Physio-Chemical and Biological Characterization of Novel HPC (Hydroxypropylcellulose):HAP (Hydroxyapatite):PLA (Poly Lactic Acid) Electrospun Nanofibers as Implantable Material for Bone Regenerative Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
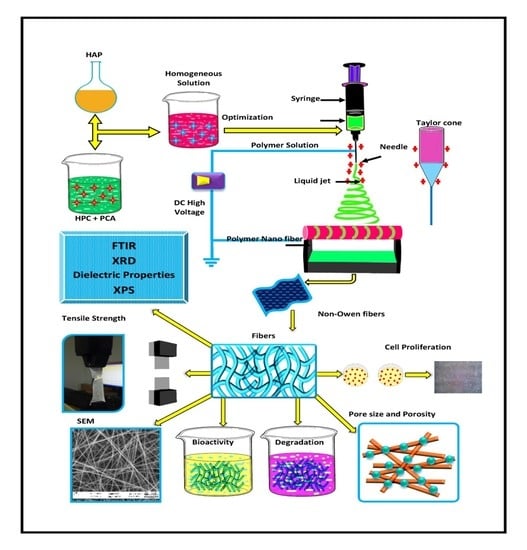
2.2.1. Preparation of Hydroxyapatite and Polymer Solution
2.2.2. Viscosity and Electrical Conductivity Analysis
2.2.3. Electrospinning
2.2.4. Physio Chemical Characterization
2.2.5. Porosity
2.2.6. Bioactivity Study
Bioactivity
In Vitro Biodegradation Properties
2.2.7. Cytotoxicity Analysis
2.2.8. Statistical Analysis
2.2.9. In Vivo Study
3. Results and Discussion
3.1. Preparation of HAP-HPC/PLA Nanofibers
3.2. Viscosity, Electrical Conductivity, and Dielectric Constant Analysis
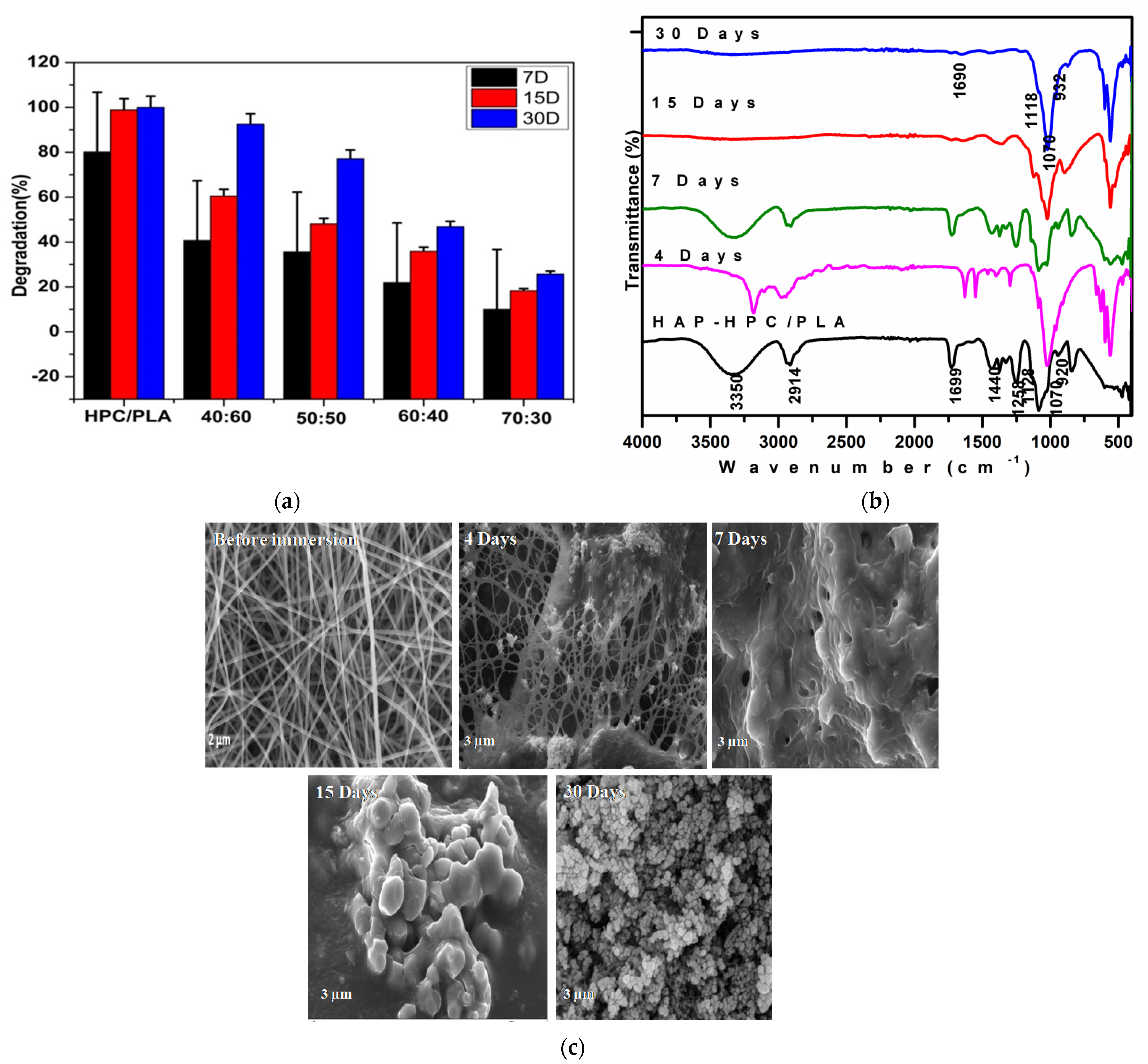
3.3. FT-IR (Fourier Transform Infrared) Analysis of HAP-HPC/PLA Nanofibers
3.4. XRD Analysis of HAP-HPC/PLA Nanofibers
3.5. Mechanical Properties
3.6. X-ray Photoelectron Spectroscopy (XPS)
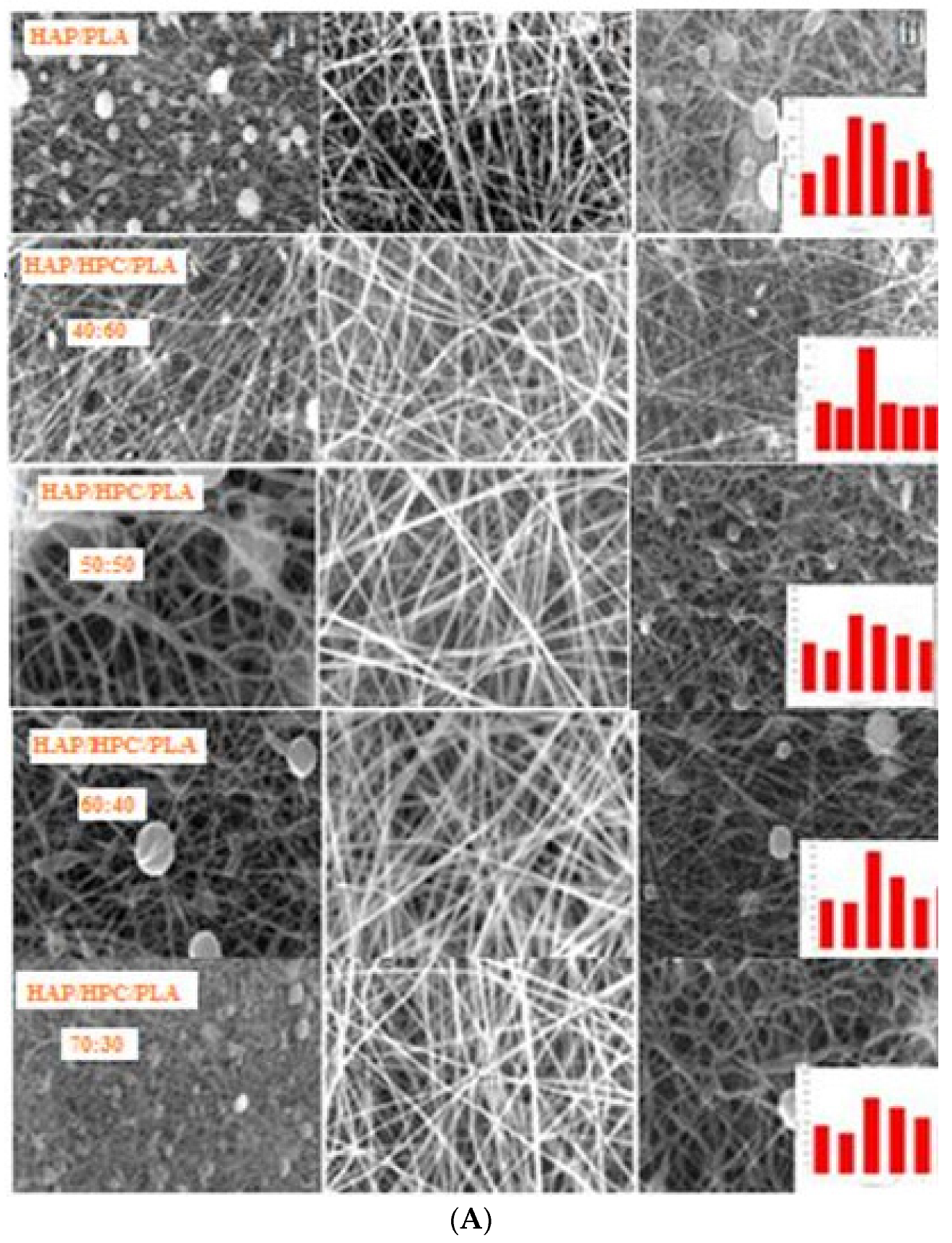
3.7. SEM Morphology and EDAX
3.8. Pore Size and Porosity
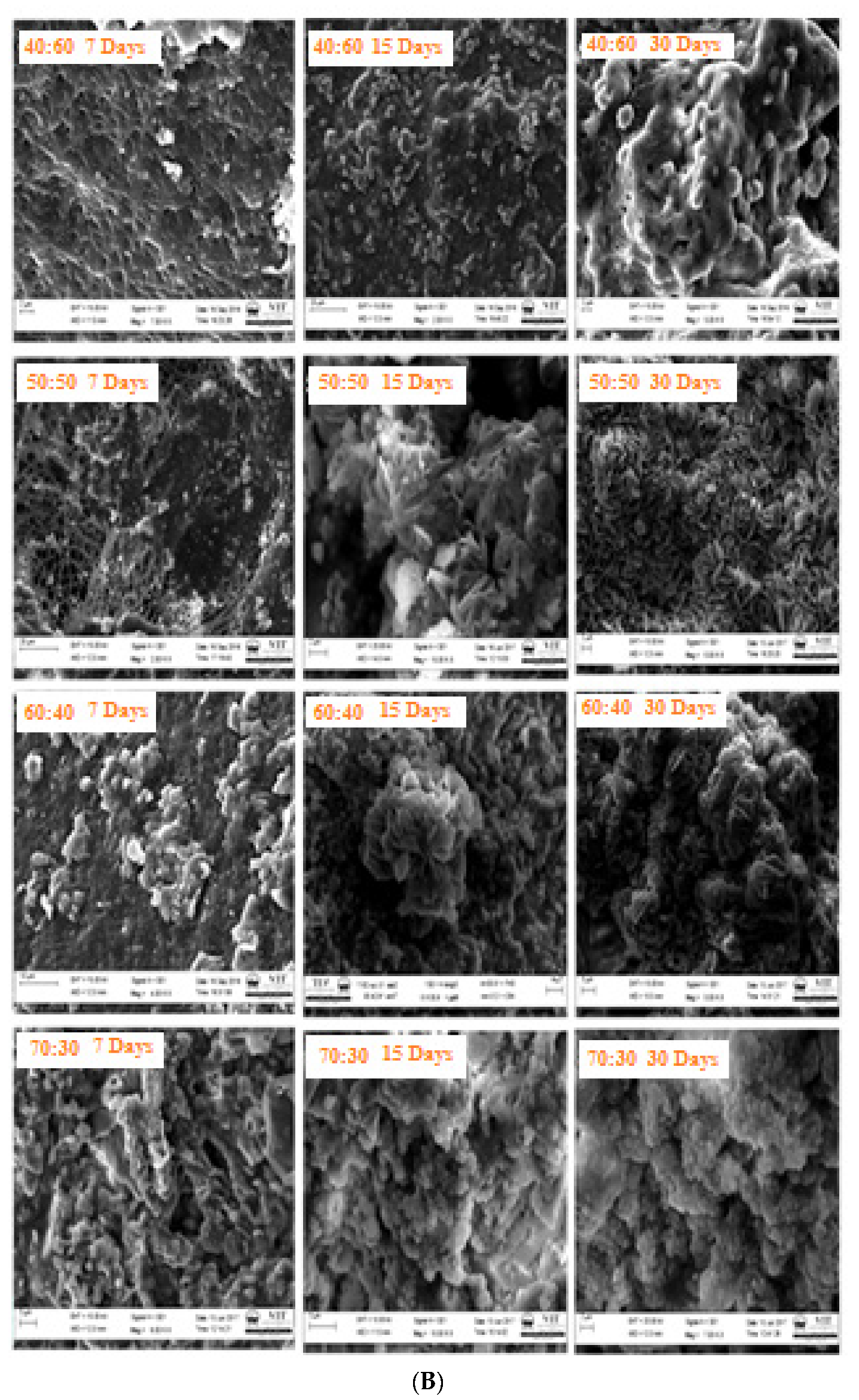
3.9. Bioactivity
3.10. Degradation
3.11. Cytotoxic Analysis
3.12. In Vivo Study
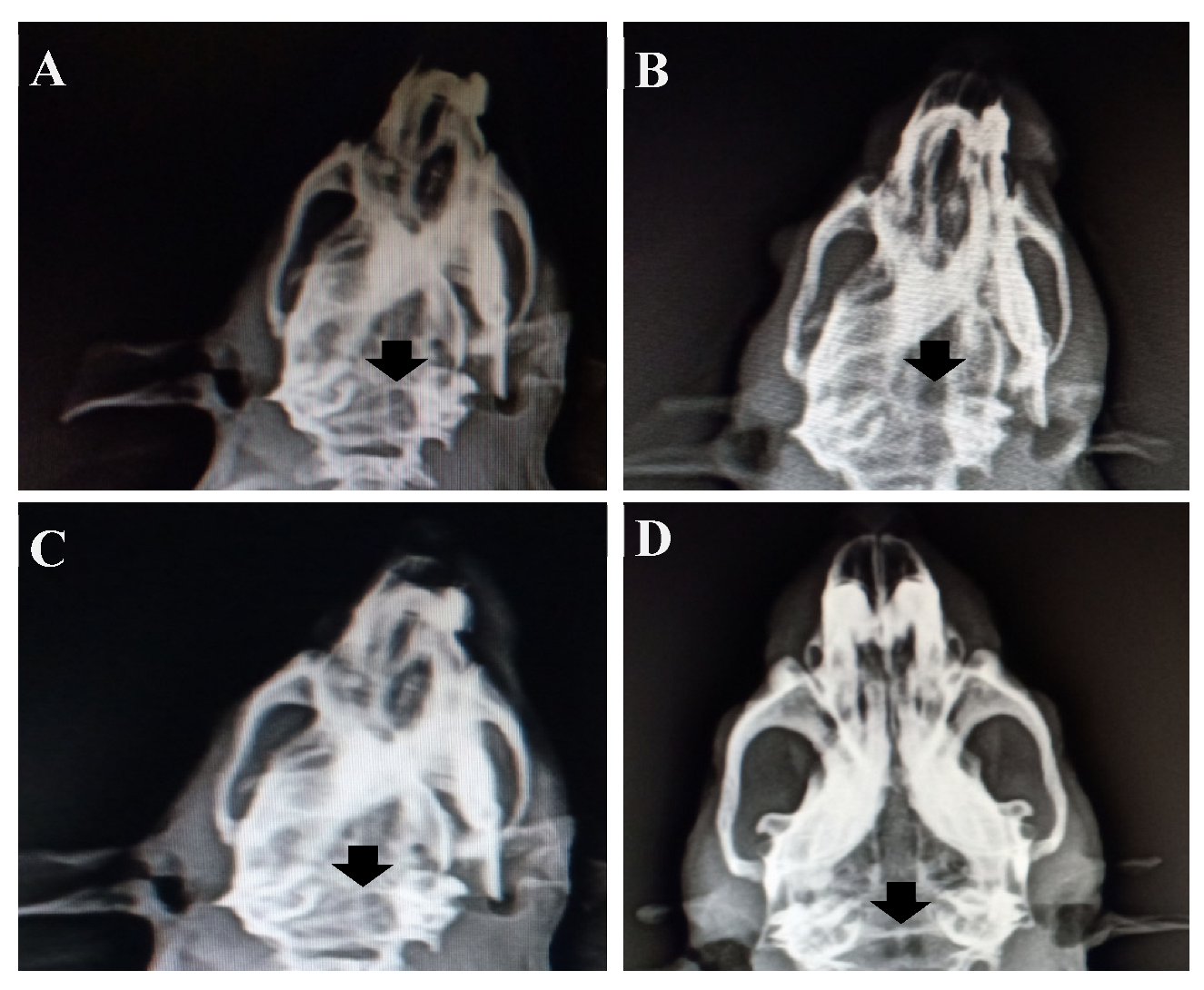
3.12.1. X-Ray Radiology Results
3.12.2. Histological Analysis
4. Conclusions
- ✓ The XRD results confirm the existence of HAP in the presence of a polymeric network and it was found that the triplet peak at 31.89°, 32.05°, and 32.20° increases with an increase in the HAP concentration in the fibrous mat.
- ✓ The mechanical property of 9.53 Mpa was obtained for the optimized composition with a high rate of HCA formation on SBF immersion, this may be due to the interconnected polymeric network and porosity of the sample which was confirmed favorable for cellular activity.
- ✓ The retention of the Ca/P ratio of HAP in the polymeric network was analyzed by XPS analysis. Finally, the biocompatibility evaluation on the MG-63 osteoblast cell line was conducted for 24 h and 48 h which deemed the material fit for biomedical applications. All the compositions revealed enhanced cell proliferation at 48 h of duration.
- ✓ In vivo animal study confirmed the effective bone formation at the 8th week of implantation of HAP-HPC-PLA grafted in the defective area with more cell differentiation when compared with the 4th week of implantation.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Lu, Q.; Yin, J.; Hu, J.; Wang, Z. Alignment of osteoblast-like cells and cell-produced collagen matrix induced by nanogrooves. Tissue Eng. 2005, 11, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.A.; Sachlos, E.; Liu, C.; Czernuszka, J. Controlling the processing of collagen-hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, 89–106. [Google Scholar] [CrossRef]
- Causa, F. A multi-functional scaffold for tissue regeneration: The need to engineer a tissue analogue. Biomaterials 2007, 28, 5093–5099. [Google Scholar] [CrossRef]
- Chemar Huntley, J.; Kristy Crews, D.; Michael Curry, L. Chemical Functionalization and Characterization of Cellulose Extracted from Wheat Straw Using Acid Hydrolysis Methodologies. Int. J. Polym. Sci. 2015, 2015, 293981. [Google Scholar] [CrossRef]
- Rodríguez, K.; Renneckar, S.; Gatenholm, P. Biomimetic calcium phosphate crystal mineralization on electrospun cellulose-based scaffolds. ACS Appl. Mater. Interfaces 2011, 3, 681–689. [Google Scholar] [CrossRef]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Trivedi, M.K. Influence of Biofield Treatment on Physicochemical Properties of Hydroxyethyl Cellulose and Hydroxypropyl Cellulose. J. Mol. Pharm. Org. Process. Res. 2015, 3, 126. [Google Scholar] [CrossRef]
- Sarode, A.L.; Malekar, S.A.; Cote, C.; Worthen, D. Hydroxypropyl cellulose stabilizes amorphous solid dispersions of the poorly water soluble drug felodipine. Carbohydr. Polym. 2014, 4, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, G.; Gong, T. Permeability characterization of hydroxypropylcellulose/ polyacrylonitrile blend membranes. e-Polymers 2013, 13, 15. [Google Scholar] [CrossRef]
- Gonzalez, E.; Shepherd, L.M.; Saunders, L.; Frey, M.W. Surface Functional Poly(lactic Acid) Electrospun Nanofibers for Biosensor Applications. Materials 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, N.; Ichioka, S.; Terada, D.; Tsuchiya, S.; Kobayashi, H. Efficacy of a poly glycolic acid (PGA)/collagen composite nanofibre scaffold on cell migration and neovascularisation in vivo skin defect model. J. Plast. Surg. Hand Surg. 2013, 47, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Wang, Y.; Zhu, Y.; Zhang, Y.; Gao, C. Poly(lactide-co-glycolide)/hydroxyapatite nanofibrous scaffolds fabricated by electrospinning for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, A.; Mary Stella, S.; Arunai Nambiraj, N.; Vijayalakshmi, U. Development of mechanically compliant 3D composite scaffolds for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2018, 106, 3267–3274. [Google Scholar] [CrossRef]
- El-Arnaouty, M.B.; Eid, M.; Salah, M.; El-Sayed Hegazy, A. Preparation and Characterization of Poly Vinyl Alcohol/Poly Vinyl Pyrrolidone/Clay Based Nanocomposite by Gamma Irradiation. J. Macromol. Sci. Part A 2012, 49, 1041–1051. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Mahanwar, P.A. Effect of Methyl Methacrylate– Acrylonitrile -Butadiene–Styrene (MABS) on the Mechanical and Thermal Properties of Poly (Methyl Methacrylate) (PMMA)-Fly Ash Cenospheres (FAC) Filled Composites. J. Miner. Mater. Charact. Eng. 2012, 11, 365–383. [Google Scholar] [CrossRef]
- Uma Maheshwari, S.; Samuel, V.K.; Nagiah, N. Fabrication and evaluation of (PVA/HAp/PCL) bilayer composites as potential scaffolds for bone tissue regeneration application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Trang, P.T.T.; Huong, H.T.; Nam, P.T.; Phuong, N.T.; Trang, N.T.T.; Hoang, T. Fabrication of poly (lactic acid)/hydroxyapatite (PLA/HAp) porous nanocomposite for bone regeneration. Int. J. Nanotechnol. 2015, 12, 391. [Google Scholar] [CrossRef]
- Martin-Pastor, M.; Stoyanov, E. Mechanism of interaction between hydroxypropyl cellulose and water in aqueous solutions: Importance of polymer chain length. J. Appl. Polym. Sci. 2020, 58, 1632–1641. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Ramakrishna, S. Green Processing of a Cationic Polyelectrolyte nanofibers in the Presence of Poly(vinyl alcohol). Int. J. Green Nanotechnol. 2011, 3, 244–249. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. Part A 2010, 94, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.; Vijayalakshmi, U. Development of cerium and silicon co-doped hydroxyapatite nanopowder and its in vitro biological studies for bone regeneration applications. Adv. Powder Technol. 2018, 29, 2792–2803. [Google Scholar] [CrossRef]
- Tighzert, W.; Habi, A.; Ajji, A.; Sadoun, T.; Daoud, F.B.-O. Fabrication and Characterization of Nanofibers Based on Poly(lactic acid)/Chitosan Blends by Electrospinning and Their Functionalization with Phospholipase A1. Fibers Polym. 2017, 18, 514–524. [Google Scholar] [CrossRef]
- Mary Stella, S.; Vijayalakshmi, U. Influence of chemically modified Luffa on the preparation of nanofiber and its biological evaluation for biomedical applications. J. Biomed. Mater. Res. Part A 2018, 107, 610–620. [Google Scholar] [CrossRef]
- Gopi, D.; Karthika, A.; Nithiya, S.; Kavitha, L. In vitro biological performance of minerals substituted hydroxyapatite coating by pulsed electrodeposition method. Mater. Chem. Phys. 2014, 144, 75–85. [Google Scholar] [CrossRef]
- Rama, M.; Vijayalakshmi, U. Influence of silk fibroin on the preparation of nanofibrous scaffolds for the effective use in oste oregenerative applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102182. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Yu, J.X. Preparation and In Vitro Degradation of PLLA Super Fiber Membrane. Adv. Mater. Res. 2013, 750–752, 221–223. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, X.; Song, H.; Hu, J.; Tang, Q.; Zhu, T.; Shen, W.; Chen, J.; Liu, H.; Heng, B.C.; et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015, 44, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Parida, C.; Das, S.C.; Dash, S.K. Mechanical Analysis of Bio Nanocomposite Prepared from Luffa cylindrica. Procedia Chem. 2012, 4, 53–59. [Google Scholar] [CrossRef][Green Version]
- He, B.; Tan, D.; Liu, T.; Wang, Z.; Zhou, H. Study on the Preparation and Anisotropic Distribution of Mechanical Properties of Well-Aligned PMIA Nanofiber Mats Reinforced Composites. J. Chem. 2017, 2017, 8274024. [Google Scholar] [CrossRef]
- Nagakane, K.; Yoshida, Y.; Hirata, I.; Fukuda, R.; Nakayama, Y.; Shirai, K.; Ogawa, T.; Suzuki, K.; Meerbeek, V.B.; Okazaki, M. Analysis of Chemical Interaction of 4-MET with Hydroxyapatite Using XPS. Dent. Mater. J. 2006, 25, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Vallet-Regı, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Mary Stella, S.; Vijayalakshmi, U. Influence of Polymer Based Scaffolds with Genipin Cross Linker for the Effective (Pore Size) Usage in Biomedical Applications. Trends Biomater. Artif. Organs 2018, 32, 83–92. [Google Scholar]
- Dridi, A.; Riahi, K.Z.; Somrani, S. Mechanism of apatite formation on a poorly crystallized calcium phosphate in a simulated body fluid (SBF) at 37 °C. J. Phys. Chem. Solids 2021, 156, 110122. [Google Scholar] [CrossRef]
- Dong, Y.; Yong, T.; Liao, S.; Chan, C.K.; Stevens, M.M.; Ramakrishna, S. Distinctive degradation behaviors of electrospun polyglycolide, poly(DL-lactide-co-glycolide), and poly(L-lactide-co-epsilon-caprolactone) nanofibers cultured with/without porcine smooth muscle cells. Tissue Eng. Part A 2010, 16, 283–298. [Google Scholar] [CrossRef]
- Gomes, S.; Rodrigues, G.; Martins, G.G.; Roberto, M.A.; Mafra, M.; Henriques, C.M.R.; Silva, J.C. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C 2015, 46, 348–358. [Google Scholar] [CrossRef]
- Liu, T.; Ding, X.; Lai, D.; Chen, Y.; Zhang, R.; Chen, J.; Feng, X.; Chen, X.; Yang, X.; Zhao, R.; et al. Enhancing in vitro bioactivity and in vivo osteogenesis of organic–inorganic nanofibrous biocomposites with novel bioceramics. J. Mater. Chem. B 2014, 2, 6293–6305. [Google Scholar] [CrossRef]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. In vivo safety evaluations of electrospun nanofibers for biomedical applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications, 1st ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 101–113. [Google Scholar] [CrossRef]
- Lopresti, F.; Pavia, F.C.; Vitrano, I.; Kersaudy-Kerhoas, M.; Brucato, V.; Carrubba, V.L. Effect of hydroxyapatite concentration and size on morpho-mechanical properties of PLA-based randomly oriented and aligned electrospun nanofibrous mats. J. Mech. Behav. Biomed. Mater. 2020, 101, 103449. [Google Scholar] [CrossRef] [PubMed]
- Pektok, E.; Nottelet, B.; Tille, J.-C.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Degradation and Healing Characteristics of Small-Diameter Poly(ε-Caprolactone) Vascular Grafts in the Rat Systemic Arterial Circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef] [PubMed]








| S.No. | Sample Ratio | Solution Preparation |
|---|---|---|
| 1 | HPC/PLA (0:100) | (Without HAP)10 mL of HPC/PLA |
| 2 | HAP-HPC/PLA (40:60) | 4 mL of HAP and 6 mL of HPC/PLA |
| 3 | HAP-HPC/PLA (50:50) | 5 mL of HAP and 5 mL of HPC/PLA |
| 4 | HAP-HPC/PLA (60:40) | 6 mL of HAP and 4 mL of HPC/PLA |
| 5 | HAP-HPC/PLA (70:30) | 7 mL of HAP and 3 mL of HPC/PLA |
| S.No. | Sample | Viscosity(cP) | Conductivity (µS/cm) | Flow Rate (1/mL) | Collector Rotation Speed (RPM) | Tip to Collector Distance (cm) | Voltage (kV) |
|---|---|---|---|---|---|---|---|
| 1 | HPC | 63 | - | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| 2 | PLA | 4519 | - | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| 3 | HPC/PLA | 3687 | 0.0018 | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| 4 | 40:60 | 2487 | 0.0023 | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| 5 | 50:50 | 1413 | 0.0024 | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| 6 | 60:40 | 1198 | 0.0036 | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| 7 | 70:30 | 786.7 | 0.0041 | 0.5 0.7 1 | 700 1000 1500 | 12 15 17 | 15 21 25 |
| S.No. | Sample | Proportional Limit (Pa) | Yield Strength (Mpa) | Tensile Strength (Mpa) | Young Modulus (Mpa) | Fracture (%) |
|---|---|---|---|---|---|---|
| 1 | HPC/PLA | 0.424 | 0.444 | 3.57 | 131.06 | 148 |
| 2 | 40:60 | 0.097 | 0.291 | 4.88 | 180.50 | 111 |
| 3 | 50:50 | 0.926 | 4.823 | 8.21 | 190.50 | 219 |
| 4 | 60:40 | 1.134 | 2.824 | 8.91 | 370.89 | 118 |
| 5 | 70:30 | 1.470 | 1.388 | 9.53 | 430.10 | 90 |
| Sample | Ca 2P | P 2p | C1s | O1s | Ca/P Ratio | ||
|---|---|---|---|---|---|---|---|
| 2p3/2 | 2p1/2 | 2p3/2 | 2p1/2 | ||||
| HAP-HPC/PLA | 348.50 | 352.51 | 136.86 | 137.31 | (a) 288.50-(C-C=O) (b) 286.73-(C-O) (c) 286.16-(C-H) (d) 285.75-(C-C/CH2) | (a) 535.68-OH (b) 534.86-P-O (c) 533.71-P-O-P | 1.66 |
| S.No. | Sample | Ca/P Ratio |
|---|---|---|
| 1 | HPC/PLA | - |
| 2 | 40:60 | 1.66 |
| 3 | 50:50 | 1.67 |
| 4 | 60:40 | 1.65 |
| 5 | 70:30 | 1.67 |
| S.No. | Sample | Pore Size (μm) | Pore Diameter (nm) | Porosity (%) |
|---|---|---|---|---|
| 1 | HPC/PLA | 4.54 | 355 ± 10 | 59.56 |
| 2 | 40:60 HAP-HPC/PLA | 7.21 | 277 ± 11 | 62.97 |
| 3 | 50:50 HAP-HPC/PLA | 9.23 | 133 ± 33 | 75.23 |
| 4 | 60:40 HAP-HPC/PLA | 11.21 | 122 ± 33 | 89.45 |
| 5 | 70:30 HAP-HPC/PLA | 15.54 | 110 ± 66 | 98.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, S.M.; Sridhar, T.M.; Ramprasath, R.; Gimbun, J.; Vijayalakshmi, U. Physio-Chemical and Biological Characterization of Novel HPC (Hydroxypropylcellulose):HAP (Hydroxyapatite):PLA (Poly Lactic Acid) Electrospun Nanofibers as Implantable Material for Bone Regenerative Application. Polymers 2023, 15, 155. https://doi.org/10.3390/polym15010155
Stella SM, Sridhar TM, Ramprasath R, Gimbun J, Vijayalakshmi U. Physio-Chemical and Biological Characterization of Novel HPC (Hydroxypropylcellulose):HAP (Hydroxyapatite):PLA (Poly Lactic Acid) Electrospun Nanofibers as Implantable Material for Bone Regenerative Application. Polymers. 2023; 15(1):155. https://doi.org/10.3390/polym15010155
Chicago/Turabian StyleStella, S. Mary, T. M. Sridhar, R. Ramprasath, Jolius Gimbun, and U. Vijayalakshmi. 2023. "Physio-Chemical and Biological Characterization of Novel HPC (Hydroxypropylcellulose):HAP (Hydroxyapatite):PLA (Poly Lactic Acid) Electrospun Nanofibers as Implantable Material for Bone Regenerative Application" Polymers 15, no. 1: 155. https://doi.org/10.3390/polym15010155
APA StyleStella, S. M., Sridhar, T. M., Ramprasath, R., Gimbun, J., & Vijayalakshmi, U. (2023). Physio-Chemical and Biological Characterization of Novel HPC (Hydroxypropylcellulose):HAP (Hydroxyapatite):PLA (Poly Lactic Acid) Electrospun Nanofibers as Implantable Material for Bone Regenerative Application. Polymers, 15(1), 155. https://doi.org/10.3390/polym15010155