Abstract
Poly(2-methoxyethyl acrylate) (PMEA) and poly(ethylene oxide) (PEO) have protein-antifouling properties and blood compatibility. ABA triblock copolymers (PMEAl-PEO11340-PMEAm (MEOMn; n is average value of l and m)) were prepared using single-electron transfer-living radical polymerization (SET-LRP) using a bifunctional PEO macroinitiator. Two types of MEOMn composed of PMEA blocks with degrees of polymerization (DP = n) of 85 and 777 were prepared using the same PEO macroinitiator. MEOMn formed flower micelles with a hydrophobic PMEA (A) core and hydrophilic PEO (B) loop shells in diluted water with a similar appearance to petals. The hydrodynamic radii of MEOM85 and MEOM777 were 151 and 108 nm, respectively. The PMEA block with a large DP formed a tightly packed core. The aggregation number (Nagg) of the PMEA block in a single flower micelle for MEOM85 and MEOM777 was 156 and 164, respectively, which were estimated using a light scattering technique. The critical micelle concentrations (CMCs) for MEOM85 and MEOM777 were 0.01 and 0.002 g/L, respectively, as determined by the light scattering intensity and fluorescence probe techniques. The size, Nagg, and CMC for MEOM85 and MEOM777 were almost the same independent of hydrophobic DP of the PMEA block.
1. Introduction
Amphipathic block copolymers form interpolymer aggregates because of the hydrophobic interactions of hydrophobic blocks in water [1]. Generally, amphipathic AB diblock copolymers form core–shell spherical polymer micelles in water. ABA triblock copolymers with two hydrophobic A blocks at both ends of the central B block form flower-like micelles caused by interpolymer aggregation in water [2]. The hydrophobic A blocks aggregate to form a core, and the hydrophilic B blocks form loop shape shells with a similar appearance to petals on the surface of the core to form flower micelles. The flower micelles are bridged when the hydrophobic A blocks in the ABA triblock copolymer are incorporated into separate cores in flower micelles. With increasing polymer concentration (Cp), the number of bridges between the flower micelles increases to form a gel [3]. The polymers increase the viscosity of the aqueous solution to form interpolymer aggregates, which can then be applied as associative thickeners with a small amount of addition [4]. For example, flower micelles formed from ABA triblock copolymers have been applied as associative thickeners [5]. Associative thickeners are used in water-based paints, coatings, personal care goods, and adhesive agents [6].
The hydrophilic shells on the surface of polymer micelles formed from amphipathic block copolymers stabilize the micelle structure and maintain their dispersion stability in solution [7]. Polymer micelles formed from high molecular weight polymers generally have a lower critical micelle concentration (CMC) and higher colloidal stability than those formed from low molecular weight surfactants [8]. The CMC of amphiphilic diblock copolymers depends on the ratio of the hydrophobic to hydrophilic block lengths. CMC decreases with increasing hydrophobic block chain length for a constant hydrophilic block chain length in the diblock copolymer [9,10,11]. Zhulina et al. [12] studied thermodynamic properties of block copolymer micelles. With ABA triblock copolymers, the CMC also decreases with increasing hydrophobic block chain length [13]. Borisov and Halperin reported theoretical models of flower micelles [14].
Single-electron transfer-living radical polymerization (SET-LRP) is a method for controlled radical polymerization using a copper catalyst [15]. A copper catalyst is widely used as an inorganic electron donor reagent for organic and polymer syntheses (Figure S1). SET-LRP can be performed at low temperatures, e.g., room temperature, because of the low activation energy [16]. Poly(ethylene oxide) (PEO) is often used as a hydrophilic block in amphipathic ABA triblock copolymers because it can form flower micelles easily [17,18]. Furthermore, PEO is widely used in biomedical and biomaterial fields owing to its biocompatibility [19]; 2-Methoxyethyl acrylate (MEA) is an acrylate monomer that can be polymerized by radical polymerization [20]. Poly(2-methoxyethyl acrylate) (PMEA) is highly blood compatible because it has a protein-antifouling effect, and platelets cannot adhere easily to PMEA [21,22]. PMEA forms an intermediate water layer on its surface to suppress protein adsorption [23]. Furthermore, PMEA can be applied as coatings on various substrates because PMEA can be soluble in organic solvents, water insoluble, transparent, and adhesive [24]. Owing to the excellent properties of PMEA, it is also used as a coating material for artificial organs [25]. Haraguchi et al. [26,27] reported protein antifouling and blood compatible coatings using amphiphilic ABA triblock copolymers composed of hydrophobic PMEA (A) and hydrophilic poly(N,N-dimethylacrylamide) (B). The hydrophobic PMEA (A) blocks show good adhesion to both organic and inorganic substrates.
In this study, ABA triblock copolymers (PMEAl-PEO11340-PMEAm (MEOMn; n is average value of l and m)) were prepared by SET-LRP to polymerize MEA using a bifunctional PEO macroinitiator at both chain ends. In particular, we are interested in the association behavior of ABA triblock copolymers with long PEO (B) block in water. MEOMn was composed of a hydrophobic PMEA (A) block and a hydrophilic PEO (B) block. In water, MEOMn formed flower micelles with a hydrophobic PMEA core and PEO loop-shaped shells (Figure 1). The associative behavior of the flower micelles formed from MEOMn in dilute aqueous solutions was examined using dynamic light scattering (DLS), static light scattering (SLS), transmission electron microscopy (TEM), and fluorescence probe technique.
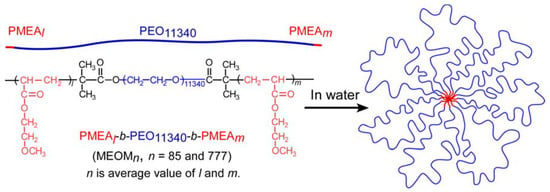
Figure 1.
Conceptual illustration of flower micelles formed from MEOMn (n = 85 and 777).
2. Materials and Methods
2.1. General
Tris(2-(dimethylamino)ethyl)amine (Me6TREN, 97%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Copper bromide (CuBr, 95.0%) was supplied by Kishida Chemical (Osaka, Japan). Ethanol (99.5%), tetrahydrofuran (THF, 99.5%), and cetylpyridinium chloride (90%) were purchased from Fujifilm Wako Pure Chemical (Osaka, Japan). All chemicals were used as received; 2-Methoxyethyl acrylate (MEA, 98.0%) from Fujifilm Wako Pure Chemical (Osaka, Japan) was treated with an inhibitor-remover prepacked column from Sigma-Aldrich (St. Louis, MO, USA) prior to use. PEO (MW = 500,000 g/mol) was purchased from Fujifilm Wako Pure Chemical (Osaka, Japan). PEO-based macroinitiator (PEO-Br) was prepared and purified in accordance with the literature [28]. Number average molecular weight (Mn(GPC)) and molecular weight distribution (Mw/Mn) estimated from gel-permeation chromatography (GPC) for PEO-Br were 4.64 × 105 g/mol and 1.23, respectively. The degree of polymerization (DP) for PEO-Br was 11,340. Pyrene (97%) from Fujifilm Wako Pure Chemical (Osaka, Japan) was recrystallized from methanol. Water was purified using an ion-exchange column system.
2.2. Preparation of MEOMn (n = 85 and 777)
MEOM85 was prepared via SET-LRP (Scheme S1). Me6TREN (2.13 mg, 3.01 μmol) was dissolved in water (1.00 mL) and stirred under an argon atmosphere for 10 min. CuBr (2.96 mg, 20.6 μmol) was then added, and the mixture was stirred for 10 min. PEO-Br (Mn(GPC) = 4.64 × 105 g/mol, 1.50 g, 3.01 μmol) and MEA (420 mg, 3.22 mmol) were dissolved in water (21.3 mL). The aqueous CuBr/Me6TREN solution was added to an aqueous PEO-Br and MEA solution under an argon atmosphere. The reaction solution was stirred for 71 h under an argon atmosphere at room temperature. The conversion of MEA was 16.8%, which was estimated by 1H nuclear magnetic resonance (NMR) spectroscopy before purification. The polymerization mixture was dialyzed against pure water for three days, and the polymer (MEOM85) was collected by freeze-drying (0.889 g, 46.3%). The DP of the PMEA block was 85, as estimated from the 1H NMR spectrum. The Mn(GPC) and Mw/Mn estimated from GPC were 5.41 × 105 g/mol and 1.17, respectively.
MEOM777 was prepared using the same procedure (2.42 g, 63.7%). The conversion of MEA before purification was 34.9%, according to 1H NMR spectroscopy. The DP of the PMEA block was 777, as estimated from the 1H NMR spectrum. The Mn(GPC) and Mw/Mn were 4.91 × 105 g/mol and 1.26, respectively.
2.3. Preparation of MEOM777 Aqueous Solution
MEOM777 (5.86 mg, 8.35 μmol) was dissolved in THF (6.02 mL), and the Cp was adjusted to 1.00 g/L. The THF solution was dialyzed against pure water for two days to remove THF. After dialysis, the aqueous solution was diluted with water to be Cp = 0.10 g/L.
2.4. Measurements
Using a Bruker (Billerica, MA, USA) DRX-500 and JEOL (Tokyo, Japan) JNM-ECZ400R at 25 °C, 1H NMR spectroscopy was performed. The water suppression by gradient-tailored excitation (Watergate) with a double pulse field gradient spin echo pulse sequence was used for the D2O solutions to suppress the water signal. Water suppression by a gradient-tailored excitation (WATERGATE) method was used for the D2O sample to reduce the water signal. The GPC measurements were conducted at 40 °C using a Shodex (Tokyo, Japan) DS-4 pump, a Shodex GF-7M column, and a Shodex RI-101 refractive index detector. THF was used as the eluent with a flow rate of 1.0 mL/min. Mn(GPC) and Mw/Mn were determined using standard polystyrene samples. The samples were analyzed by attenuated total reflection-Fourier-transform infrared (ATR-FTIR, FT/IR-4200, Jasco, Tokyo, Japan) spectroscopy. DLS measurements were performed at 25 °C using a Malvern (Malvern, UK) Zetasizer nano ZS at a scattering angle of 173°. The data were analyzed using a Malvern (Malvern, UK) Zetasizer Software package 7.11 to determine the hydrodynamic radius (Rh), light scattering intensity (LSI), and polydispersity (PDI). SLS measurements were taken at 25 °C using an Otsuka Electronics (Osaka, Japan) DLS-7000. The weight-average molecular weight (Mw(SLS)) was calculated from Debye plots. The refractive index increment (dn/dCp) was determined using an Otsuka Electronics (Osaka, Japan) DRM-3000 differential refractometer at 25 °C. Transmission electron microscopy (TEM, JEM-2100, Jeol, Tokyo, Japan) was performed at an acceleration voltage of 160 kV. The TEM samples were prepared by placing a drop of the sample solution on a copper grid coated with a form bar, and the samples were stained with a sodium phosphotungstate aqueous solution. The samples were dried for one day under reduced pressure. Fluorescence measurements were taken using a Hitachi High-Tech (Tokyo, Japan) F-2500 fluorescence spectrophotometer. The pyrene aqueous solutions (6.0 ×10−7 M) were excited at 334 nm; the excitation and emission slit widths were 20 and 2.5 nm, respectively.
3. Results and Discussion
3.1. Characterization
MEOMn was prepared by SET-LRP using MEA and PEO-Br macroinitiator. The 1H NMR spectra for PEO-Br and MEOMn were measured in CDCl3 (Figure 2). The terminal groups in PEO-Br could not be observed clearly because of the low signal intensity. The integral intensities of the PMEA pendant methylene protons at 3.3 ppm (e) and the PEO main chain methylene protons at 3.5–4.0 ppm (f) were compared to estimate the DP (NMR = n) of one end of the PMEA block in MEOMn. The DP(NMR) values for MEOM85 and MEOM777 were 85 and 777, respectively (Table 1).
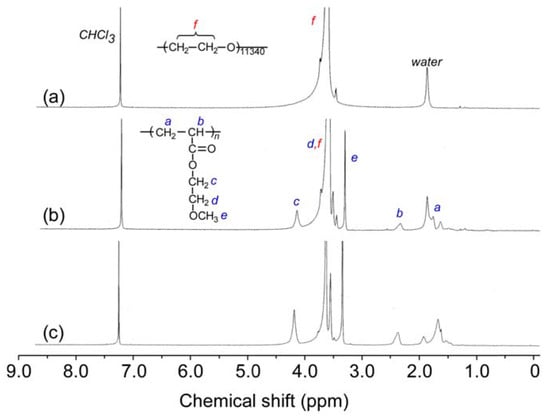
Figure 2.
1H NMR spectra of (a) PEO-Br, (b) MEOM85, and (c) MEOM777 in CDCl3.

Table 1.
Characteristics of polymers.
The theoretical DP(theo) and number average molecular weight (Mn(theo)) can be calculated from the following equations:
where [M]0 and [Br]0 are the initial concentrations of the monomer and bromine atoms at the PEO-Br chain ends, respectively, and Mm and MPEO are the molecular weights of the monomer and PEO, respectively. The DP(theo) values of MEOM85 and MEOM777 were 86 and 1020, respectively. These theoretical values were close to the DP(NMR) values. GPC was performed for MEOMn using THF as an eluent (Figure S2). The structure of MEOMn could be controlled because the Mw/Mn values estimated from GPC were less than 1.3. However, the retention time for the GPC elution curves of MEOMn was similar to that of PEO-Br. Unexpected interactions may have occurred between the MEOMn and GPC column, and polystyrene was used as the standard that may have impeded a correct estimation of the Mn(GPC) [29].
ATR-FTIR was performed to characterize the chemical structure of MEOMn (Figure S3). The C=O vibration stretching peak was observed at 1700 cm−1 for MEOMn, whereas the peak could be observed for PEO-Br. The peak intensity at 1700 cm−1 increased with increasing DP of the PMEA block in MEOMn. These results confirmed that MEOMn had been prepared.
3.2. Association Behavior of MEOMn
The Rh distributions of the flower micelles formed from MEOMn in water were examined by DLS (Figure 3). MEOM777 could not dissolve directly in water because of its long hydrophobic PMEA blocks. Therefore, an aqueous solution was prepared to dialyze the THF solution of MEOM777 against water. In contrast, MEOM85 could dissolve directly in water. The Rh values of flower micelles obtained after directly dissolving them in water and after the dialysis method were compared to confirm the difference between the preparation methods of the MEOM85 aqueous solutions (Figure S4). The Rh values for MEOM85 prepared by direct dissolution in water and the dialysis method were 151 and 144 nm, respectively, which are similar. Therefore, flower micelles formed from MEOM85 regardless of the solution preparation method. The association state of MEOM85 that was easily soluble in water reaches the lowest association energy independently of the dissolution methods. Unless noted otherwise, the MEOM85 aqueous solution was prepared using the direct dissolution method. The Rh distributions for MEOMn in water were unimodal. The Rh values for MEOM85 and MEOM777 were 144 and 108 nm, respectively. The DP of the hydrophobic PMEA block in MEOM777 was larger than that in MEOM85, but Rh of MEOM777 was smaller than that of MEOM85. As the DP of the PMEA block in MEOMn increased, the hydrophobic interaction became stronger to form a more compact core of the flower micelle. The PDI values for MEOM85 and MEOM777 were 0.166 and 0.211, respectively. MEOM85 formed more uniformly sized flower micelles than MEOM777.
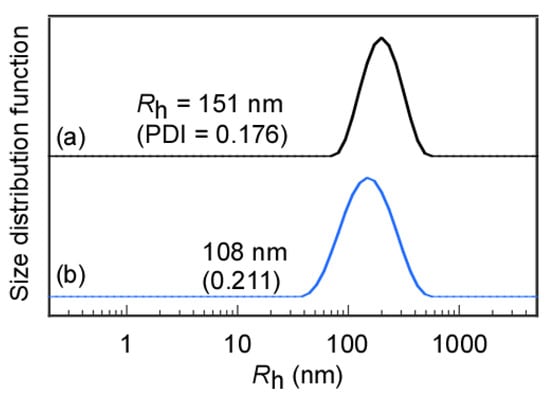
Figure 3.
Hydrodynamic radius (Rh) distributions of (a) MEOM85 and (b) MEOM777 in water at Cp = 0.1 g/L.
The structure of flower micelles formed from MEOMn in water was confirmed by SLS (Figure S5). The apparent weight-average molecular weight (Mw(SLS)) and radius of gyration (Rg) were obtained from the SLS measurements. The refractive index increment (dn/dCp) required to determine Mw(SLS) was obtained using a differential refractometer. The dn/dCp values for MEOM85 and MEOM777 were 0.138 and 0.426 mL/g, respectively. The number of PMEA chains (Nagg) forming a single flower micelle was calculated from the equation, Nagg = 2Mw(SLS)/(Mn(NMR) × Mw/Mn). The Nagg values for MEOM85 and MEOM777 were 156 and 164, respectively (Table 2). The DP of the PMEA block in MEOM777 was approximately nine times larger than that in MEOM85, but both Nagg values were close. The interface between the core and shell was sterically crowded with the PEO chains because the DP of the PEO block forms a loop-shaped shell. It was unlikely that the Nagg value would increase above a certain number because of the congestion of the PEO shell chains on the core–shell interface. The Rg values for MEOM85 and MEOM777 were 141 and 164 nm, respectively. From the Rh and Rg values, the flower micelles formed from MEOM85 and MEOM777 have similar size. The Rg/Rh ratios for MEOM85 and MEOM777 were 0.934 and 1.52, respectively. These Rg/Rh ratios were close to one, suggesting that the shape of flower micelles was spherical [30]. The density (ΦH) of the micelle can be calculated from Equation (3) [31]:
where NA is Avogadro’s number. The ΦH values for MEOM85 and MEOM777 were 5.47 × 10−3 and 2.29 × 10−2 g/mL, respectively. MEOM777 with a long PMEA chain formed a tightly packed core because the ΦH value of MEOM777 was larger than that of MEOM85; 1H NMR spectroscopy of MEOMn was performed in D2O (Figure S6). The PEO signals were observed, but the PMEA signals were not. This observation suggests that the motion of PMEA was restricted due to the formation of the core, but the motion of PEO was not restricted.

Table 2.
Characteristics of MEOMn flower-like micelles in water.
TEM of MEOM85 and MEOM777 in water (Figure 4) revealed spherical aggregates. The radii (RTEM) of MEOM85 and MEOM777 estimated from TEM were 42.4 and 59.2 nm, respectively. These RTEM values were smaller than Rh and Rg obtained from light scattering measurements. The shells formed from PEO were not observed because PEO cannot be stained by sodium phosphotungstate. The core formed by the association of the PMEA blocks could be stained, as observed by TEM. Therefore, the cores observed by TEM were separated a certain distance due to the unstained PEO loop shells that cannot be observed by TEM.
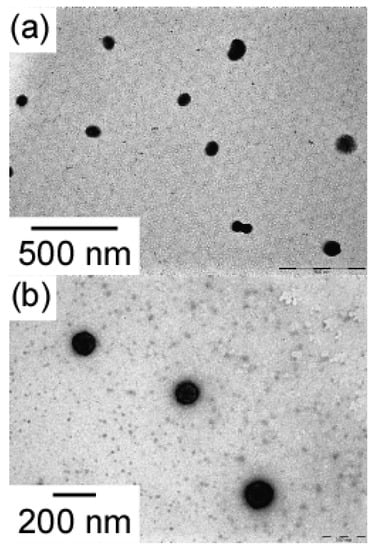
Figure 4.
TEM images for (a) MEOM85 and (b) MEOM777 in water at Cp = 0.1 g/L.
3.3. Critical Micelle Concentration (CMC) of MEOMn
To determine the CMC of flower micelles, the LSI for MEOMn aqueous solutions was measured as a function of Cp (Figure 5). The ratio (I/I0) of the LSI of the solution (I) to the solvent (I0) was plotted as a function of Cp. The CMC was estimated from the inflection point of the slope [32]. The CMC values for MEOM85 and MEOM777 were calculated to be 0.01 and 0.002 g/L, respectively (Table 3).
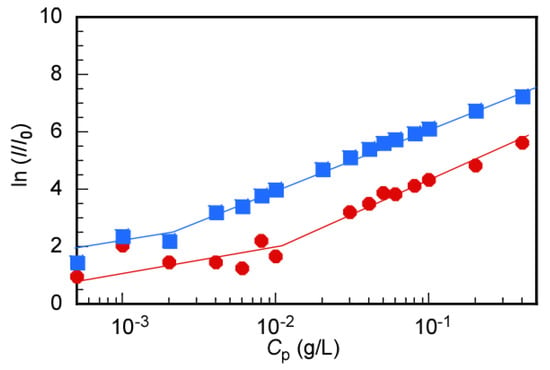
Figure 5.
Light scattering intensity (LSI) ratio (I/I0) as a function of polymer concentration (Cp) for MEOM85 (●) and MEOM777 (■) in aqueous solutions; I0 is LSI of water, and I is LSI of the polymer solution.

Table 3.
Critical micelle concentration (CMC) of MEOMn.
The CMC of MEOMn was also estimated using pyrene as a hydrophobic fluorescence probe (Figure 6). The intensity ratio (I3/I1) of the first (I1) to third vibronic peak (I3) of the pyrene fluorescence spectrum depends on the microenvironmental polarity around the pyrene molecule [33]. I3/I1 increased with decreasing microenvironmental polarity. I3/I1 was plotted as a function of Cp to determine the CMC (CMC(Em)) (Figure 6). CMC(Em) was calculated from the intersections of the two tangents of the plots. The CMC(Em) values for MEOM85 and MEOM777 were 0.01 and 0.0015 g/L, respectively. The emission maximum wavelength of the 0–0 band in the pyrene excitation spectrum shifts to a longer wavelength when the microenvironment around the pyrene molecule becomes hydrophobic [34]. The 0–0 band maximum wavelengths of the aqueous solutions in the presence and absence of MEOMn were 338 and 335 nm, respectively. The CMC (CMC(Ex)) was determined from a plot of I338/I335 vs. Cp, where I338 and I335 are the emission intensities at 338 and 335 nm, respectively. The CMC(Ex) values for MEOM85 and MEOM777 were 0.01 and 0.002 g/L, respectively. The CMC values estimated from the LSI and fluorescence probe methods were similar. These observations suggest that hydrophobic anticancer drugs can be encapsulated in the core above the CMC.
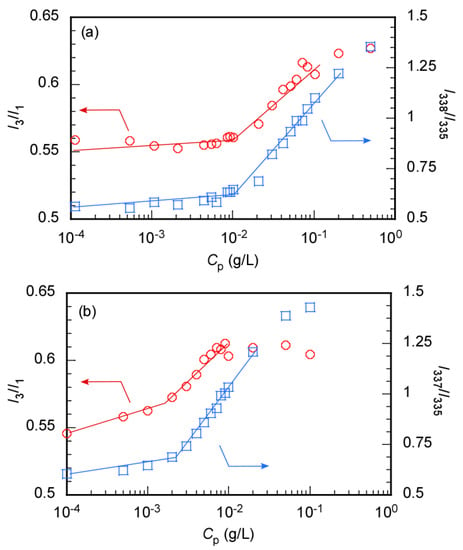
Figure 6.
Pyrene fluorescence intensity ratio (I3/I1, 〇) and excitation intensity ratio (I338/I335, □) as a function of the polymer concentration (Cp) for (a) MEOM85 and (b) MEOM777 in aqueous solutions; I3 and I1 are the third and first vibronic peak intensities of pyrene fluoresce, and I338 and I335 are the peak intensities at 338 and 335 nm in the excitation spectra of pyrene.
4. Conclusions
Amphiphilic ABA triblock copolymers, MEOMn, were prepared via SET-LRP using a bifunctional PEO-Br macroinitiator. MEOM85 and MEOM777 were prepared with different DP of the hydrophobic PMEA blocks at the central PEO chain ends. The DP of the PMEA block in MEOM777 was approximately nine times larger than that of MEOM85. The Rh values for flower micelles formed from MEOM85 and MEOM777 in water were 151 and 108 nm, respectively. The hydrophobic PMEA blocks with a large DP in MEOM777 associated to form a densely packed core due to the strong hydrophobic interactions. The Nagg values for flower micelles formed from MEOM85 and MEOM777 were similar. The CMC for MEOM85 and MEOM777 were 0.01 and 0.002 g/L, respectively. The CMC of MEOM777 was smaller than that of MEOM85 because of the strong hydrophobic interactions of MEOM777. These results may come from much larger DP of the PEO block than that of the PMEA blocks. The PEO blocks that formed the loop shells of flower micelles and the PMEA blocks that formed the core were both biocompatible. Therefore, the biocompatible flower micelles formed from MEOMn may have applications as novel drug delivery carriers. We believe that the chemical design of MEOMn can be applied for coating on the various biomedical devices.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/polym14091678/s1, Scheme S1. Synthesis of MEOMn (n = 85 and 777); Figure S1. Mechanism of single-electron transfer-living radical polymerization (SET-LRP); M; monomer, P; polymer, Cu; copper, X; halogen, L; ligand, kact; activation, kdeact; deactivation, kp; propagation, kt; termination; Figure S2. Gel-permeation chromatography (GPC) elution curves of PEO11340-Br (black line), MEOM85 (red line), and MEOM777 (blue line) using THF as an eluent with a flow rate of 1.0 mL/min at 40 °C; Figure S3. Attenuated total reflection (ATR) Fourier-transform infrared (FTIR) spectra for (a) PEO11340-Br, (b) MEOM85, and (c) MEOM777; Figure S4. Hydrodynamic radius (Rh) distributions of MEOM85 aqueous solution prepared by directly dissolution (blue line) and by dialysis from THF solution against aqueous solution (red line); the final polymer concentration (Cp) was adjusted to 0.1 g/L; Figure S5. Debye plots for (a) MEOM85 and (b) MEOM777 in water; Figure S6. Water suppression by gradient-tailored excitation (WATERGATE) 1H NMR spectroscopy in D2O for (a) MEOM85 and (b) MEOM777 at 25 °C.
Author Contributions
Conceptualization and experimental design: K.H. and S.-i.Y.; Experimental work and data analysis: Y.M. and E.O.; writing: Y.M., K.H., and S.-i.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by KAKENHI grants (21H02005, 21K19931, 21H05027, 21H05535) from the Japan Society for the Promotion of Science (JSPS), JSPS Bilateral Joint Research Projects (JPJSBP120203509), the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (20214044)”, the International Collaborative Research Program of Institute for Chemical Research, Kyoto University (2022–121), and MEXT Promotion of Distinctive Joint Research Center Program (JPMXP 0621467946).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, Y.; Van Nostrum, C.F.; Hennink, W.E. Interfacially hydrazone cross-linked thermosensitive polymeric micelles for acid-triggered release of paclitaxel. ACS Biomater. Sci. Eng. 2015, 1, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Yamamoto, T.; Tezuka, Y. Topology-directed control on thermal stability: Micelles formed from linear and cyclized amphiphilic block copolymers. J. Am. Chem. Soc. 2010, 132, 10251–10253. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Larson, R.G. Multiscale molecular dynamics simulations of model hydrophobically modified ethylene oxide urethane micelles. J. Phys. Chem. B 2015, 119, 12540–12551. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.J.; Brown, R.G.; Glass, J.E.; Eley, R.R. Synthesis, characterization, and solution rheology of model hydrophobically-modified, water-soluble ethoxylated urethanes. Langmuir 1994, 10, 3027–3034. [Google Scholar] [CrossRef]
- Maiti, S.; Chatterji, P.R. Transition from normal to flower like micelles. J. Phys. Chem. B 2000, 104, 10253–10257. [Google Scholar] [CrossRef]
- Vorobyova, O.; Yekta, A.; Winnik, M.A.; Lau, W. Fluorescent probe studies of the association in an aqueous solution of a hydrophobically modified poly(ethylene oxide). Macromolecules 1998, 31, 8998–9007. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. “Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- De Graaf, A.J.; Boere, K.W.M.; Kemmink, J.; Fokkink, R.G.; Van Nostrum, C.F.; Rijkers, D.T.S.; Van Der Gucht, J.; Wienk, H.; Baldus, M.; Mastrobattista, E.; et al. Looped structure of flower like micelles revealed by 1H NMR relaxometry and light scattering. Langmuir 2011, 27, 9843–9848. [Google Scholar] [CrossRef]
- Kelarakis, A.; Yang, Z.; Pousia, E.; Nixon, S.K.; Price, C.; Booth, C.; Hamley, I.W.; Castelletto, V.; Fundin, J. Association properties of diblock copolymers of propylene oxide and ethylene oxide in aqueous solution. The effect of P and E block lengths. Langmuir 2001, 17, 8085–8091. [Google Scholar] [CrossRef]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Atanase, L.I.; Riess, G. Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances. Polymers 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhulina, E.B.; Adam, M.; LaRue, I.; Sheiko, S.S.; Rubinstein, M. Diblock Copolymer Micelles in a Dilute Solution. Macromolecules 2005, 38, 5330–5351. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsiue, G.H. New amphiphilic poly(2-ethyl-2-oxazoline)/poly(L-lactide) triblock copolymers. Biomacromolecules 2003, 4, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Borisov, O.V.; Halperin, A. Micelles of polysoaps. Langmuir 1995, 11, 2911–2919. [Google Scholar] [CrossRef]
- Zhang, N.; Samanta, S.R.; Rosen, B.M.; Percec, V. Single electron transfer in radical ion and radical-mediated organic, materials and polymer synthesis. Chem. Rev. 2014, 114, 5848–5958. [Google Scholar] [CrossRef]
- Lligadas, G.; Grama, S.; Percec, V. Single-electron transfer living radical polymerization platform to practice, develop, and invent. Biomacromolecules 2017, 18, 2981–3008. [Google Scholar] [CrossRef]
- Najafi, M.; Kordalivand, N.; Moradi, M.A.; Van Den Dikkenberg, J.; Fokkink, R.; Friedrich, H.; Sommerdijk, N.A.J.M.; Hembury, M.; Vermonden, T. Native chemical ligation for cross-linking of flower-like micelles. Biomacromolecules 2018, 19, 3766–3775. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.T.; Oh, Y.T.; Oh, N.M.; Kim, K.; Lee, D.H.; Lee, E.S. A smart flower-like polymeric micelle for pH-triggered anticancer drug release. Int. J. Pharm. 2009, 375, 163–169. [Google Scholar] [CrossRef]
- Baek, A.; Baek, Y.M.; Kim, H.M.; Jun, B.H.; Kim, D.E. Polyethylene glycol-engrafted graphene oxide as biocompatible materials for peptide nucleic acid delivery into cells. Bioconjug. Chem. 2018, 29, 528–537. [Google Scholar] [CrossRef]
- Tanaka, M.; Motomura, T.; Kawada, M.; Anzai, T.; Kasori, Y.; Shiroya, T.; Shimura, K.; Onishi, M.; Mochizuki, A. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)-relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials 2000, 21, 1471–1481. [Google Scholar] [CrossRef]
- Steinhauer, W.; Hoogenboom, R.; Keul, H.; Moeller, M. Copolymerization of 2-hydroxyethyl acrylate and 2-methoxyethyl acrylate via RAFT: Kinetics and thermoresponsive properties. Macromolecules 2010, 43, 7041–7047. [Google Scholar] [CrossRef]
- Tanaka, M.; Mochizuki, A.; Ishii, N.; Motomura, T.; Hatakeyama, T. Study of blood compatibility with poly(2-methoxyethyl acrylate). Relationship between water structure and platelet compatibility in poly(2-methoxyethylacrylate-co-2-hydroxyethylmethacrylate). Biomacromolecules 2002, 3, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Qiu, Q.; Shen, W.; An, Z. Aqueous dispersion polymerization of 2-methoxyethyl acrylate for the synthesis of biocompatible nanoparticles using a hydrophilic RAFT polymer and a redox initiator. Macromolecules 2011, 44, 5237–5245. [Google Scholar] [CrossRef]
- Liu, G.; Jin, Q.; Liu, X.; Lv, L.; Chen, C.; Ji, J. Biocompatible vesicles based on PEO-b-PMPC/α-cyclodextrin inclusion complexes for drug delivery. Soft Matter 2011, 7, 662–669. [Google Scholar] [CrossRef]
- Mueller, X.M.; Jegger, D.; Augstburger, M.; Horisberger, J.; Von Segesser, L.K. Poly2-methoxyethylacrylate (PMEA) coated oxygenator: An ex vivo study. Int. J. Artif. Organs 2002, 25, 223–229. [Google Scholar] [CrossRef]
- Haraguchi, K.; Kubota, K.; Takada, T.; Mahara, S. Highly protein-resistant coatings and suspension cell culture thereon from amphiphilic block copolymers Prepared by RAFT polymerization. Biomacromolecules 2014, 15, 1992–2003. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T.; Mizuno, T.; Kubota, K. Antithrombogenic properties of amphiphilic block copolymer coatings: Evaluation of hemocompatibility using whole blood. ACS Biomater. Sci. Eng. 2015, 1, 352–362. [Google Scholar] [CrossRef]
- Filippov, S.K.; Bogomolova, A.; Kaberov, L.; Velychkivska, N.; Starovoytova, L.; Cernochova, Z.; Rogers, S.E.; Lau, W.M.; Khutoryanskiy, V.V.; Cook, M.T. Internal nanoparticle structure of temperature-responsive self-assembled PNIPAM-b-PEG-b-PNIPAM triblock copolymers in aqueous solutions: NMR, SANS, and light scattering studies. Langmuir 2016, 32, 5314–5323. [Google Scholar] [CrossRef] [Green Version]
- Meier, M.A.R.; Lohmeijer, B.G.G.; Schubert, U.S. Characterization of defined metal-containing supramolecular block copolymers. Macromol. Rapid Commun. 2003, 24, 852–857. [Google Scholar] [CrossRef]
- Akcasu, A.Z.; Han, C.C. Molecular weight and temperature dependence of polymer dimensions in solution. Macromolecules 1979, 12, 276–280. [Google Scholar] [CrossRef]
- Tahara, Y.; Sakiyama, M.; Takeda, S.; Nishimura, T.; Mukai, S.A.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Self-assembled nanogels of cholesterol-bearing hydroxypropyl cellulose: A thermoresponsive building block for nanogel tectonic materials. Langmuir 2016, 32, 12283–12289. [Google Scholar] [CrossRef] [PubMed]
- Topel, Ö.; Çakir, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic and intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Xu, J.P.; Ji, J.; Chen, W.D.; Shen, J.C. Novel biomimetic surfactant: Synthesis and micellar characteristics. Macromol. Biosci. 2005, 5, 164–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).