A Study on the Dual Thermo- and pH-Responsive Behaviors of Well-Defined Star-like Block Copolymers Synthesize by Combining of RAFT Polymerization and Thiol-Ene Click Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
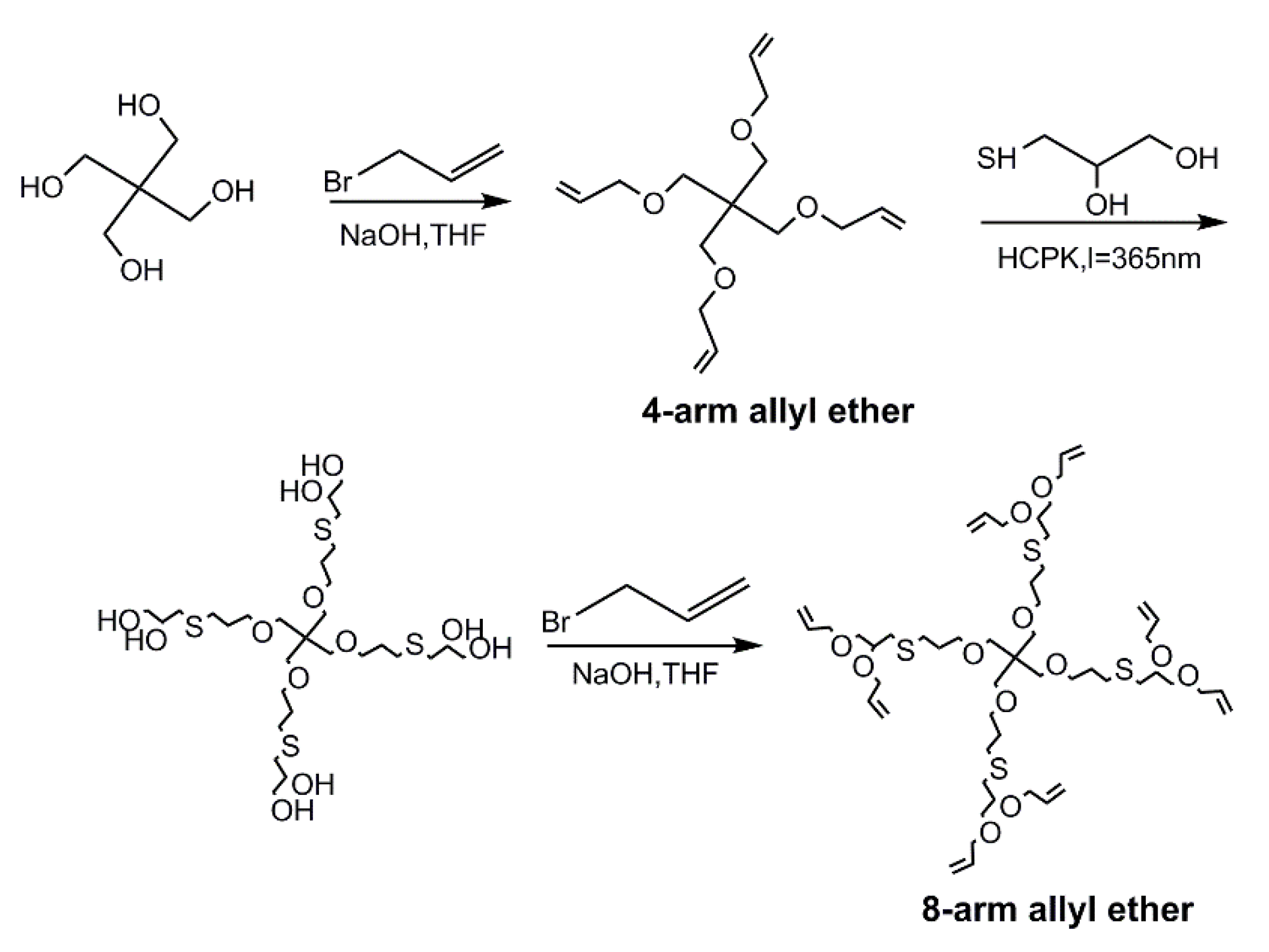
2.2. Preparation of 4-, 8-, and 12-Functionalized Cores via PITE Click Reaction
2.3. Preparation of PDMAEMA-b-PtBA Block Copolymer Arms via RAFT Polymerization
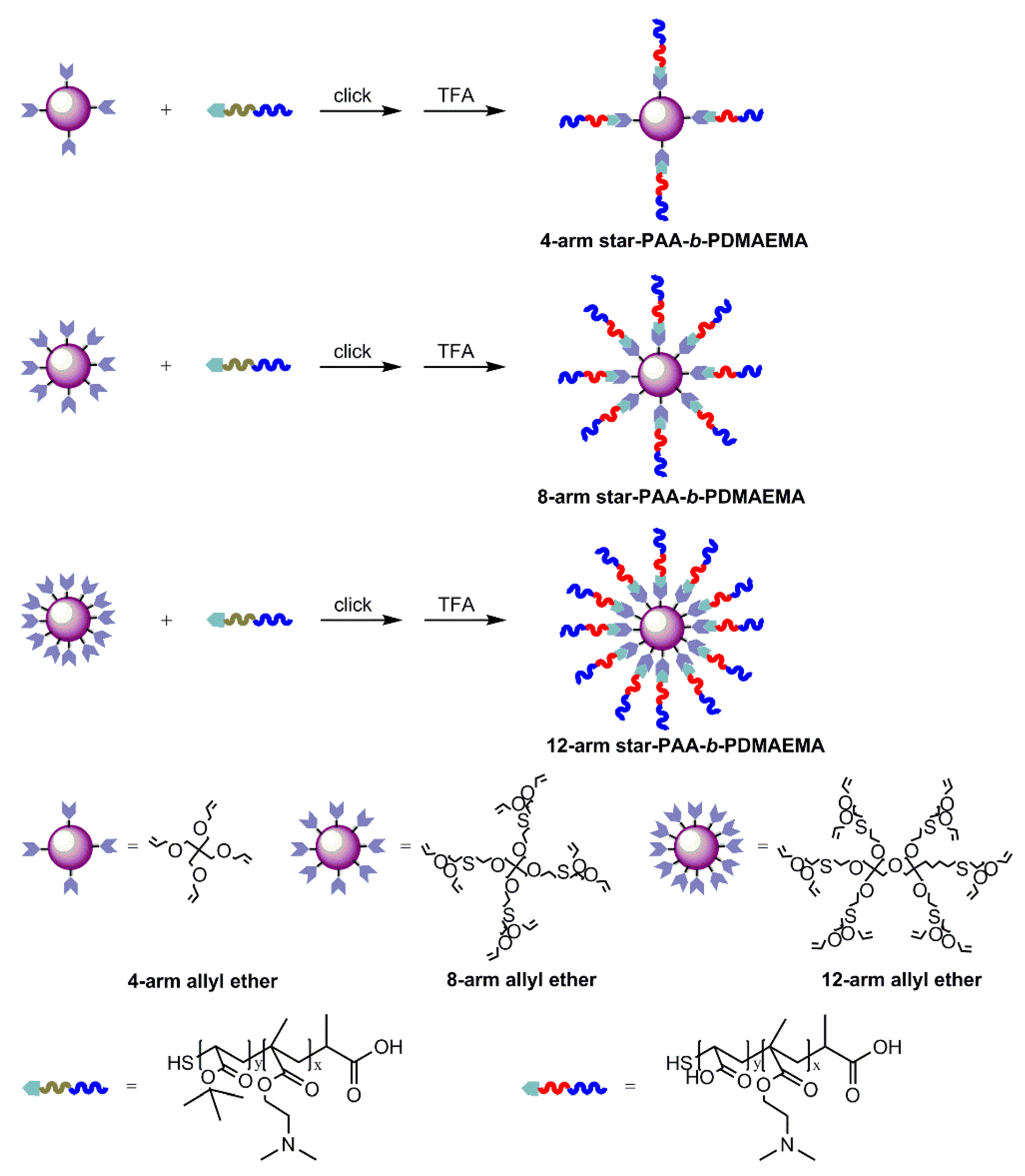
2.4. Preparation of Star-like Block Copolymers via PITE Click Reaction
2.5. Characterization
3. Results and Discussion
3.1. Preparation of Star-like Block Copolymers with Reverse-Ordered Blocks
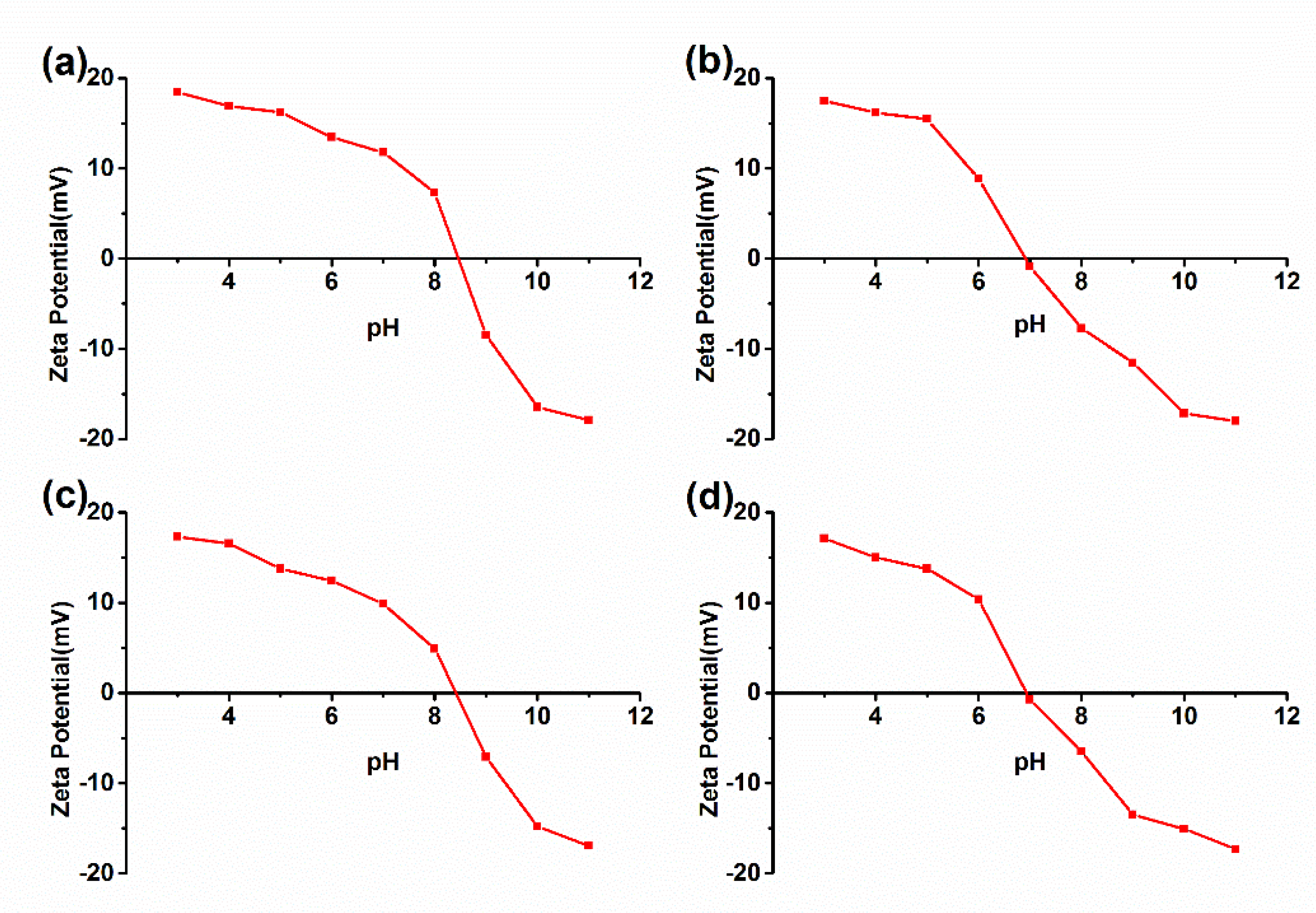
3.2. The Isoelectric Point (IEP) of the Multi-Arm Star-like Block Copolymers
3.3. pH- and Thermo-Responsive Behaviors of the Star-like Block Copolymer Solutions
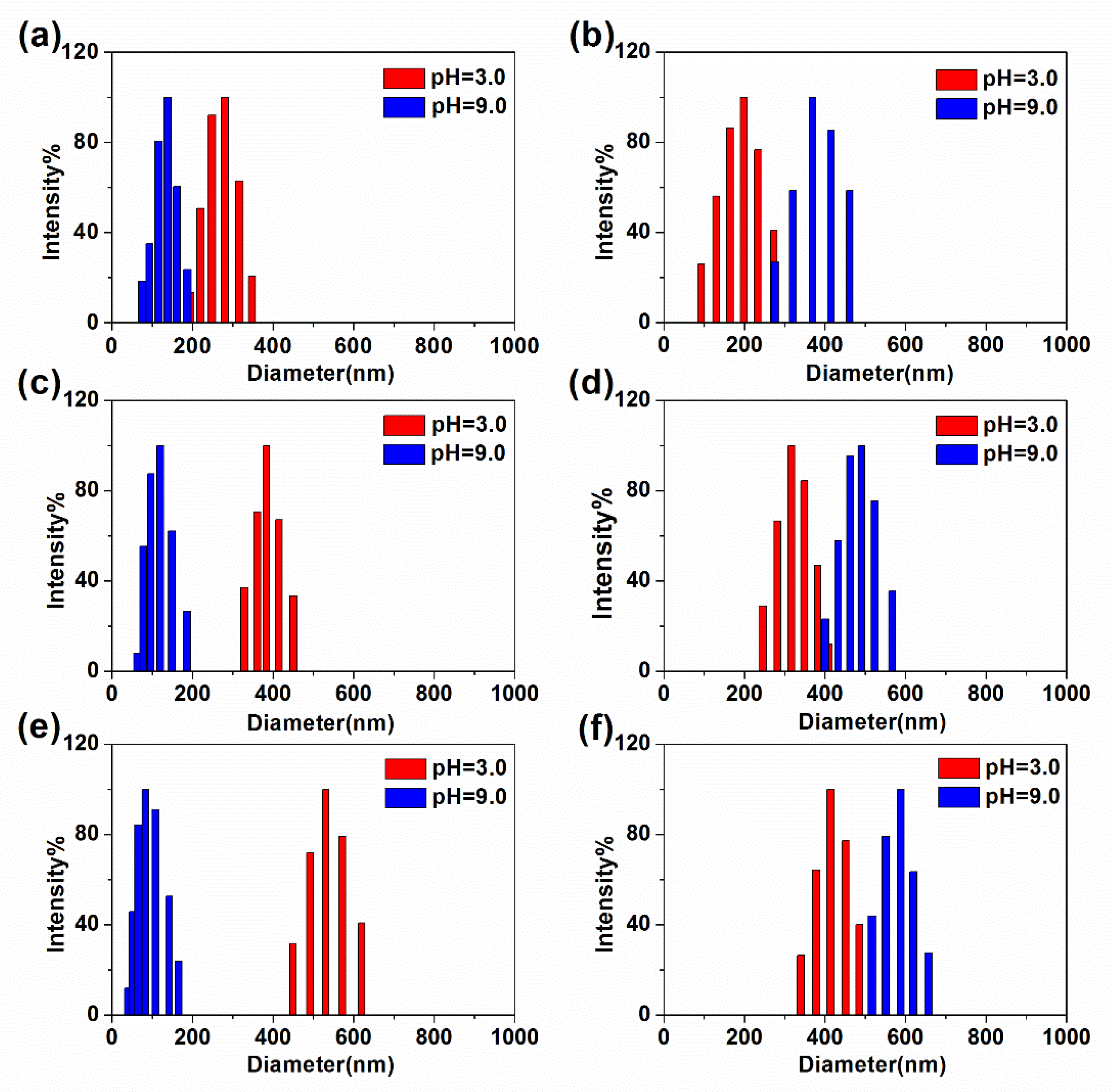
3.3.1. pH-Responsive Self-Assembly of Star-like PAA-b-PDMAEMA
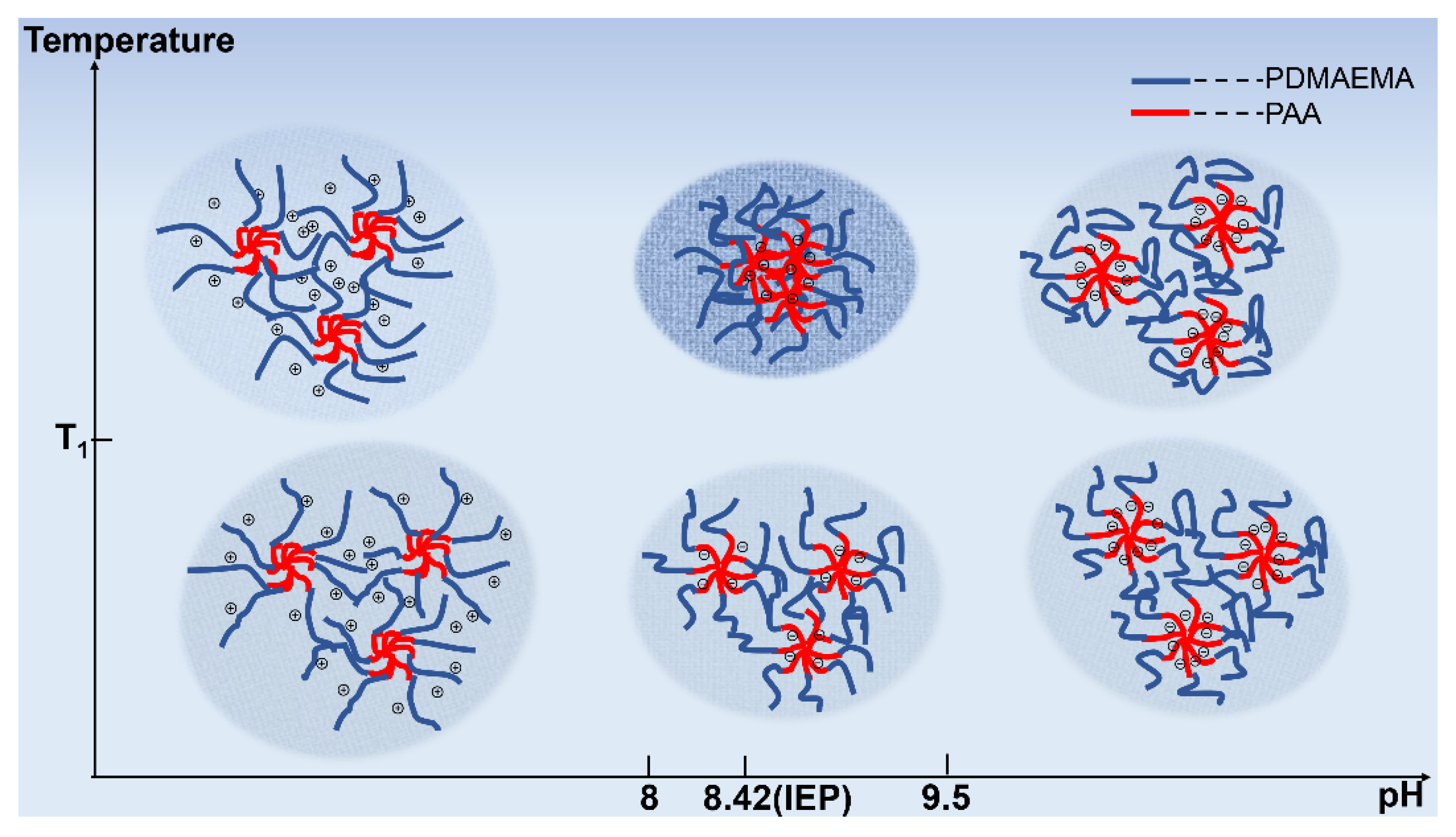
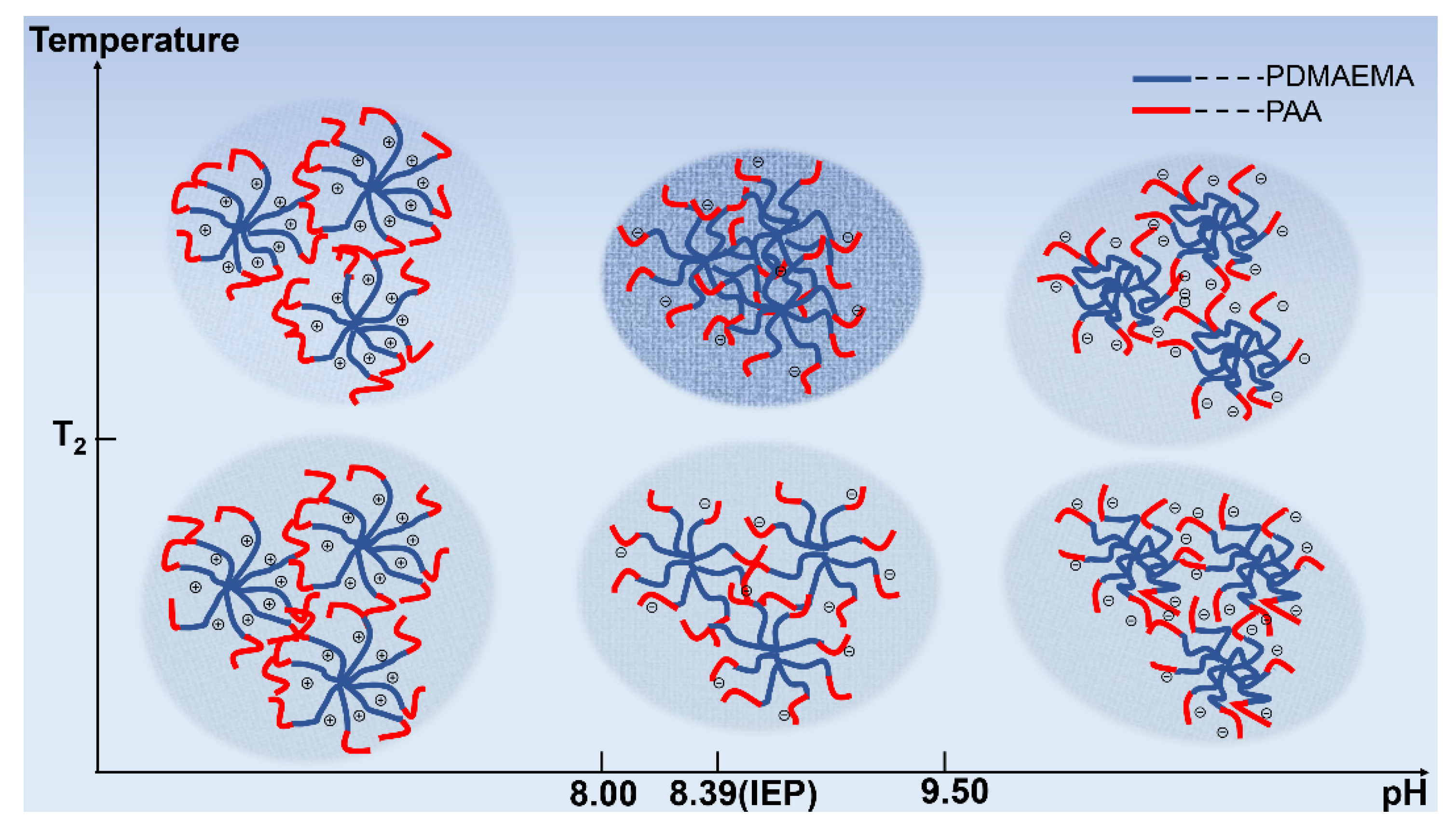
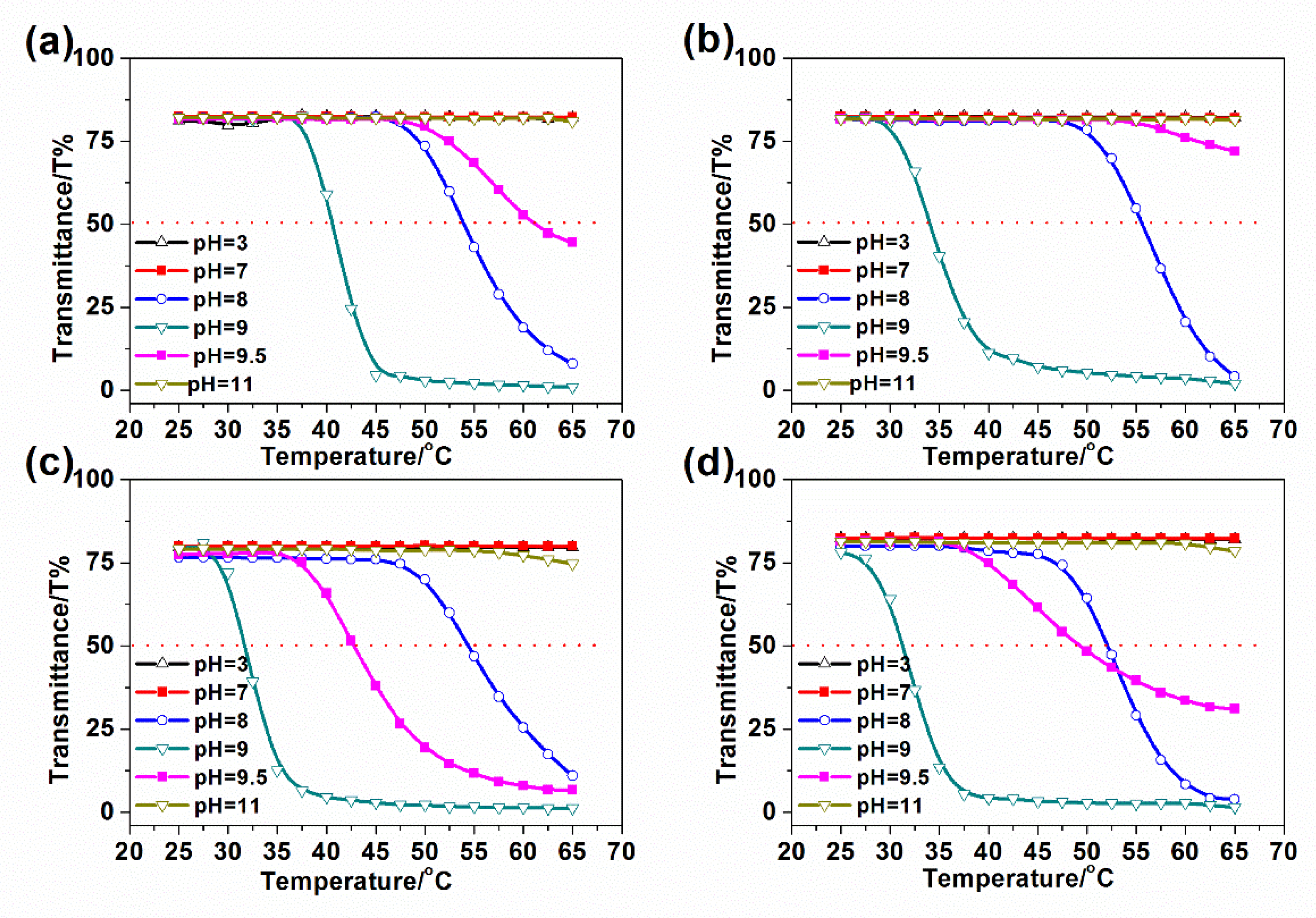
3.3.2. The Effect of Block Sequence and Arm Number on pH-Tuned Thermo-Responsive Behaviors of Star-like Block Copolymers
3.3.3. The Effect of Block Sequence and Arm Number on pH-Tuned Thermo-Responsive Behaviors of Star-like Block Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Li, M.; Li, X.; Shang, Y.; Liu, H. Stimuli-responsive and micellar behaviors of star-shaped poly[2-(dimethylamino)ethyl methacrylate]-b-poly[2-(2-methoxyethoxy)ethyl methacrylate] with a β-cyclodextrin core. React. Funct. Polym. 2017, 116, 77–86. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yang, F.; Shen, H.; You, Y.; Wu, D. Self-Assembly Behavior of a Linear-Star Supramolecular Amphiphile Based on Host–Guest Complexation. Langmuir 2014, 30, 13014–13020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, Q.; Kang, Y.; Zhou, L.; Zhou, H.; Yuan, J.; Wu, S. Fabrication of thermo-responsive hydrogels from star-shaped copolymer with a biocompatible β-cyclodextrin core. Polymer 2012, 53, 3719–3725. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- He, J.; Zhang, W.; Lv, C.; Chen, R.; Wang, L.; Wang, Y.; Pan, X. Thermo- and pH-responsive star-like polymers synthesized by photoATRP. Polymer 2021, 215, 123345. [Google Scholar] [CrossRef]
- Jana, S.; Uchman, M. Poly(2-oxazoline)-based stimulus-responsive (Co)polymers: An overview of their design, solution properties, surface-chemistries and applications. Prog. Polym. Sci. 2020, 106, 101252. [Google Scholar] [CrossRef]
- Grubbs, R.B.; Grubbs, R.H. 50th Anniversary Perspective: Living Polymerization—Emphasizing the Molecule in Macromolecules. Macromolecules 2017, 50, 6979–6997. [Google Scholar] [CrossRef]
- Braunecker, W.; Matyjaszewski, K. Erratum to: “Controlled/living radical polymerization: Features, developments and perspectives”. Prog. Polym. Sci. 2008, 33, 165. [Google Scholar] [CrossRef]
- Xu, J.; Jung, K.; Atme, A.; Shanmugam, S.; Boyer, C. A Robust and Versatile Photoinduced Living Polymerization of Conjugated and Unconjugated Monomers and Its Oxygen Tolerance. J. Am. Chem. Soc. 2014, 136, 5508–5519. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Rosselgong, J.; Williams, E.G.L.; Le, T.P.; Grusche, F.; Hinton, T.M.; Tizard, M.; Gunatillake, P.; Thang, S.H. Core Degradable Star RAFT Polymers: Synthesis, Polymerization, and Degradation Studies. Macromolecules 2013, 46, 9181–9188. [Google Scholar] [CrossRef]
- Kobben, S.; Ethirajan, A.; Junkers, T. Synthesis of Degradable Poly (methyl methacrylate) Star Polymers via RAFT Copolymerization with Cyclic Ketene Acetals. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1633–1641. [Google Scholar] [CrossRef]
- Herfurth, C.; Laschewsky, A.; Noirez, L.; von Lospichl, B.; Gradzielski, M. Thermoresponsive (star) block copolymers from one-pot sequential RAFT polymerizations and their self-assembly in aqueous solution. Polymer 2016, 107, 422–433. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; Li, Z.; Choi, B.; Mulder, R.J.; Feng, A.; Moad, G.; Thang, S.H. Versatile Approach for Preparing PVC-Based Mikto-Arm Star Additives Based on RAFT Polymerization. Macromolecules 2020, 53, 4465–4479. [Google Scholar] [CrossRef]
- Boschmann, D.; Vana, P. Z-RAFT Star Polymerizations of Acrylates: Star Coupling via Intermolecular Chain Transfer to Polymer. Macromolecules 2007, 40, 2683–2693. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Liu, Q.; Li, S.; Perrier, S.; Zhao, Y. Facile Synthesis of Hyperbranched and Star-Shaped Polymers by RAFT Polymerization Based on a Polymerizable Trithiocarbonate. Macromolecules 2011, 44, 2034–2049. [Google Scholar] [CrossRef]
- Cakir Yigit, N.; Tunca, U.; Hizal, G.; Durmaz, H. Heterofunctionalized Multiarm Star Polymers via Sequential Thiol- para -Fluoro and Thiol-Ene Double “Click” Reactions. Macromol. Chem. Phys. 2016, 217, 636–645. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Yin, B.; Jiang, X. Click Chemistry-Mediated Nanosensors for Biochemical Assays. Theranostics 2016, 6, 969–985. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.S.; Yang, X.K.; Zhu, W.; Zou, L.; Zhang, K.; Chen, Y.M.; Xi, F. Strain-promoted azide-alkyne cycloaddition “click” as a conjugation tool for building topological polymers. Polymer 2014, 55, 4812–4819. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.-R.; Zhao, X.; Wei, Z.-H.; Huang, Y.-P.; Liu, Z.-S. Robust immobilized enzyme reactor based on trimethylolpropane trimethacrylate organic monolithic matrix through “thiol-ene” click reaction. Eur. Polym. J. 2020, 124, 109456. [Google Scholar] [CrossRef]
- Sitterli, A.; Heinze, T. Studies about reactive ene-functionalized dextran derivatives for Thiol-ene click reactions. React. Funct. Polym. 2019, 136, 66–74. [Google Scholar] [CrossRef]
- Winkler, R.; Pellet-Rostaing, S.; Arrachart, G. Efficient and multi-function compatible click-reaction of organosilanes. Tetrahedron Lett. 2020, 61, 152145. [Google Scholar] [CrossRef]
- Shen, X.; Liu, P.; He, C.; Xia, S.; Liu, J.; Cheng, F.; Suo, H.; Zhao, Y.; Chen, L. Surface PEGylation of polyacrylonitrile membrane via thiol-ene click chemistry for efficient separation of oil-in-water emulsions. Sep. Purif. Technol. 2021, 255, 117418. [Google Scholar] [CrossRef]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Harvison, M.A.; Lowe, A.B.J.M.R.C. Combining RAFT Radical Polymerization and Click/Highly Efficient Coupling Chemistries: A Powerful Strategy for the Preparation of Novel Materials. Macromol. Rapid Commun. 2011, 32, 779–800. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, K.; Huang, J.; Zhao, Q.; Cao, S. Synthesis of Diverse α,ω-Telechelic Polystyrenes with Di- and Tri-functionality via Tandem or One-Pot Strategies Combining Aminolysis of RAFT-Polystyrene and Thiol-Ene “Click” Reaction. RSC Adv. 2015, 5, 44571–44577. [Google Scholar] [CrossRef]
- Fu, C.; Huang, Z.; Hawker, C.; Moad, G.; Xu, J.; Boyer, C. RAFT-mediated, visible light-initiated single unit monomer insertion and its application in the synthesis of sequence-defined polymers. Polym. Chem. 2017, 8, 4637–4643. [Google Scholar] [CrossRef]
- Sulistio, A.; Lowenthal, J.; Blencowe, A.; Bongiovanni, M.N.; Ong, L.; Gras, S.L.; Zhang, X.; Qiao, G.G. Folic Acid Conjugated Amino Acid-Based Star Polymers for Active Targeting of Cancer Cells. Biomacromolecules 2011, 12, 3469–3477. [Google Scholar] [CrossRef]
- Chen, L.; Feng, W.; Zhou, X.; Yin, Z.; He, C. Thermo-and pH dual-responsive mesoporous silica nanoparticles for controlled drug release. J. Control. Release 2015, 213, e69–e70. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Gadad, A. Development and Evaluation of High Oxaliplatin Loaded CS-g-PNIPAAm Co-Polymeric Nanoparticles for Thermo and pH Responsive Delivery. Indian J. Pharm. Educ. Res. 2018, 52, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Hooshyar, Z.; Bardajee, G. A novel dual thermo- and pH-responsive silver nanocomposite hydrogel as a drug delivery system. J. Iran. Chem. Soc. 2016, 14, 541–549. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of novel biocompatible thermo-sensitive graft copolymers based on poly(ε-caprolactone), poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Micellization of pH-stimulable poly(2-vinylpyridine)-b-poly(ethylene oxide) copolymers and their complexation with anionic surfactants. J. Colloid Interface Sci. 2013, 395, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.-T.; Tai, P.-Y.; Cheng, C.-C. Dual CO2/Temperature-Responsive Diblock Copolymers Confer Controlled Reversible Emulsion Behavior. Polym. Chem. 2019, 10, 2641–2646. [Google Scholar] [CrossRef]
- Yuan, W.; Zou, H.; Guo, K.; Wang, A.; Ren, J. Supramolecular amphiphilic star-branched copolymer: From LCST-UCST transition to temperature-fluorescence responses. J. Mater. Chem. 2012, 22, 24783–24791. [Google Scholar] [CrossRef]
- Yu, C.; Wang, L.; Xu, Z.; Teng, W.; Wu, Z.-M.; Xiong, D. Smart micelles self-assembled from four-arm star polymers as potential drug carriers for pH-triggered DOX release. J. Polym. Res. 2020, 27, 111. [Google Scholar] [CrossRef]
- Iatridi, Z.; Tsitsilianis, C. pH responsive MWCNT–star terpolymer nanohybrids. Soft Matter 2013, 9, 185–193. [Google Scholar] [CrossRef]
- Quick, A.S.; Fischer, J.; Richter, B.; Pauloehrl, T.; Trouillet, V.; Wegener, M.; Barner-Kowollik, C. Preparation of Reactive Three-Dimensional Microstructures via Direct Laser Writing and Thiol-ene Chemistry. Macromol. Rapid Commun. 2013, 34, 335–340. [Google Scholar] [CrossRef]
- Zimm, B.; Stockmayer, W. The Dimensions of Chain Molecules Containing Branches and Rings. J. Chem. Phys. 1949, 17, 1301–1314. [Google Scholar] [CrossRef]
- Sakai, K.; Vamvakaki, M.; Smith, E.G.; Wanless, E.J.; Armes, S.P.; Biggs, S. Adsorption characteristics of zwitterionic diblock copolymers at the silica/aqueous solution interface. J. Colloid Interface Sci. 2008, 317, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J.; Hughes, R.J.; Smith, E.G.; Pham, B.T.T.; Hawkett, B.S.; Gilbert, R.G.; Serelis, A.K.; Such, C.H. Effective ab Initio Emulsion Polymerization under RAFT Control. Macromolecules 2002, 35, 9243–9245. [Google Scholar] [CrossRef]



| Sample | Mn,th(a) (g mol −1) | Mn,app(b) or Mn,star (c) (g/mol) | PDI (b) | n (DMAEMA)/ n (tBA) (d) | Arm Number (c) |
|---|---|---|---|---|---|
| CTA-PDMAEMA4 | 835 | 864 | 1.04 | - | - |
| HS-PtBA2-b-PDMAEMA4 | 965 | 1138 | 1.03 | 2.09 | - |
| 4-Star-PtBA2-b-PDMAEMA4 | 4256 | 4999 | 1.23 | 2.09 | 4.13 |
| 8-Star-PtBA2-b-PDMAEMA4 | 8877 | 9282 | 1.30 | 2.09 | 7.24 |
| 12-Star-PtBA2-b-PDMAEMA4 | 12,547 | 13,997 | 1.18 | 2.09 | 10.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Huang, D.; Wang, X.; Zhang, C. A Study on the Dual Thermo- and pH-Responsive Behaviors of Well-Defined Star-like Block Copolymers Synthesize by Combining of RAFT Polymerization and Thiol-Ene Click Reaction. Polymers 2022, 14, 1695. https://doi.org/10.3390/polym14091695
Xue Y, Huang D, Wang X, Zhang C. A Study on the Dual Thermo- and pH-Responsive Behaviors of Well-Defined Star-like Block Copolymers Synthesize by Combining of RAFT Polymerization and Thiol-Ene Click Reaction. Polymers. 2022; 14(9):1695. https://doi.org/10.3390/polym14091695
Chicago/Turabian StyleXue, Yan, Dan Huang, Xinyong Wang, and Chunquan Zhang. 2022. "A Study on the Dual Thermo- and pH-Responsive Behaviors of Well-Defined Star-like Block Copolymers Synthesize by Combining of RAFT Polymerization and Thiol-Ene Click Reaction" Polymers 14, no. 9: 1695. https://doi.org/10.3390/polym14091695
APA StyleXue, Y., Huang, D., Wang, X., & Zhang, C. (2022). A Study on the Dual Thermo- and pH-Responsive Behaviors of Well-Defined Star-like Block Copolymers Synthesize by Combining of RAFT Polymerization and Thiol-Ene Click Reaction. Polymers, 14(9), 1695. https://doi.org/10.3390/polym14091695





