Lornoxicam-Loaded Chitosan-Decorated Nanoemulsion: Preparation and In Vitro Evaluation for Enhanced Transdermal Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Formulation Studies
2.2.1. Solubility Studies
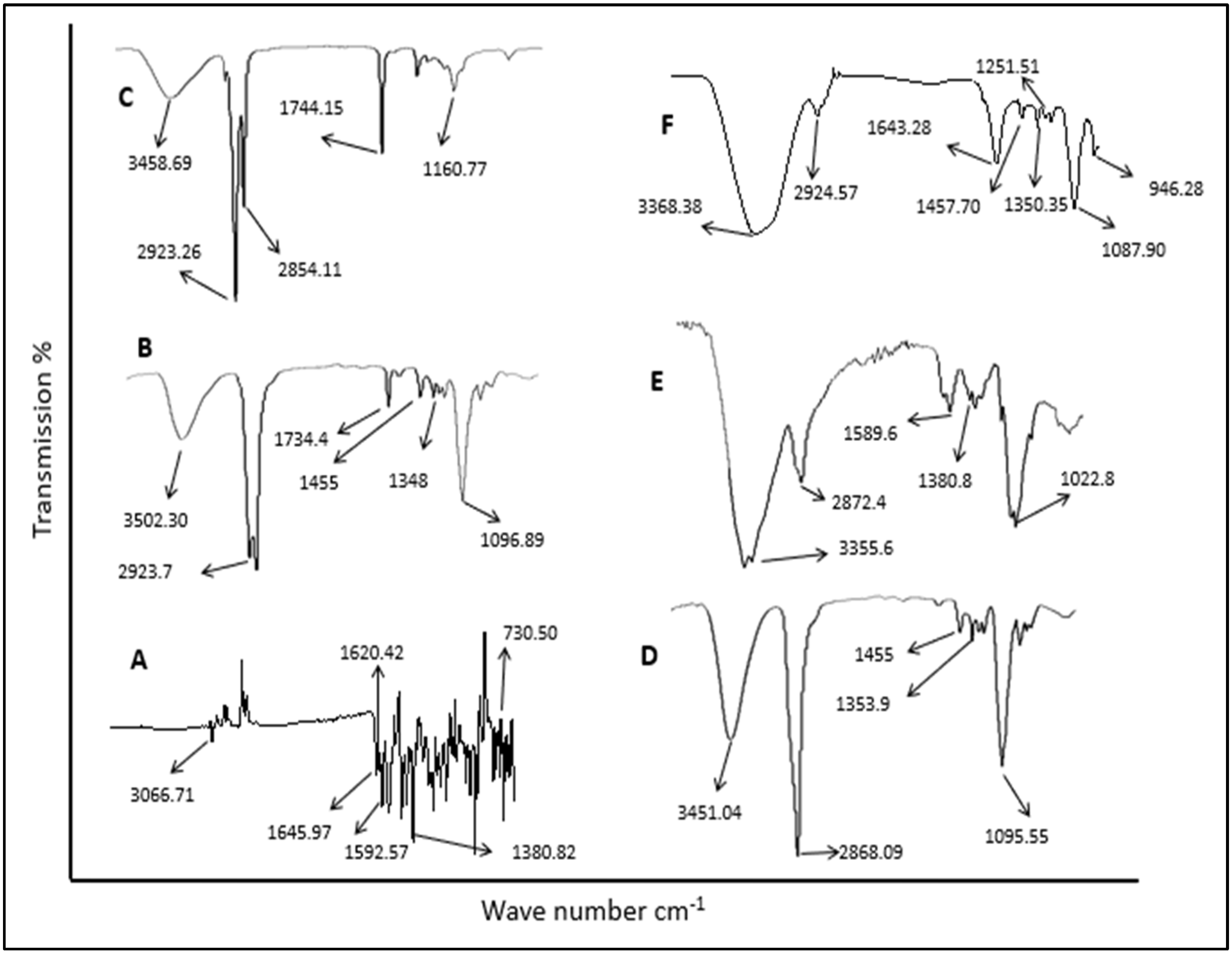
2.2.2. Drug Excipient Compatibility Study (ATR-FTIR Analysis)
2.3. Preparation of LRX-NE Formulations with and without Chitosan
2.4. Physico-Chemical Characterization of LRX-NE and C-LRX NE Formulations
2.4.1. Thermodynamic Stability Assessment of LRX-NE Formulations with and without Chitosan
- Heating–cooling cycle
- b.
- Centrifugation
- c.
- Freeze–thaw cycle
2.4.2. pH Measurements
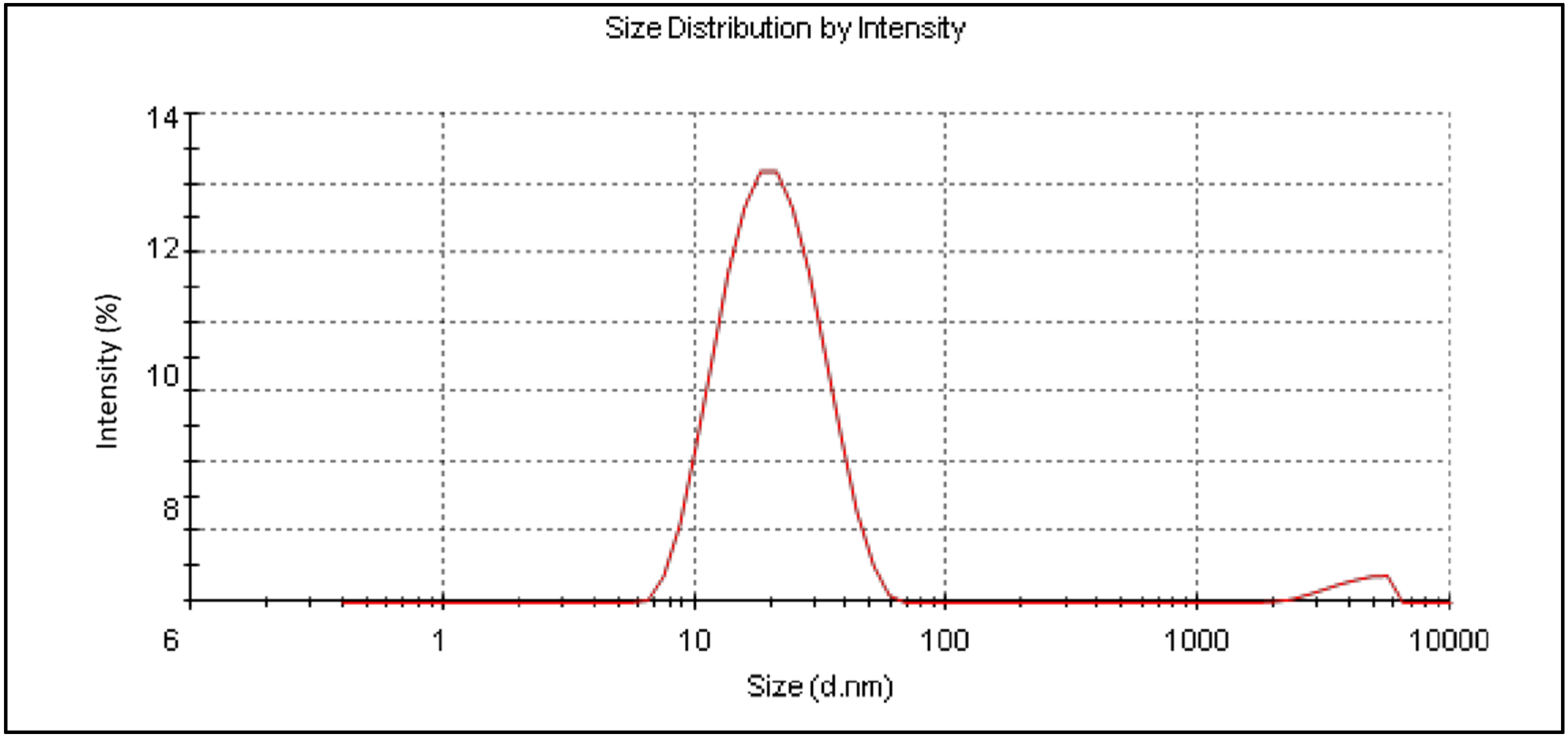
2.4.3. Globule Size Measurement
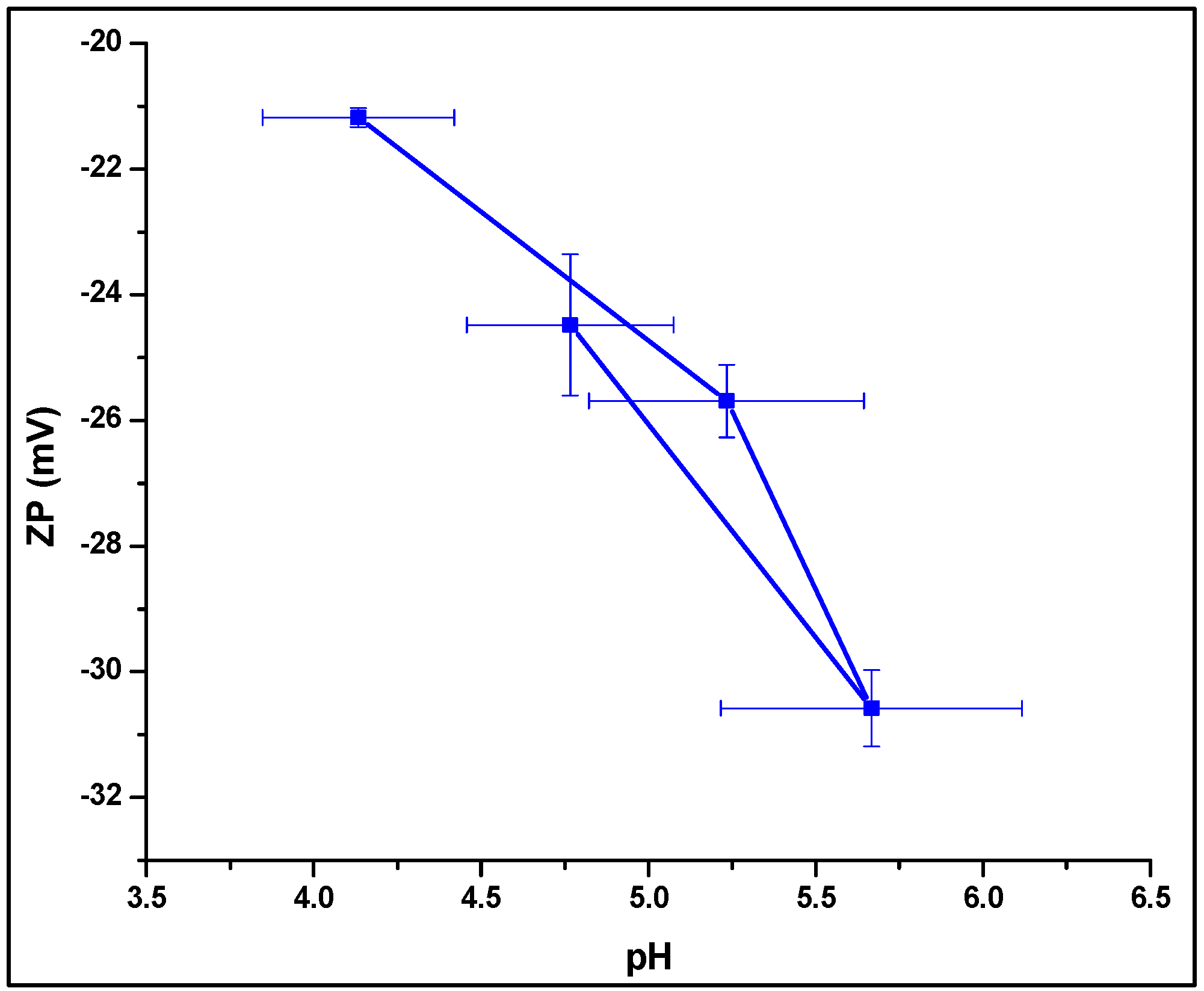
2.4.4. Zeta Potential Analysis
2.4.5. Drug Content and Drug Entrapment Efficiency
2.4.6. Viscosity and Surface Tension Measurement
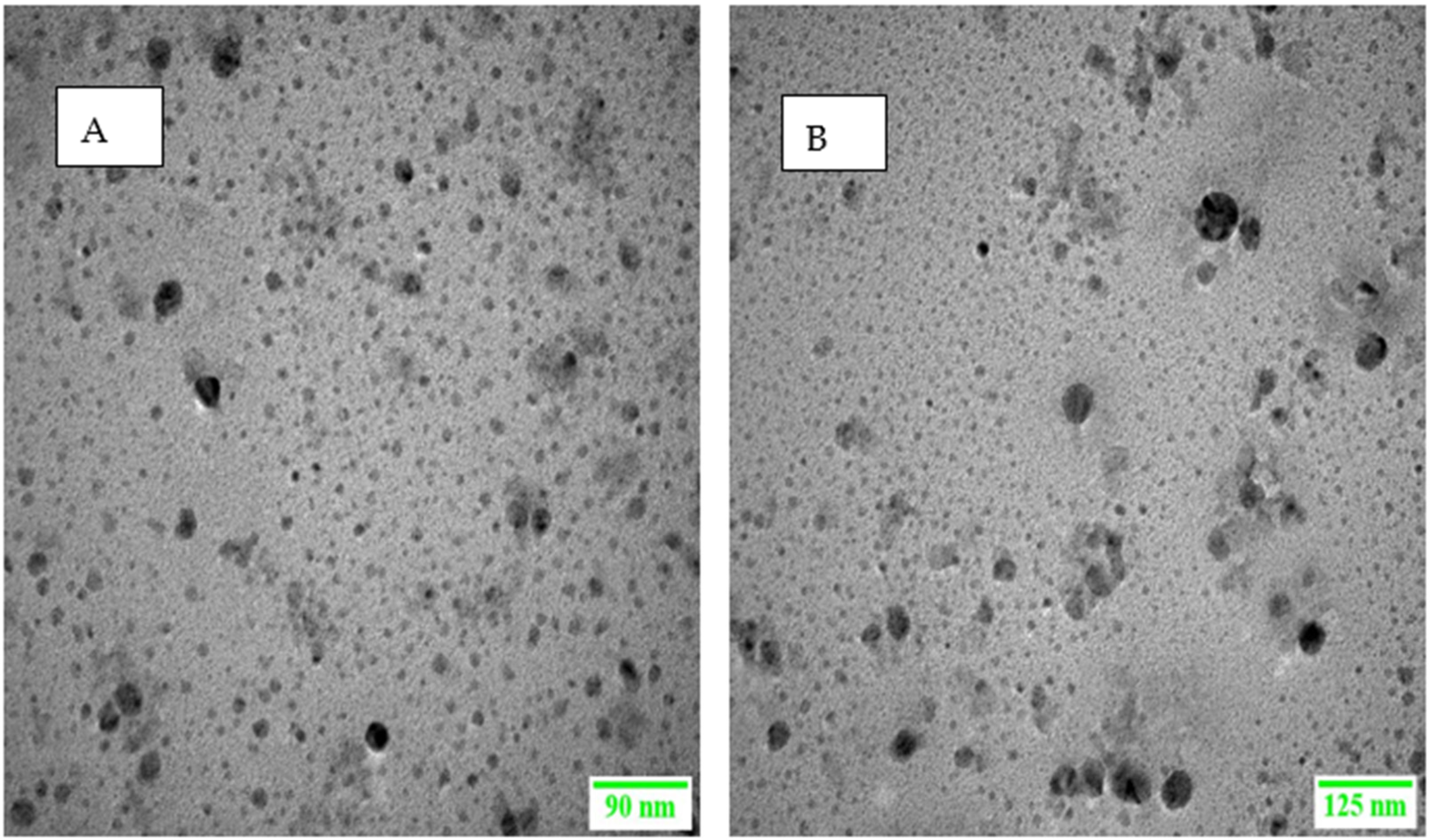
2.4.7. Surface Morphology
2.4.8. In Vitro Drug Release and Kinetic Model Profiling
2.4.9. Permeation Studies
2.4.10. Permeation Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Solubility Study
3.2. ATR-FTIR Analysis
3.3. Preparation of LRX-NE Formulations with and without Chitosan
3.4. Physicochemical Characterization of LRX-NE with and without Chitosan
3.4.1. Thermodynamic Stability
3.4.2. Surface Morphology Analysis
3.4.3. Globule Size Measurement
3.4.4. Zeta Potential Analysis
3.4.5. Viscosity and Surface Tension Measurement
3.4.6. pH Measurement
3.4.7. Drug Entrapment Efficiency
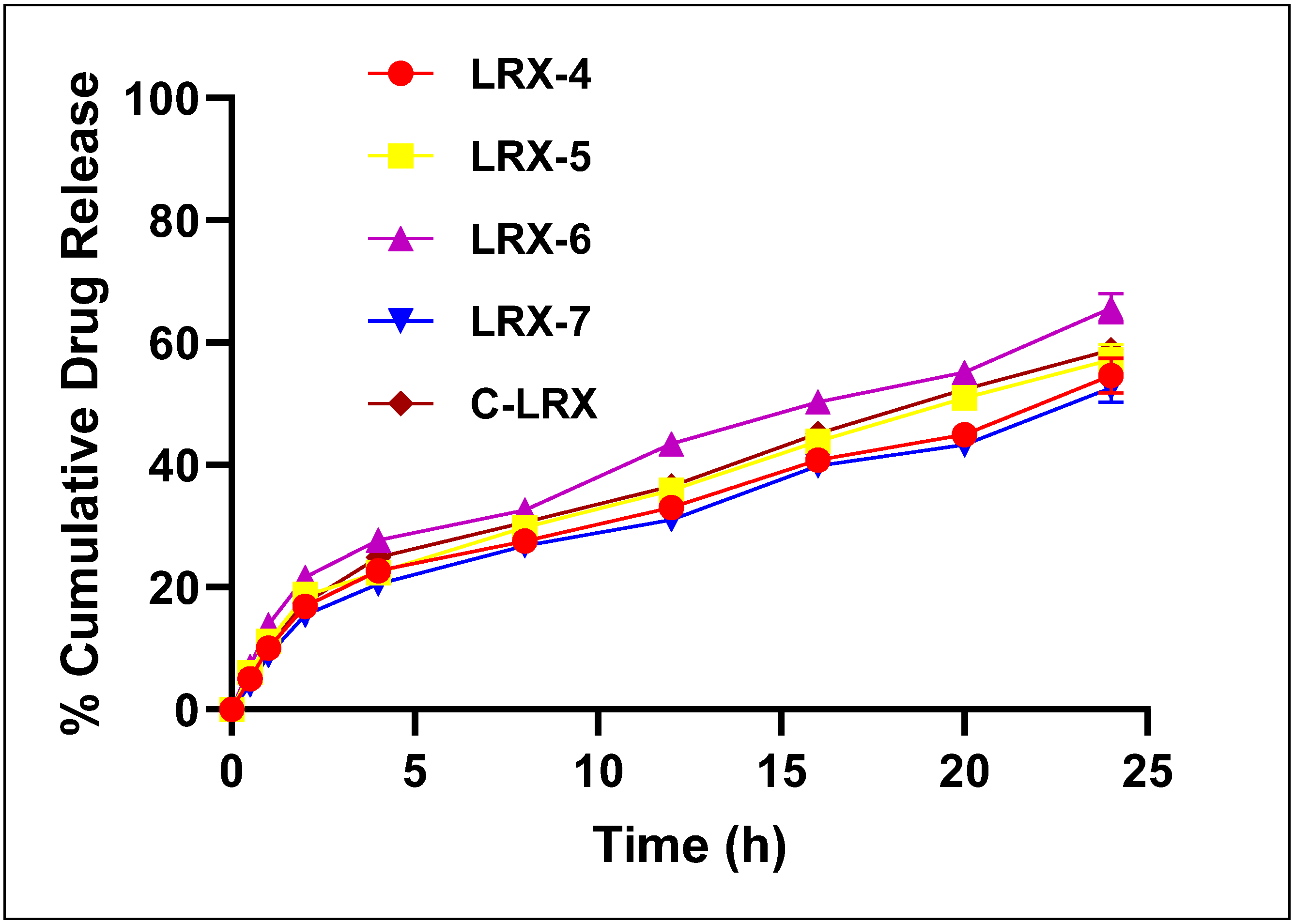
3.4.8. In Vitro Drug Release
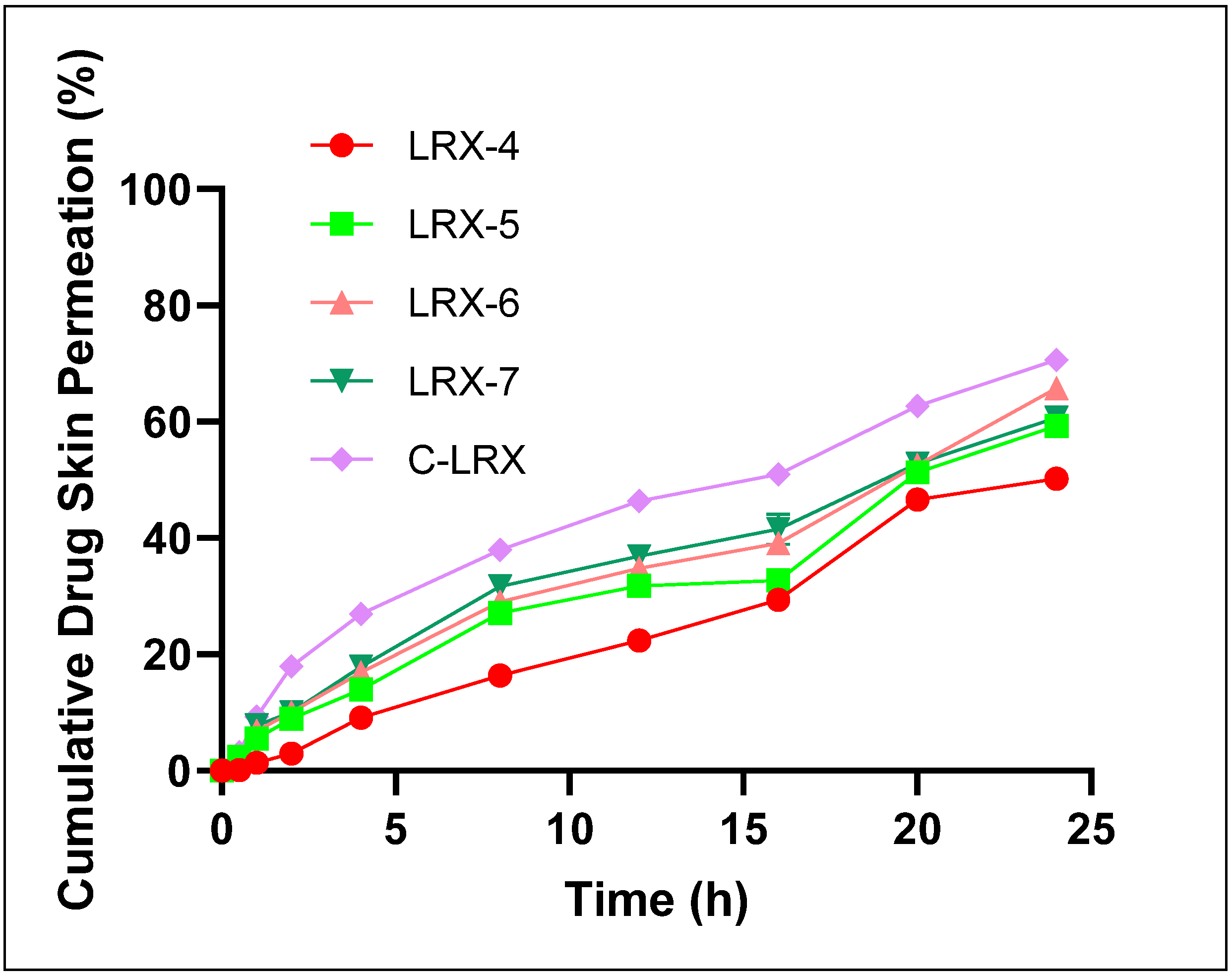
3.4.9. Ex Vivo Permeation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salama, A.; El-Hashemy, H.A.; Darwish, A.B. Formulation and optimization of lornoxicam-loaded bilosomes using 23 full factorial design for the management of osteoarthritis in rats: Modulation of MAPK/Erk1 signaling pathway. J. Drug Deliv. Sci. Technol. 2022, 69, 103175. [Google Scholar] [CrossRef]
- Stoicea, N.; Fiorda-Diaz, J.; Joseph, N.; Shabsigh, M.; Arias-Morales, C.; Gonzalez-Zacarias, A.A.; Mavarez-Martinez, A.; Marjoribanks, S.; Bergese, S. Advanced Analgesic Drug Delivery and Nanobiotechnology. Drugs 2017, 77, 1069–1076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhowmik, D.; Pusupoleti, K.R.; Duraivel, S.; Sampath Kumar, K. Recent Approaches in Transdermal Drug Delivery System. Pharma Innov. 2013, 2, 99–108. Available online: www.thepharmajournal.com (accessed on 7 December 2021).
- Kaul, S.; Jain, N.; Nagaich, U. Ultra deformable vesicles for boosting transdermal delivery of 2-arylpropionic acid class drug for management of musculoskeletal pain. J. Pharm. Investig. 2022, 52, 217–231. [Google Scholar] [CrossRef]
- Kirkby, M.; Hutton, A.R.; Donnelly, R.F. Microneedle Mediated Transdermal Delivery of Protein, Peptide and Antibody Based Therapeutics: Current Status and Future Considerations. Pharm. Res. 2020, 37, 1–18. [Google Scholar] [CrossRef]
- Parada, L.; Marstein, J.P.; Danilov, A. Tolerability of the COX-1/COX-2 inhibitor lornoxicam in the treatment of acute and rheumatic pain. Pain Manag. 2016, 6, 445–454. [Google Scholar] [CrossRef]
- Hashmat, D.; Shoaib, M.H.; Ali, F.R.; Siddiqui, F. Lornoxicam controlled release transdermal gel patch: Design, characterization and optimization using co-solvents as penetration enhancers. PLoS ONE 2020, 15, e0228908. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Yang, C.; Ayaril, N.; Szekely, G. Solvent-Resistant Thin-Film Composite Membranes from Biomass-Derived Building Blocks: Chitosan and 2,5-Furandicarboxaldehyde. ACS Sustain. Chem. Eng. 2021, 10, 998–1007. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Talukder, M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Atkinson, J.P.; Maibach, H.I. Chemical Penetration Enhancers: Classification and Mode of Action; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–27. [Google Scholar]
- Lopes, L.B. Overcoming the Cutaneous Barrier with Microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef]
- Kalayanappa, S.; Goli, D. Design and in vitro evaluation of a novel sustained release double layered tablets of lornoxicam by using semi synthetic polymers. Indian J. Pharm. Educ. Res. 2015, 49, 281–291. [Google Scholar] [CrossRef]
- Emara, L.H.; Abdou, A.R.; El-Ashmawy, A.A.; Mursi, N.M. Preparation and evaluation of metronidazole sustained release floating tablets. Int. J. Pharm. Pharm. Sci. 2014, 6, 198–204. [Google Scholar]
- Dasgupta, S.; Ghosh, S.; Ray, S.; Kaurav, S.; Mazumder, B. In vitro & in vivo Studies on Lornoxicam Loaded Nanoemulsion Gels for Topical Application. Curr. Drug Deliv. 2014, 11, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S. Nanoemulsion Formulations for Tumor-Targeted Delivery. Nanotechnol. Cancer Ther. 2006, 723–739. [Google Scholar] [CrossRef]
- Rashid, S.A.; Bashir, S.; Ullah, H.; Khan, D.H.; Shah, P.A.; Danish, M.Z.; Khan, M.H.; Mahmood, S.; Sohaib, M.; Irfan, M.M.; et al. Development, characterization and optimization of methotrexate-olive oil nano-emulsion for topical application. Pak. J. Pharm. Sci. 2021, 34, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Fradique, R.; Vallejo, M.; Correia, T.; Miguel, S.A.P.; Correia, I. In vitro evaluation of hEnSCs derived osteoblast like cells behavior on gelatin/collagen/bioglass nanofibers scaffolds. Mater. Sci. Eng. C 2015, 55, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Wong, T.W. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Jawalkar, S.S.; Aminabhavi, T.M. Controlled release of cephalexin through gellan gum beads: Effect of formulation parameters on entrapment efficiency, size, and drug release. Eur. J. Pharm. Biopharm. 2006, 63, 249–261. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Aburahma, M.H. Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J. Liposome Res. 2014, 25, 107–121. [Google Scholar] [CrossRef]
- Kumbhar, D.; Wavikar, P.; Vavia, P. Niosomal gel of lornoxicam for topical delivery: In vitro assessment and pharmacodynamic activity. AAPS PharmSciTech 2013, 14, 1072–1082. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; De Luca, M.; Ragno, G.; Picci, N.; Muzzalupo, R. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Int. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Jan, S.U.; Khan, N.; Hussain, A.; Khan, G.M. Formulation and in vitro evaluation of clotrimazole gel containing almond oil and tween 80 as penetration enhancer for topical application. Pak. J. Pharm. Sci. 2013, 26, 617–622. [Google Scholar]
- Hussain, A.; Khan, G.M.; Shah, S.U.; Shah, K.U.; Khan, N.; Wahab, A.; Rehman, A.-U. Development of a novel ketoprofen transdermal patch: Effect of almond oil as penetration enhancers on in-vitro and ex-vivo penetration of ketoprofen through rabbit skin. Pak. J. Pharm. Sci. 2012, 25, 227–232. [Google Scholar] [PubMed]
- Joseph, J.; Hari, B.N.V.; Devi, D.R. Experimental optimization of Lornoxicam liposomes for sustained topical delivery. Eur. J. Pharm. Sci. 2018, 112, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.V.; Urade, M.N. Formulation and Evaluation of Chitosan Based Transdermal Patches of Lornoxicam for Prolonged Drug Release and To Study the Effect of Permeation Enhancer. Indian J. Pharm. Educ. Res. 2019, 53, 88–96. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Rodrigues, F.; Diniz, L.; Sousa, R.; Honorato, T.; Simão, D.; Araújo, C.; Gonçalves, T.; Rolim, L.; Goto, P.; Tedesco, A.; et al. Preparation and Characterization of Nanoemulsion Containing a Natural Naphthoquinone. Química Nova 2018, 41, 756–761. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Madan, J.R.; Ghuge, N.P.; Dua, K. Formulation and evaluation of proniosomes containing lornoxicam. Drug Deliv. Transl. Res. 2016, 6, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, S.; Basri, M.; Shamsudin, N.; Basri, H. Stability of Positively Charged Nanoemulsion Formulation Containing Steroidal Drug for Effective Transdermal Application. J. Chem. 2014, 2014, 748680. [Google Scholar] [CrossRef]
- Akhtar, N.; Ahmad, M.; Khan, H.M.S.; Akram, J.; Gulfishan, G.; Mahmood, A.; Uzair, M. Formulation and characterization of a multiple emulsion containing 1% L-ascorbic acid. Bull. Chem. Soc. Ethiop. 2010, 24, 1–10. [Google Scholar] [CrossRef]
- Masoumi, H.R.F.; Basri, M.; Samiun, W.S.; Izadiyan, Z.; Lim, C.J. Enhancement of encapsulation efficiency of nanoemulsion-containing aripiprazole for the treatment of schizophrenia using mixture experimental design. Int. J. Nanomed. 2015, 10, 6469–6476. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Rajendra, M. Design and evaluation of transdermal films of lornoxicam. Int. J. Pharma Bio Sci. 2011, 2, 54–62. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.641.4577&rep=rep1&type=pdf (accessed on 16 January 2022).
- Shuwaili, A.H.A.L.; Rasool, B.K.A.; Abdulrasool, A.A. Optimization of elastic transfersomes formulations for transdermal delivery of pentoxifylline. Eur. J. Pharm. Biopharm. 2016, 102, 101–114. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.; Zhang, J.; Wang, A.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Szumała, P. Structure of Microemulsion Formulated with Monoacylglycerols in the Presence of Polyols and Ethanol. J. Surfactants Deterg. 2015, 18, 97–106. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Liu, K.-S.; Sung, K.; Al-Suwayeh, S.A.; Ku, M.-C.; Chu, C.-C.; Wang, J.-J.; Fang, J.-Y. Enhancement of transdermal apomorphine delivery with a diester prodrug strategy. Eur. J. Pharm. Biopharm. 2011, 78, 422–431. [Google Scholar] [CrossRef]
- Acharya, D.P.; Hartley, P.G. Progress in microemulsion characterization. Curr. Opin. Colloid Interface Sci. 2012, 17, 274–280. [Google Scholar] [CrossRef]

| Ingredients | Solubility (mg mL−1) Mean ± SD | |
|---|---|---|
| Oils | Almond oil | 0.035 ± 0.004 |
| Coconut oil | 0.029 ± 0.003 | |
| Olive oil | 0.011 ± 0.007 | |
| Sesame oil | 0.0312 ± 0.002 | |
| Sunflower oil | 0.048 ± 0.006 | |
| Surfactants | Cremophor RH 40 | 5.05 ± 0.056 |
| Tween 80 | 3.33 ± 0.037 | |
| Co-surfactants | DMSO | 7.00 ± 0.067 |
| Ethanol | 0.085 ± 0.018 | |
| PBS (pH 7.4) | 6.1 ± 0.021 | |
| PEG 400 | 4.132 ± 0.02 | |
| Water | 0.025 ± 0.008 | |
| Formulation Code | Oil | Surfactant (S) | Co-Surfactant (Co-S) | S: Co-S Ratio | Parts of Oil | Parts of Surfactant | (Oil: S Mix) % |
|---|---|---|---|---|---|---|---|
| LRX-1 | Almond oil | Tween 80 | Ethanol | 2:1 | 9 | 1 | 9.0 |
| LRX-2 | 8 | 2 | 4.0 | ||||
| LRX-3 | 7 | 3 | 2.3 | ||||
| LRX-4 | 6 | 4 | 1.5 | ||||
| LRX-5 | 5 | 5 | 1.0 | ||||
| LRX-6 | 4 | 6 | 0.7 | ||||
| LRX-7 | 3 | 7 | 0.4 | ||||
| LRX-8 | 2 | 8 | 0.3 | ||||
| LRX-9 | 1 | 9 | 0.1 | ||||
| C-LRX (2%) | 4 | 6 | 0.7 |
| F. Codes | Temperature | Color | Odor Change | Phase Separation | Centrifugation Stability | Thermodynamic Test |
|---|---|---|---|---|---|---|
| Blank | 4 °C | White | No change | Nil | Stable | Passed |
| 25 °C | White | No change | Nil | Stable | Passed | |
| 45 °C | White | No change | Nil | Stable | Passed | |
| Optimized LRX-NE | 4 °C | Pale Yellow | No change | Nil | Stable | Passed |
| 25 °C | Pale Yellow | No change | Nil | Stable | Passed | |
| 45 °C | Pale Yellow | No change | Nil | Stable | Passed | |
| C-LRXNE | 4 °C | Yellow | No change | Nil | Stable | Passed |
| 25 °C | Yellow | No change | Nil | Stable | Passed | |
| 45 °C | Yellow | No change | Nil | Stable | Passed |
| Formulation Code | Globule Size (nm ± SD) | PDI | ZP (mV ± SD) | Viscosity (mPa.s ± SD) | Surface Tension (Dynescm−1) | pH Mean ± SD | Drug Entrapment Efficiency (%) Mean ± SD |
|---|---|---|---|---|---|---|---|
| LRX-4 | 168.4 ± 43.2 | 0.45 ± 0.03 | −21.18 ± 0.15 | 85.21 ± 2.14 | 56.34 ± 3.04 | 4.73 ± 0.29 | 48.96 ± 5.71 |
| LRX-5 | 125.8 ± 36.5 | 0.25 ± 0.04 | −25.69 ± 0.58 | 62.19 ± 3.18 | 39.59 ± 4.08 | 5.23 ± 0.41 | 62.95 ± 1.90 |
| LRX-6 | 78.6 ± 11.7 | 0.24 ± 0.02 | −29.58 ± 0.61 | 48.47 ± 2.12 | 26.46 ± 2.29 | 5.67 ± 0.45 | 71.91 ± 3.17 |
| LRX-7 | 63.3 ± 15.6 | 0.31 ± 0.06 | −24.48 ± 1.12 | 24.38 ± 3.97 | 35.01 ± 4.77 | 4.47 ± 0.31 | 57.58 ± 6.58 |
| C-LRX | 101.3 ± 24.51 | 0.27 ± 0.04 | +20.29 ± 2.10 | 58.12 ± 5.09 | 31.05 ± 3.57 | 5.95 ± 0.35 | 65.25 ± 4.89 |
| Formulations | Power Law Kinetic Model | |||
|---|---|---|---|---|
| K ± SD | R2 | N | Release Mechanism | |
| LRX-4 | 0.263 ± 0.131 | 0.8176 | 0.432 | Fickian Diffusion |
| LRX-5 | 0.032 ± 0.024 | 0.9657 | 0.501 | Anomalous non-Fickian Diffusion |
| LRX-6 | 0.179 ± 0.015 | 0.9751 | 0.597 | Anomalous non-Fickian Diffusion |
| LRX-7 | 0.021 ± 0.001 | 0.9122 | 0.405 | Fickian Diffusion |
| C-LRX NE | 0.089 ± 0.156 | 0.9976 | 0.583 | Anomalous non-Fickian Diffusion |
| Formulation Code | Steady-State Flux Jss ± S (µg cm−2 hr−1) | Permeability Coefficient Kp ± SD (cm hr−1) × 10−2 | Enhancement Ratio (ER) |
|---|---|---|---|
| LRX-4 | 45.63 ± 3.15 | 0.35 ± 0.019 | 1.63 |
| LRX-5 | 95.63 ± 5.67 | 2.36 ± 0.15 | 3.21 |
| LRX-6 | 210.16 ± 7.52 | 2.26 ± 0.077 | 6.85 |
| LRX-7 | 121.25 ± 3.65 | 1.12 ± 0.015 | 4.22 |
| C-LRX NE | 229.18 ± 9.25 | 2.49 ± 0.127 | 7.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.U.; Shah, S.U.; Rashid, S.A.; Naseem, F.; Shah, K.U.; Farid, A.; Hakeem, K.R.; Kamli, M.R.; Althubaiti, E.H.; Alamoudi, S.A. Lornoxicam-Loaded Chitosan-Decorated Nanoemulsion: Preparation and In Vitro Evaluation for Enhanced Transdermal Delivery. Polymers 2022, 14, 1922. https://doi.org/10.3390/polym14091922
Khan RU, Shah SU, Rashid SA, Naseem F, Shah KU, Farid A, Hakeem KR, Kamli MR, Althubaiti EH, Alamoudi SA. Lornoxicam-Loaded Chitosan-Decorated Nanoemulsion: Preparation and In Vitro Evaluation for Enhanced Transdermal Delivery. Polymers. 2022; 14(9):1922. https://doi.org/10.3390/polym14091922
Chicago/Turabian StyleKhan, Rahman Ullah, Shefaat Ullah Shah, Sheikh Abdur Rashid, Faiza Naseem, Kifayat Ullah Shah, Arshad Farid, Khalid Rehman Hakeem, Majid Rasool Kamli, Eman Hillal Althubaiti, and Soha A. Alamoudi. 2022. "Lornoxicam-Loaded Chitosan-Decorated Nanoemulsion: Preparation and In Vitro Evaluation for Enhanced Transdermal Delivery" Polymers 14, no. 9: 1922. https://doi.org/10.3390/polym14091922
APA StyleKhan, R. U., Shah, S. U., Rashid, S. A., Naseem, F., Shah, K. U., Farid, A., Hakeem, K. R., Kamli, M. R., Althubaiti, E. H., & Alamoudi, S. A. (2022). Lornoxicam-Loaded Chitosan-Decorated Nanoemulsion: Preparation and In Vitro Evaluation for Enhanced Transdermal Delivery. Polymers, 14(9), 1922. https://doi.org/10.3390/polym14091922







