Design and Fabrication of Nanofibrous Dura Mater with Antifibrosis and Neuroprotection Effects on SH-SY5Y Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. NDM Characterization
2.4. Encapsulation Efficiency (EE)
2.5. In Vitro TMP Release Profiles
2.6. Extraction of NDM Immersion Solution
2.7. Cell Culture
2.8. OGD Challenge
2.9. Cell Viability
2.10. Lactate Dehydrogenase (LDH) Release Assay
2.11. Cell Morphology
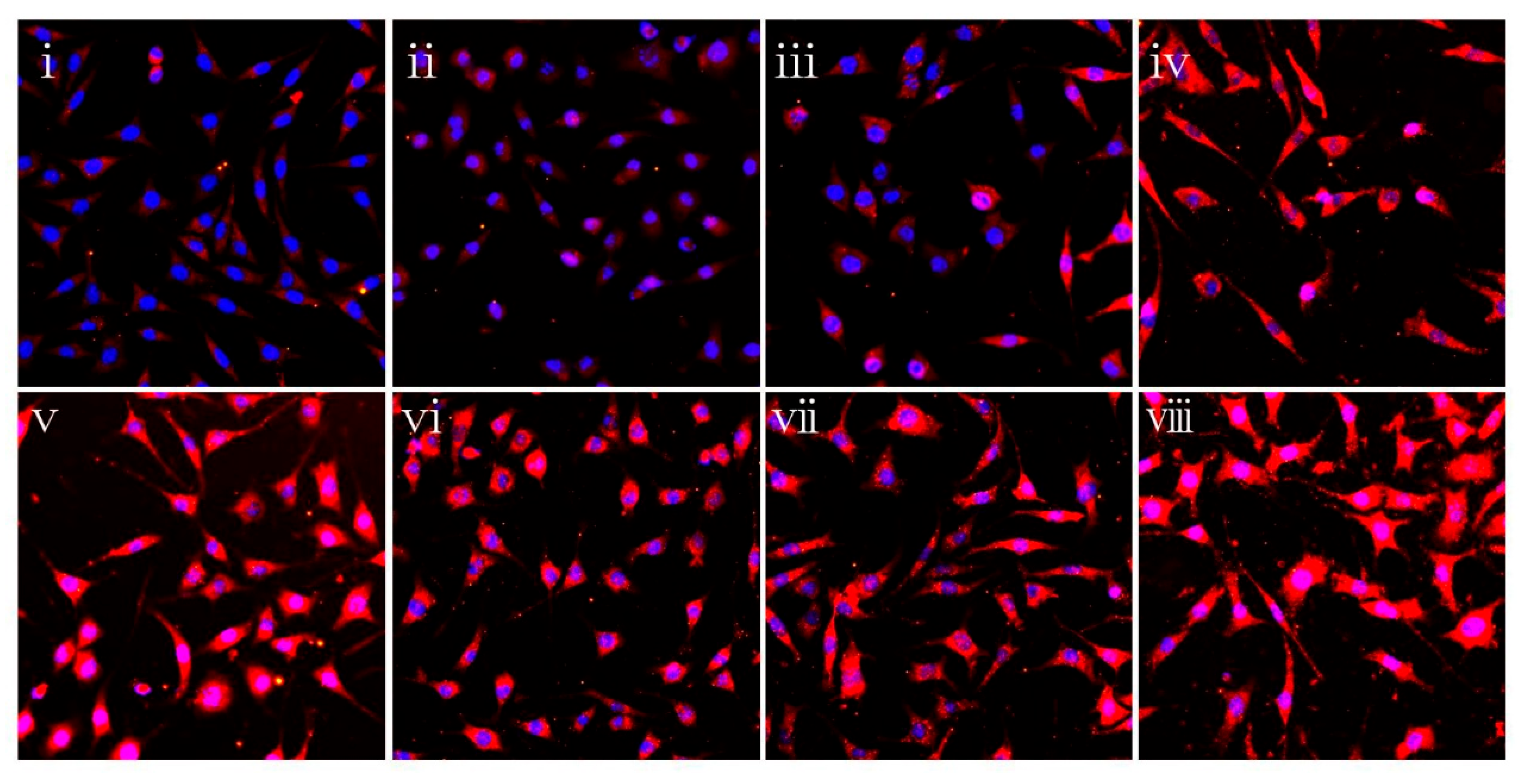
2.12. Immunofluorescence
2.13. Statistical Analysis
3. Results and Discussion
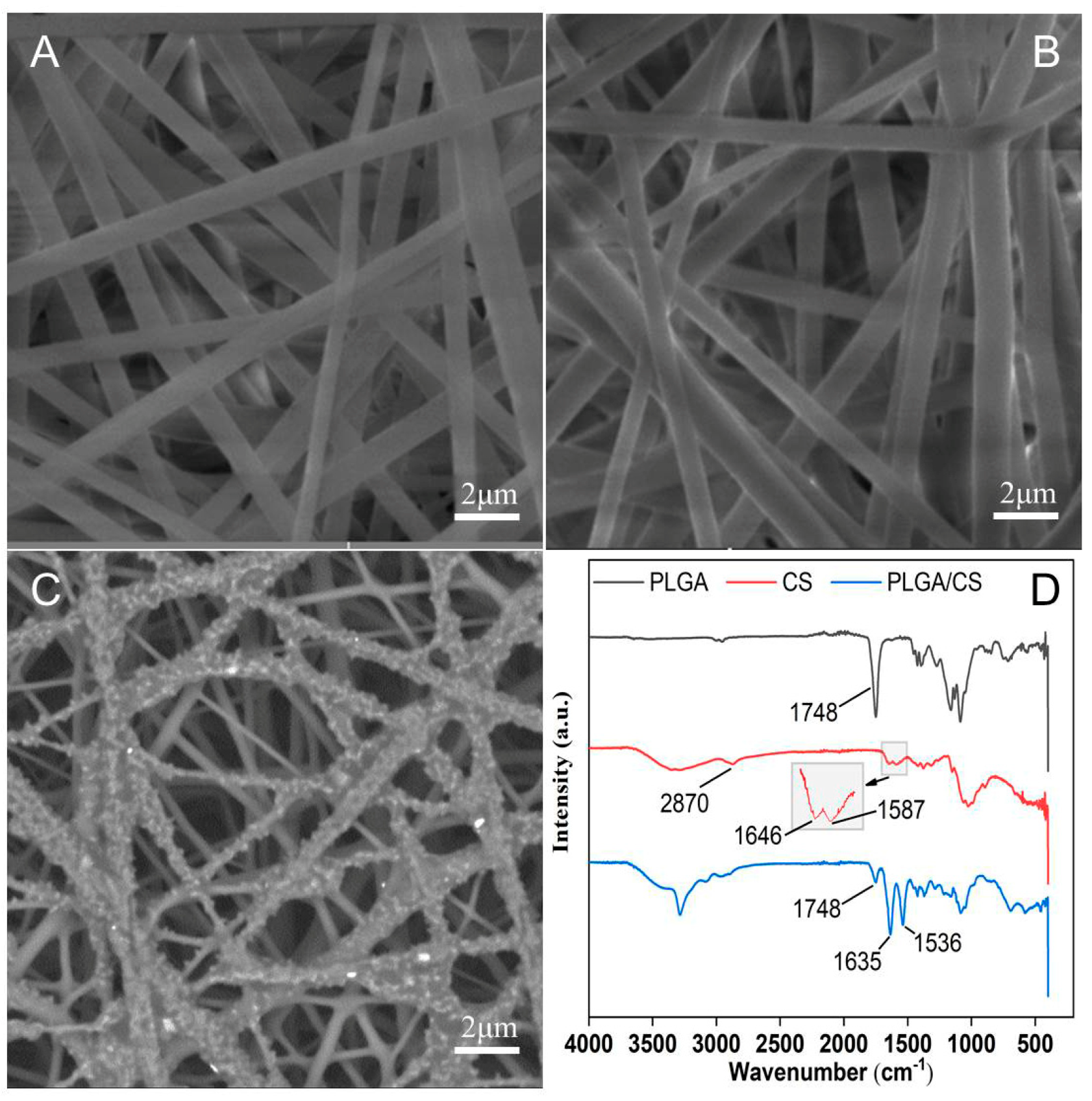
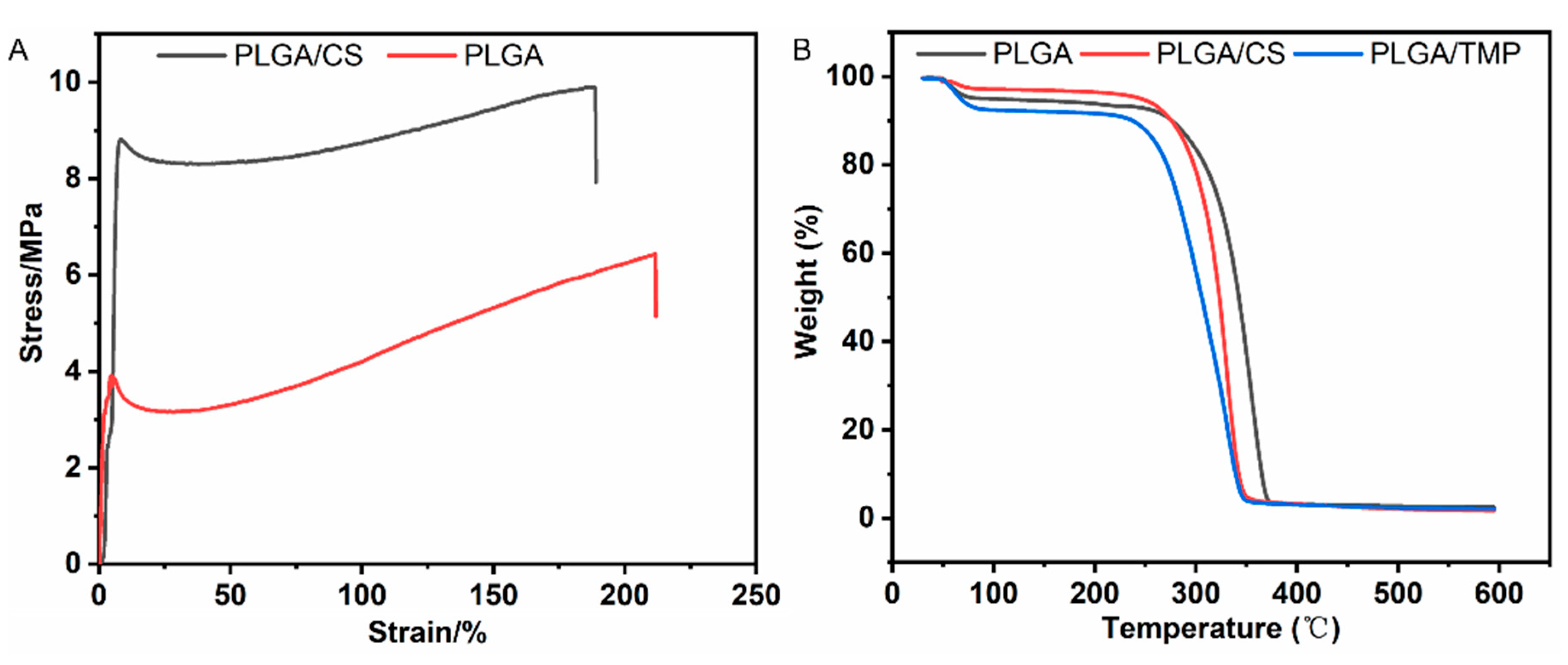
3.1. NDM Characterization
3.2. Encapsulation Efficiency and In Vitro TMP Release Profiles
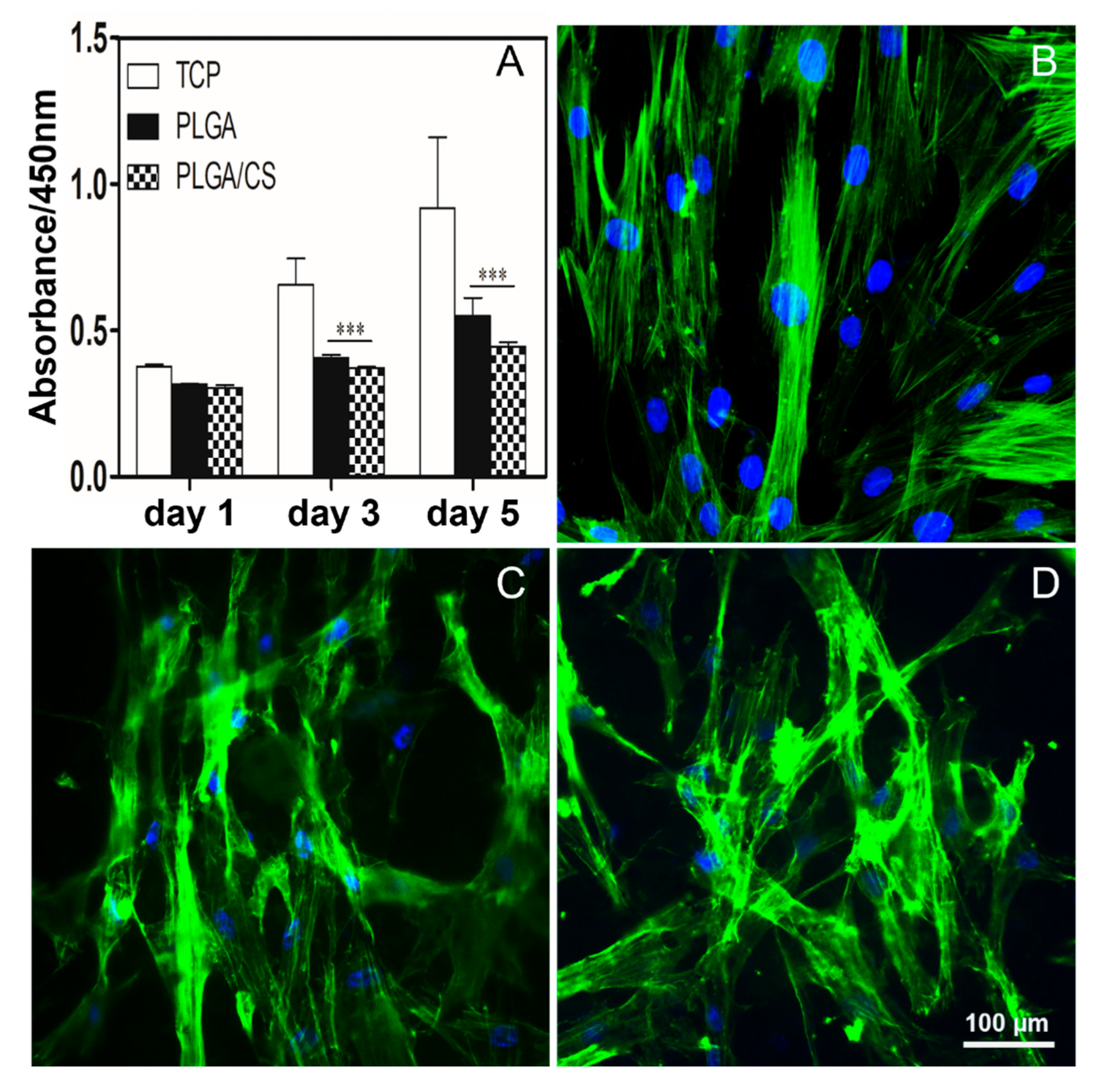
3.3. PLGA/CS NDMs Inhibit the Excessive Proliferation of Fibroblasts
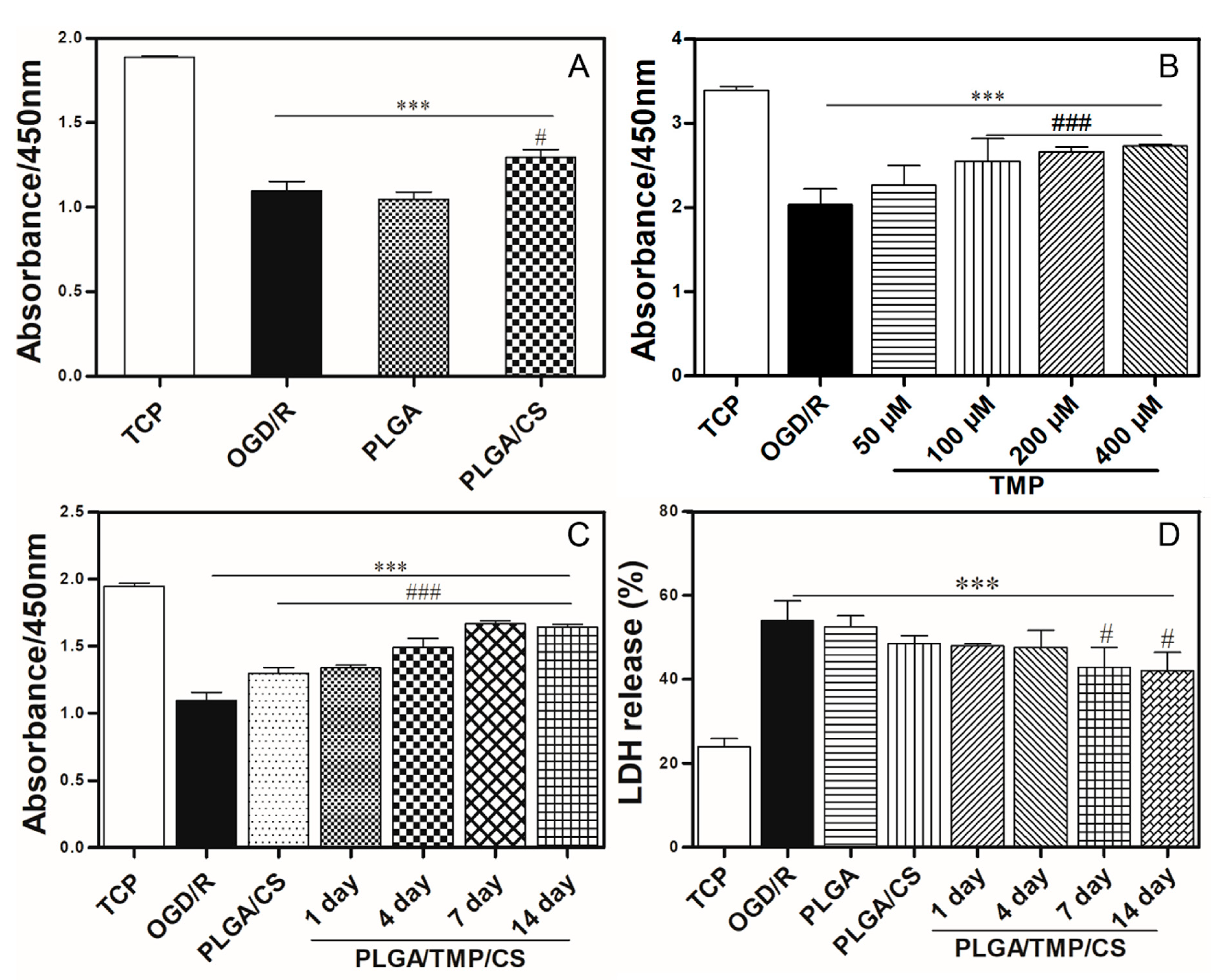
3.4. PLGA/CS NDMs Promote the Survival of OGD-Treated SH-SY5Y Cells
3.5. The Working Concentration of TMP with Neuroprotective Effect
3.6. PLGA/TMP/CS NDMs Promote the Survival of OGD-Treated SH-SY5Y Cells
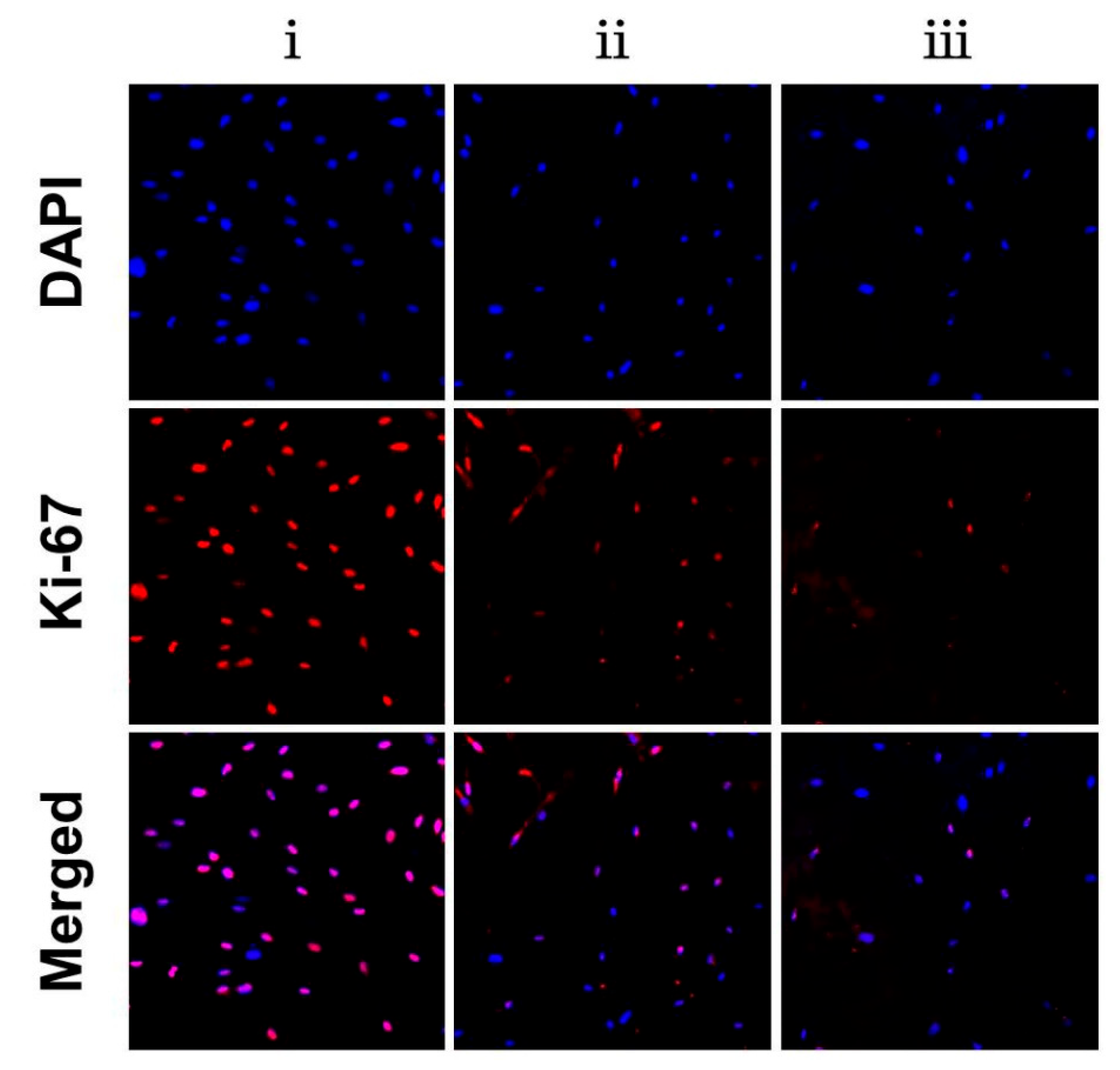
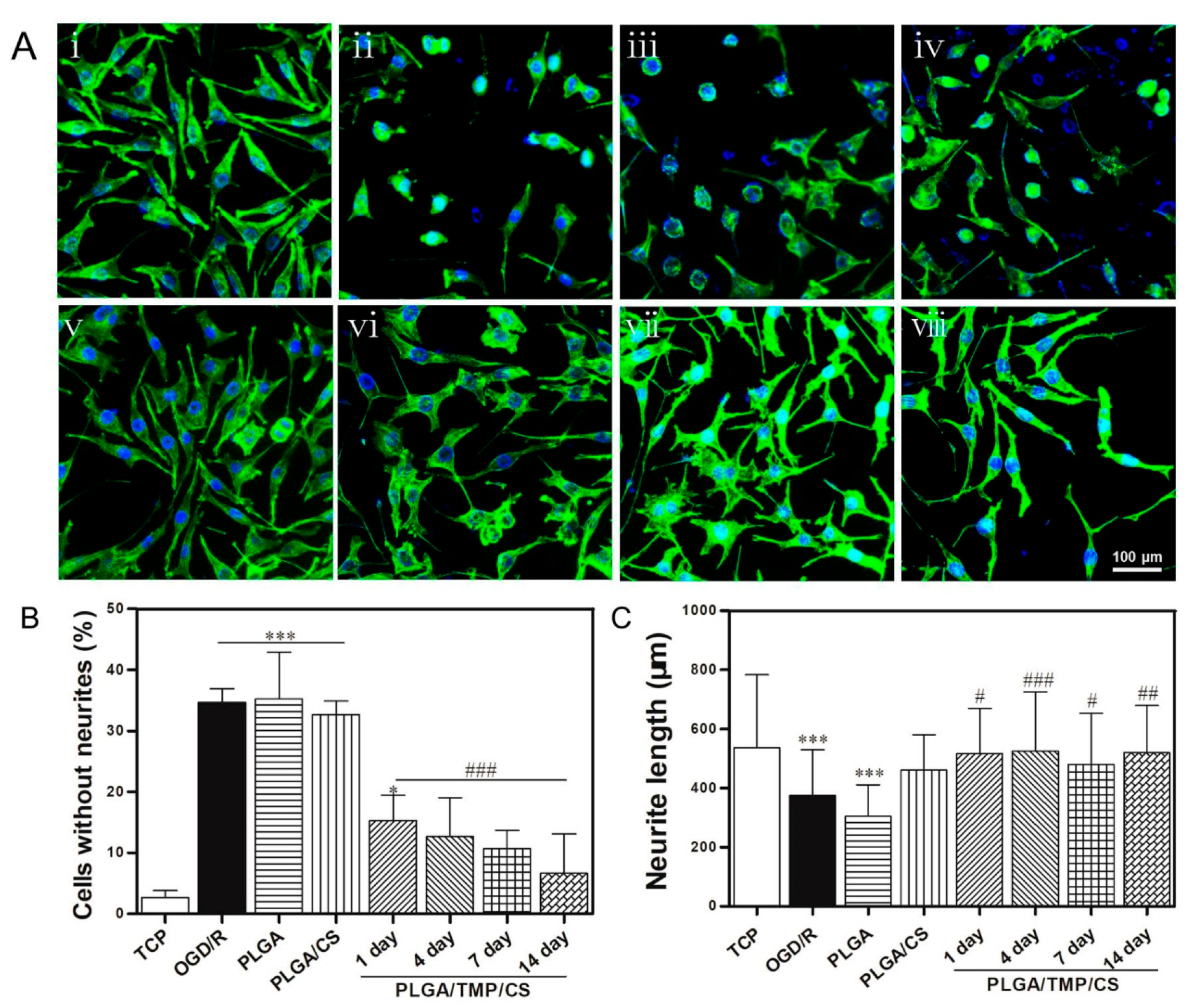
3.7. PLGA/TMP/CS NDM Promote Nerve Repair
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suwanprateeb, J.; Luangwattanawilai, T.; Theeranattapong, T.; Suvannapruk, W.; Chumnanvej, S.; Hemstapat, W. Bilayer oxidized regenerated cellulose/poly ε-caprolactone knitted fabric-reinforced composite for use as an artificial dural substitute. J. Mater. Sci. Mater. Electron. 2016, 27, 122. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ma, X.; Chen, S.; Tao, M.; Yuan, L.; Jing, Y. Bacterial Cellulose Membranes Used as Artificial Substitutes for Dural Defection in Rabbits. Int. J. Mol. Sci. 2014, 15, 10855–10867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramot, Y.; Harnof, S.; Klein, I.; Amouyal, N.; Steiner, M.; Manassa, N.N.; Bahar, A.; Rousselle, S.; Nyska, A. Local Tolerance and Biodegradability of a Novel Artificial Dura Mater Graft Following Implantation Onto a Dural Defect in Rabbits. Toxicol. Pathol. 2020, 48, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Miyamoto, S.; Nagata, I.; Kikuchi, H.; Ikada, Y.; Iwata, H.; Yamamoto, K. Development of a dural substitute from synthetic bioabsorbable polymers. J. Neurosurg. 1997, 86, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Parlato, C.; Di Nuzzo, G.; Luongo, M.; Parlato, R.S.; Accardo, M.; Cuccurullo, L.; Moraci, A. Use of a collagen biomatrix (TissuDura®) for dura repair: A long-term neuroradiological and neuropathological evaluation. Acta Neurochir. 2010, 153, 142–147. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Guo, H.-F.; Ying, D.-J. Multilayer scaffold of electrospun PLA-PCL-collagen nanofibers as a dural substitute. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1359–1366. [Google Scholar] [CrossRef]
- Pogorielov, M.; Kravtsova, A.; Reilly, G.C.; Deineka, V.; Tetteh, G.; Kalinkevich, O.; Pogorielova, O.; Moskalenko, R.; Tkach, G. Experimental evaluation of new chitin–chitosan graft for duraplasty. J. Mater. Sci. Mater. Med. 2017, 28, 34. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Mooney, D. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials 1996, 17, 115–124. [Google Scholar] [CrossRef]
- Gelperina, S.; Maksimenko, O.; Khalansky, A.; Vanchugova, L.; Shipulo, E.; Abbasova, K.; Berdiev, R.; Wohlfart, S.; Chepurnova, N.; Kreuter, J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: Influence of the formulation parameters. Eur. J. Pharm. Biopharm. 2010, 74, 157–163. [Google Scholar] [CrossRef]
- Wohlfart, S.; Khalansky, A.S.; Gelperina, S.; Maksimenko, O.; Bernreuther, C.; Glatzel, M.; Kreuter, J. Efficient Chemotherapy of Rat Glioblastoma Using Doxorubicin-Loaded PLGA Nanoparticles with Different Stabilizers. PLoS ONE 2011, 6, e19121. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, P.; Kong, L. Chitosan-Modified PLGA Nanoparticles with Versatile Surface for Improved Drug Delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Nawrotek, K.; Tylman, M.; Rudnicka, K.; Gatkowska, J.; Wieczorek, M. Epineurium-mimicking chitosan conduits for peripheral nervous tissue engineering. Carbohydr. Polym. 2016, 152, 119–128. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Park, H.-N.; Kim, K.-H.; Lee, M.-H.; Choi, Y.S.; Park, Y.-J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Han, S.-B.; et al. Biological Evaluation of Chitosan Nanofiber Membrane for Guided Bone Regeneration. J. Periodontol. 2005, 76, 1778–1784. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, H.; Lv, C.; Xing, X. The systematic effects of chitosan on fibroblasts derived from hypertrophic scars and keloids. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 520. [Google Scholar] [CrossRef]
- Zhou, J. Reduction in postoperative adhesion formation and re-formation after an abdominal operation with the use of N, O-carboxymethyl chitosan. Surgery 2004, 135, 307–312. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Li, Q.; Youn, H.-Y.; Kim, D.Y. Oral Administration of Chitosan Attenuates Bleomycin-induced Pulmonary Fibrosis in Rats. In Vivo 2019, 33, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Huang, Z.; Pu, X.; Yin, G.; Zhang, J. Fabrication of Chitosan/Polypyrrole-coated poly(L-lactic acid)/Polycaprolactone aligned fibre films for enhancement of neural cell compatibility and neurite growth. Cell Prolif. 2019, 52, e12588. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Gong, J.; Yang, L.; Niu, C.; Ni, X.; Wang, Y.; Peng, S.; Gu, X.; Sun, C.; et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 2017, 134, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Wang, X.; Yan, X.; Miao, Y.; Long, Y.-Z.; Yin, H.-L.; Sun, B.; Song, G. Fabrication and biocompatibility of poly(l-lactic acid) and chitosan composite scaffolds with hierarchical microstructures. Mater. Sci. Eng. C 2016, 64, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Masoomikarimi, M.; Bastami, F.; Jafarisani, M.; Alizadeh, M.; Mehrabi, M.; Salehi, M. Fabrication and Characterization of Nanofibrous Poly (L-Lactic Acid)/Chitosan-Based Scaffold by Liquid–Liquid Phase Separation Technique for Nerve Tissue Engineering. Mol. Biotechnol. 2021, 63, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.K.T.; Shan, J.J.; Chiu, K.W. Tetramethylpyrazine, a Calcium Antagonist. Planta Med. 1996, 62, 431–435. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, J.; Cao, C.; Jia, Z.; Zhou, N.; Han, S.; Wang, Y.; Xu, Y.; Cui, H. Tetramethylpyrazine promotes SH-SY5Y cell differentiation into neurons through epigenetic regulation of Topoisomerase IIβ. Neuroscience 2014, 278, 179–193. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Q.; Ge, J.; Tan, Z. Protective effects of tetramethylpyrazine on rat retinal cell cultures. Neurochem. Int. 2008, 52, 1176–1187. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Pan, X.; Georgakilas, A.G.; Chen, P.; Hu, H.; Yang, Y.; Tian, S.; Xia, L.; Zhang, J.; Cai, X.; et al. Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits glioma by down regulating chemokine receptor CXCR4 expression. Cancer Lett. 2013, 336, 281–289. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Lin, Y.-Y.; Chou, S.-C.; Tsai, T.-H.; Kao, L.-S.; Hsu, S.-Y.; Cheng, F.-C.; Shih, Y.-H.; Cheng, H.; Wang, J.-Y. Tetramethylpyrazine inhibits activities of glioma cells and glutamate neuro-excitotoxicity: Potential therapeutic application for treatment of gliomas. Neuro-Oncol. 2008, 10, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-Y.; Jia, Y.-H.; Sun, W.-G.; Tang, Y.; An, G.-S.; Ni, J.-H.; Jia, H.-T. Stabilization of mitochondrial function by tetramethylpyrazine protects against kainate-induced oxidative lesions in the rat hippocampus. Free Radic. Biol. Med. 2010, 48, 597–608. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Q.-H.; Yu, X.-D.; Jiang, D.-M. Additive effect of tetramethylpyrazine and deferoxamine in the treatment of spinal cord injury caused by aortic cross-clamping in rats. Spinal Cord 2010, 49, 302–306. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Liu, Y.; Qi, C.; Qiu, F.; Chen, X.; Zhang, J.; Yang, P. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol. Res. 2010, 32, 547–555. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Chen, X.; Zhang, H.; Shi, Q.; Zhang, J.; Yang, P. Tetramethylpyrazine promotes proliferation and differentiation of neural stem cells from rat brain in hypoxic condition via mitogen-activated protein kinases pathway in vitro. Neurosci. Lett. 2010, 474, 26–31. [Google Scholar] [CrossRef]
- Feng, J.; Li, F.; Zhao, Y.; Feng, Y.; Abe, Y. Brain pharmacokinetics of tetramethylpyrazine after intranasal and intravenous administration in awake rats. Int. J. Pharm. 2009, 375, 55–60. [Google Scholar] [CrossRef]
- Xia, H.; Cheng, Z.; Cheng, Y.; Xu, Y. Investigating the passage of tetramethylpyrazine-loaded liposomes across blood-brain barrier models in vitro and ex vivo. Mater. Sci. Eng. C 2016, 69, 1010–1017. [Google Scholar] [CrossRef]
- Xia, H.; Jin, H.; Cheng, Y.; Cheng, Z.; Xu, Y. The Controlled Release and Anti-Inflammatory Activity of a Tetramethylpyrazine-Loaded Thermosensitive Poloxamer Hydrogel. Pharm. Res. 2019, 36, 52. [Google Scholar] [CrossRef]
- Wu, T.; Huang, C.; Li, D.; Yin, A.; Liu, W.; Wang, J.; Chen, J.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. A multi-layered vascular scaffold with symmetrical structure by bi-directional gradient electrospinning. Colloids Surf. B Biointerfaces 2015, 133, 179–188. [Google Scholar] [CrossRef]
- Reis, K.; Sperling, L.; Teixeira, C.; Sommer, L.; Colombo, M.; Koester, L.; Pranke, P. VPA/PLGA microfibers produced by coaxial electrospinning for the treatment of central nervous system injury. Braz. J. Med Biol. Res. 2020, 53, e8993. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Zhang, Y.; Zhao, X.; Shao, L.; Liu, G.; Sun, C.; Xu, R.; Zhang, Z. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 2021, 93, 312–321. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.; Gao, M.; Zhang, R.; Gao, J.; Sun, J.; Chai, Q.; Wu, T.; Liang, K.; Chen, P.; et al. Super-Assembled Periodic Mesoporous Organosilica Frameworks for Real-Time Hypoxia-Triggered Drug Release and Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 50246–50257. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Jia, Y.; Zhang, H.; Tang, T. Hydroxypropyltrimethyl Ammonium Chloride Chitosan Functionalized-PLGA Electrospun Fibrous Membranes as Antibacterial Wound Dressing: In Vitro and In Vivo Evaluation. Polymers 2017, 9, 697. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Rao, S.; Zhu, J.; Wang, Z.; Zhang, Y.; Duan, Y.; Chen, X.; Yin, J. Nanoporous multilayer poly(l-glutamic acid)/chitosan microcapsules for drug delivery. Int. J. Pharm. 2012, 427, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xue, J.; Jia, L.; Du, Q.; Niu, J.; Zhang, D. Surface-modified PLGA nanoparticles with chitosan for oral delivery of tolbutamide. Colloids Surfaces B Biointerfaces 2018, 161, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, J.; Scholze, M.; Waddell, J.N.; Ondruschka, B.; Hammer, N. Mechanical Properties of Human Dura Mater in Tension—An Analysis at an Age Range of 2 to 94 Years. Sci. Rep. 2019, 9, 16655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Shi, G.; Tang, J.; Cheng, R.; Shen, X.; Gu, Y.; Wu, L.; Xi, K.; Zhao, Y.; Cui, W.; et al. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci. Adv. 2020, 6, eabc2036. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Lin, H.; Cao, L.; Cheng, J.; Dong, J.; Yu, L.; Ding, J. Efficacy of Poly(d,l-Lactic Acid-co-Glycolic acid)-Poly(Ethylene Glycol)-Poly(d,l-Lactic Acid-co-Glycolic Acid) Thermogel As a Barrier to Prevent Spinal Epidural Fibrosis in a Postlaminectomy Rat Model. Clin. Spine Surg. A Spine Publ. 2017, 30, E283–E290. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Liu, H.; Yin, J.; Cui, L.; Chen, Z. The prevention effect of poly (l-glutamic acid)/chitosan on spinal epidural fibrosis and peridural adhesion in the post-laminectomy rabbit model. Eur. Spine J. 2014, 23, 2423–2431. [Google Scholar] [CrossRef]
- Chen, X.-G.; Wang, Z.; Liu, W.-S.; Park, H.-J. The effect of carboxymethyl-chitosan on proliferation and collagen secretion of normal and keloid skin fibroblasts. Biomaterials 2002, 23, 4609–4614. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Sun, C.; Hu, W.; Zhao, J.; Li, G.; Zhang, L.; Liu, M.; Liu, Y.; Ding, F.; et al. Chitosan Degradation Products Promote Nerve Regeneration by Stimulating Schwann Cell Proliferation via miR-27a/FOXO1 Axis. Mol. Neurobiol. 2014, 53, 28–39. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, L.; Liu, S.; Wang, X. Tetramethylpyrazine Protects Neurons from Oxygen-Glucose Deprivation-Induced Death. Med Sci. Monit. 2017, 23, 5277–5282. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Fu, Y.; Sun, H.; Liu, X. Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life 2017, 70, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, T.; Wu, L.; Zhou, X.; Gu, J.; Li, C.; Liu, W.; Long, C.; Yang, X.; Shan, L.; et al. Neuroprotective Effect and Mechanism of Action of Tetramethylpyrazine Nitrone for Ischemic Stroke Therapy. Neuromol. Med. 2018, 20, 97–111. [Google Scholar] [CrossRef]
- Cai, X.; Yang, Y.; Chen, P.; Ye, Y.; Liu, X.; Wu, K.; Yu, M. Tetramethylpyrazine Attenuates Transdifferentiation of TGF-β2–Treated Human Tenon’s Fibroblasts. Investig. Opthalmology Vis. Sci. 2016, 57, 4740–4748. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Hernández, J.D.; Balderas-Márquez, J.E.; Carranza, M.; Luna, M.; Martínez-Moreno, C.G.; Arámburo, C. Growth Hormone (GH) Enhances Endogenous Mechanisms of Neuroprotection and Neuroplasticity after Oxygen and Glucose Deprivation Injury (OGD) and Reoxygenation (OGD/R) in Chicken Hippocampal Cell Cultures. Neural Plast. 2021, 2021, 9990166. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Wu, T.; Cui, Y.; Zhao, R.; Wan, Q.; Xu, R. Design and Fabrication of Nanofibrous Dura Mater with Antifibrosis and Neuroprotection Effects on SH-SY5Y Cells. Polymers 2022, 14, 1882. https://doi.org/10.3390/polym14091882
Zhao Z, Wu T, Cui Y, Zhao R, Wan Q, Xu R. Design and Fabrication of Nanofibrous Dura Mater with Antifibrosis and Neuroprotection Effects on SH-SY5Y Cells. Polymers. 2022; 14(9):1882. https://doi.org/10.3390/polym14091882
Chicago/Turabian StyleZhao, Zhiyuan, Tong Wu, Yu Cui, Rui Zhao, Qi Wan, and Rui Xu. 2022. "Design and Fabrication of Nanofibrous Dura Mater with Antifibrosis and Neuroprotection Effects on SH-SY5Y Cells" Polymers 14, no. 9: 1882. https://doi.org/10.3390/polym14091882
APA StyleZhao, Z., Wu, T., Cui, Y., Zhao, R., Wan, Q., & Xu, R. (2022). Design and Fabrication of Nanofibrous Dura Mater with Antifibrosis and Neuroprotection Effects on SH-SY5Y Cells. Polymers, 14(9), 1882. https://doi.org/10.3390/polym14091882






