Phyto Synthesis of Manganese-Doped Zinc Nanoparticles Using Carica papaya Leaves: Structural Properties and Its Evaluation for Catalytic, Antibacterial and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Reagents
2.2. Collection and Preparation of C. papaya Leaves Extract
2.3. Green Synthesis of C. papaya-Manganese–Zinc Nanoparticles (C. papaya-Mn-Zn NPs)
2.4. Analysis of Green Synthesized Mn-Zn Bimetal Oxide Nanoparticles
2.5. Anti-Oxidant Activity
2.5.1. DPPH
2.5.2. Hydrogen Peroxide (H2O2) Radical Scavenging Assay
2.5.3. Hydroxyl-Radical Scavenging Activity
2.5.4. Ferric Reducing Antioxidant Power Assay
2.6. Antibacterial Activity
2.7. Photocatalytic Activity of C. papaya-Mn-Zn NPs
2.8. Conversion of p-Nitrophenol to p-Aminophenol
3. Results and Discussion
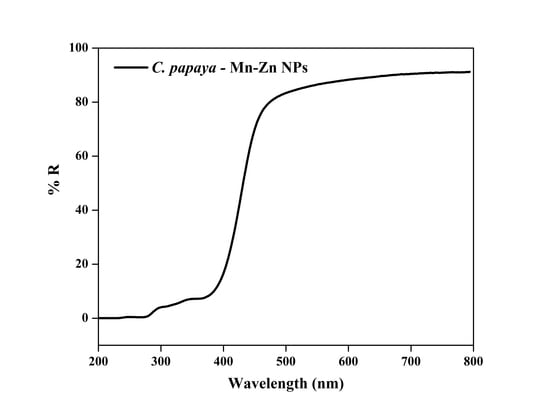
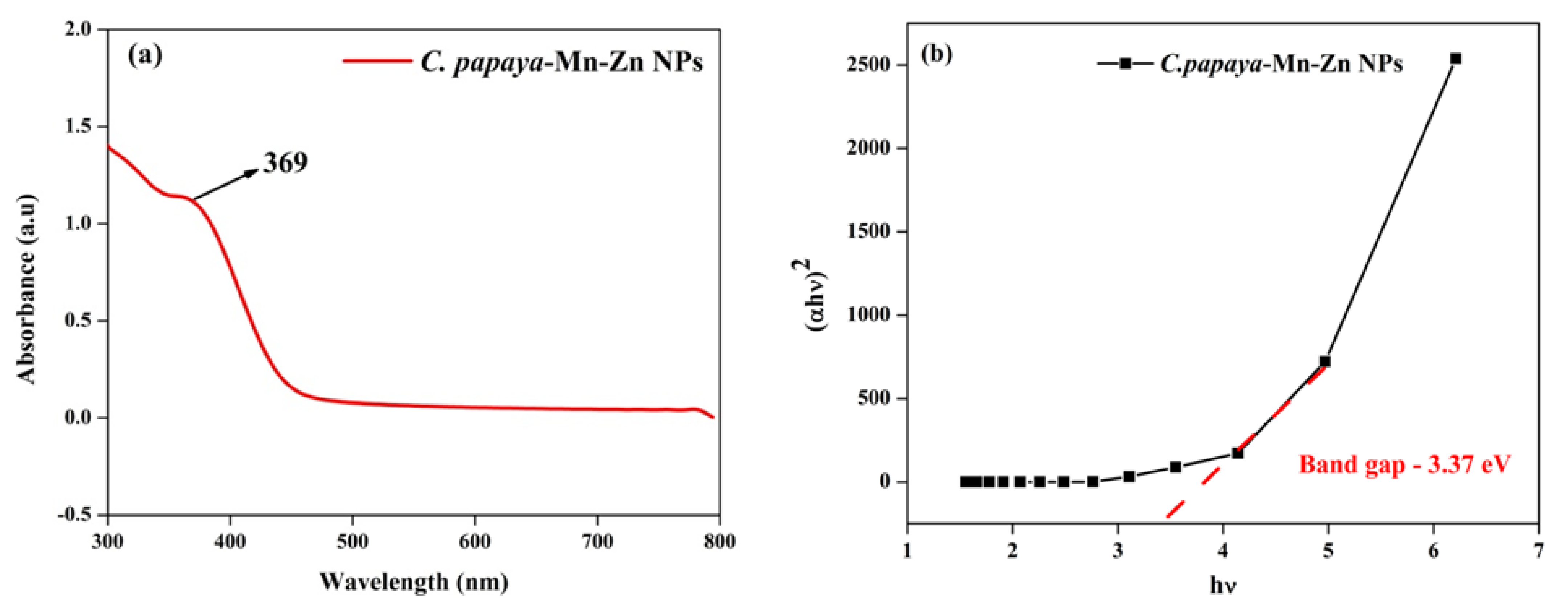
3.1. UV-Vis Spectra Analysis
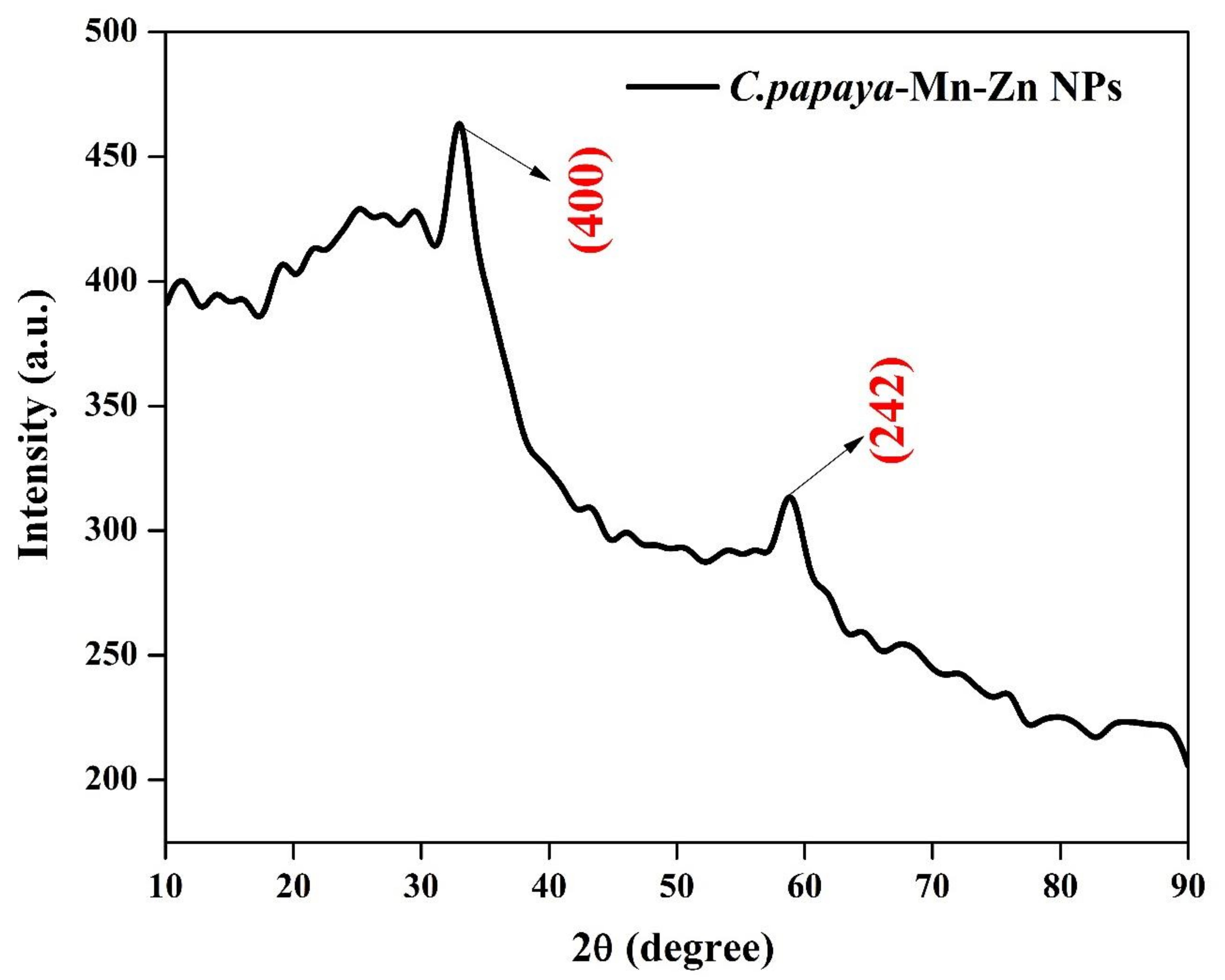
3.2. XRD Analysis
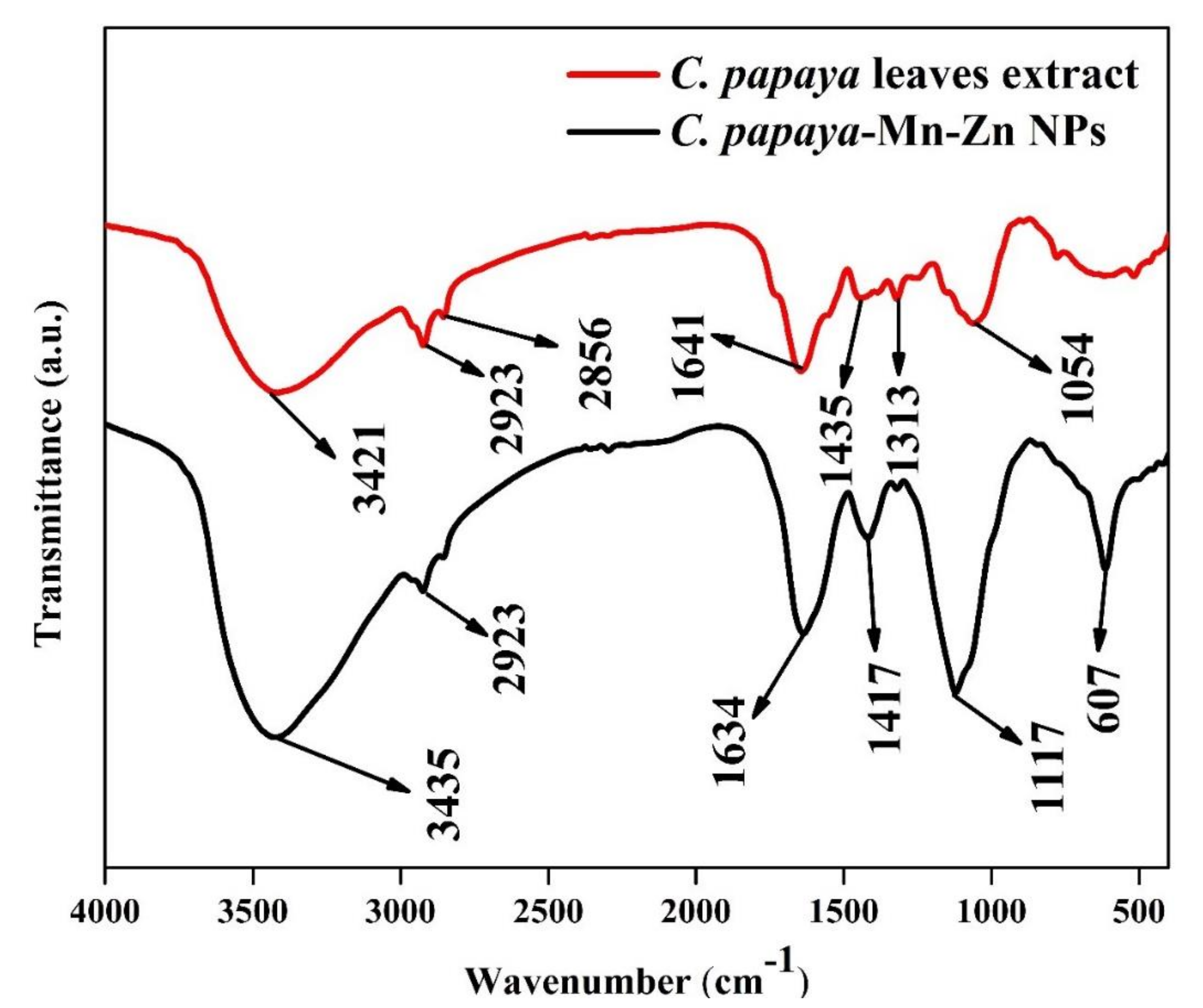
3.3. FT-IR Analysis
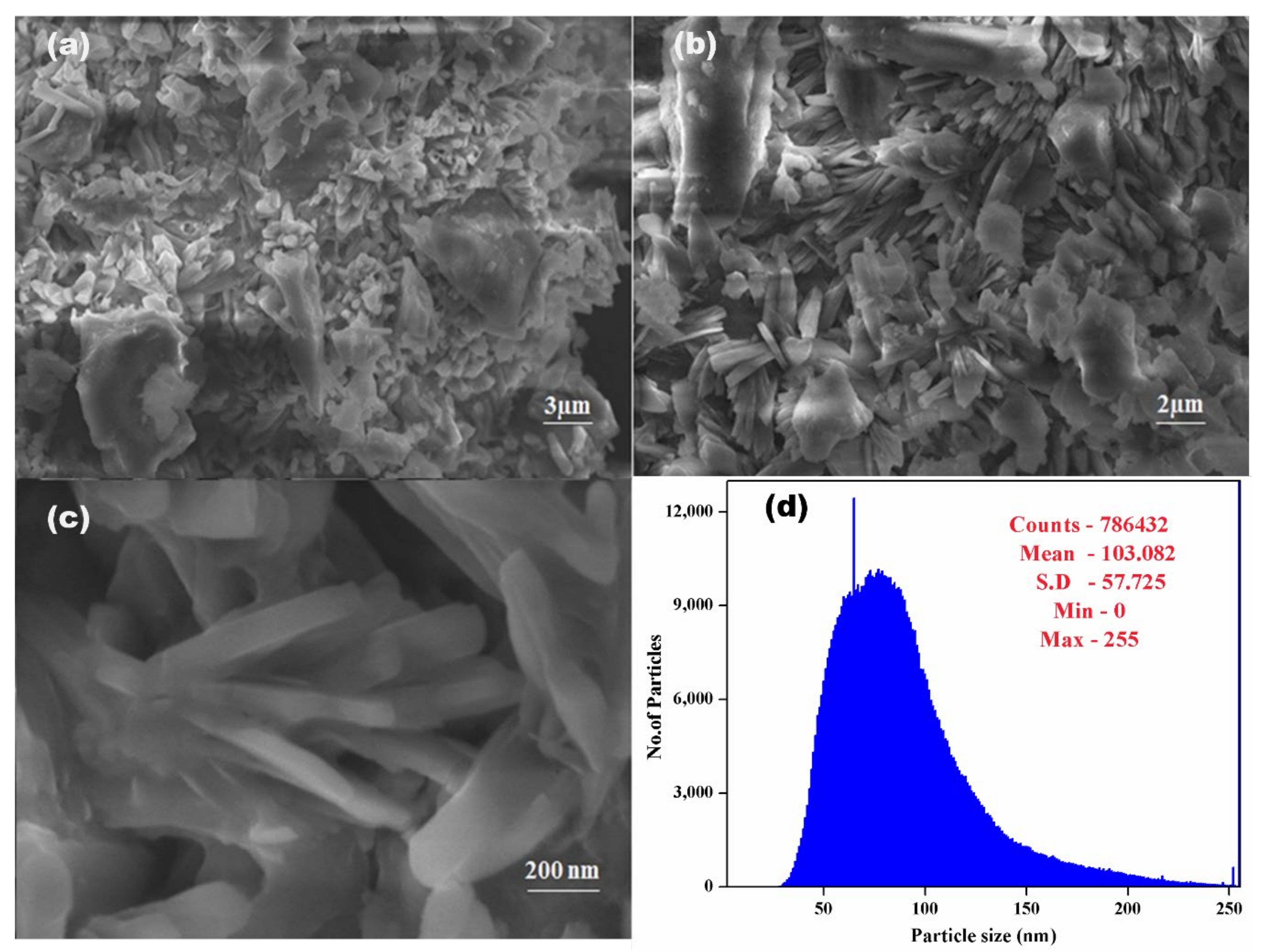
3.4. SEM-EDX
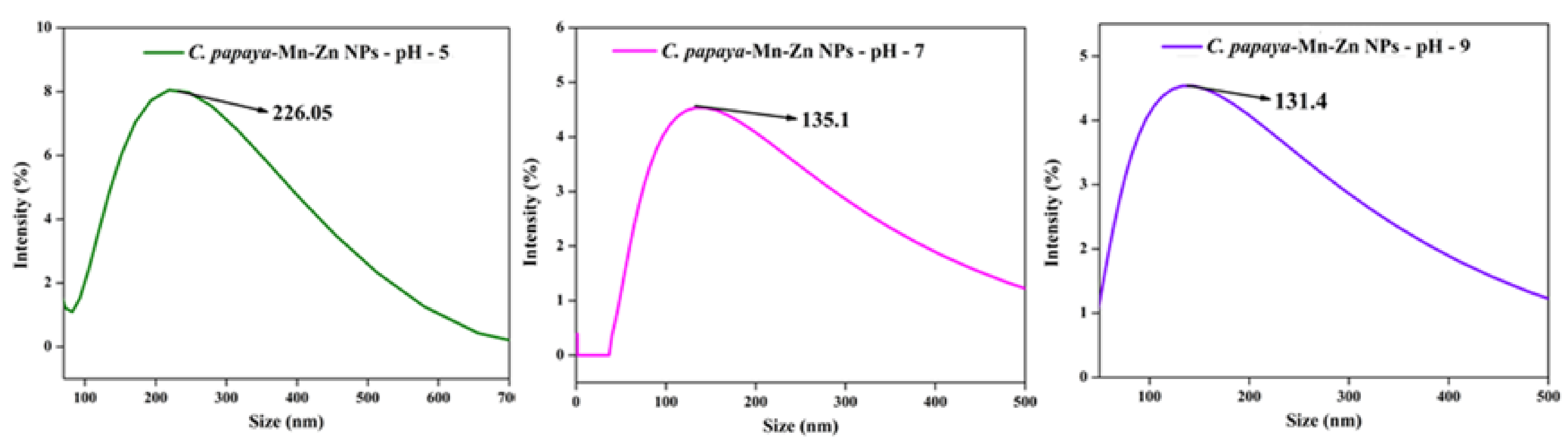
3.5. DLS Analysis
3.6. Effect of pH on Optical Spectra and DLS
3.7. Antioxidant Activity
3.7.1. DPPH Scavenging Assay
3.7.2. Hydrogen Peroxide-Scavenging Assay
3.7.3. Hydroxyl-Radical Scavenging Activity
3.7.4. Ferric-Reducing Antioxidant Power (FRAP)
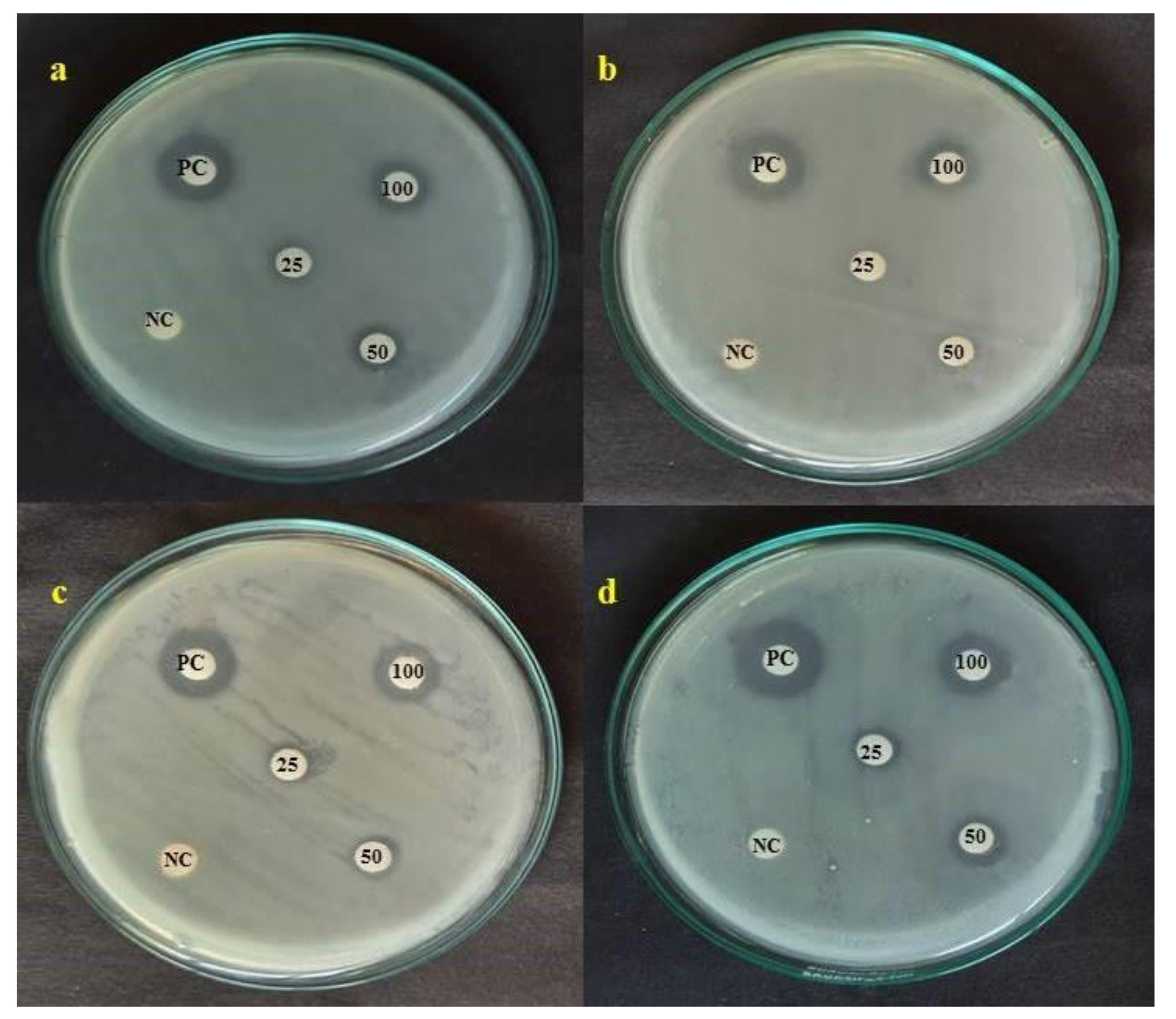
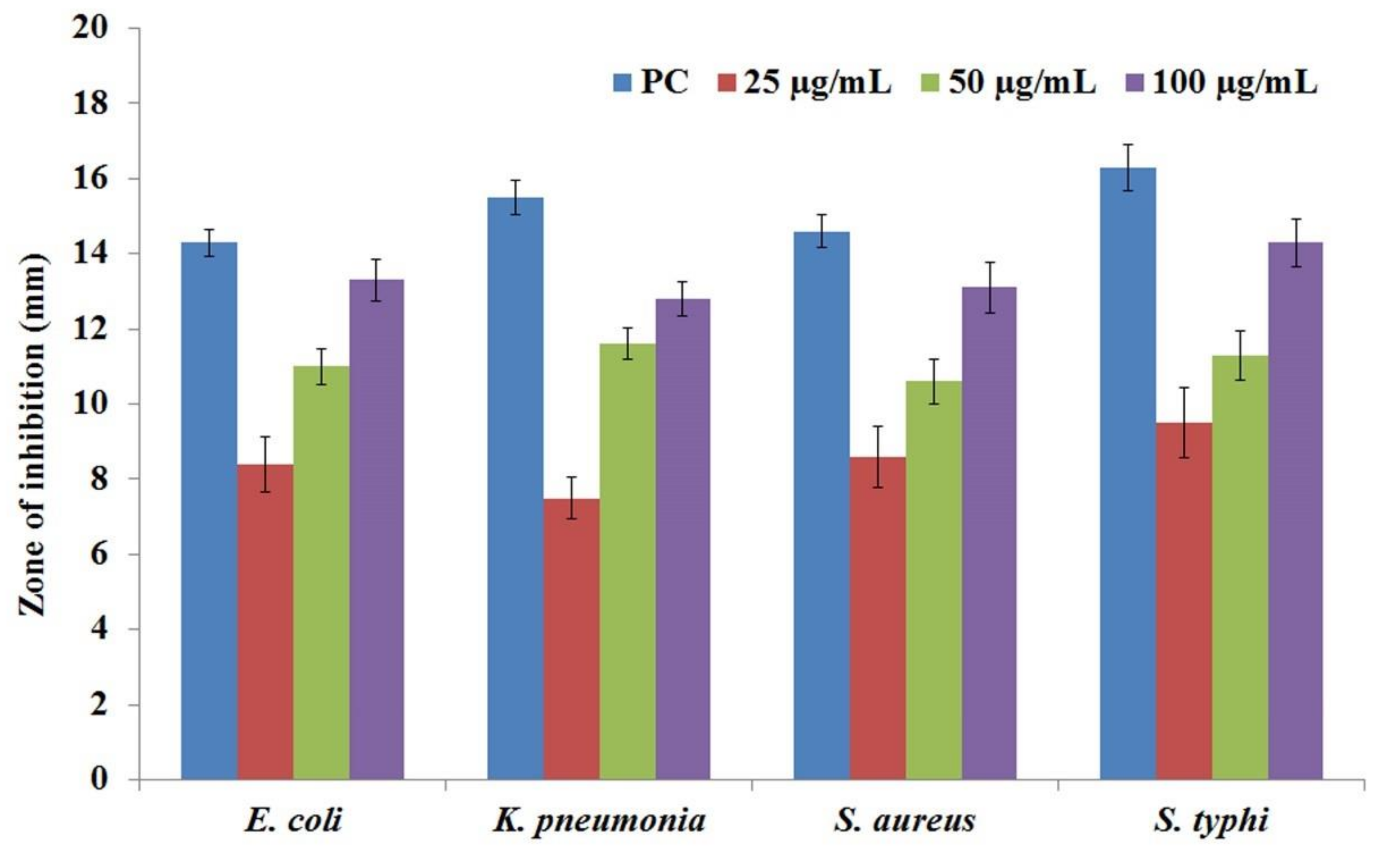
3.8. Antibacterial Activity
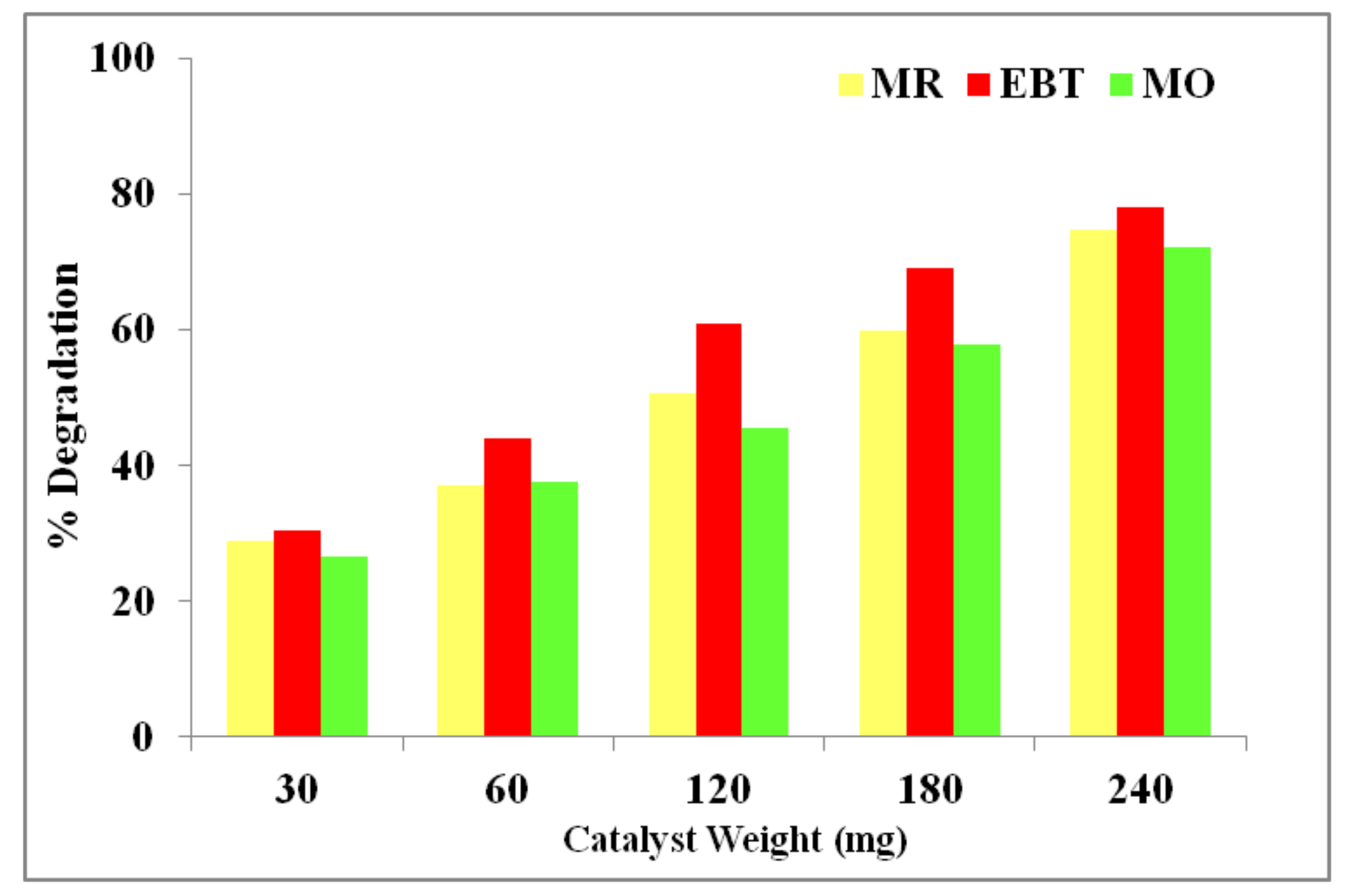
3.9. Photodegradation Activity
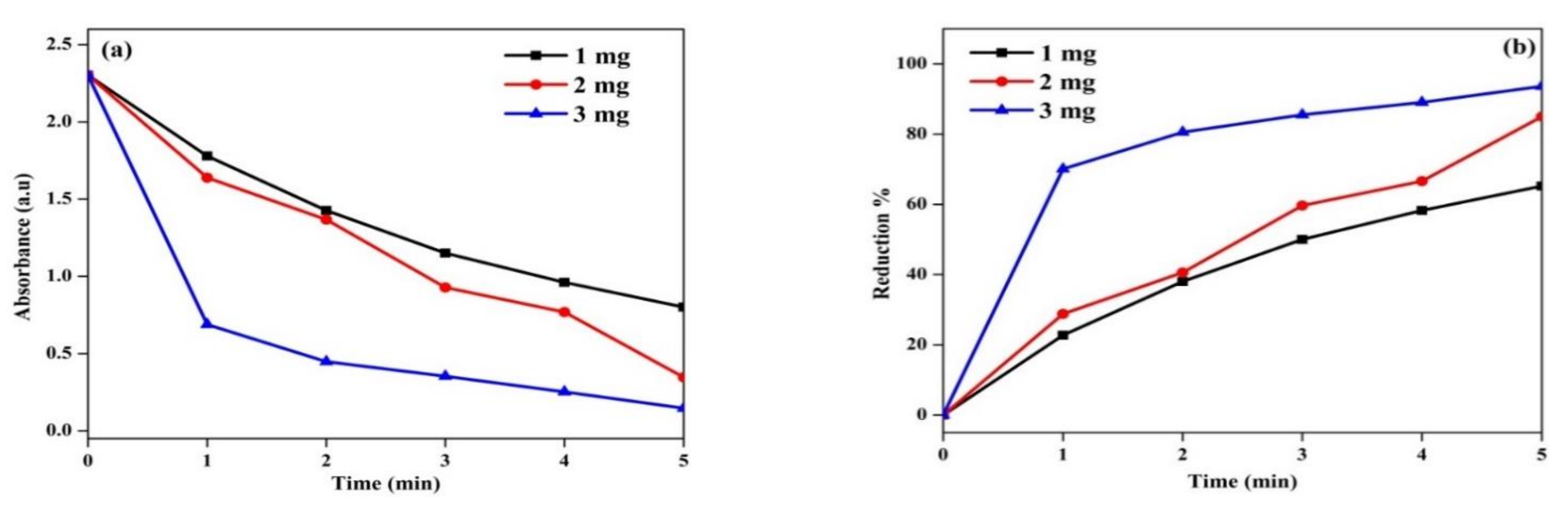
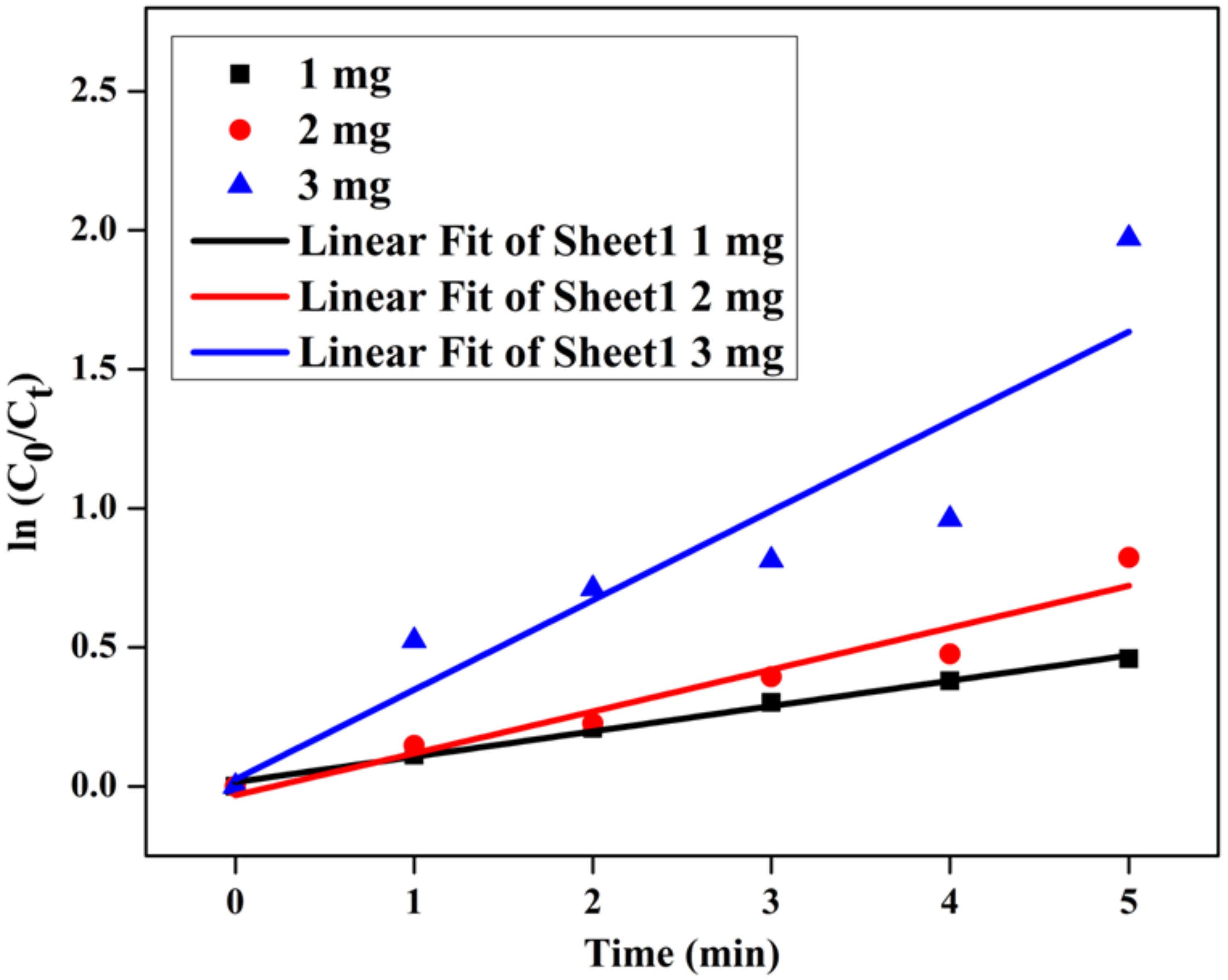
3.10. Catalytic Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Ben Slama, H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Li, N.; Zhang, F.; Wang, H.; Hou, S. Catalytic Degradation of 4-Nitrophenol in Polluted Water by Three-Dimensional Gold Nanoparticles/Reduced Graphene Oxide Microspheres. Eng. Sci. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Verdine, J.C. Metal oxides in heterogeneous oxidation catalysis: State of the art and challenges for a more sustainable world. ChemSusChem 2019, 12, 577–588. [Google Scholar]
- Zhou, S.; Du, Z.; Li, X.; Zhang, Y.; He, Y.; Zhang, Y. Degradation of methylene blue by anatural manganese oxides: Kinetics and transformation products. R. Soc. Open Sci. 2019, 6, 190351. [Google Scholar] [CrossRef] [Green Version]
- Hoseinpour, V.; Ghaemi, N. Green synthesis of manganese nanoparticles: Applications and future perspective—A review. J. Photochem. Photobiol. B Biol. 2018, 189, 234–243. [Google Scholar] [CrossRef]
- Devi, S.M.; Nivetha, A.; Prabha, I. Role of citric acid/glycine-reinforced nanometal oxide for the enhancement of physio-chemical specifications in catalytic properties. J. Supercond. Nov. Magn. 2020, 33, 3893–3901. [Google Scholar] [CrossRef]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Green synthesis and characterization of manganese nanoparticles using natural plant extracts and its evaluation of antimicrobial activity. J. Appl. Pharm. Sci. 2015, 5, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.W.; Khatoon, U.; Qurashi, A. Synthesis and characterization of Cu-SnO2 nanoparticles deposited on glass using ultrasonic spray pyrolysis and their H2S sensing properties. Curr. Nanosci. 2012, 8, 919–924. [Google Scholar] [CrossRef]
- Prasad, K.S.; Patra, A. Green synthesis of MnO2 nanorods using Phyllanthus amarus plant extract and their fluorescence studies. Green Process Synth. 2017, 6, 549–554. [Google Scholar] [CrossRef]
- Alam, M.W.; Wang, Z.; Naka, S.; Okada, H. Performance enhancement of top-contact pentacene-based organic thin-film transistors with bilayer WO3/Au electrodes. Jpn. J. Appl. Phys. 2013, 52, 03BB08. [Google Scholar] [CrossRef]
- Zhou, J.; Xua, N.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Exp. Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.L.; Lai, C.W.; Hamid, S.B.A. Tunable band gap energy of Mn-doped ZnO nanoparticles using the co-precipitation technique. J. Nanomater. 2014, 2014, 371720. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.W.; Xu, Z.; Zhang, F.J.; Zhao, S.L.; Lu, L.F. Structure, optical and magnetic properties of Mn-doped ZnO films prepared by sputtering. Int. J. Min. Met. Mater. 2010, 17, 475–480. [Google Scholar] [CrossRef]
- Sangeetha, R.; Muthukumaran, S.; Ashokkumar, M. Structural, optical, dielectric and antibacterial studies of Mn doped Zn0.96Cu0.04O nanoparticles. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 144, 1–7. [Google Scholar] [CrossRef]
- Dhanemozhi, A.C.; Rajeswari, V.; Sathyajothi, S. Green Synthesis of Zinc Oxide Nanoparticle Using Green Tea Leaf Extract for Supercapacitor Application. Mater. Today Proc. 2017, 4, 660–667. [Google Scholar] [CrossRef]
- Subbiah, R.; Muthukumaran, S.; Raja, V. Phyto-assisted synthesis of Mn and Mg Co-doped ZnO nanostructures using Carica papaya leaf extract for photocatalytic applications. BioNanoScience 2021, 11, 1127–1141. [Google Scholar] [CrossRef]
- Pallavi, S.; Nainika, T.; Trisha, S.; Aishwarya, G.; Sargam, V. Phyotochemical screening and analysis of Carica papaya, Agave Americana and piper nigrum. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1786–1794. [Google Scholar]
- Rajagopal, G.; Nivetha, A.; Ilango, S.; Muthudevi, G.P.; Prabha, I.; Arthimanju, R. Phytofabrication of selenium nanoparticles using Azolla pinnata: Evaluation of catalytic properties in oxidation, antioxidant and antimicrobial activities. J. Environ. Chem. Eng. 2021, 9, 105483. [Google Scholar] [CrossRef]
- Juan, D.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2012, 1826, 443–457. [Google Scholar]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2019, 14, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Herrmann, C.; Kömpe, K.; Haase, M.; Schlücker, S. Synthesis of bifunctional Au/Pt/Au core/shell nanoraspberries for in situ SERS monitoring of platinum-catalyzed reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Bashir, W.; Kanwal, S.; Iqbal, A. Synthesis, characterization and evaluation of biological activities of manganese-doped zinc oxide nanoparticles. Trop. J. Pharm. Res. 2017, 16, 2331–2339. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Amsaveni, P.; Nivetha, A.; Sakthivel, C.; Philip, C.S.; Prabha, I. Effectiveness of surfactants for unique hierarchical Mn2O3 as enhanced oxidative catalysts, antibacterial agents, and photocatalysts. J. Phys. Chem. Solids 2020, 144, 109249. [Google Scholar] [CrossRef]
- Dhanalakshmi, A.; Natarajan, B.; Ramadas, V.; Palanimurugan, A.; Thanikaikarasan, S. Structural, morphological, optical and antibacterial activity of rod-shaped zinc oxide and manganese doped zinc oxide nanoparticles. Pramana—J. Phys. 2016, 87, 57. [Google Scholar] [CrossRef]
- Khan, M.M.; Harunsani, M.H.; Tan, A.L.; Hojamberdiev, M.; Azamay, S.; Ahmad, N. Antibacterial activities of zinc oxide and Mn-doped ZnO synthesized using Melastoma malabathricum (L.) leaf extract. Bioprocess Biosyst. Eng. 2020, 43, 1499–1508. [Google Scholar] [CrossRef]
- Abdollahi, Y.; Abdullah, A.H.; Zainal, Z.; Yosof, N.A. Synthesis and characterization of manganses doped ZnO nanoparticles. Int. J. Basic Appl. Sci. 2011, 11, 62–69. [Google Scholar]
- Thakur, D.; Sharma, A.; Awasthi, A.; Rana, D.S.; Singh, D.; Pandey, S.; Thakur, S. Manganese-Doped Zinc Oxide Nanostructures as Potential Scaffold for Photocatalytic and Fluorescence Sensing Applications. Chemosensors 2020, 8, 120. [Google Scholar] [CrossRef]
- Das, P.; Ghosh, S.; Baskey, M. Heterogeneous catalytic reduction of 4-nitroaniline by RGO-Ni nanocomposite for water resource management. J. Mater. Sci. Mater. Electron. 2019, 30, 19731–19737. [Google Scholar] [CrossRef]
- Kayani; Nazir, Z.; Anjum, M.; Riaz, S.; Naseem, S.; Zeeshan, T. Role of Mn in biological, optical, and magnetic properties ZnO nano-particles. Appl. Phys. A 2020, 126, 1–17. [Google Scholar] [CrossRef]
- Bonifacio, M.A.R.; de Lucena Lira, H. Nanoparticles of ZnO doped with Mn: Structural and morphological characteristics. Mater. Res. 2017, 20, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Eltarahony, M.; Zaki, S.; ElKady, M.; Abd-El-Haleem, D. Biosynthesis, Characterization of Some Combined Nanoparticles, and Its Biocide Potency against a Broad Spectrum of Pathogens. J. Nanomater. 2018, 2018, 5263814. [Google Scholar] [CrossRef] [Green Version]
- Khoshnamvand, M.; Huo, C.; Liu, J. Silver nanoparticles synthesized using Allium ampeloprasum L. leaf extract: Characterization and performance in catalytic reduction of 4-nitrophenol and antioxidant activity. J. Mol. Struct. 2019, 1175, 90–96. [Google Scholar] [CrossRef]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Azeez, M.A.; Yekeen, T.A.; Akinboro, A. Evaluation of some biosynthesized silver nanoparticles for biomedical applications: Hydrogen peroxide scavenging, anticoagulant and thrombolytic activities. J. Clust. Sci. 2017, 28, 1379–1392. [Google Scholar] [CrossRef]
- Sundar, M.; Suresh, S.; Lingakumar, K. Influence of Caralluma adscendens Var. attenuata cold cream on UV-B damaged skin epidermal cells: A novel approach. 3 Biotech 2021, 11, 155. [Google Scholar]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.-K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 82–89. [Google Scholar] [CrossRef]
- Insoo, K.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Preparation and characterization of positively surface charged zinc oxide nanoparticles against bacterial pathogens. Microb. Pathog. 2020, 149, 104290. [Google Scholar]
- Rajagopal, G.; Manivannan, N.; Sundararajan, M.; Kumar, A.G.; Senthilkumar, S.; Mathivanan, N.; Ilango, S. Biocompatibility assessment of silver chloride nanoparticles derived from Padina gymnospora and its therapeutic potential. Nano Express 2021, 2, 010010. [Google Scholar] [CrossRef]
- Saeed, M.; Ahmad, A.; Boddula, R.; Ul Haq, A.; Azhar, A. Ag@ MnxOy: An effective catalyst for photo-degradation of rhodamine B dye. Environ. Chem. Lett. 2018, 16, 287–294. [Google Scholar] [CrossRef]
- Lashanizadegan, M.; Esfandiari, Z. Evaluation performance of Fe-Mn-Ce-O mixed metal oxides and Fe-Mn-Ce-O/Montmorillonite for adsorption of azo dyes in aqueous solution and oxidation reaction. Mater. Res. Express 2019, 6, 125028. [Google Scholar] [CrossRef]
- Mondal, A.; Mondal, A.; Adhikary, B.; Mukherjee, D.K. Cobalt nanoparticles as reusable catalysts for reduction of 4-nitrophenol under mild conditions. Bull. Mater. Sci. 2017, 40, 321–328. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.W.; Al Qahtani, H.S.; Aamir, M.; Abuzir, A.; Khan, M.S.; Albuhulayqah, M.; Mushtaq, S.; Zaidi, N.; Ramya, A. Phyto Synthesis of Manganese-Doped Zinc Nanoparticles Using Carica papaya Leaves: Structural Properties and Its Evaluation for Catalytic, Antibacterial and Antioxidant Activities. Polymers 2022, 14, 1827. https://doi.org/10.3390/polym14091827
Alam MW, Al Qahtani HS, Aamir M, Abuzir A, Khan MS, Albuhulayqah M, Mushtaq S, Zaidi N, Ramya A. Phyto Synthesis of Manganese-Doped Zinc Nanoparticles Using Carica papaya Leaves: Structural Properties and Its Evaluation for Catalytic, Antibacterial and Antioxidant Activities. Polymers. 2022; 14(9):1827. https://doi.org/10.3390/polym14091827
Chicago/Turabian StyleAlam, Mir Waqas, Hassan S. Al Qahtani, Muhammad Aamir, Alaaedeen Abuzir, Muhammad Shuaib Khan, Maryam Albuhulayqah, Shehla Mushtaq, Noushi Zaidi, and Ambikapathi Ramya. 2022. "Phyto Synthesis of Manganese-Doped Zinc Nanoparticles Using Carica papaya Leaves: Structural Properties and Its Evaluation for Catalytic, Antibacterial and Antioxidant Activities" Polymers 14, no. 9: 1827. https://doi.org/10.3390/polym14091827
APA StyleAlam, M. W., Al Qahtani, H. S., Aamir, M., Abuzir, A., Khan, M. S., Albuhulayqah, M., Mushtaq, S., Zaidi, N., & Ramya, A. (2022). Phyto Synthesis of Manganese-Doped Zinc Nanoparticles Using Carica papaya Leaves: Structural Properties and Its Evaluation for Catalytic, Antibacterial and Antioxidant Activities. Polymers, 14(9), 1827. https://doi.org/10.3390/polym14091827