A Preliminary Investigation of Embedding In Vitro HepaRG Spheroids into Recombinant Human Collagen Type I for the Promotion of Liver Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Maintenance
2.2. Spheroid Culture
2.3. Embedding of Spheroids into Collagen
2.4. Live/Dead Staining
2.5. Immunofluorescence
2.6. qPCR
2.7. Statistical Analysis
3. Results
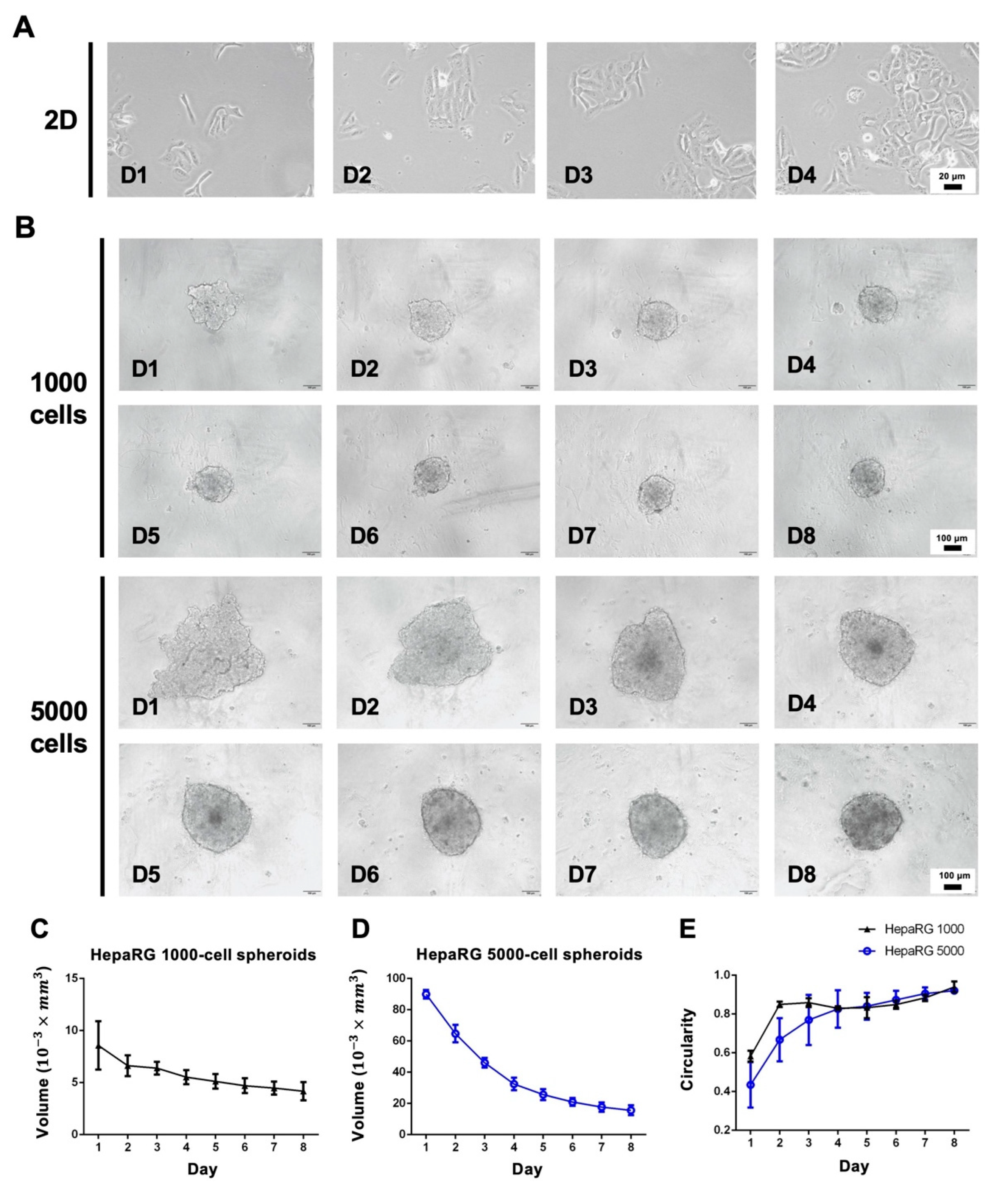
3.1. Morphology Assessment of 2D and 3D Cultures
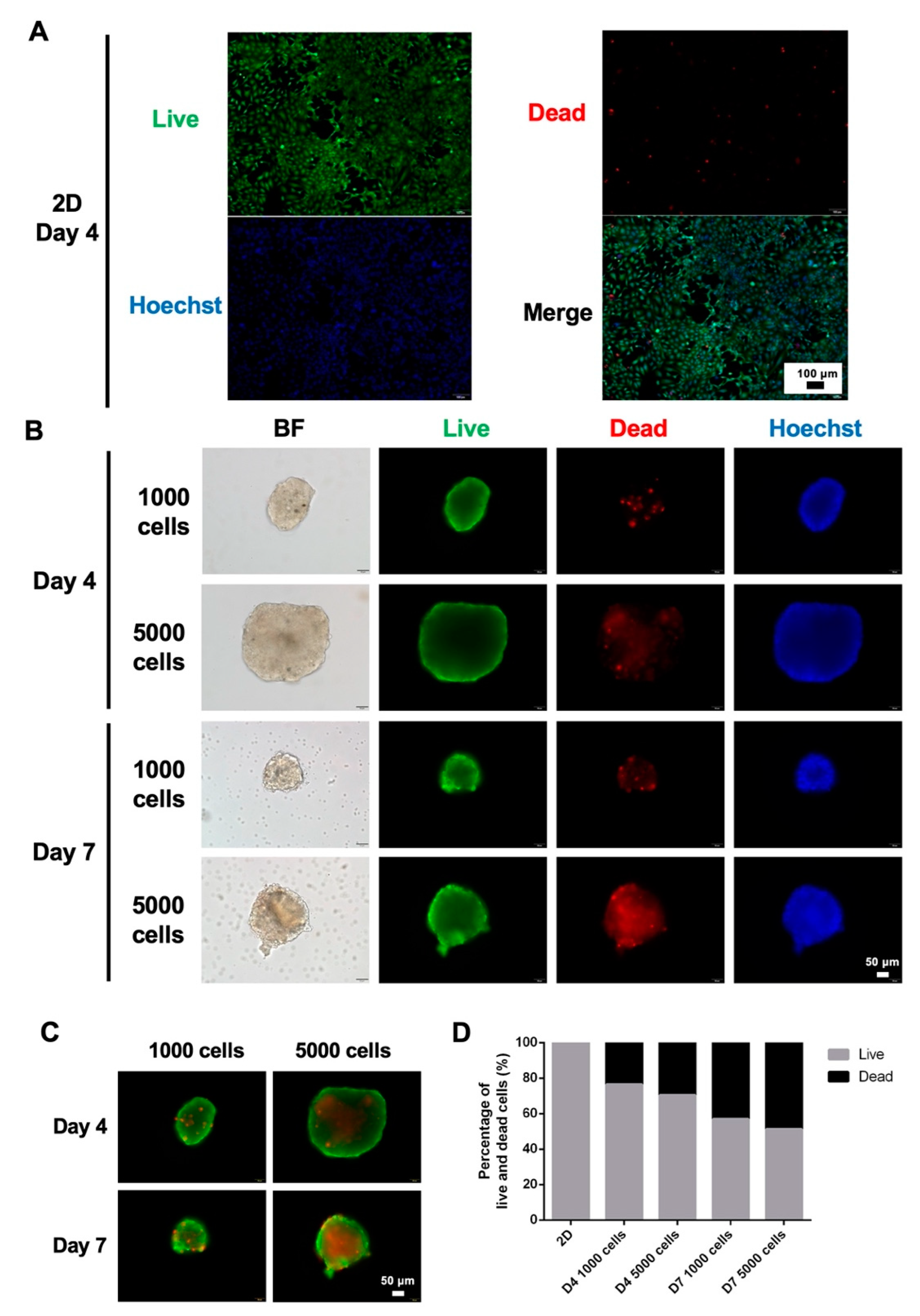
3.2. Viability of 2D and 3D Cultures
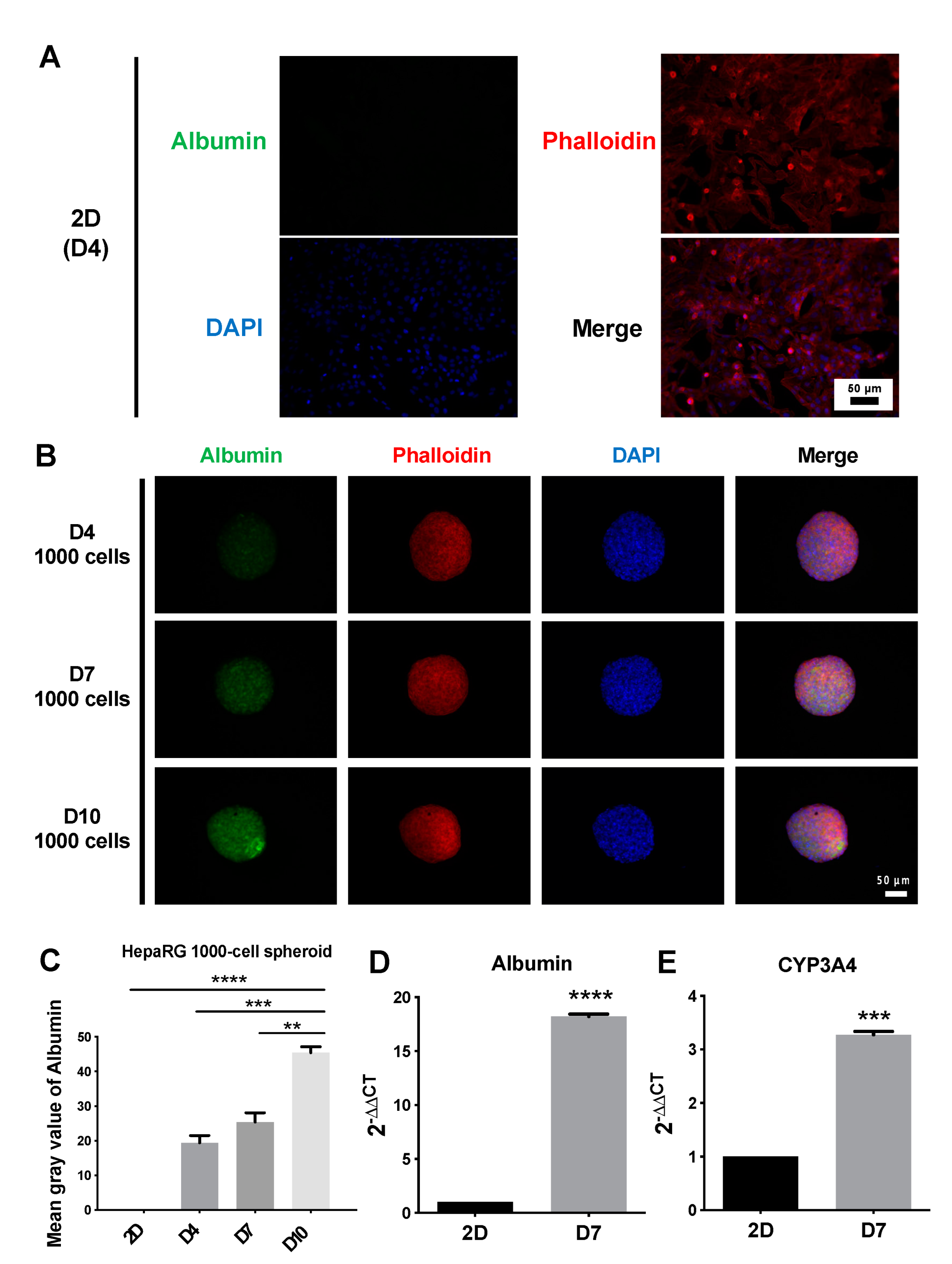
3.3. Structure and Functional Validation of 2D and 3D Cultures
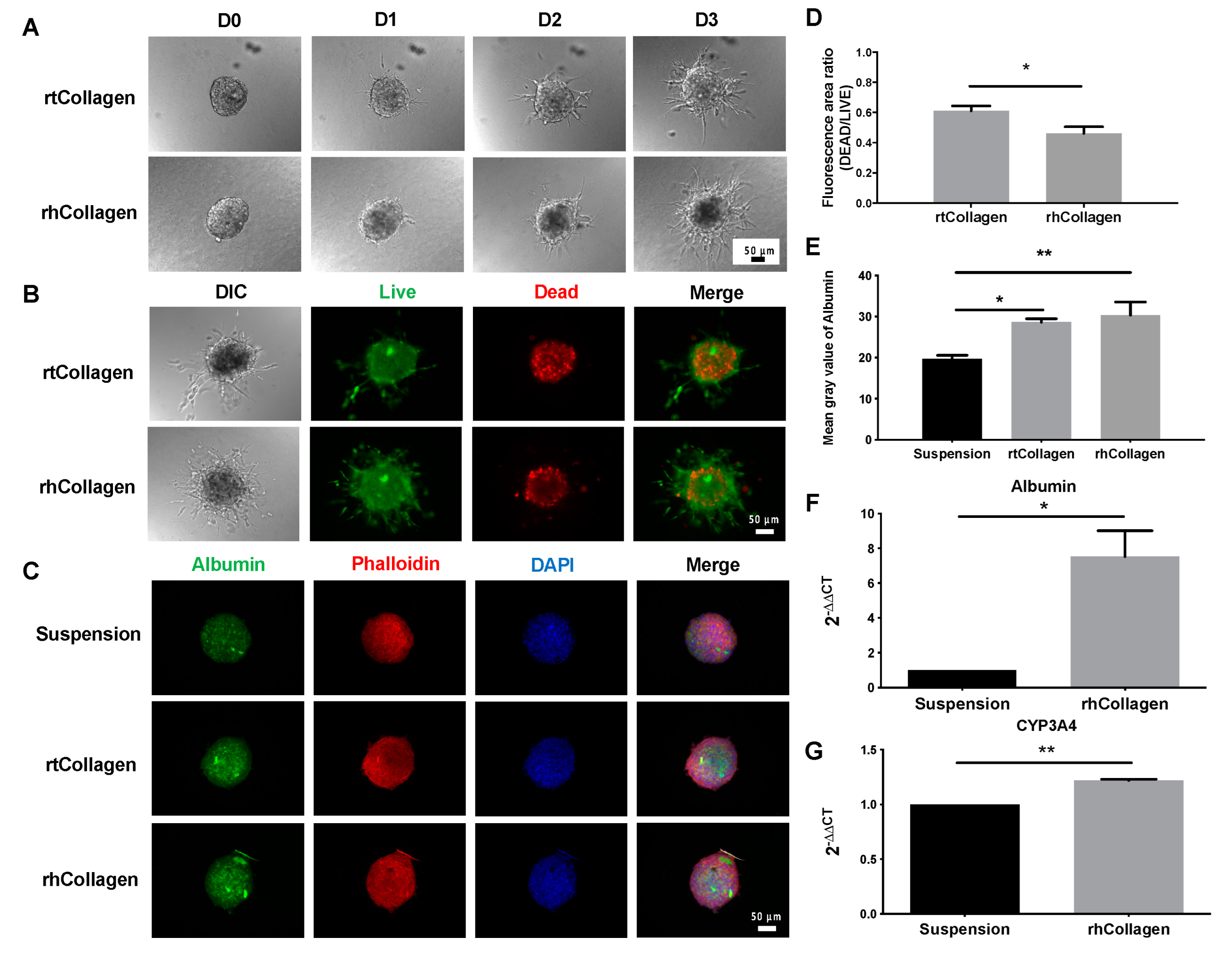
3.4. Function of Collagen I in 3D Spheroid Cultures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, S.D.; Yuen, G.; Tu, T.; Budzinska, M.A.; Spring, K.; Bryant, K.; Shackel, N.A. In Vitro Models of the Liver: Disease Modeling, Drug Discovery and Clinical Applications. In Hepatocellular Carcinoma [Internet]; Tirnitz-Parker, J.E.E., Ed.; Codon Publications: Brisbane, Australia, 2019; Chapter 3. [Google Scholar]
- Lin, R.Z.; Chang, H.Y. Recent Advances in Three-Dimensional Multicellular Spheroid Culture for Biomedical Research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chen, Y.C.; Wu, K.W.; Hsu, W.C.; Lin, H.P.; Chang, H.C.; Lee, Y.C.; Wang, Y.K.; Tu, T.Y. Simple In-House Fabrication of Microwells for Generating Uniform Hepatic Multicellular Cancer Aggregates and Discovering Novel Therapeutics. Materials 2019, 12, 3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Kuo, C.; Tu, T. A Highly Reproducible Micro U-Well Array Plate Facilitating High-Throughput Tumor Spheroid Culture and Drug Assessment. Glob. Chall. 2021, 5, 2000056. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chen, M.C.; Wu, K.W.; Tu, T.Y. A Facile Approach for Rapid Prototyping of Microneedle Molds, Microwells and Micro-through-Holes in Various Substrate Materials Using CO2 Laser Drilling. Biomedicines 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Kuo, Y.-T.; Tsao, C.-H.; Shyong, Y.-J.; Shih, S.-H.; Tu, T.-Y. Development of an In Vitro 3D Model for Investigating Ligamentum Flavum Hypertrophy. Biol. Proced. Online 2020, 22, 20. [Google Scholar] [CrossRef]
- Bierwolf, J.; Volz, T.; Lütgehetmann, M.; Allweiss, L.; Riecken, K.; Warlich, M.; Fehse, B.; Kalff, J.C.; Dandri, M.; Pollok, J.-M. Primary Human Hepatocytes Repopulate Livers of Mice After In Vitro Culturing and Lentiviral-Mediated Gene Transfer. Tissue Eng. Part A 2016, 22, 742–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Antona, C.; Donato, M.T.; Boobis, A.; Edwards, R.J.; Watts, P.S.; Castell, J.V.; Gómez-Lechón, M.J. Cytochrome P450 Expression in Human Hepatocytes and Hepatoma Cell Lines: Molecular Mechanisms That Determine Lower Expression in Cultured Cells. Xenobiotica 2002, 32, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.J.; Lechón, M.J.G.; Houston, J.B.; Hallifax, D.; Brown, H.S.; Maurel, P.; Kenna, J.G.; Gustavsson, L.; Lohmann, C.; Skonberg, C.; et al. Primary Hepatocytes: Current Understanding of the Regulation of Metabolic Enzymes and Transporter Proteins, and Pharmaceutical Practice for the Use of Hepatocytes in Metabolism, Enzyme Induction, Transporter, Clearance, and Hepatotoxicity Studies. Drug Metab. Rev. 2007, 39, 159–234. [Google Scholar] [CrossRef]
- Gunness, P.; Mueller, D.; Shevchenko, V.; Heinzle, E.; Ingelman-Sundberg, M.; Noor, F. 3D Organotypic Cultures of Human Heparg Cells: A Tool for in Vitro Toxicity Studies. Toxicol. Sci. 2013, 133, 67–78. [Google Scholar] [CrossRef]
- Guillouzo, A.; Corlu, A.; Aninat, C.; Glaise, D.; Morel, F.; Guguen-Guillouzo, C. The Human Hepatoma HepaRG Cells: A Highly Differentiated Model for Studies of Liver Metabolism and Toxicity of Xenobiotics. Chem.-Biol. Interact. 2007, 168, 66–73. [Google Scholar] [CrossRef]
- Kanebratt, K.P.; Andersson, T.B. Evaluation of HepaRG Cells as an In Vitro Model for Human Drug Metabolism Studies. Drug Metab. Dispos. 2008, 36, 1444–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaiahgari, S.C.; Waidyanatha, S.; Dixon, D.; DeVito, M.J.; Paules, R.S.; Ferguson, S.S. Three-Dimensional (3D) HepaRG Spheroid Model with Physiologically Relevant Xenobiotic Metabolism Competence and Hepatocyte Functionality for Liver Toxicity Screening. Toxicol. Sci. 2017, 159, 124–136. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Asghar, M.W.; Rong, Y.; Doschak, M.R.; Kiang, T.K.L. Advanced In Vitro HepaRG Culture Systems for Xenobiotic Metabolism and Toxicity Characterization. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Zhou, X.; Han, J.; Huang, C.Y.; Nashed, A.; Khatri, S.; Mattson, G.; Fukunishi, T.; Zhang, H.; Hibino, N. In Vivo Therapeutic Applications of Cell Spheroids. Biotechnol. Adv. 2018, 36, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.S.; Wong, J.Y.; Gupta, K.; Yu, H.; Ananthanarayanan, A.; Nugraha, B.; Liu, Z.; Huang, X.; Lee, F.; Sun, M. Cleavable Cellulosic Sponge for Functional Hepatic Cell Culture and Retrieval. Biomaterials 2019, 201, 16–32. [Google Scholar] [CrossRef]
- Bedossa, P.; Paradis, V. Liver Extracellular Matrix in Health and Disease. J. Pathol. 2003, 200, 504–515. [Google Scholar] [CrossRef]
- Matsui, H.; Takeuchi, S.; Osada, T.; Fujii, T.; Sakai, Y. Enhanced Bile Canaliculi Formation Enabling Direct Recovery of Biliary Metabolites of Hepatocytes in 3D Collagen Gel Microcavities. Lab Chip 2012, 12, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Hendriks, D.F.G.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Guo, C.; Woodhead, J.L.; St Claire, R.L.; Watkins, P.B.; Siler, S.Q.; Howell, B.A.; Brouwer, K.L.R. Sandwich-Cultured Hepatocytes as a Tool to Study Drug Disposition and Drug-Induced Liver Injury. J. Pharm. Sci. 2016, 105, 443–459. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Cuvellier, M.; Ezan, F.; Carteret, J.; Bruyère, A.; Legagneux, V.; Nesslany, F.; Baffet, G.; Langouët, S. DMSO-Free Highly Differentiated HepaRG Spheroids for Chronic Toxicity, Liver Functions and Genotoxicity Studies. Arch. Toxicol. 2022, 96, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Ezan, F.; Cuvellier, M.; Bruyère, A.; Legagneux, V.; Langouët, S.; Baffet, G. Generation of Proliferating Human Adult Hepatocytes Using Optimized 3D Culture Conditions. Sci. Rep. 2021, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, F.; Singh, N.H.; Tan, E.K.F.; Xing, J.; Li, H.; Hissette, S.; Manesh, S.; Fulwood, J.; Gupta, K.; Ng, C.W.; et al. Tethered Primary Hepatocyte Spheroids on Polystyrene Multi-Well Plates for High-Throughput Drug Safety Testing. Sci. Rep. 2020, 10, 4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, P.L.; Su, J.; Yan, M.; Meng, F.; Glaser, S.S.; Alpini, G.D.; Green, R.M.; Sosa-Pineda, B.; Shah, R.N. Complex Bile Duct Network Formation within Liver Decellularized Extracellular Matrix Hydrogels. Sci. Rep. 2018, 8, 12220. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, H.; Xu, Y.; Gao, Y.; Tan, P.; Zhao, R.; Liu, Z.; Tang, Y.; Zhu, X.; Bao, C.; et al. The Biological Effect of Recombinant Humanized Collagen on Damaged Skin Induced by UV-Photoaging: An in Vivo Study. Bioact. Mater. 2022, 11, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Win Naing, M. Production of Recombinant Collagen: State of the Art and Challenges. Eng. Biol. 2017, 1, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Rojkind, M.; Giambrone, M.A.; Biempica, L. Collagen Types in Normal and Cirrhotic Liver. Gastroenterology 1979, 76, 710–719. [Google Scholar] [CrossRef]
- Asthana, A.; Kisaalita, W.S. Microtissue Size and Hypoxia in HTS with 3D Cultures. Drug Discov. Today 2012, 17, 810–817. [Google Scholar] [CrossRef]
- Hercog, K.; Štampar, M.; Štern, A.; Filipič, M.; Žegura, B. Application of Advanced HepG2 3D Cell Model for Studying Genotoxic Activity of Cyanobacterial Toxin Cylindrospermopsin. Environ. Pollut. 2020, 265, 114965. [Google Scholar] [CrossRef] [PubMed]
- Kessel, S.; Cribbes, S.; Bonasu, S.; Rice, W.; Qiu, J.; Chan, L.L.Y. Real-Time Viability and Apoptosis Kinetic Detection Method of 3D Multicellular Tumor Spheroids Using the Celigo Image Cytometer. Cytom. Part A 2017, 91, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Tzanakakis, E.S.; Hansen, L.K.; Hu, W.S. The Role of Actin Filaments and Microtubules in Hepatocyte Spheroid Self-Assembly. Cell Motil. Cytoskelet. 2001, 46, 175–189. [Google Scholar] [CrossRef]
- Anthérieu, S.; Chesné, C.; Li, R.; Camus, S.; Lahoz, A.; Picazo, L.; Turpeinen, M.; Tolonen, A.; Uusitalo, J.; Guguen-Guillouzo, C.; et al. Stable expression, activity and inducibility of cytochromes p450 in differentiated heparg cells. Drug Metab. Dispos. 2010, 38, 516–525. [Google Scholar] [CrossRef]
- Pasqua, M.; Pereira, U.; Messina, A.; De Lartigue, C.; Vigneron, P.; Dubart-Kupperschmitt, A.; Legallais, C. HepaRG Self-Assembled Spheroids in Alginate Beads Meet the Clinical Needs for Bioartificial Liver. Tissue Eng.-Part A 2020, 26, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Chang, L.-C.; Su, G.-L.; Chang, P.-Y.; Hsu, H.-F.; Lee, C.-L.; Li, J.-R.; Liao, M.-C.; Thangudu, S.; Treekoon, J.; et al. Chemical Structure and Shape Enhance MR Imaging-Guided X-Ray Therapy Following Marginative Delivery. ACS Appl. Mater. Interfaces 2022, 14, 13056–13069. [Google Scholar] [CrossRef]
- Tu, T.-Y.; Shen, Y.-P.; Lim, S.-H.; Wang, Y.-K. A Facile Method for Generating a Smooth and Tubular Vessel Lumen Using a Viscous Fingering Pattern in a Microfluidic Device. Front. Bioeng. Biotechnol. 2022, 10, 877480. [Google Scholar] [CrossRef]
- Tu, T.-Y.; Wang, Z.; Bai, J.; Sun, W.; Peng, W.K.; Huang, R.Y.; Thiery, J.P.; Kamm, R.D. Rapid prototyping of concave microwells for the formation of 3D multicellular cancer aggregates for drug screening. Adv. Healthc. Mater. 2014, 3, 609–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, F.-C.; Wang, Y.-K.; Cheng, M.-Y.; Tu, T.-Y. A Preliminary Investigation of Embedding In Vitro HepaRG Spheroids into Recombinant Human Collagen Type I for the Promotion of Liver Differentiation. Polymers 2022, 14, 1923. https://doi.org/10.3390/polym14091923
Liao F-C, Wang Y-K, Cheng M-Y, Tu T-Y. A Preliminary Investigation of Embedding In Vitro HepaRG Spheroids into Recombinant Human Collagen Type I for the Promotion of Liver Differentiation. Polymers. 2022; 14(9):1923. https://doi.org/10.3390/polym14091923
Chicago/Turabian StyleLiao, Fang-Chun, Yang-Kao Wang, Ming-Yang Cheng, and Ting-Yuan Tu. 2022. "A Preliminary Investigation of Embedding In Vitro HepaRG Spheroids into Recombinant Human Collagen Type I for the Promotion of Liver Differentiation" Polymers 14, no. 9: 1923. https://doi.org/10.3390/polym14091923
APA StyleLiao, F.-C., Wang, Y.-K., Cheng, M.-Y., & Tu, T.-Y. (2022). A Preliminary Investigation of Embedding In Vitro HepaRG Spheroids into Recombinant Human Collagen Type I for the Promotion of Liver Differentiation. Polymers, 14(9), 1923. https://doi.org/10.3390/polym14091923






