] | |
| S8 | −CH2−C(CH3)(O•)−CH2− | - | 2.65 | 0.08 | 2.0057 | ||
| •O−C(CH3)3 (ref) | - | 2.68 | N/A | N/A | DBPO/nitroso-t- butane/benzene | [49] | |
| S9 | •CH(OH)− | 0.19 | 1.49 | 0.06 | 2.0057 | ||
| •CH(OH)CH3 (ref) | 0.2 | 1.488 | N/A | N/A | lactic acid, dibenzoyl peroxide/DMSO+H2O/MNP | [50] | |
| S10 | •CH(OH)− | 1.06 | 1.03 | 0.18 | 2.0038 | ||
| N/A | N/A | N/A | N/A | N/A | N/A | ||
| S11 | •CH(O) | 0.09 | 0.59 | 0.05 | 2.0059 | ||
| •CH(O) (ref) | 0.14 | 0.69 | N/A | 2.0072 | CHI3/2-nitroso-2- methylpropane (MNP) | [51] | |
| S12 | •C(O)– | - | 0.75 | 0.06 | 2.0064 | ||
| •C(O)(CH2)4CH3 (ref) | - | 0.78 | N/A | N/A | cyclohexanol/Lead tetra- acetate (LTA)/MNP | [52] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.A.; Lim, H.M.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Okubayashi, S.; Hosoda, S.; Sato, T. Spin Trapping Analysis of Radical Intermediates on the Thermo-Oxidative Degradation of Polypropylene. Polymers 2023, 15, 200. https://doi.org/10.3390/polym15010200
Nguyen TA, Lim HM, Kinashi K, Sakai W, Tsutsumi N, Okubayashi S, Hosoda S, Sato T. Spin Trapping Analysis of Radical Intermediates on the Thermo-Oxidative Degradation of Polypropylene. Polymers. 2023; 15(1):200. https://doi.org/10.3390/polym15010200
Chicago/Turabian StyleNguyen, Thu Anh, Hui Ming Lim, Kenji Kinashi, Wataru Sakai, Naoto Tsutsumi, Satoko Okubayashi, Satoru Hosoda, and Tetsu Sato. 2023. "Spin Trapping Analysis of Radical Intermediates on the Thermo-Oxidative Degradation of Polypropylene" Polymers 15, no. 1: 200. https://doi.org/10.3390/polym15010200
APA StyleNguyen, T. A., Lim, H. M., Kinashi, K., Sakai, W., Tsutsumi, N., Okubayashi, S., Hosoda, S., & Sato, T. (2023). Spin Trapping Analysis of Radical Intermediates on the Thermo-Oxidative Degradation of Polypropylene. Polymers, 15(1), 200. https://doi.org/10.3390/polym15010200








