Aqueous Strippable Polymer Coating for Highly Efficient Primary Radioactive Uranium Decontamination with Versatility on Diversified Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
2.3.1. Surface Decontamination of the Polychlorinated Biphenyl (PCB) Contamination Solutions
2.3.2. Surface Decontamination of the Radioactive Contamination Solution
2.3.3. Morphology of the Strippable Film
2.3.4. Mechanical Properties of the Strippable Film
3. Results and Discussion
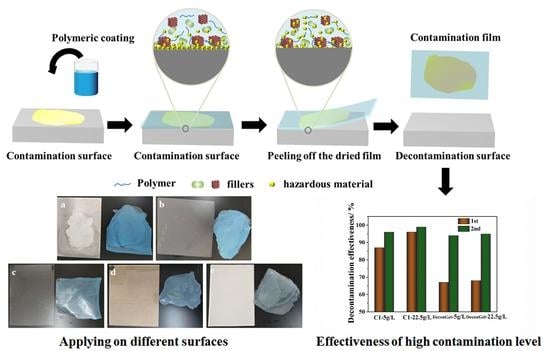
3.1. Decontamination Process of the Polymer Strippable Coating
3.2. Surface Decontamination of Polychlorinated Biphenyls (PCBs) of the Polymer Strippable Coating
3.3. Performance and Stability of Surface Decontamination of Uranium Compounds for the Polymer Strippable Coating
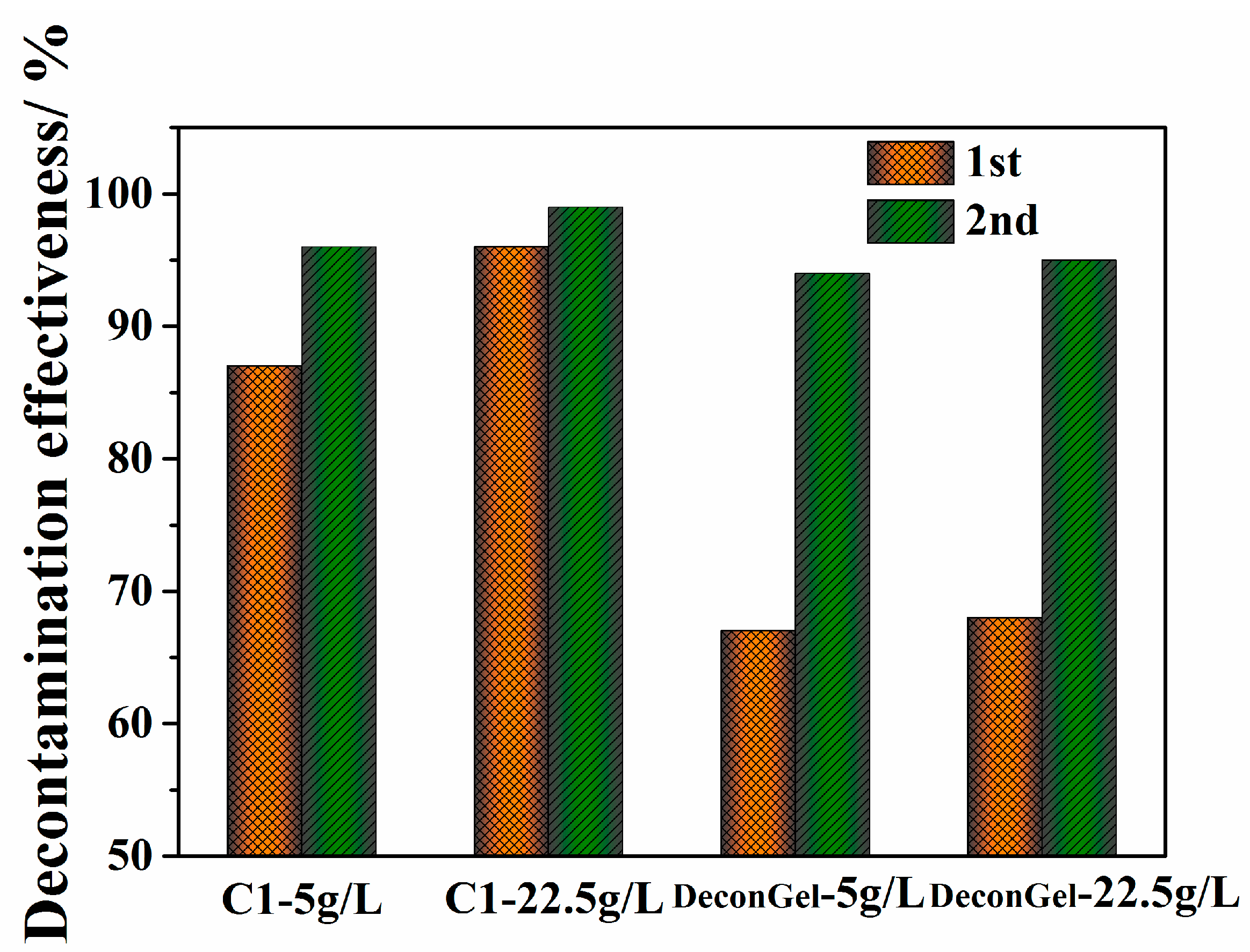
3.4. Comparison of Surface Decontamination of High Uranium Levels for the Polymer Strippable Coating
3.5. Surface Decontamination of the Uranium Compounds for the Strippable Coatings on Various Substrates
3.6. Mechanical Performance of the Strippable Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, L.; Zhao, L.; Zhang, T. Strippable coatings for radioactive contamination removal: A short review and perspectives. J. Radioanal. Nucl. Chem. 2021, 330, 29–36. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Jian, Y.; Luo, G.; Wang, M.; Hu, B.; Liu, T.; Li, J.; Yuan, Y.; Wang, N. Interlayer spacing adjusted zirconium phosphate with 2D ion channels for highly efficient removal of uranium contamination in radioactive effluent. Chem. Eng. J. 2022, 429, 132265. [Google Scholar] [CrossRef]
- Zhang, L.; Lai, J.L.; Zhang, Y.; Luo, X.G.; Li, Z.G. Degradation of Uranium-Contaminated Decontamination Film by UV Irradiation and Microbial Biodegradation. Microb. Ecol. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.N.; Jorgensen, B.; McClaugherty, D.L.; Kippenberger, A. Smart Polymeric Coatings for Surface Decontamination. Ind. Eng. Chem. Res. 2001, 40, 3540–3546. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, W.S.; Kim, G.N.; Park, H.M.; Park, U.R.; Moon, J.K. Decontamination of uranium-contaminated concrete. J. Radioanal. Nucl. Chem. 2013, 298, 973–980. [Google Scholar] [CrossRef]
- Wagle, P.G.; Tamboli, S.S.; More, A.P. Peelable coatings: A review. Prog. Org. Coat. 2021, 150, 106005. [Google Scholar] [CrossRef]
- Yang, H.M.; Yoon, I.H.; Lee, Y. Poly(vinyl alcohol)-borax complex-based spray coating for the decontamination of radioactive Cs from wide-area surfaces. Chem. Eng. J. 2020, 402, 126299. [Google Scholar] [CrossRef]
- Pulpea, D.; Rotariu, T.; Toader, G.; Pulpea, G.B.; Neculae, V.; Teodorescu, M. Decontamination of radioactive hazardous materials by using novel biodegradable strippable coatings and new generation complexing agents. Chemosphere 2020, 258, 127227. [Google Scholar] [CrossRef]
- Yang, H.M.; Park, C.W.; Lee, K.W. Polymeric coatings for surface decontamination and ecofriendly volume reduction of radioactive waste after use. Prog. Nucl. Energy 2018, 104, 67–74. [Google Scholar] [CrossRef]
- Liu, R.L.; Li, Y.T.; Zhou, Y.L.; Zhang, H.Y.; Zhang, Q.P.; Zheng, J.; Wang, S.Q. Fabrication of Poly(methyl methacrylate)-block-Poly(methacrylic acid) Diblock Copolymer as a Self-embrittling Strippable Coating for Radioactive Decontamination. Chem. Lett. 2016, 45, 793–794. [Google Scholar] [CrossRef]
- Zhang, H.; Xi, H.; Li, Z.; Pan, X.; Wang, Y.; Chen, C.; Lin, X.; Luo, X. The stability and decontamination of surface radioactive contamination of biomass-based antifreeze foam. Colloid Surf. A 2021, 624, 126774. [Google Scholar] [CrossRef]
- Toader, G.; Stănescu, P.O.; Zecheru, T.; Rotariu, T.; El-Ghayoury, A.; Teodorescu, M. Water-based strippable coatings containing bentonite clay for heavy metal surface decontamination. Arab. J. Chem. 2019, 12, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Gurau, D.; Deju, R. The use of chemical gel for decontamination during decommissioning of nuclear facilities. Radiat. Phys. Chem. 2015, 106, 371–375. [Google Scholar] [CrossRef]
- Rao, S.V.S.; Lal, K.B. Surface decontamination studies using polyvinyl acetate based strippable polymer. J. Radioanal. Nucl. Chem. 2004, 260, 35–42. [Google Scholar] [CrossRef]
- Behringer, D.L.; Smith, D.L.; Katona, V.R.; Lewis, A.T.; Hernon-Kenny, L.A.; Crenshaw, M.D. Demonstration of spread-on peel-off consumer products for sampling surfaces contaminated with pesticides and chemical warfare agent signatures. Forensic Sci. Int. 2014, 241, 7–14. [Google Scholar] [CrossRef]
- Gurau, D.; Deju, R. Radioactive decontamination technique used in decommissioning of nuclear facilities. Rom. J. Phys. 2014, 59, 912–919. [Google Scholar]
- Yang, H.M.; Park, C.W.; Lee, K.W.; Lee, B.S.; Kim, I.; Yoon, I.H. Polyvinyl alcohol-borate hydrogel containing Prussian blue for surface decontamination. J. Radioanal. Nucl. Chem. 2018, 316, 955–962. [Google Scholar] [CrossRef]
- Yang, H.M.; Hwang, K.S.; Park, C.W.; Lee, K.W. Polyvinyl alcohol-borate hydrogel containing magnetic adsorbent for surface decontamination. Ann. Nucl. Energy 2017, 109, 359–364. [Google Scholar] [CrossRef]
- Yang, H.M.; Jang, S.C.; Hong, S.B.; Lee, K.W.; Roh, C.; Huh, Y.S.; Seo, B.K. Prussian blue-functionalized magnetic nanoclusters for the removal of radioactive cesium from water. J. Alloys Compd. 2016, 657, 387–393. [Google Scholar] [CrossRef]
- Nie, X.; Hu, X.; Liu, C.; Xia, X.; Dong, F. Decontamination of uranium contained low-level radioactive wastewater from UO2 fuel element industry with vacuum membrane distillation. Desalination 2021, 516, 115226. [Google Scholar] [CrossRef]
- Chang, S.; Lee, H.K.; Kang, H.B.; Kim, T.J.; Park, S.; Jeon, H. Decontamination of Uranium-Contaminated Soil by Acid Washing with Uranium Recovery. Water Air Soil Pollut. 2021, 232, 415. [Google Scholar] [CrossRef]
- Selvakumar, R.; Ramadoss, G.; Mridula, P.M.; Rajendran, K.; Thavamani, P.; Ravi, N.; Megharaj, M. Challenges and complexities in remediation of uranium contaminated soils: A review. J. Environ. Radioact. 2018, 192, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.L.; Tan, Z.Y.; Liao, Y.T.; Feng, Y.J. Application of SO42–/TiO2 solid superacid in decontaminating radioactive pollutants. J. Environ. Radioact. 2006, 87, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Zhao, L.; Wang, S.; Li, J. Research on nuclear emergency decontamination technology based on strippable coating. J. Radioanal. Nucl. Chem. 2019, 322, 1049–1054. [Google Scholar] [CrossRef]
- Yoon, I.H.; Jung, C.H.; Yoon, S.B.; Kim, C.; Kim, S.; Yang, H.B.; Moon, J.K.; Choi, W.K. Structure and stability of decontamination foam in concentrated nitric acid and silica nanoparticles by image analysis. Ann. Nucl. Energy 2016, 95, 102–108. [Google Scholar] [CrossRef]
- Natali, I.; Carretti, E.; Angelova, L.; Baglioni, P.; Weiss, R.G.; Dei, L. Structural and Mechanical Properties of “Peelable” Organoaqueous Dispersions with Partially Hydrolyzed Poly(vinyl acetate)-Borate Networks: Applications to Cleaning Painted Surfaces. Langmuir 2011, 27, 13226–13235. [Google Scholar] [CrossRef]
- He, Z.Y.; Li, Y.T.; Zhang, Q.P.; Li, Y.J.; Liu, D.L.; Xiao, Z.Q.; Zhang, S.F.; Zhou, Y.L.; Luo, D.L. Study on the Influencing Factors in the Process of Surface Strippable Decontaminant. Coatings 2020, 10, 649. [Google Scholar] [CrossRef]
- Zeng, R.; Jin, B.K.; Yang, Z.H.; Guan, R.; Quan, C. Preparation of a modified crosslinked chitosan/polyvinyl alcohol blended affinity membrane for purification of His-tagged protein. J. Appl. Polym. Sci. 2018, 136, 47347. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, S.; Chen, S.; Guo, Z.; Xin, Y. Effect of pH, ionic strength and humic substances on the adsorption of Uranium (VI) onto Na-rectorite. J. Radioanal. Nucl. Chem. 2011, 287, 557–565. [Google Scholar] [CrossRef]
- Lozano, J.C.; Rodriguez, P.B.; Tome, F.V.; Calvo, C.P. Enhancing uranium solubilization in soils by citrate, EDTA, and EDDS chelating amendments. J. Hazard. Mater. 2011, 198, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S.D.; Norvell, W.A.; Kochian, L.V. The effect of acidification and chelating agents on the solubilization of uranium from contaminated soil. J. Environ. Qual. 1998, 27, 1486–1494. [Google Scholar] [CrossRef]
- PCBs-Decontamination-DeconGel. Available online: https://www.decongel.com/technical-product-information/ (accessed on 15 March 2022).
- Pan, B.O.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005. [Google Scholar] [CrossRef] [PubMed]










| Samples | C1 | C2 |
|---|---|---|
| component | PVA | PVA |
| CaCO3 | TiO2 | |
| modified chitosan | modified starch | |
| EDTA-2Na | EDTA-2Na |
| Sample | Contaminant Level before Decontamination/Bq cm−1 | Contaminant Level after Decontamination/Bq cm−1 | Decontamination Effectiveness/% |
|---|---|---|---|
| C1 | 0.93 | 0.01 | 98.9 ± 0.1 |
| C2 | 0.95 | 0.03 | 96.8 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Yang, W.; Yue, R.; Chen, Y. Aqueous Strippable Polymer Coating for Highly Efficient Primary Radioactive Uranium Decontamination with Versatility on Diversified Surface. Polymers 2022, 14, 1656. https://doi.org/10.3390/polym14091656
Xue Y, Yang W, Yue R, Chen Y. Aqueous Strippable Polymer Coating for Highly Efficient Primary Radioactive Uranium Decontamination with Versatility on Diversified Surface. Polymers. 2022; 14(9):1656. https://doi.org/10.3390/polym14091656
Chicago/Turabian StyleXue, Yang, Wuxinchen Yang, Renliang Yue, and Yunfa Chen. 2022. "Aqueous Strippable Polymer Coating for Highly Efficient Primary Radioactive Uranium Decontamination with Versatility on Diversified Surface" Polymers 14, no. 9: 1656. https://doi.org/10.3390/polym14091656
APA StyleXue, Y., Yang, W., Yue, R., & Chen, Y. (2022). Aqueous Strippable Polymer Coating for Highly Efficient Primary Radioactive Uranium Decontamination with Versatility on Diversified Surface. Polymers, 14(9), 1656. https://doi.org/10.3390/polym14091656