Fabrication and Performance of Phase Change Thermoregulated Fiber from Bicomponent Melt Spinning
Abstract
:1. Introduction
2. Materials and Methods
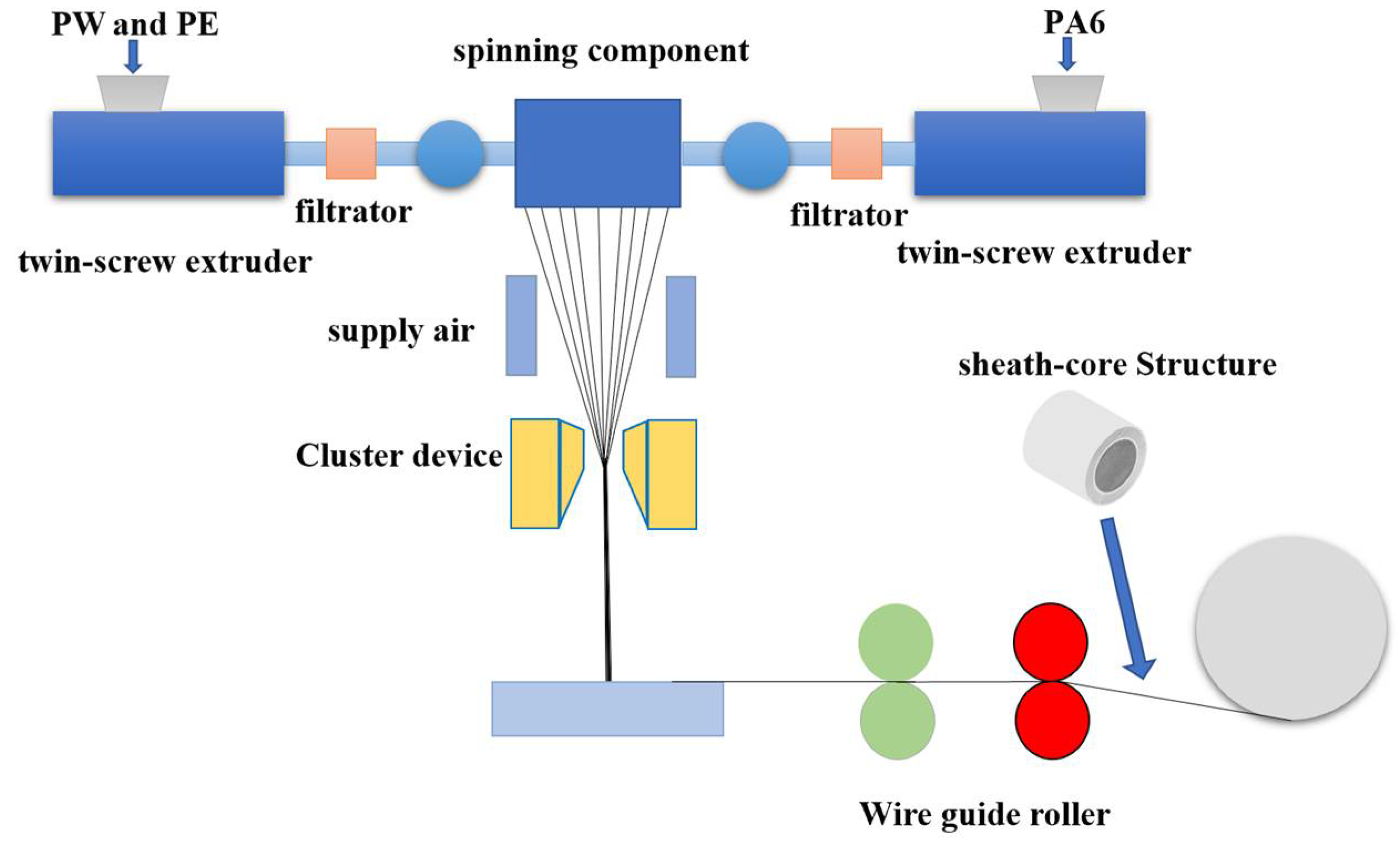
2.1. Preparation of Phase Change Thermoregulated Fiber
2.2. Testing and Characterization
3. Results and Discussion
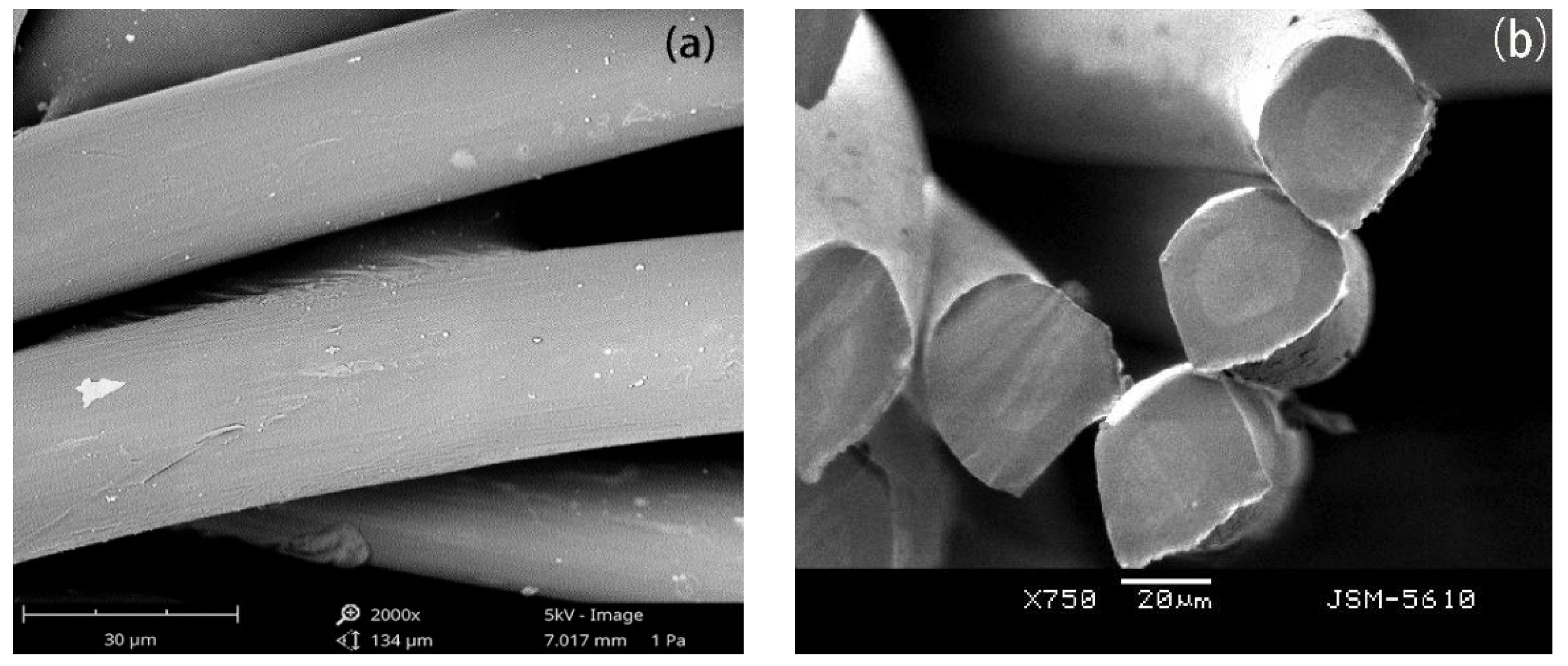
3.1. Morphology of the PCTF
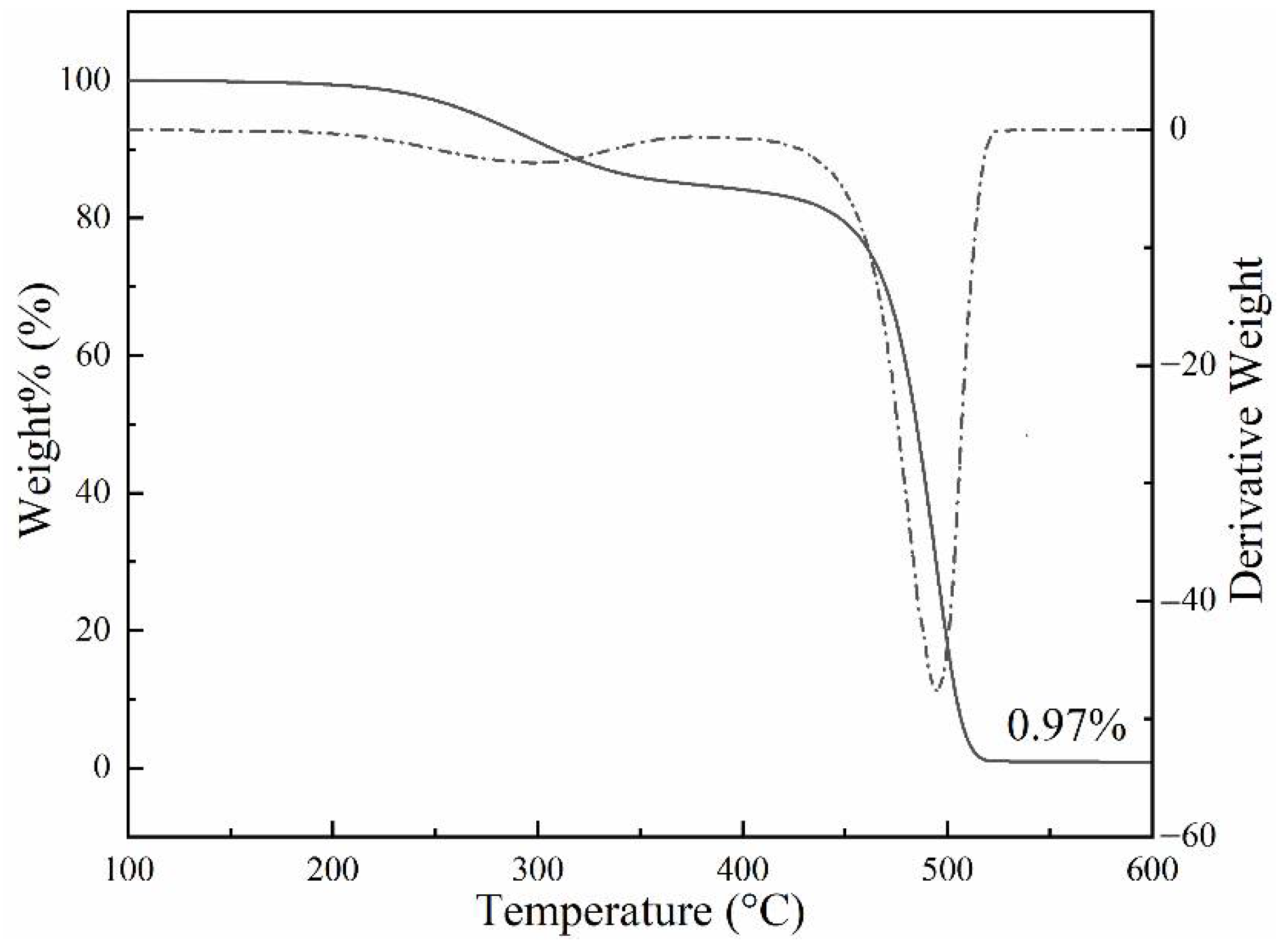
3.2. Thermogravimetric Analysis of the PCTF
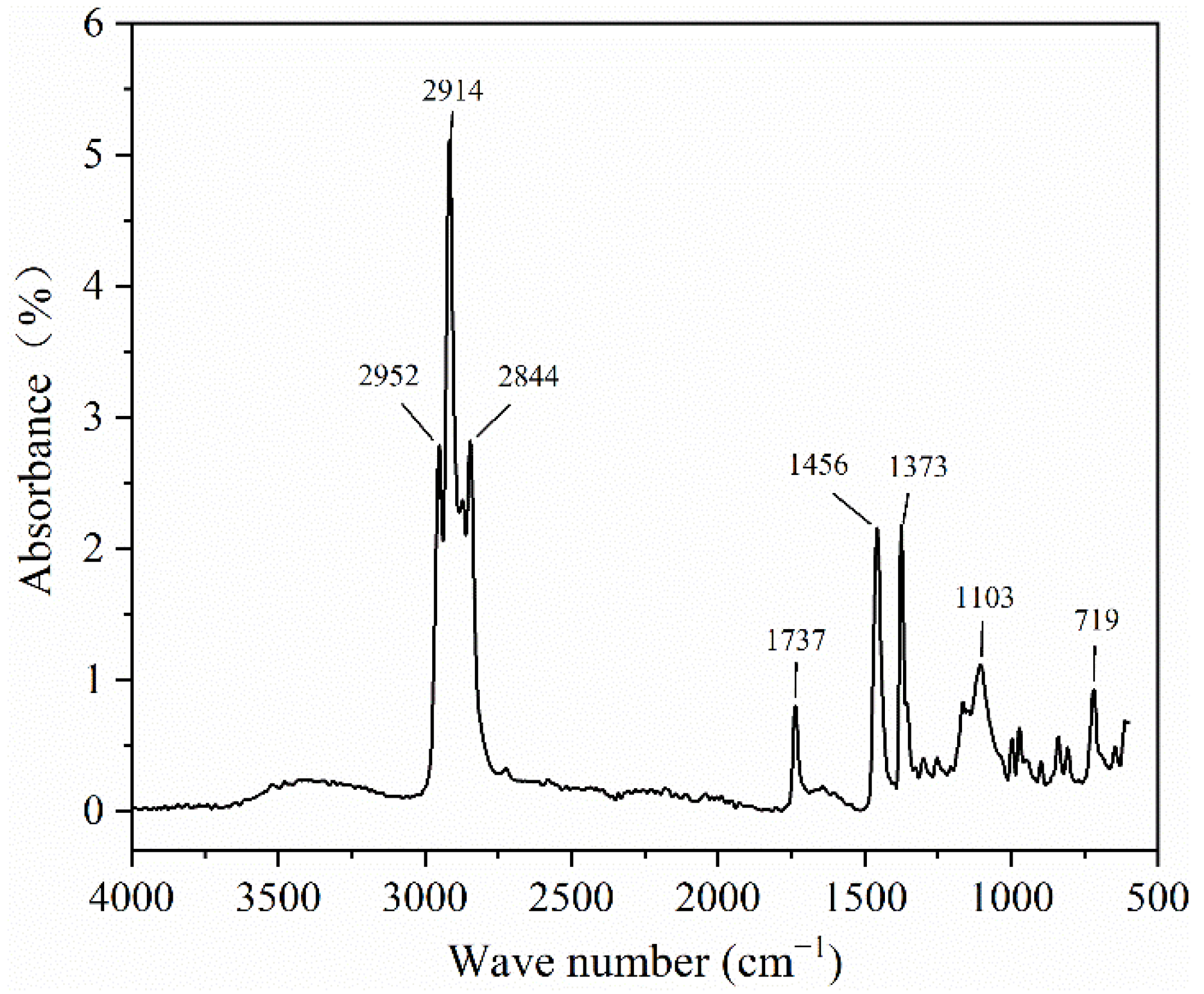
3.3. Infrared Spectra of the PCTF
3.4. XRD Analysis of the PCTF
3.5. DSC Analysis of the PCTF
3.6. Mechanical Properties of the PCTF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.X.; Meng, J.Y.; Tang, X.F.; Shi, H.F. Thermo-Regulated Fiber and Preparation Method Thereof. U.S. Patent Application No. 14/412,184, 28 May 2015. [Google Scholar]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef] [Green Version]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Q.-H.; Xin, J.-W.; Li, X.; Sun, B.-Y.; Yang, J.-Y. Research status and application of phase change materials. Cailiao Gongcheng-J. Mater. Eng. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Sun, D.; Ashraf, M.; Rehman, A.; Safdar, F.; Basit, A.; Maqsood, H.S. Phase change materials, their synthesis and application in textiles—A review. J. Text. Inst. 2018, 110, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Zhang, X.; Ji, J.; Han, L.; Ding, X.; Xie, W. Application and research progress of phase change materials in biomedical field. Biomater. Sci. 2021, 9, 5762–5780. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Kang, K.-W.; Choi, C.-W.; Jin, J.-W. A Wet-Spinning Process for Producing Carbon Nanotube/Polyvinylidene Fluoride Fibers Having Highly Consistent Electrical and Mechanical Properties. Polymers 2021, 13, 4048. [Google Scholar] [CrossRef]
- Kakiage, M.; Fukagawa, D. Preparation of ultrahigh-molecular-weight polyethylene fibers by combination of melt-spinning and melt-drawing. Mater. Today Commun. 2019, 23, 100864. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.; Jiang, Z.; Jiang, S.; Zhang, Q.; Xiong, R.; Huang, C. Green Electrospun Nanofibers and Their Application in Air Filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-Spun Fibers for Textile Applications. Materials 2020, 13, 4298. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Rodríguez, J.F.; Carmona, M.; Romero, A.; Sánchez, P. Thermal and morphological stability of polystyrene microcapsules containing phase-change materials. J. Appl. Polym. Sci. 2010, 120, 291–297. [Google Scholar] [CrossRef]
- Rao, Z.H.; Wang, S.H.; Zhang, Y.L.; Zhang, G.Q.; Zhang, J.Y. Thermal Properties of Paraffin/Nano-AlN Phase Change Energy Storage Materials. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 2281–2286. [Google Scholar] [CrossRef]
- Trigui, A.; Karkri, M.; Krupa, I. Thermal conductivity and latent heat thermal energy storage properties of LDPE/wax as a shape-stabilized composite phase change material. Energy Convers. Manag. 2014, 77, 586–596. [Google Scholar] [CrossRef]
- Fang, Y.; Wei, H.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Preparation and Thermal Performance of Silica/n-Tetradecane Microencapsulated Phase Change Material for Cold Energy Storage. Energy Fuels 2016, 30, 9652–9657. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Tong, X.; Li, N.; Zeng, M.; Wang, Q. Organic phase change materials confined in carbon-based materials for thermal properties enhancement: Recent advancement and challenges. Renew. Sustain. Energy Rev. 2019, 108, 398–422. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R. Thermal cycling test of few selected inorganic and organic phase change materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Zhang, Z.; Tang, G.; Song, G. Preparation, thermal property, and thermal stability of microencapsulated n-octadecane with poly(stearyl methacrylate) as shell. J. Therm. Anal. 2014, 118, 1441–1449. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Feng, J. Systematic investigation on shape stability of high-efficiency SEBS/paraffin form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2013, 118, 54–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Tang, B.; Lu, R.; Zhang, S. Form-stable phase change materials with high phase change enthalpy from the composite of paraffin and cross-linking phase change structure. Appl. Energy 2016, 184, 241–246. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, S.; Liu, C.; Situ, Y.; Liu, J.; Huang, H. Preparation of PE-EPDM based phase change materials with great mechanical property, thermal conductivity and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2019, 200, 109988. [Google Scholar] [CrossRef]
- Guang-Hua, N.; Jian-Ping, W.; Yan, W.; He, W.; Wei, L.; Xing-Xiang, Z. Preparation and Properties of Nanoencapsulated Phase Change Materials Containing Polyaniline. Acta Phys.-Chim. Sin. 2014, 30, 338–344. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Ioniţǎ, S.; Lincu, D.; Berger, D.; Matei, C. A Review of Composite Phase Change Materials Based on Porous Silica Nanomaterials for Latent Heat Storage Applications. Molecules 2021, 26, 241. [Google Scholar] [CrossRef]
- JianShe, H.; Chao, Y.; Xu, Z.; Jiao, Z.; JinXing, D. Structure and thermal properties of expanded graphite/paraffin composite phase change material. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 41, 86–93. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Cherif, C.; Tran, N.H.A.; Kirsten, M.; Bruenig, H.; Vogel, R. Environmentally friendly and highly productive bi-component melt spinning of thermoregulated smart polymer fibres with high latent heat capacity. Express Polym. Lett. 2018, 12, 203–214. [Google Scholar] [CrossRef]
- Kong, W.; Fu, X.; Liu, Z.; Zhou, C.; Lei, J. A facile synthesis of solid-solid phase change material for thermal energy storage. Appl. Therm. Eng. 2017, 117, 622–628. [Google Scholar] [CrossRef]
- Krupa, I.; Miková, G.; Luyt, A. Phase change materials based on low-density polyethylene/paraffin wax blends. Eur. Polym. J. 2007, 43, 4695–4705. [Google Scholar] [CrossRef]
- Krupa, I.; Miková, G.; Luyt, A. Polypropylene as a potential matrix for the creation of shape stabilized phase change materials. Eur. Polym. J. 2007, 43, 895–907. [Google Scholar] [CrossRef]
- Ito, A.; Hioki, K.; Kono, K.; Hiejima, Y.; Nitta, K.-H. Effects of Liquid Paraffin on Dynamic Mechanical Properties of Linear High-Density Polyethylene. Macromolecules 2020, 53, 8459–8466. [Google Scholar] [CrossRef]
- Aleksandrova, E.A.; Khadisova, Z.T.; Aklimadova, K.K.; Makhmudova, L.S.; Abubalcarova, A.S. Structuraland Mechanical Properties of Polymer—Paraffin Composites. Chem. Technol. Fuels Oils 2019, 55, 404–411. [Google Scholar] [CrossRef]
- Xi, P.; Gu, X.; Cheng, B.; Wang, Y. Preparation and characterization of a novel polymeric based solid–solid phase change heat storage material. Energy Convers. Manag. 2009, 50, 1522–1528. [Google Scholar] [CrossRef]
- Shen, L.; Severn, J.; Bastiaansen, C.W. Drawing behavior and mechanical properties of ultra-high molecular weight polyethylene blends with a linear polyethylene wax. Polymer 2018, 153, 354–361. [Google Scholar] [CrossRef]
- Elnahas, H.; Abdou, S.M.; El-Zahed, H.; Abdeldaym, A. Structural, morphological and mechanical properties of gamma irradiated low density polyethylene/paraffin wax blends. Radiat. Phys. Chem. 2018, 151, 217–224. [Google Scholar] [CrossRef]
- Resch-Fauster, K.; Feuchter, M. Thermo-physical characteristics, mechanical performance and long-term stability of high temperature latent heat storages based on paraffin-polymer compounds. Thermochim. Acta 2018, 663, 34–45. [Google Scholar] [CrossRef]
- Kim, S.; Moon, H.; Kim, J. Thermal characterizations of the paraffin wax/low density polyethylene blends as a solid fuel. Thermochim. Acta 2015, 613, 9–16. [Google Scholar] [CrossRef]
- Akishino, J.; Cerqueira, D.; Silva, G.; Swinka-Filho, V.; Munaro, M. Morphological and thermal evaluation of blends of polyethylene wax and paraffin. Thermochim. Acta 2016, 626, 9–12. [Google Scholar] [CrossRef]
- Zhang, P.; Song, L.; Lu, H.; Wang, J.; Hu, Y. The influence of expanded graphite on thermal properties for paraffin/high density polyethylene/chlorinated paraffin/antimony trioxide as a flame retardant phase change material. Energy Convers. Manag. 2010, 51, 2733–2737. [Google Scholar] [CrossRef]
- Krupa, I.; Nógellová, Z.; Spitalsky, Z.; Janigová, I.; Boh, B.; Sumiga, B.; Kleinová, A.; Karkri, M.; AlMaadeed, M.A. Phase change materials based on high-density polyethylene filled with microencapsulated paraffin wax. Energy Convers. Manag. 2014, 87, 400–409. [Google Scholar] [CrossRef]
- GB/T 27761-2011; Standard Test Method of Mass Loss and Residue Measurement Validation of Thermogravimetric Analyzers. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2012.
- GB/T 6040-2019; General Rules for Infrared Analysis. State Administration for Martket Regulation, Standardization Administration People’s Republic of China: Beijing, China, 2020.
- Lee, J.-Y.; Kim, K.-J. MEG Effects on Hydrolysis of Polyamide 66/Glass Fiber Composites and Mechanical Poperty Changes. Molecules 2019, 24, 755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parres, F.; Crespo, J.E.; Nadal-Gisbert, A. Thermal degradation analysis of polyamide 6 processed at different cycles using sequential pyrolysis. J. Appl. Polym. Sci. 2009, 114, 713–719. [Google Scholar] [CrossRef]
- Grinys, T.; Tamulevicius, S.; Prosycevas, I.; Meskinis, S. XRD Analysis of Plasma Sprayed YSZ-NiO-Ni Ceramic Coatings. Plasma Processes Polym. 2007, 4, S181–S184. [Google Scholar] [CrossRef]


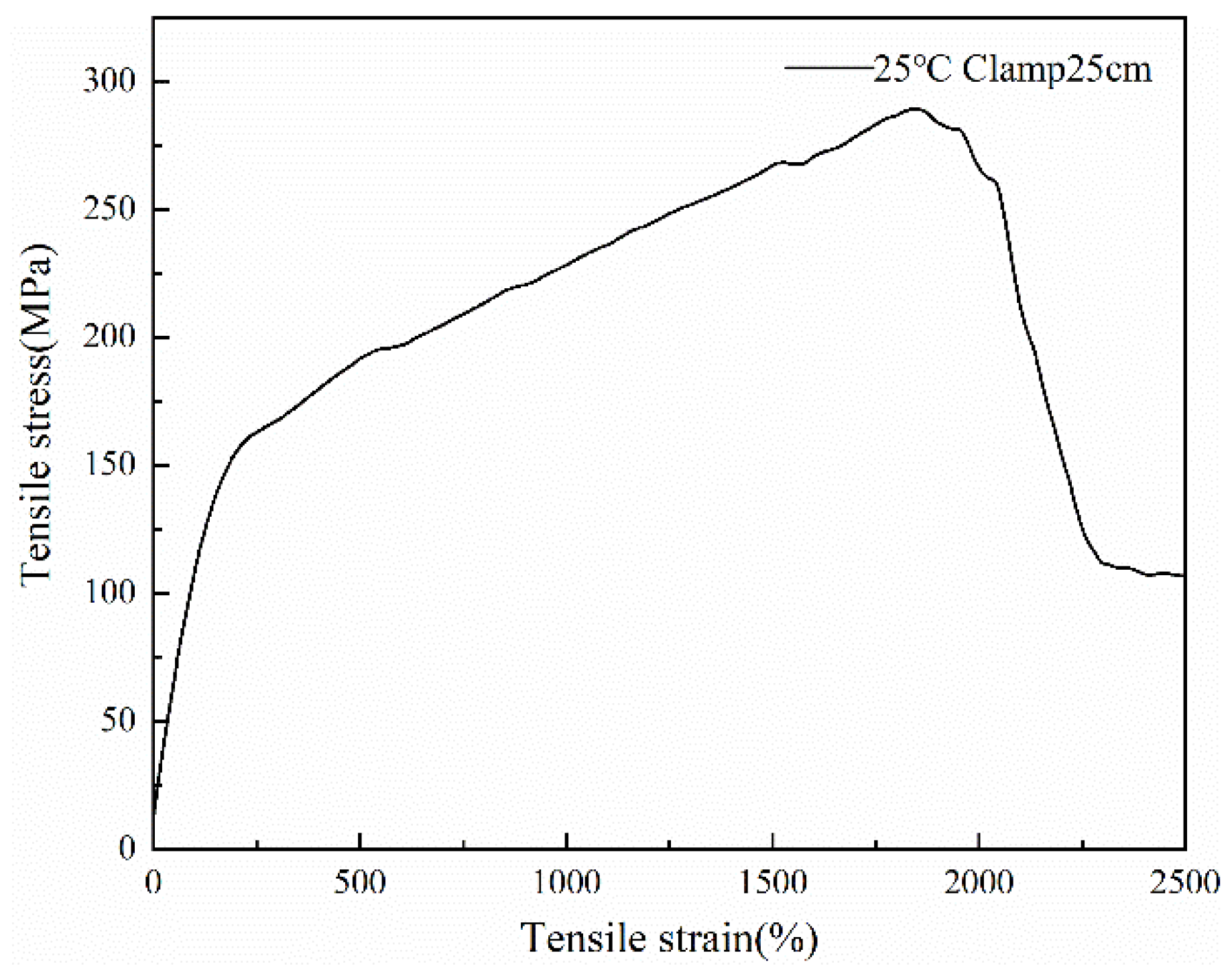
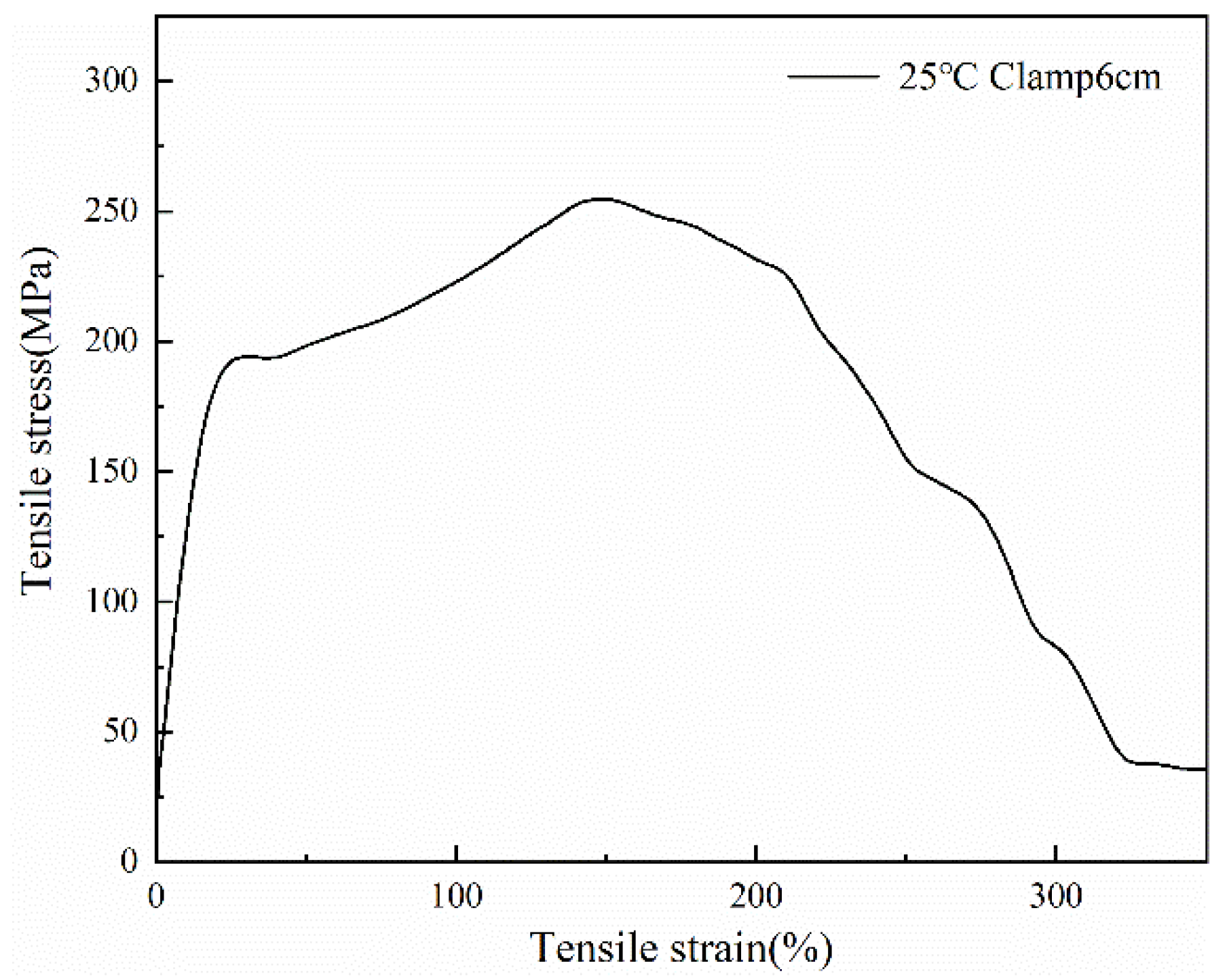
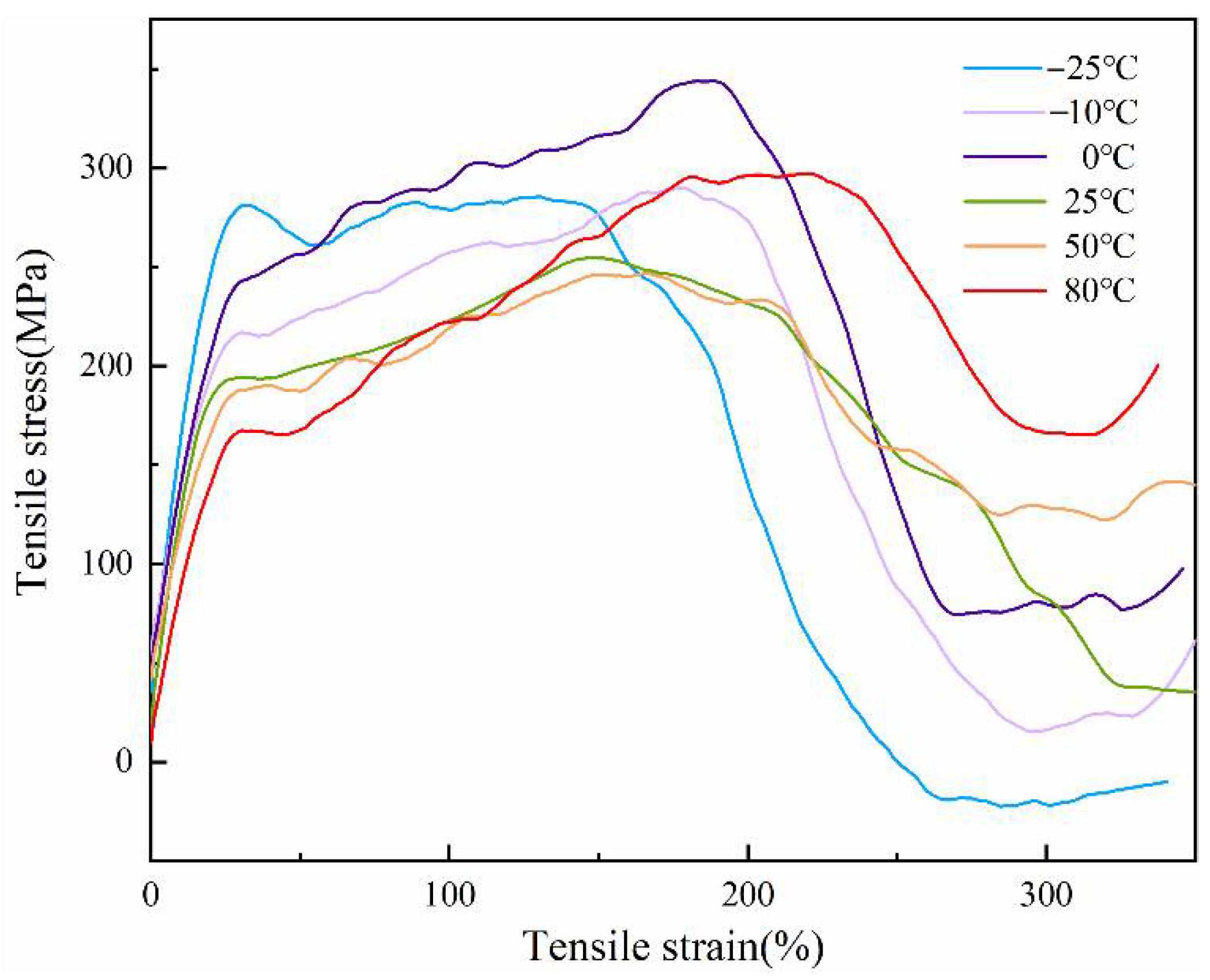
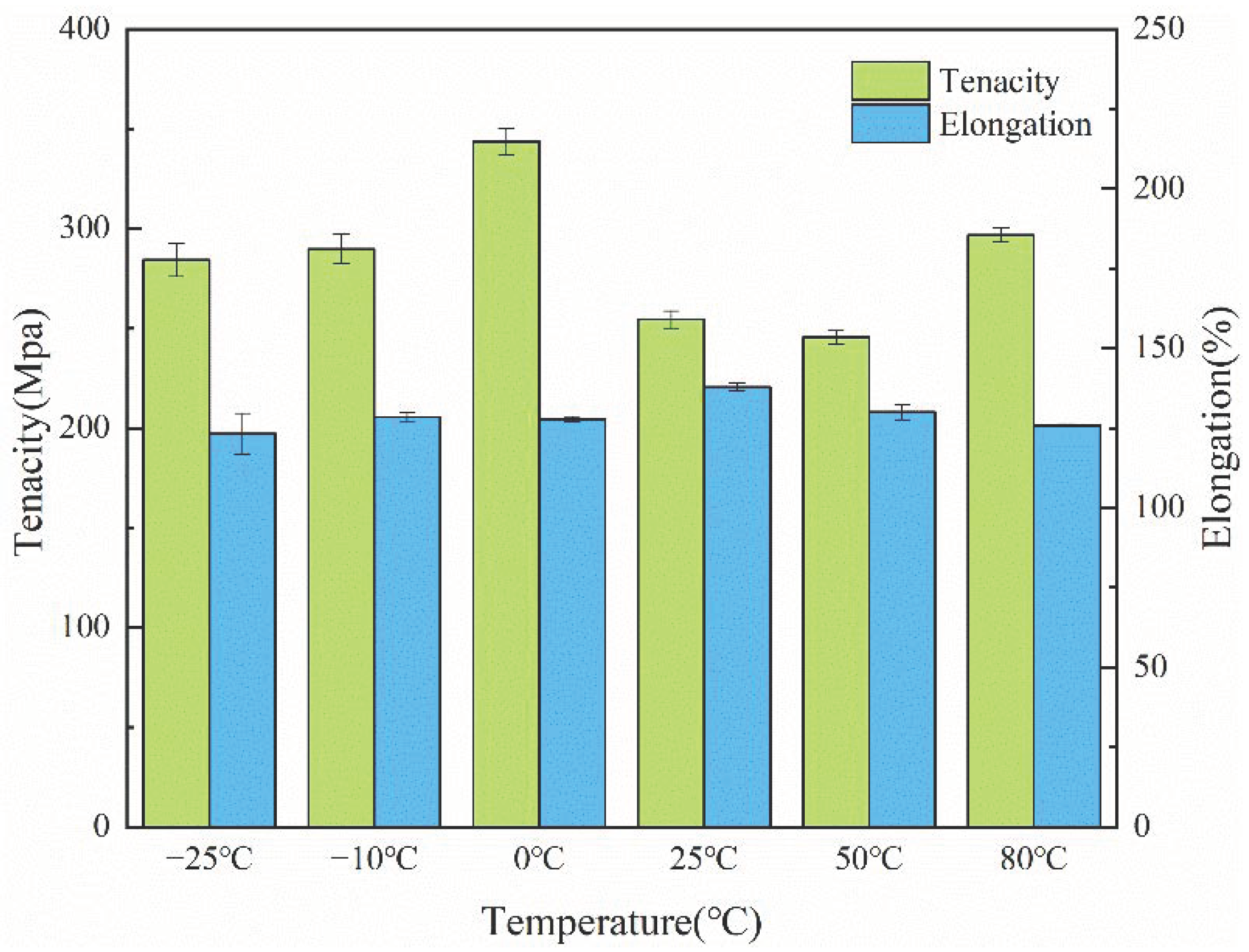
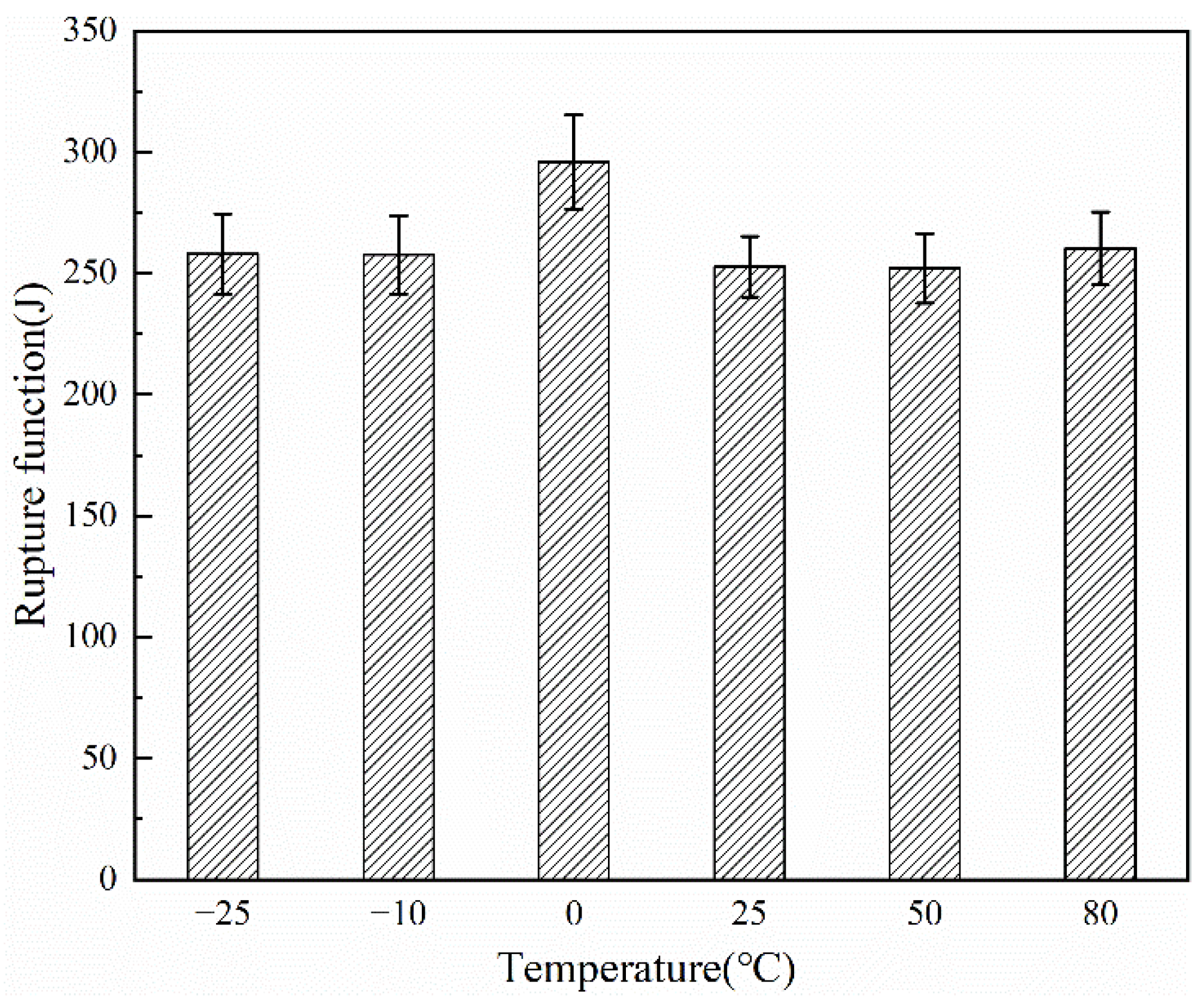
| Temperature (°C) | Young’s Modulus (MPa) | Tenacity (MPa) | Elongation (%) | Fracture Work (J) |
| −25 | 20.57 ± 0.61 | 284.34 ± 8.47 | 197.13 ± 10.29 | 258.02 ± 16.51 |
| −10 | 13.74 ± 0.45 | 289.73 ± 7.48 | 205.49 ± 2.51 | 257.61 ± 16.05 |
| 0 | 14.95 ± 0.47 | 343.59 ± 6.63 | 204.52 ± 1.21 | 295.90 ± 19.45 |
| 25 | 18.15 ± 0.42 | 254.54 ± 4.31 | 220.58 ± 2.08 | 252.71 ± 12.47 |
| 50 | 12.65 ± 0.32 | 245.63 ± 3.28 | 208.08 ± 3.78 | 252.15 ± 14.36 |
| 80 | 11.86 ± 0.37 | 296.77 ± 3.67 | 201.35 ± 0.56 | 260.20 ± 15.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Hu, D.; Yao, J.; Wang, Y.; Zhang, G.; Křemenáková, D.; Militky, J.; Wiener, J.; Li, L.; Zhu, G. Fabrication and Performance of Phase Change Thermoregulated Fiber from Bicomponent Melt Spinning. Polymers 2022, 14, 1895. https://doi.org/10.3390/polym14091895
Liu Z, Hu D, Yao J, Wang Y, Zhang G, Křemenáková D, Militky J, Wiener J, Li L, Zhu G. Fabrication and Performance of Phase Change Thermoregulated Fiber from Bicomponent Melt Spinning. Polymers. 2022; 14(9):1895. https://doi.org/10.3390/polym14091895
Chicago/Turabian StyleLiu, Zenan, Diefei Hu, Juming Yao, Yan Wang, Guoqing Zhang, Dana Křemenáková, Jiri Militky, Jakub Wiener, Li Li, and Guocheng Zhu. 2022. "Fabrication and Performance of Phase Change Thermoregulated Fiber from Bicomponent Melt Spinning" Polymers 14, no. 9: 1895. https://doi.org/10.3390/polym14091895
APA StyleLiu, Z., Hu, D., Yao, J., Wang, Y., Zhang, G., Křemenáková, D., Militky, J., Wiener, J., Li, L., & Zhu, G. (2022). Fabrication and Performance of Phase Change Thermoregulated Fiber from Bicomponent Melt Spinning. Polymers, 14(9), 1895. https://doi.org/10.3390/polym14091895








