Development of an Atomic-Oxygen-Erosion-Resistant, Alumina-Fiber-Reinforced, Fluorinated Polybenzoxazine Composite for Low-Earth Orbital Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
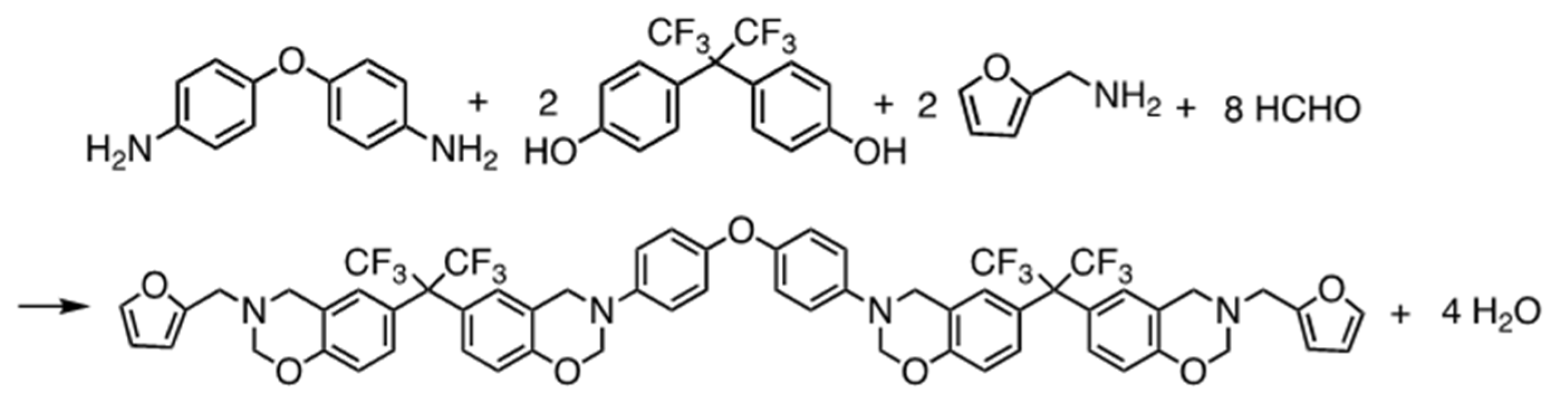
2.2. Monomer Synthesis
2.3. Preparation of BAF-oda-fu with POSS Additive
2.4. Polymerization Procedure
2.5. Preparation of Composite Samples
2.6. Characterization
3. Results and Discussions
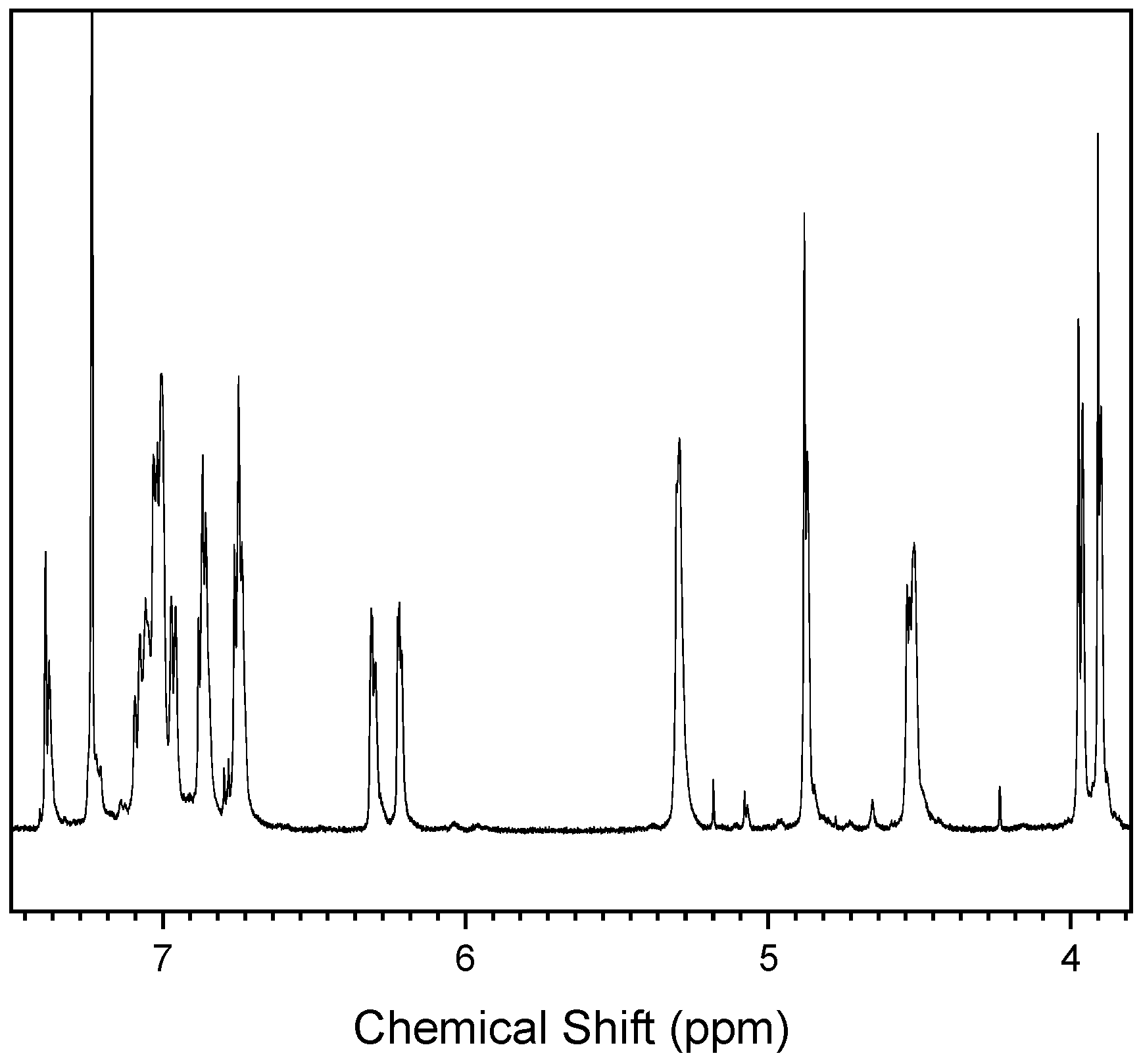
3.1. Synthesis and Characterization of BAF-oda-fu
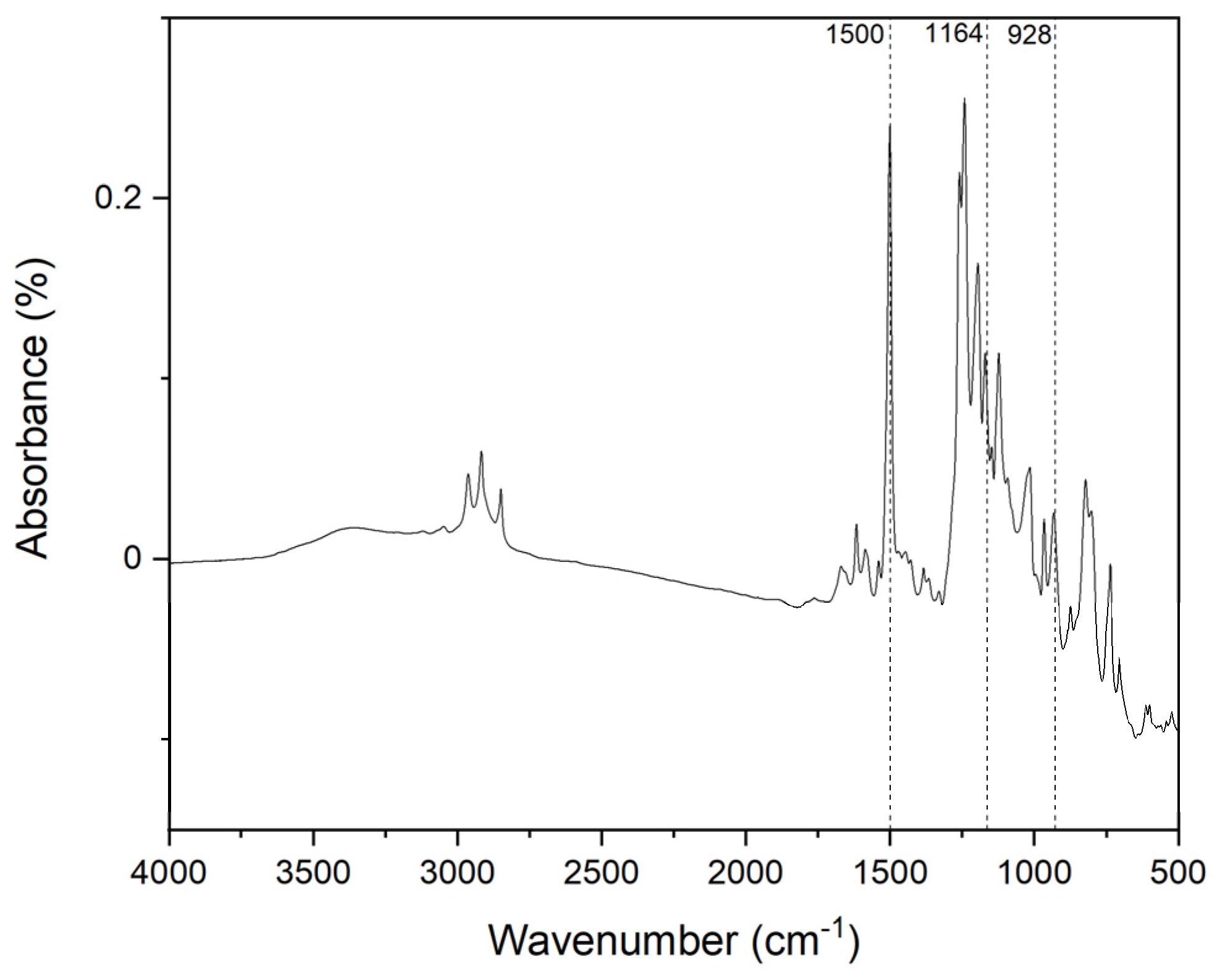
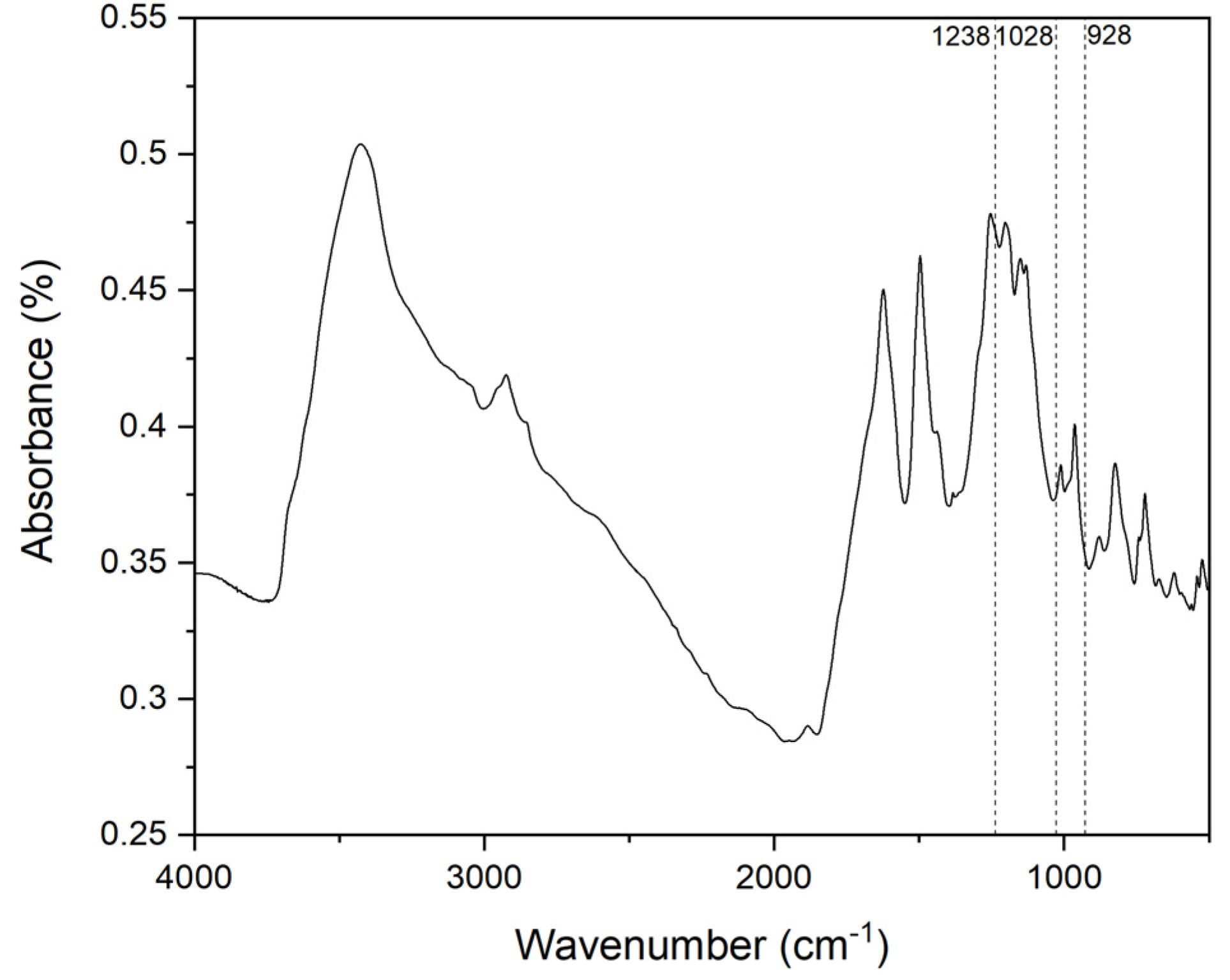
3.2. Polymerization Behavior of BAF-oda-fu
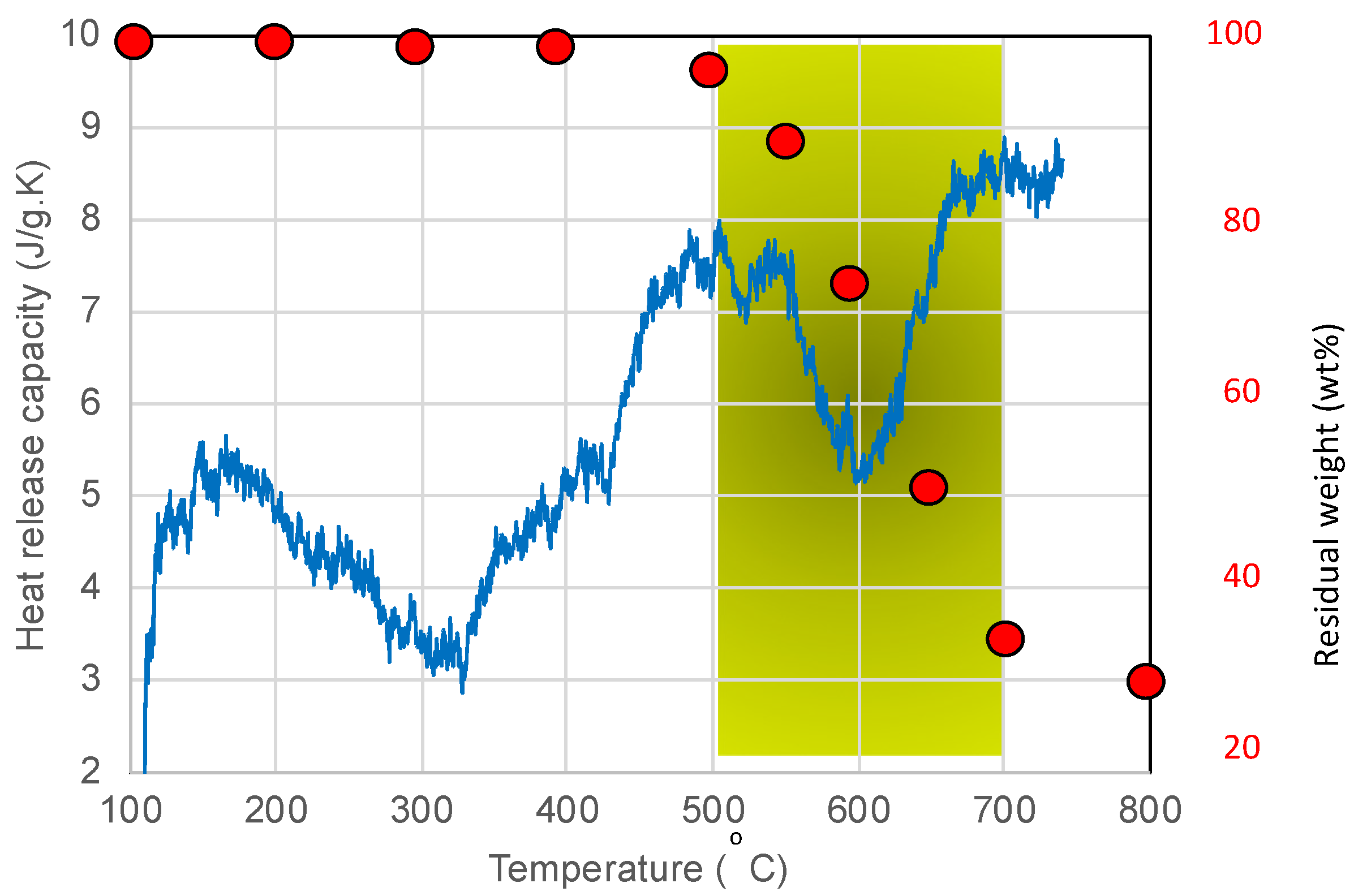
3.3. Thermal Analysis
3.4. Dynamic Mechanical Analysis of Composite Samples
3.5. Density Measurements and Composite Void Analysis
3.6. Ground-Based Atomic-Oxygen Erosion Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines-New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Ishida, H.; Agag, T. Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; 1083p. [Google Scholar]
- Iguchi, D.; Ohashi, S.; Abarro, G.; Yin, X.; Han, L.; Winroth, S.; Scott, C.; Gleydura, M.; Jin, L.; Kanagasegar, N.; et al. Development of Hydrogen-rich Benzoxazine Resins with Low Polymerization Temperature for Space Radiation Shielding. ACS Omega 2018, 3, 11569–11581. [Google Scholar] [CrossRef] [PubMed]
- Winroth, S.; Scott, C.; Ishida, H. Structure and performance of benzoxazine composites for space radiation shielding. Molecules 2020, 25, 4346. [Google Scholar] [CrossRef]
- Gohel, A.; Makwana, R. Multi-layered shielding materials for high energy space radiation. Radiat. Phys. Chem. 2022, 197, 110131. [Google Scholar] [CrossRef]
- Cha, J.H.; Jang, W.H.; Kuimar, S.K.S.; Noh, J.E.; Choi, J.S.; Kim, C.G. Functionalized multi-walled carbon nanotuibes/hydrogen-rich benzoxazine nanocomposites for cosmic radiation shielding with enhanced mechanical properties and space environment resistance. Compos. Sci. Technol. 2022, 228, 109634. [Google Scholar] [CrossRef]
- Kanchanasopa, M.; Yanumet, N.; Hemvichian, K.; Ishida, H. The effect of polymerization conditions on the density and Tg of bisphenol-A and hexafluoroisopropylidene-containing polybenzoxazines. Polym. Polym. Compos. 2001, 9, 367–375. [Google Scholar] [CrossRef]
- Liu, J.P.; Ishida, H. High yield synthesis of fluorinated benzoxazine monomers and their molecular characterization. Polym. Polym. Compos. 2002, 10, 191–204. [Google Scholar] [CrossRef]
- Su, Y.C.; Chang, F.C. Synthesis and characterization of fluorinated polybenzoxazine material with low dielectric constant. Polymer 2003, 44, 7989–7996. [Google Scholar] [CrossRef]
- Velez-Herrera, P.; Doyama, K.; Abe, H.; Ishida, H. Synthesis and characterization of highly fluorinated polymer with the benzoxazine moiety on the main chain and low dielectric constant. Macromolecules 2008, 41, 9704–9714. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Lee, H.H.; Chang, H.C.; Hwang, K.Y.; Tu, A.P.; Su, W.C. Fluorinated benzoxazines and the structure-property relationship of resulting polybenzoxazines. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4970–4983. [Google Scholar] [CrossRef]
- Qu, L.; Xin, Z. Preparation and surface properties of novel low surface free energy fluorinated silane-functional polybenzoxazine films. Langmuir 2011, 27, 8365–8370. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Ko, T.; Kim, K.; Choi, S.W.; Park, J.O.; Kim, K.H.; Pak, C.; Chang, H.; Lee, J.C. Poly[2,2’-(m-phenylene)-5,5’-bibenzimidazole] and poly[6-fluoro-3-(pyridin-2-yl)-3,4-dihydro-2H-benzoxazine] based polymer electrolyte membranes for fuel cells at elevated temperature. Macromol. Res. 2012, 20, 1181–1190. [Google Scholar] [CrossRef]
- Raza, A.; Si, Y.; Wang, X.F.; Ren, T.; Ding, B.; Yu, J.Y.; Al-Theyab, S.S. Novel fluorinated polybenzoxazine-silica films: Chemical synthesis and superhydrophobicity. RSC Adv. 2012, 2, 12804–12811. [Google Scholar] [CrossRef]
- Demir, K.D.; Kiskan, B.; Latthe, S.S.; Demirel, A.L.; Yagci, Y. Thermally curable fluorinated main chain benzoxazine polyethers via Ullmann coupling. Polym. Chem. 2013, 4, 2106–2114. [Google Scholar] [CrossRef]
- He, X.Y.; Wang, J.; Wang, Y.D.; Liu, C.J.; Liu, W.B.; Yang, L. Synthesis, thermal properties and curing kinetics of fluorine diamine-based benzoxazine containing ester groups. Eur. Polym. J. 2013, 49, 2759–2768. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, F.R.; Yuan, Q.L.; Zhou, Y.; Du, L. Synthesis of novel imide-functionalized fluorinated benzoxazines and properties of their thermosets. High Perform. Polym. 2013, 25, 677–684. [Google Scholar] [CrossRef]
- Wang, J.; Ren, T.T.; Wang, Y.D.; He, X.Y.; Liu, W.B.; Shen, X.D. Synthesis, curing behavior and thermal properties of fluorine-containing benzoxazines based on linear and branched butylamines. React. Funct. Polym. 2014, 74, 22–30. [Google Scholar] [CrossRef]
- Parveen, A.S.; Thirukumaran, P.; Sarojadevi, M. Low dielectric materials from fluorinated polybenzoxazines. Polym. Adv. Technol. 2014, 25, 1538–1545. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Feng, T.T.; Ramdani, N.; Li, Y.; Xu, X.D.; Liu, W.B. Synthesis, curing behavior, and thermal properties of fluorine-based benzoxazine-endcapped copoly(ether ketone ketone)s. J. Therm. Anal. Calor. 2015, 119, 1913–1921. [Google Scholar] [CrossRef]
- Feng, T.T.; Wanbg, J.; Wang, H.; Ramdani, N.; Zu, L.W.; Liu, W.B.; Xu, X.D. Copolymerization of fluorine-based main-chain benzoxazine and their high performance thermosets. Polym. Adv. Technol. 2015, 26, 581–588. [Google Scholar] [CrossRef]
- Wu, J.B.; Xi, Y.; McCandless, G.T.; Xie, Y.H.; Menon, R.; Patel, Y.; Yang, D.J.; Iacono, S.T.; Novak, B.M. Synthesis and characterization of partially fluorinated polybenzoxazine resins utilizing octafluorocyclopentene as a versatile building block. Macromolecules 2015, 48, 6087–6095. [Google Scholar] [CrossRef]
- Pattharasiriwong, P.; Rimdusit, S. Characterizations of fluorine-containing polybenzoxazine prepared by solventless procedure. Key Eng. Mater. 2015, 659, 368–372. [Google Scholar] [CrossRef]
- Situ, Y.; Zhu, Z.W.; Huang, H. Catalytic effect of trifluoroacetamido group on thermally induced ring-opening polymerization of 1,3-benzoxazine and formation of arylamine Mannich bridge structure. High Perform. Polym. 2016, 28, 271–280. [Google Scholar] [CrossRef]
- Feng, T.T.; Wang, J.; Pan, L.; Derradji, M.; Ramdani, N.; Liu, W.B.; Zhou, H.R. Tunable properties of novel tetra-functional fluorine-based benzoxazines from mixed amines: Synthesis, characterization and curing kinetics. Thermochim. Acta 2016, 633, 1–11. [Google Scholar] [CrossRef]
- Rivera, A.; Rojas, J.J.; Rios-Motta, J.; Bolte, M. Crystal structure and C—H⋯F hydrogen bonding in the fluorinated bis-benzoxazine: 3,3′-(ethane-1,2-di yl)bis (6-fluoro-3,4-di hydro-2H-1,3-benzoxazine). Acta Cryst. Sect. E 2016, 72, 1509–1511. [Google Scholar] [CrossRef] [PubMed]
- Pattharasiriwong, P.; Jubsilp, C.; More, P.; Rimdusit, S. Dielectric and thermal behaviors of fluorine-containing dianhydride-modified polybenzoxazine: A molecular design flexibility. J. Appl. Polym. Sci. 2017, 134, 45204. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wang, R.R.; An, Q.; Su, X.H.; Li, C.Y.; Shen, S.S.; Huo, G.Y. The F···H hydrogen bonding effect on the dynamic mechanical and shape memory properties of a fluorine-containing polybenzoxazine. Macromol. Chem. Phys. 2017, 218, 1700050. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Tkachenko, I.M.; Lobko, E.V.; Shekera, O.V.; Syrovets, A.P.; Shevchenko, V.V. Low dielectric material from novel core-fluorinated polybenzoxazine. Mendeleev Commun. 2017, 27, 41–43. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Tkachenko, I.M.; Bliznyuk, V.N.; Lobko, E.V.; Shekera, O.V.; Shevchenko, V.V. Synthesis and characterization of fluorinated isomeric polybenzoxazines from core-fluorinated diamine-based benzoxazines. Polymer 2018, 145, 62–69. [Google Scholar] [CrossRef]
- Kobzar, Y.L.; Tkachenko, I.M.; Bliznyuk, V.N.; Shevchenko, V.V. Fluorinated polybenzoxazines as advanced phenolic resins for leading-edge applicatios. React. Funct. Polym. 2018, 133, 71–92. [Google Scholar] [CrossRef]
- Chen, S.J.; Ren, D.X.; Li, B.; Chen, L.; Xu, M.Z.; Liu, X.B. Benzoxazine containing fluorinated aromatic ether nitrile linkage: Preparation, curing kinetics and dielectric properties. Polymers 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, R.X.; You, Q.Q.; Fu, W.J.; Guo, W.S. Synthesis of SCF3-containing benzoxazines and oxazolines via a photoredox-catalyzed radical trifluoromethylthiolation-cyclization of olefinic amides. Asian J. Org. Chem. 2019, 8, 2002–2005. [Google Scholar] [CrossRef]
- Kurinchyslvan, S.; Chandramohan, A.; Hariharan, A.; Gomathipriya, P.; Alagar, M. Cardanol-based benzoxazine-terminated graphene oxide-reinforced fluorinated benzoxazine hybrid composites for low K applications. Compos. Interfaces 2020, 28, 737–751. [Google Scholar] [CrossRef]
- Ren, D.X.; Xu, M.Z.; Chen, S.J.; Xu, X.Q.; Zhang, S.A.; Han, M.G.; Liu, X.B. Curing reaction and properties of a kind of fluorinated phthalonitrile containing benzoxazine. Eur. Polym. J. 2021, 159, 110715. [Google Scholar]
- Fan, X.T.; Liu, Z.X.; Huang, J.G.; Han, D.; Qiao, Z.H.; Liu, H.H.; Du, J.; Yan, H.; Ma, Y.Y.; Zhang, C.Y.; et al. Synthesis, curing mechanism, thermal stability, and surface properties of fluorinated polybenzoxazines for coating applications. Adv. Compos. Hybrid Mater. 2022, 5, 322–334. [Google Scholar] [CrossRef]
- Finckenor, M.M. Materials for Spacecraft. In Aerospace Materials and Applications; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2018; p. 403. [Google Scholar]
- Avcu, S.; Celik, B. Structural Material Selection and Processing for Low Earth Orbit Spacecraft Regarding Atomic Oxygen Effects. In Proceedings of the International Conference on Recent Advances in Space Technologies, Istanbul, Turkey, 20–22 November 2003; Kurnaz, S., Ince, F., Onbasioglu, S., Eds.; Institute of Electrical and Electronics Engineers: Manhattan, NY, USA, 2003; pp. 589–594. [Google Scholar]
- Banks, B.A.; Demko, R. Atomic Oxygen Protection of Materials in Low Earth Orbit; NASA Technical Reports Server, NASA/TM-2002-211360; Office of Aviation Research: Washington, DC, USA, 1 February 2002; pp. 1–12. [Google Scholar]
- Uchida, S.; Kawauchi, T. Polymer alloys of high-molecular-weight benzoxazine and epoxy resin. High Perform. Polym. 2014, 26, 846–855. [Google Scholar] [CrossRef]
- Liu, Y.; Chou, C. High performance benzoxazine monomers and polymers containing furan groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5267–5282. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15N isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. 2017, 121, 6269–6282. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resinds, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar] [CrossRef]
- Machado, I.; Rachita, E.; Fuller, E.; Calado, V.M.A.; Ishida, H. Very high-char yielding elastomers based on the copolymers of a catechol/furfurylamine benzoxazine and polydimethylsiloxane oligomers. ACS Agric. Sci. Technol. 2021, 9, 16637–16650. [Google Scholar] [CrossRef]
- Zhan, Z.M.; Yan, H.Q.; Yin, P.; Cheng, J.; Fang, Z.P. Synthesis and properties of a novel bio-based benzoxazine resin with excellent low-temperature curing ability. Polym. Int. 2019, 69, 355–362. [Google Scholar] [CrossRef]
- Dai, J.; Teng, N.; Peng, Y.; Liu, Y.; Cao, L.; Zhu, J.; Liu, X. Biobased benzoxazine derived from daidzein and furfurylamine: Microwave-assisted synthesis and thermal properties investigation. ChemSusChem 2018, 11, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Zeng, M.; Feng, Z.; Chen, J.; Huang, Y.; Xu, Q. A facile method for the preparation of furfurylamine based benzoxazine resin with high-frequency low dielectric constants and ultra-low dielectric losses. J. Mater. Sci. Mater. Electron. 2019, 30, 8358–8370. [Google Scholar] [CrossRef]
- Yang, R.; Han, M.; Hao, B.; Zhang, K. Biobased high-performance tri-furan functional bis-benzoxazine resin derived from renewable guaiacol, furfural and furfurylamine. Eur. Polym. J. 2020, 131, 109706. [Google Scholar] [CrossRef]
- Wang, C.F.; Sun, J.Q.; Liu, X.D.; Sudo, A.; Endo, T. Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine. Green Chem. 2012, 14, 2799–2806. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.H.; Lin, C.H.; Juang, T.Y. High-performance bio-based benzoxazines derived from phosphinated biphenols and furfurylamine. Eur. Polym. J. 2018, 108, 48–56. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. The influence of acid-treated multi-walled carbon nanotubes on the surface morphology and thermal properties of alanine-based poly (amide-imide)/MWCNT nanocomposites system. Colloid Polym. Sci. 2015, 293, 333–339. [Google Scholar] [CrossRef]
- Lion, R.E.; Janssens, M.L. Polymer Flammability; DOT/FAA/AR-05/14; Office of Aviation Research: Washington, DC, USA, 2005. [Google Scholar]
- Banks, B.A.; Rutledge, S.K.; Brady, J.A.; Merrow, J.E. Atomic oxygen effects on materials (N89-23540). In NASA SDIO Space Environmental Effects on Materials Workshop, Part 1; Langley Research Center: Hampton, VA, USA, 1989. [Google Scholar]



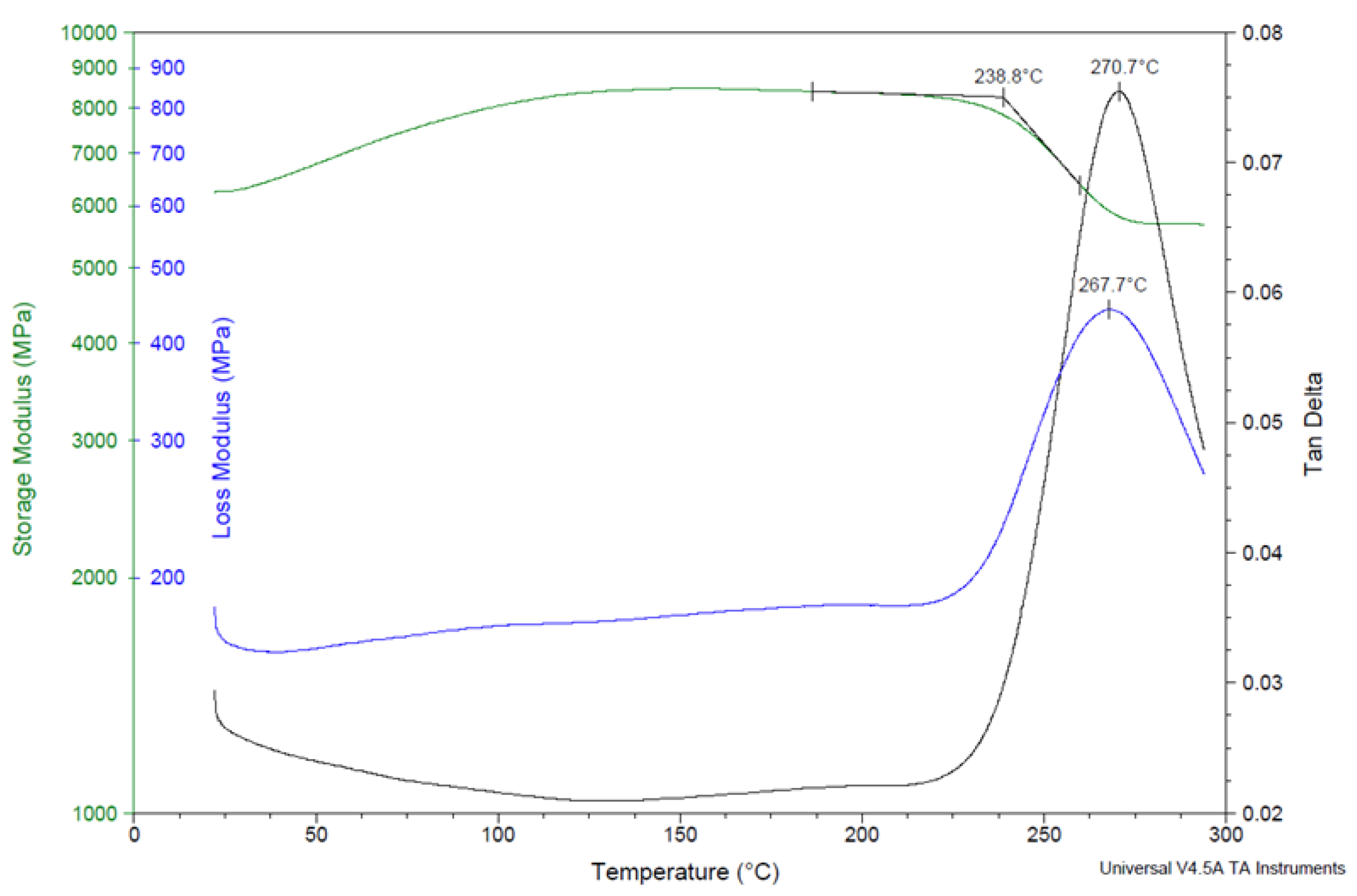
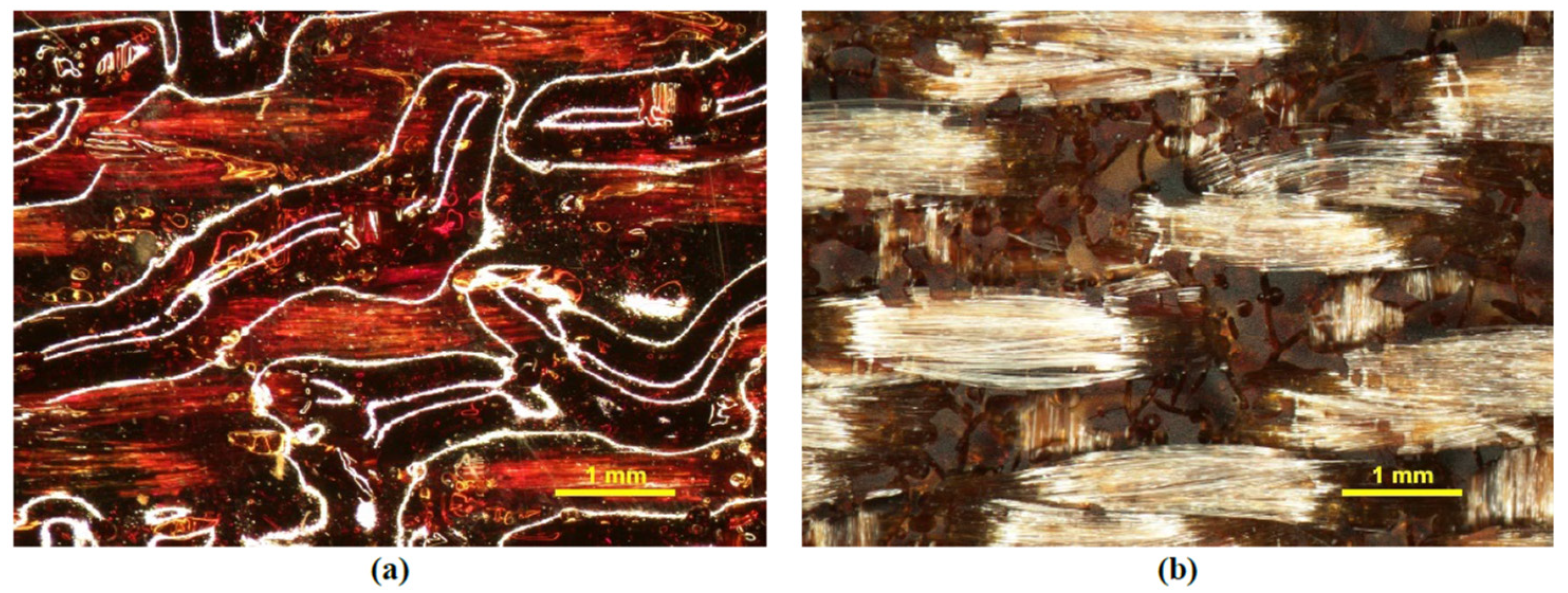
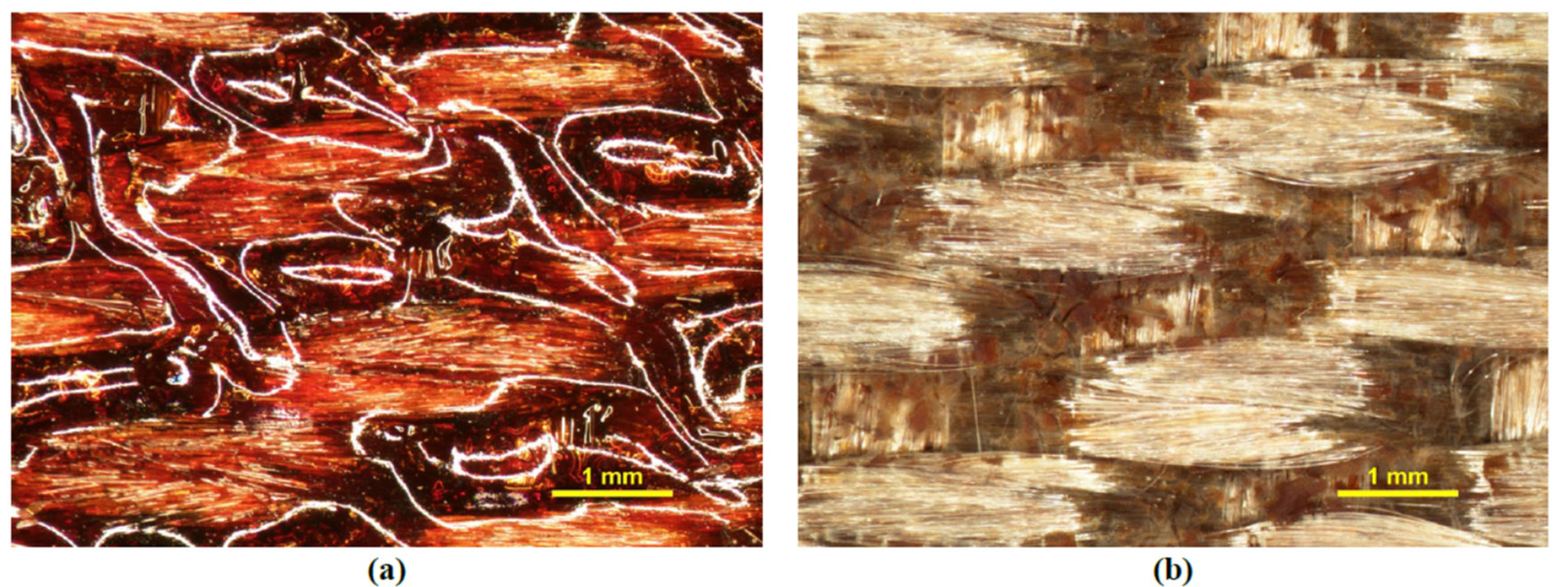
| Sample | Cure Schedule | Post-Cure Heat Treatment | E’max | Glass Transition Temperature | ||
|---|---|---|---|---|---|---|
| DMA Tg | E” Peak | Tan δ Peak | ||||
| Nextel 312/ poly(BAF-oda-fu) composite, no heat treatment | 2 h at 200 °C | None | 8470 MPa (at 153 °C) | 239 °C | 268 °C | 271 °C |
| Nextel 312/ poly(BAF-oda-fu) composite, heat treated | 2 h at 200 °C | 30 min at 240 °C | 10670 MPa (at 261 °C) | 273 °C | 299 °C | 310 °C |
| Nextel 312/ poly(BAF-oda-fu-POSS) composite, heat treated | 2 h at 200 °C | 30 min at 240 °C | 12550 MPa (at 254 °C) | 308 °C | 336 °C | Peak not observed |
| Material | Sample ID | Density (g/cm3) | Surface Area (cm2) | Mass Loss (g) | Effective Fluence (atoms/cm2 × 1021) | Ground-Based Atomic Oxygen Erosion Yield (cm3/atom × 10−24) |
|---|---|---|---|---|---|---|
| Nextel 312/poly(BAF-oda-fu) composite, heat treated | A1 | 1.61 | 8.64 | 0.1353 | 2.20 | 4.43 |
| A2 | 1.68 | 8.85 | 0.1440 | 2.30 | 4.20 | |
| Nextel 312/poly(BAF-oda-fu-POSS) composite, heat treated | B1 | 1.64 | 8.73 | 0.1120 | 1.99 | 3.93 |
| B2 | 1.65 | 8.79 | 0.0923 | 1.57 | 4.06 |
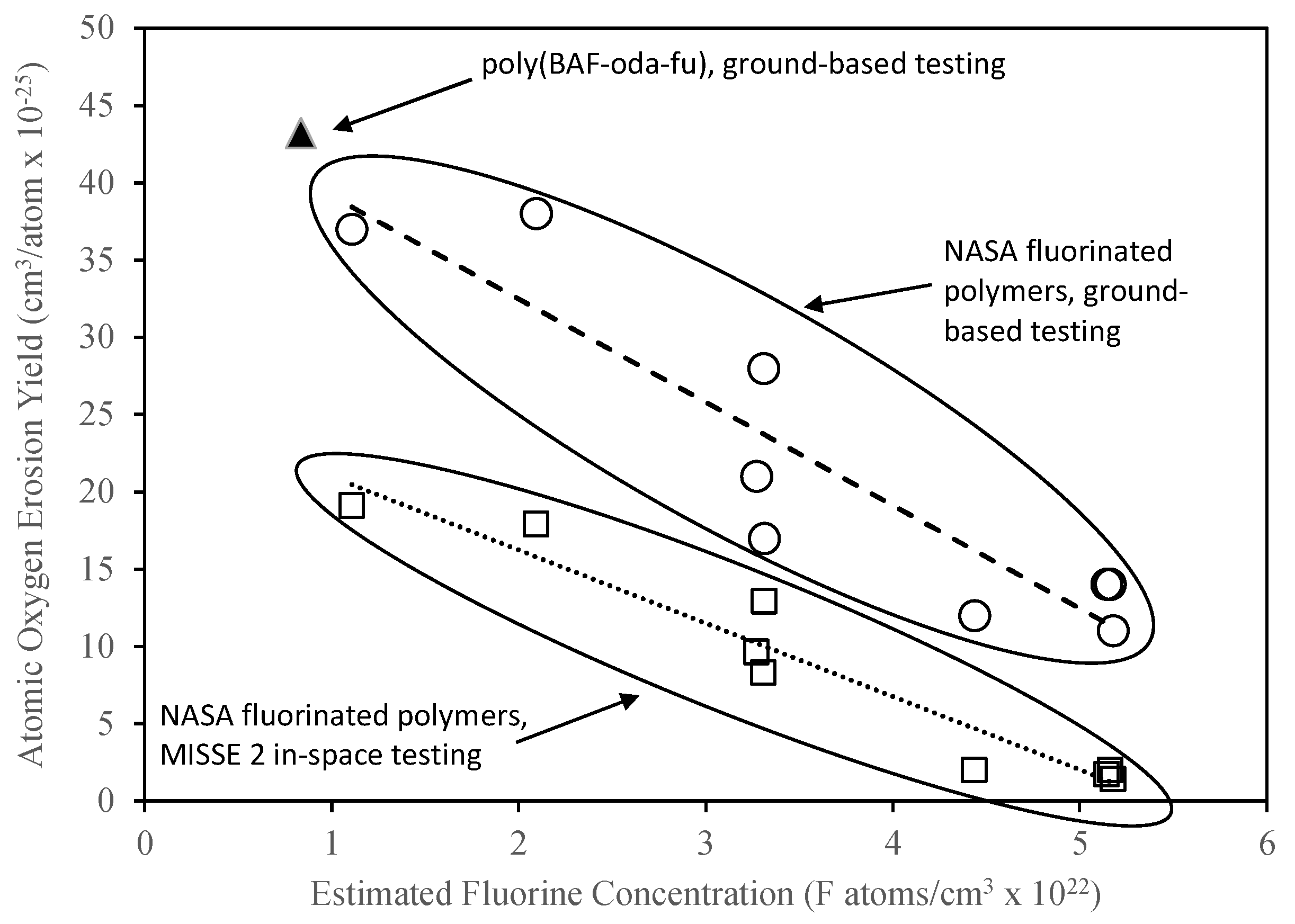
| Polymer Type | Repeat Unit | Molar Mass (g/mol) | Density (g/cm3) | Atomic Oxygen Erosion Yield (cm3/atom × 10−25) | Estimated Fluorine Concentration (F atoms/cm3 × 1022) | |
|---|---|---|---|---|---|---|
| Ground-Based RF Plasma Asher | In-Space MISSE 2 | |||||
| Poly(BAF-oda-fu) | C62H54F12N4O7 | 1195.09 | 1.38 | 43.2* | Not Tested | 0.83 |
| Polyimide (fluorinated) | C46H22O6N2F12 | 926.66 | 1.42 | 37.0 | 19.1 | 1.11 |
| Ethylene-chlorotrifluoroethylene | C4H4ClF3 | 144.52 | 1.68 | 38.0 | 17.9 | 2.10 |
| Ethylene-tetrafluoroethylene | C4H4F4 | 128.07 | 1.74 | 21.0 | 9.6 | 3.27 |
| Chlorotrifluoroethylene | C2ClF3 | 116.47 | 2.13 | 28.0 | 8.3 | 3.31 |
| Polyvinylidene fluoride | C2H2F2 | 64.03 | 1.76 | 17.0 | 12.9 | 3.31 |
| Amorphous fluoropolymer | C19O6F32 | 932.15 | 2.15 | 12.0 | 2.0 | 4.44 |
| Perfluoroalkoxy copolymer resin | C203OF406 | 10167.52 | 2.14 | 14.0 | 1.7 | 5.14 |
| Fluorinated ethylene propylene | C11F22 | 550.08 | 2.14 | 14.0 | 2.0 | 5.16 |
| Polytetrafluoroethylene | C2F4 | 100.02 | 2.15 | 11.0 | 1.4 | 5.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oppenheimer, L.; Ramkumar, M.; Machado, I.; Scott, C.; Winroth, S.; Ishida, H. Development of an Atomic-Oxygen-Erosion-Resistant, Alumina-Fiber-Reinforced, Fluorinated Polybenzoxazine Composite for Low-Earth Orbital Applications. Polymers 2023, 15, 112. https://doi.org/10.3390/polym15010112
Oppenheimer L, Ramkumar M, Machado I, Scott C, Winroth S, Ishida H. Development of an Atomic-Oxygen-Erosion-Resistant, Alumina-Fiber-Reinforced, Fluorinated Polybenzoxazine Composite for Low-Earth Orbital Applications. Polymers. 2023; 15(1):112. https://doi.org/10.3390/polym15010112
Chicago/Turabian StyleOppenheimer, Leah, Malavika Ramkumar, Irlaine Machado, Chris Scott, Scott Winroth, and Hatsuo Ishida. 2023. "Development of an Atomic-Oxygen-Erosion-Resistant, Alumina-Fiber-Reinforced, Fluorinated Polybenzoxazine Composite for Low-Earth Orbital Applications" Polymers 15, no. 1: 112. https://doi.org/10.3390/polym15010112
APA StyleOppenheimer, L., Ramkumar, M., Machado, I., Scott, C., Winroth, S., & Ishida, H. (2023). Development of an Atomic-Oxygen-Erosion-Resistant, Alumina-Fiber-Reinforced, Fluorinated Polybenzoxazine Composite for Low-Earth Orbital Applications. Polymers, 15(1), 112. https://doi.org/10.3390/polym15010112









