Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review
Abstract
1. Introduction
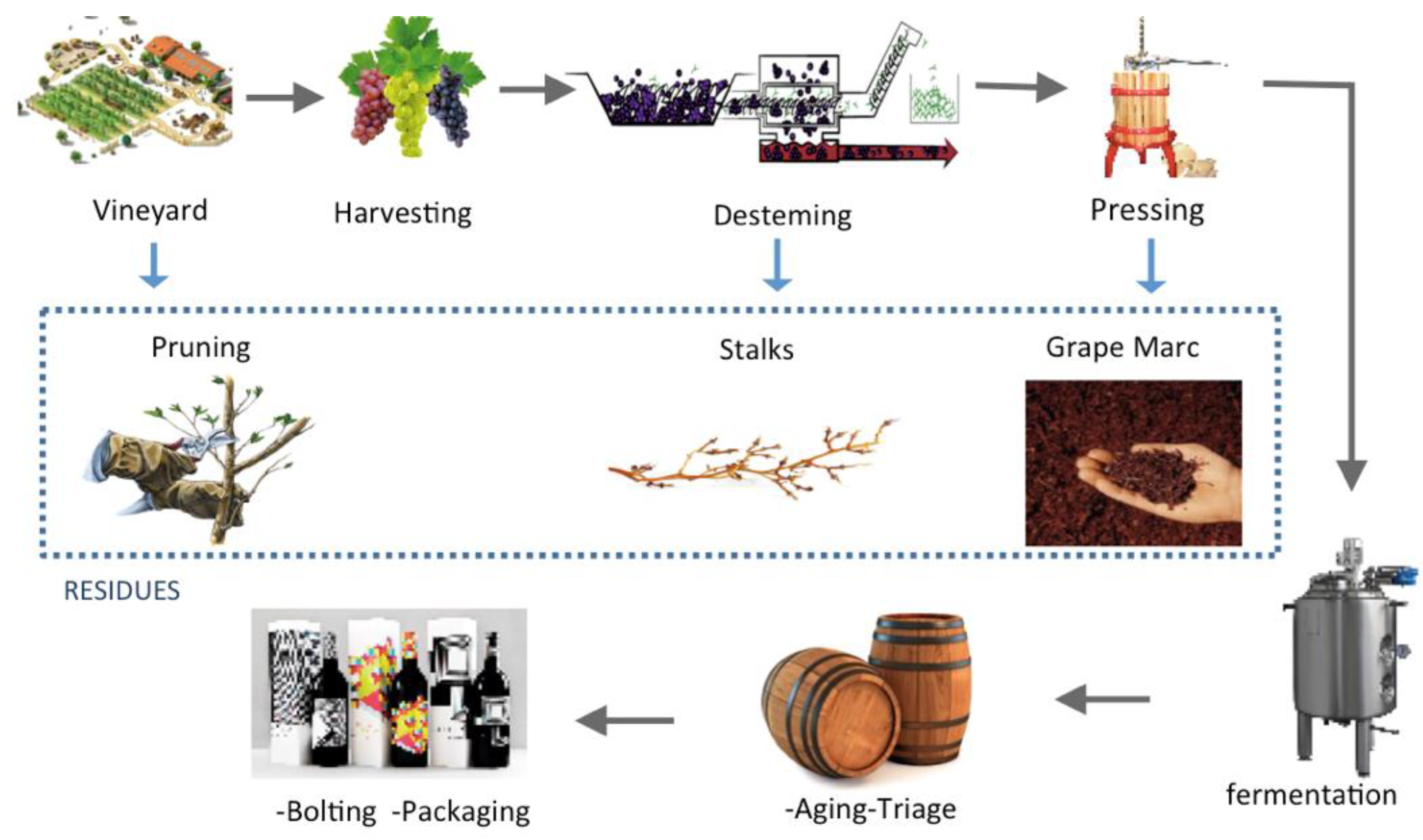
2. Biomass Residues from Grape and Wine Manufacturing
2.1. Grape Marc
2.2. Grape Stalk
2.3. Vine-Pruning Residue
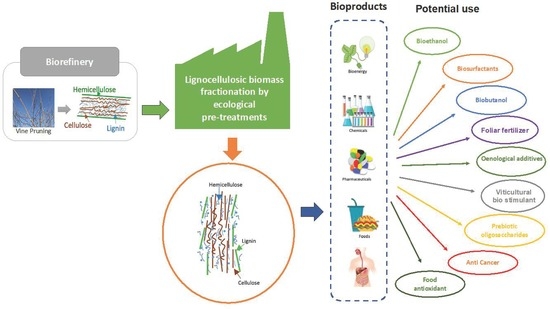
3. Fractionation of Lignocellulosic Biomass for its Revalorisation: A Biorefinery Approach
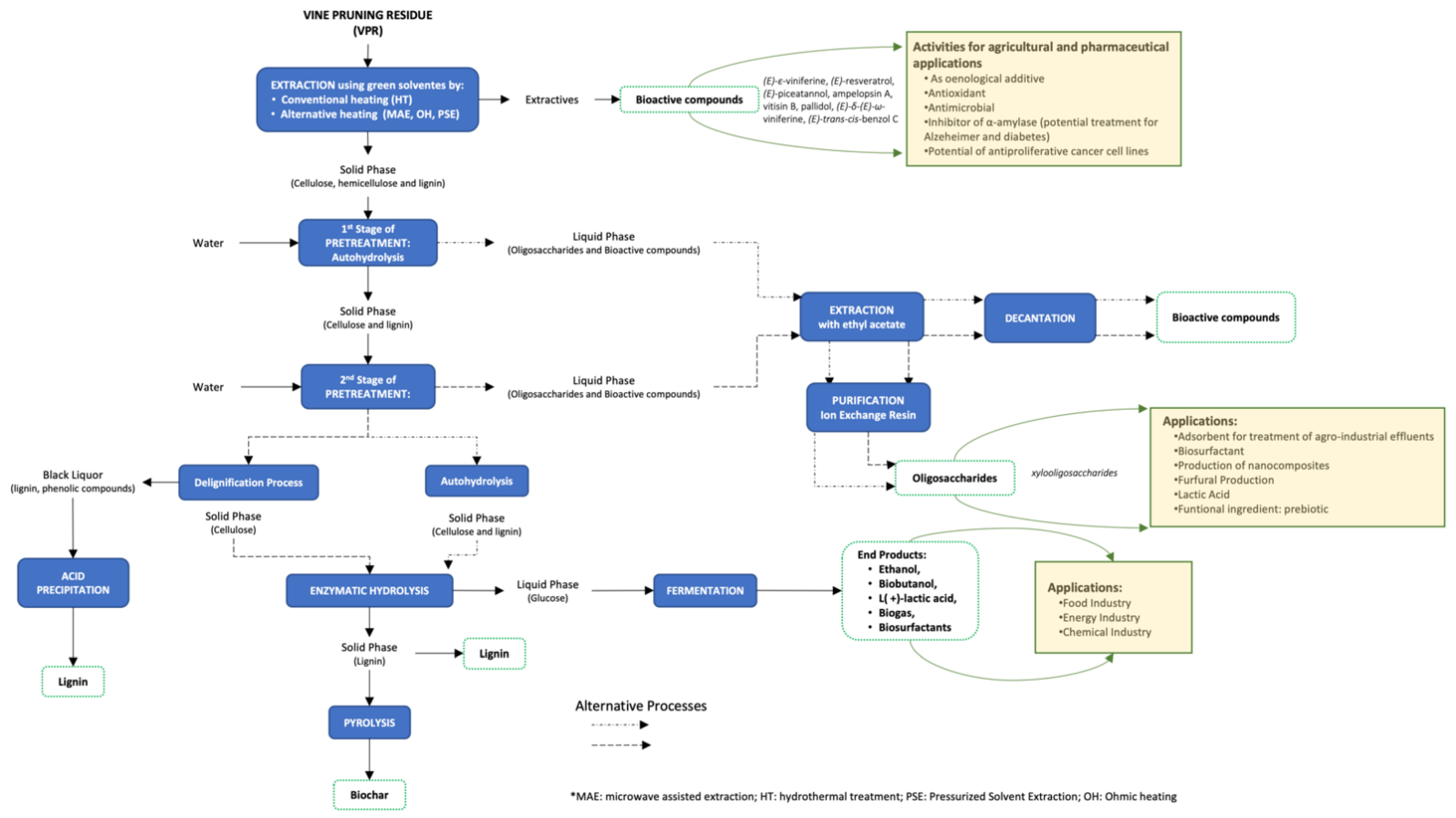
3.1. Pre-Treatment as First Step of a Biorefinery for Valorisation of Vine-Pruning Residues
- Simple and economical operation;s
- Limited requirements of energy, process water, and chemicals;
- Limited corrosion;
- Ability to alter the structure of lignocellulosic material;
- Selectivity towards polysaccharide losses;
- High recovery of valuable hemicellulose-derived products;
- Limited production of undesired degradation products;
- Production of substrates with high cellulose content and susceptibility towards enzymatic hydrolysis;
- Generation of high-quality lignin or lignin-derived products; and
- Limited generation of waste.
3.2. Phenolic Extraction and Composition of Vine Pruning
3.3. Applications of VPR Extracts in Agriculture and Pharmacological Properties
3.4. Application of Polysaccharides from Vine Pruning in the Production of Bioproducts
3.5. Potential of Vine Pruning for the Production of Biofuels
3.6. A Biorefinery Approach for the Integral Valorisation of Vine-Pruning Residues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment Updated Bioeconomy Strategy. 2018. Available online: https://knowledge4policy.ec.europa.eu/publication/sustainable-bioeconomy-europe-strengthening-connection-between-economy-society_en (accessed on 18 March 2019).
- Black, M.J.J.; Sadhukhan, J.; Day, K.; Drage, G.; Murphy, R.J.J. Developing Database Criteria for the Assessment of Biomass Supply Chains for Biorefinery Development. Chem. Eng. Res. Des. 2016, 107, 253–262. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic Biomass: A Sustainable Bioenergy Source for the Future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef] [PubMed]
- OIV. International Organisation of Vine and Wine. IOV Statistical Report on World Vitiviniculture; United Nations Food and Agriculture Organization (FAO): Paris, France, 2013. [Google Scholar]
- Gullón, P.; González-Muñoz, M.J.; van Gool, M.P.; Schols, H.A.; Hirsch, J.; Ebringerová, A.; Parajó, J.C. Structural Features and Properties of Soluble Products Derived from Eucalyptus Globulus Hemicelluloses. Food Chem. 2011, 127, 1798–1807. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery Based on Olive Biomass. State of the Art and Future Trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.L.; Barral, M.T.T.; Cruz, J.M.M.; Moldes, A.B.B. Valorization of Winery Waste vs. the Costs of Not Recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Koutinas, A.A.; Stamatelatou, K.; Mubofu, E.B.; Matharu, A.S.; Kopsahelis, N.; Pfaltzgraff, L.A.; Clark, J.H.; Papanikolaou, S.; Kwan, T.H.; et al. Current and Future Trends in Food Waste Valorization for the Production of Chemicals, Materials and Fuels: A Global Perspective. Biofuels Bioprod. Biorefining 2014, 8, 686–715. [Google Scholar] [CrossRef]
- OIV 2017 World Viticulture Situation. 2017 World Vitiviniculture Situat. OIV Stat. Rep. World Vitiviniculture; United Nations Food and Agriculture Organization (FAO): Paris, France, 2017; Volume 20. [Google Scholar]
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Viticulture in Portugal: A Review of Recent Trends and Climate Change Projections. OENO One 2017, 51, 61–69. [Google Scholar] [CrossRef]
- OIV. 2018 World Vitiviniculture Situation: OIV Statistical Report on World Vitiviniculture; United Nations Food and Agriculture Organization (FAO): Paris, France, 2018. [Google Scholar]
- APA. Portuguese National Inventory Report on Greenhouse Gases, 1990–2012; Portuguese Enviromental Agency: Amadora, Portugal, 2014. [Google Scholar]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Zafrilla, P.; Gonzalo-Diago, A.; Guerrero, R.F.; Cantos-Villar, E. Replacement of Sulfur Dioxide by Hydroxytyrosol in White Wine: Influence on Both Quality Parameters and Sensory. LWT Food Sci. Technol. 2016, 65, 214–221. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Cayuela, J.M.; Zafrilla, P.; Piñeiro, Z.; Moreno-Rojas, J.M.; Mulero, J.; Puertas, B.; Giron, F.; Guerrero, R.F.; et al. Valorization of Grape Stems. Ind. Crops Prod. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Alonso, J.L.; Labidi, J.; Gullón, P. Vine Shoots as New Source for the Manufacture of Prebiotic Oligosaccharides. Carbohydr. Polym. 2019, 207, 34–43. [Google Scholar] [CrossRef]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral Valorization of Vine Pruning Residue by Sequential Autohydrolysis Stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef]
- Torchio, F.; Urcan, D.E.; Lin, L.; Gerbi, V.; Giacosa, S.; Río Segade, S.; Pop, N.; Lambri, M.; Rolle, L. Influence of Different Withering Conditions on Phenolic Composition of Avanà, Chatus and Nebbiolo Grapes for the Production of “Reinforced” Wines. Food Chem. 2016, 194, 247–256. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Laginhas, C.; Carrott, P.J.M.; Carrott, M.M.L.R. Thermal Conversion of a Novel Biomass Agricultural Residue (Vine Shoots) into Activated Carbon Using Activation with CO2. J. Anal. Appl. Pyrolysis 2010, 87, 8–13. [Google Scholar] [CrossRef]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of Suitability of Vine Shoots for Hemicellulosic Oligosaccharides Production through Aqueous Processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef]
- Rivas, S.; López, L.; Vila, C.; Parajó, J.C. Organosolv Processing of Vine Shoots: Fractionation and Conversion of Hemicellulosic Sugars into Platform Chemicals by Microwave Irradiation. Bioresour. Technol. 2021, 342, 125967. [Google Scholar] [CrossRef]
- Gloria, A.; Contri, A.; Grotta, L.; Carluccio, A.; Robbe, D.; Ianni, A.; Vignola, G.; Martino, G. Effect of Dietary Grape Marc on Fresh and Refrigerated Boar Semen. Anim. Reprod. Sci. 2019, 205, 18–26. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Xavier, A.M.R.B.; Evtuguin, D.V.; Lopes, L.P.C. Integrated Utilization of Grape Skins from White Grape Pomaces. Ind. Crops Prod. 2013, 49, 286–291. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Lagazzo, A.; Garbarino, G.; Carnasciali, M.M.; Perego, P.; Busca, G. Pyrolysis of Grape Marc before and after the Recovery of Polyphenol Fraction. Fuel Process. Technol. 2016, 153, 121–128. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for Recycling and Valorization of Grape Marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical Composition of Grape Stalks of Vitis Vinifera, L. from Red Grape Pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of Grape Stalks as a Bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape Stalk Valorization for Fermentation Purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Ioannidou, S.P.; Margellou, A.G.; Petala, M.D.; Triantafyllidis, K.S. Pretreatment/Fractionation and Characterization of Winery Waste Streams within an Integrated Biorefinery Concept. Sustain. Chem. Pharm. 2022, 27, 100670. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, P.; Andrés, M.A.; Labidi, J. Coproduction of Lignin and Glucose from Vine Shoots by Eco-Friendly Strategies: Toward the Development of an Integrated Biorefinery. Bioresour. Technol. 2017, 244, 328–337. [Google Scholar] [CrossRef]
- Çetin, E.S.; Altinöz, D.; Tarçan, E.; Baydar, N.G. Chemical Composition of Grape Canes. Ind. Crops Prod. 2011, 34, 994–998. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of Polyphenol-Rich Grape by-Products in Monogastric Nutrition. A Review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Zhang, N.; Hoadley, A.; Patel, J.; Lim, S.; Li, C. Sustainable Options for the Utilization of Solid Residues from Wine Production. Waste Manag. 2017, 60, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape Marc as a Source of Carbohydrates for Bioethanol: Chemical Composition, Pre-Treatment and Saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Mannitol Bioproduction from Surplus Grape Musts and Wine Lees. LWT 2021, 151, 112083. [Google Scholar] [CrossRef]
- Campanella, D.; Rizzello, C.G.; Fasciano, C.; Gambacorta, G.; Pinto, D.; Marzani, B.; Scarano, N.; De Angelis, M.; Gobbetti, M. Exploitation of Grape Marc as Functional Substrate for Lactic Acid Bacteria and Bifidobacteria Growth and Enhanced Antioxidant Activity. Food Microbiol. 2017, 65, 25–35. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the Extraction Process of Natural Antioxidants from Chardonnay Grape Marc Using Microwave-Assisted Extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef]
- Ibn Ferjani, A.; Jeguirim, M.; Jellali, S.; Limousy, L.; Courson, C.; Akrout, H.; Thevenin, N.; Ruidavets, L.; Muller, A.; Bennici, S. The Use of Exhausted Grape Marc to Produce Biofuels and Biofertilizers: Effect of Pyrolysis Temperatures on Biochars Properties. Renew. Sustain. Energy Rev. 2019, 107, 425–433. [Google Scholar] [CrossRef]
- Teixeira, N.; Mateus, N.; de Freitas, V.; Oliveira, J. Wine Industry By-Product: Full Polyphenolic Characterization of Grape Stalks. Food Chem. 2018, 268, 110–117. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.À.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical Characterization of Different Granulometric Fractions of Grape Stalks Waste. Ind. Crops Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Portinho, R.; Zanella, O.; Féris, L.A. Grape Stalk Application for Caffeine Removal through Adsorption. J. Environ. Manag. 2017, 202, 178–187. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Díaz-Fierros, F.; Barral, M.T. Trace Metals in River Bed Sediments: An Assessment of Their Partitioning and Bioavailability by Using Multivariate Exploratory Analysis. J. Environ. Manag. 2010, 91, 2471–2477. [Google Scholar] [CrossRef]
- Dávila, I.; Robles, E.; Egüés, I.; Labidi, J.; Gullón, P. The Biorefinery Concept for the Industrial Valorization of Grape Pro-cessing By-Products. In Handbook of Grape Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 29–53. ISBN 9780128098707. [Google Scholar]
- Verdini, F.; Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Cravotto, G.; Bassani, A. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Appl. Sci. 2021, 11, 4693. [Google Scholar] [CrossRef]
- Garita-Cambronero, J.; Hijosa-Valsero, M.; Paniagua-García, A.I.; Díez-Antolínez, R. Revisiting the Production of L(+)-Lactic Acid from Vine Shoots: Bioconversion Improvements by Employing Thermotolerant Bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 9385–9402. [Google Scholar] [CrossRef]
- Cotana, F.; Barbanera, M.; Foschini, D.; Lascaro, E.; Buratti, C. Preliminary Optimization of Alkaline Pretreatment for Ethanol Production from Vineyard Pruning. Energy Procedia 2015, 82, 389–394. [Google Scholar] [CrossRef][Green Version]
- Romaní, A.; Tomaz, P.D.; Garrote, G.; Teixeira, J.A.; Domingues, L. Combined Alkali and Hydrothermal Pretreatments for Oat Straw Valorization within a Biorefinery Concept. Bioresour. Technol. 2016, 220, 323–332. [Google Scholar] [CrossRef] [PubMed]
- El Achaby, M.; El Miri, N.; Hannache, H.; Gmouh, S.; Ben youcef, H.; Aboulkas, A. Production of Cellulose Nanocrystals from Vine Shoots and Their Use for the Development of Nanocomposite Materials. Int. J. Biol. Macromol. 2018, 117, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Effects of Enzymatic Hydrolysis and Ultrasounds Pretreatments on Corn Cob and Vine Trimming Shoots for Biogas Production. Bioresour. Technol. 2016, 221, 130–138. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive Compounds Recovery Optimization from Vine Pruning Residues Using Conventional Heating and Microwave-Assisted Extraction Methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Kantar, S.; Afif, C.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Selective Multistage Extraction Process of Biomolecules from Vine Shoots by a Combination of Biological, Chemical, and Physical Treatments. Comptes Rendus Chim. 2018, 21, 581–589. [Google Scholar] [CrossRef]
- Max, B.; Salgado, J.M.; Cortés, S.; Domínguez, J.M. Extraction of Phenolic Acids by Alkaline Hydrolysis from the Solid Residue Obtained after Prehydrolysis of Trimming Vine Shoots. J. Agric. Food Chem. 2010, 58, 1909–1917. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Dávila, I.; Labidi, J.; Gullón, P. Antioxidant and Antimicrobial Activities of Extracts Obtained from the Refining of Autohydrolysis Liquors of Vine Shoots. Ind. Crops Prod. 2017, 107, 105–113. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-Assisted Extraction of Polyphenols from Vine Shoot Cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A Comparative Study of Physical Pretreatments for the Extraction of Polyphenols and Proteins from Vine Shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque De Castro, M.D. Comparison of Accelerated Methods for the Extraction of Phenolic Compounds from Different Vine-Shoot Cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese Vine Shoot Wastes as Natural Resources of Bioactive Compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Marrufo-Curtido, A.; Vela, C.; Palma, M. Microwave-Assisted Extraction of Stilbenes from Woody Vine Material. Food Bioprod. Process. 2017, 103, 18–26. [Google Scholar] [CrossRef]
- Zachová, Z.; Tříska, J.; Vrchotová, N.; Balík, J.; Sajfrtová, M.; Sovová, H. Combining High-Pressure Methods for Extraction of Stilbenes from Grape Cane. J. Supercrit. Fluids 2018, 142, 38–44. [Google Scholar] [CrossRef]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis Vinifera, L. Pinot Noir Grape Cane Extract: Isolation, Characterization, in Vitro Antioxidant Capacity and Anti-Proliferative Effect on Cancer Cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.; Genisheva, Z.; Carvalho, A.C.; Pereira-Wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic Heating Polyphenolic Extracts from Vine Pruning Residue with Enhanced Biological Activity. Food Chem. 2020, 316, 125298. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Winemaking with Vine-Shoots. Modulating the Composition of Wines by Using Their Own Resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Pardo, F.; Gonzalo, L.A.; Salinas, M.R. Moscatel Vine-Shoot Extracts as a Grapevine Biostimulant to Enhance Wine Quality. Food Res. Int. 2017, 98, 40–49. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Garde-Cerdán, T.; Zalacain, A.; Garcia, R.; Cabrita, M.J.; Salinas, M.R. Vine-Shoot Waste Aqueous Extract Applied as Foliar Fertilizer to Grapevines: Effect on Amino Acids and Fermentative Volatile Content. Food Chem. 2016, 197, 132–140. [Google Scholar] [CrossRef]
- Vecino, X.; Rodríguez-López, L.; Gudiña, E.J.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Vineyard Pruning Waste as an Alternative Carbon Source to Produce Novel Biosurfactants by Lactobacillus Paracasei. J. Ind. Eng. Chem. 2017, 55, 40–49. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Pérez-Rodríguez, N.; de Souza Oliveira, R.P.; Huerta, B.E.B.; Domínguez, J.M. Production of Biosurfactants from Vine-Trimming Shoots Using the Halotolerant Strain Bacillus Tequilensis ZSB10. Ind. Crops Prod. 2016, 79, 258–266. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Maciel, T.C.; Gudiña, E.; Miguel, E.C.; Rodrigues, L.R.; Rodrigues, S. Multivariate Analysis as a Tool for Selecting the Vine Pruning Pretreatment towards the Highest Enzymatic Hydrolysis Yield. Biomass Bioenergy 2020, 140, 105653. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Labidi, J.; Gullón, P. Multiproduct Biorefinery from Vine Shoots: Bio-Ethanol and Lignin Production. Renew. Energy 2019, 142, 612–623. [Google Scholar] [CrossRef]
- Buratti, C.; Barbanera, M.; Lascaro, E. Ethanol Production from Vineyard Pruning Residues with Steam Explosion Pretreatment. Environ. Prog. Sustain. Energy 2015, 34, 802–809. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Villagrasa, S.; Cruz, J.M.; Moldes, A.B. Kinetic and Morphology Study of Alginate-Vineyard Pruning Waste Biocomposite vs. Non Modified Vineyard Pruning Waste for Dye Removal. J. Environ. Sci. 2015, 38, 158–167. [Google Scholar] [CrossRef]
- Giorio, C.; Pizzini, S.; Marchiori, E.; Piazza, R.; Grigolato, S.; Zanetti, M.; Cavalli, R.; Simoncin, M.; Soldà, L.; Badocco, D.; et al. Sustainability of Using Vineyard Pruning Residues as an Energy Source: Combustion Performances and Environmental Impact. Fuel 2019, 243, 371–380. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-Microporous Adsorbents Prepared from Vine Shoots-Derived Biochar with High CO2 uptake and CO2/N2 selectivity. Chem. Eng. J. 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Azuara, M.; Sáiz, E.; Manso, J.A.; García-Ramos, F.J.; Manyà, J.J. Study on the Effects of Using a Carbon Dioxide Atmosphere on the Properties of Vine Shoots-Derived Biochar. J. Anal. Appl. Pyrolysis 2017, 124, 719–725. [Google Scholar] [CrossRef]
- Garita-Cambronero, J.; Paniagua-García, A.I.; Hijosa-Valsero, M.; Díez-Antolínez, R. Biobutanol Production from Pruned Vine Shoots. Renew. Energy 2021, 177, 124–133. [Google Scholar] [CrossRef]
- Rivas, S.; Parajó, J.C.; Carral, C.; Álvarez Bermúdez, X. Single-Stage Fractionation of Vine Shoots Using Microwave Heating. Appl. Sci. 2021, 11, 7954. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of Toasting on Non-Volatile and Volatile Vine-Shoots Low Molecular Weight Phenolic Compounds. Food Chem. 2016, 204, 499–505. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Electrical, Mechanical, and Chemical Effects of High-Voltage Electrical Discharges on the Polyphenol Extraction from Vine Shoots. Innov. Food Sci. Emerg. Technol. 2015, 31, 60–66. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, T.; Rodríguez, I.; Ramil, M.; Cela, R. Assessment of Alcoholic Distillates for the Extraction of Bioactive Polyphenols from Grapevine Canes. Ind. Crops Prod. 2018, 111, 99–106. [Google Scholar] [CrossRef]
- Max, B.; Torrado, A.M.; Moldes, A.B.; Converti, A.; Domínguez, J.M. Ferulic Acid and P-Coumaric Acid Solubilization by Alkaline Hydrolysis of the Solid Residue Obtained after Acid Prehydrolysis of Vine Shoot Prunings: Effect of the Hydroxide and PH. Biochem. Eng. J. 2009, 43, 129–134. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H.; Parajó, J.C. Production of Antioxidants by Non-Isothermal Autohydrolysis of Lignocellulosic Wastes. LWT Food Sci. Technol. 2011, 44, 436–442. [Google Scholar] [CrossRef]
- Nitsos, C.; Matsakas, L.; Triantafyllidis, K.; Rova, U.; Christakopoulos, P. Investigation of Different Pretreatment Methods of Mediterranean-Type Ecosystem Agricultural Residues: Characterisation of Pretreatment Products, High-Solids Enzymatic Hydrolysis and Bioethanol Production. Biofuels 2018, 9, 545–558. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Parajó, J.C. Bioethanol Production from Autohydrolyzed Eucalyptus Globulus by Simultaneous Saccharification and Fermentation Operating at High Solids Loading. Fuel 2012, 94, 305–312. [Google Scholar] [CrossRef]
- Dorosh, O.; Rodrigues, F.; Delerue-Matos, C.; Moreira, M.M. Increasing the Added Value of Vine-Canes as a Sustainable Source of Phenolic Compounds: A Review. Sci. Total Environ. 2022, 830, 154600. [Google Scholar] [CrossRef]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis Vinifera) Subcritical Water Extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef]
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of Resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products: Sustainable Solutions; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 267–292. ISBN 9780128098714. [Google Scholar]
- Alexandru, L.; Binello, A.; Mantegna, S.; Boffa, L.; Cravotto, G.; Chemat, F.; Cravotto, G. Efficient Green Extraction of Polyphenols from Post-Harvested Agro-Industry Vegetal Sources in Piedmont. Comptes Rendus Chim. 2014, 17, 212–217. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and Health – A Comprehensive Review of Human Clinical Trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Xu, Y. Resveratrol Protects Vascular Smooth Muscle Cells against High Glucose-Induced Oxidative Stress and Cell Proliferation in Vitro. Med. Sci. Monit. Basic Res. 2014, 20, 82–92. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine Cane’s Waste Is a Source of Bioactive Stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Gullón, B.; Teixeira, J.A.; Botelho, C.M.; Yáñez, R. Exploiting the Potential of Bioactive Molecules Extracted by Ultrasounds from Avocado Peels—Food and Nutraceutical Applications. Antioxidants 2021, 10, 1475. [Google Scholar] [CrossRef]
- Okur, M.E.; Karadağ, A.E.; Özhan, Y.; Sipahi, H.; Ayla, Ş.; Daylan, B.; Kültür, Ş.; Demirci, B.; Demirci, F. Anti-Inflammatory, Analgesic and in Vivo-in Vitro Wound Healing Potential of the Phlomis Rigida Labill. Extract. J. Ethnopharmacol. 2021, 266, 113408. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent Selection for Efficient Extraction of Bioactive Compounds from Grape Pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Bidanchi, R.M.; Lalrindika, L.; Khushboo, M.; Bhanushree, B.; Dinata, R.; Das, M.; Nisa, N.; Lalrinzuali, S.; Manikandan, B.; Saeed-Ahmed, L.; et al. Antioxidative, Anti-Inflammatory and Anti-Apoptotic Action of Ellagic Acid against Lead Acetate Induced Testicular and Hepato-Renal Oxidative Damages and Pathophysiological Changes in Male Long Evans Rats. Environ. Pollut. 2022, 302, 119048. [Google Scholar] [CrossRef] [PubMed]
- García-Niño, W.R.; Zazueta, C. Ellagic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Liver Protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, Ellagic Acid and Their Derived Metabolites: A Review about Source, Metabolism, Functions and Health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, X.; Men, X.; Xu, Z.; Wang, T. In Vitro and in Vivo Antioxidant Activities of Three Major Polyphenolic Compounds in Pomegranate Peel: Ellagic Acid, Punicalin, and Punicalagin. J. Integr. Agric. 2017, 16, 1808–1818. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Gómez-Alonso, S.; Hermosín-Gutierrez, I.; Mena-Morales, A.; García-Romero, E.; Salinas, M.R.; Zalacain, A. Vine-Shoot Tannins: Effect of Post-Pruning Storage and Toasting Treatment. J. Agric. Food Chem. 2018, 66, 5556–5562. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Sánchez-Vioque, R.; Santana-Méridas, O.; Martín-Bejerano, M.; Alonso, G.L.L.; Salinas, M.R.R.; Zalacain, A. A Potential Use of Vine-Shoot Wastes: The Antioxidant, Antifeedant and Phytotoxic Activities of Their Aqueous Extracts. Ind. Crops Prod. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Jesus, M.S.; Carvalho, A.C.; Teixeira, J.A.; Domingues, L.; Pereira-Wilson, C. Ohmic Heating Extract of Vine Pruning Residue Has Anti-Colorectal Cancer Activity and Increases Sensitivity to the Chemotherapeutic Drug 5-FU. Foods 2020, 9, 1102. [Google Scholar] [CrossRef]
- Mamaní, A.; Maturano, Y.; Mestre, V.; Montoro, L.; Gassa, L.; Deiana, C.; Sardella, F. Valorization of Olive Tree Pruning. Application for Energy Storage and Biofuel Production. Ind. Crops Prod. 2021, 173, 114082. [Google Scholar] [CrossRef]
- Romero-García, J.M.; López-Linares, J.C.; Contreras, M.D.M.; Romero, I.; Castro, E. Exploitation of Olive Tree Pruning Biomass through Hydrothermal Pretreatments. Ind. Crops Prod. 2022, 176, 114425. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Romero, I.; Moya, M.; Castro, E. Olive-Derived Biomass as a Renewable Source of Value-Added Products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Álvarez, C.; Manzanares, P.; Moreno, A.D. Fermentation Strategies for the Efficient Use of Olive Tree Pruning Biomass from a Flexible Biorefinery Approach. Fuel 2020, 277, 118171. [Google Scholar] [CrossRef]
- Domínguez, E.; Romaní, A.; Domingues, L.; Garrote, G. Evaluation of Strategies for Second Generation Bioethanol Production from Fast Growing Biomass Paulownia within a Biorefinery Scheme. Appl. Energy 2017, 187, 777–789. [Google Scholar] [CrossRef]
- Bello, M.; Jiddah-kazeem, B.; Fatoki, T.H.; Ibukun, E.O.; Akinmoladun, A.C. Antioxidant Property of Eucalyptus Globulus Labill. Extracts and Inhibitory Activities on Carbohydrate Metabolizing Enzymes Related to Type-2 Diabetes. Biocatal. Agric. Biotechnol. 2021, 36, 102111. [Google Scholar] [CrossRef]
- Romaní, A.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus Wood by Glycerol-Organosolv Pretreatment within the Biorefinery Concept: An Integrated and Intensified Approach. Renew. Energy 2016, 95, 1–9. [Google Scholar] [CrossRef]
- Costa, C.E.; Romaní, A.; Cunha, J.T.; Johansson, B.; Domingues, L. Integrated Approach for Selecting Efficient Saccharomyces Cerevisiae for Industrial Lignocellulosic Fermentations: Importance of Yeast Chassis Linked to Process Conditions. Bioresour. Technol. 2017, 227, 24–34. [Google Scholar] [CrossRef]
- Oksman, K.; Etang, J.A.; Mathew, A.P.; Jonoobi, M. Cellulose Nanowhiskers Separated from a Bio-Residue from Wood Bioethanol Production. Biomass Bioenergy 2011, 35, 146–152. [Google Scholar] [CrossRef]

| Residues | Cellulose | Hemicellulose | Lignin | Ashes | Proteins | Extractives | Estimated Production | Estimated Availability in World (Million t/Year) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Grape marc | 20.8 | 2.5 | 29.8 | 4.2–7.8 | 12.1–18.8 | 39.1 | 20–30% | 10.5–13.1 | [22,23,24,25] |
| Stalks | 20–36 | 21–24.5 | 17.4–34 | 3.9–7 | 6.1 | 1.7–2.3 | 3–7.5% | 2.2–3.1 | [26,27,28,29] |
| Vine prunings | 32.9–39.9 | 5.8–27 | 26.7–46.8 | 2.6–3.3 | 2.0–2.7 | 3.1–16.6 | - | 2–4 | [17,19,21,30,31,32] |
| Treatments | Products | Reference |
|---|---|---|
| CHE and MAE * | Phenolic compounds | [51] |
| Acid and alkaline hydrolysis | Phenolic compounds | [53] |
| Autohydrolysis | Phenolic compounds | [54] |
| CHE * and β-cyclodextrin | Phenolic compounds | [55] |
| Alkaline hydrolysis and HVED * | Phenolic compounds | [56] |
| SHLE, MAE, and UAE * | Phenolic compounds | [57] |
| CHE and MAE * | Phenolic compounds | [58] |
| MAE * | Phenolic compounds | [59] |
| Enzymatic hydrolysis, alkaline hydrolysis, and HVED * | Phenolic compounds and proteins | [52] |
| PSE and PFE * | Phenolic compounds | [60] |
| CHE * | Phenolic compounds | [61] |
| OH * | Phenolic compounds | [62] |
| - | Oenological additives | [63] |
| CHE, SLDE, PSE, and MAE * | Viticultural bio stimulant | [64] |
| CHE * | Foliar fertilizer | [65] |
| Autohydrolysis | Prebiotic oligosaccharides | [16] |
| Hydrolysis acid | Biosurfactants | [66] |
| Dilute acid hydrolysis, delignification, and enzymatic hydrolysis | Biosurfactants | [67] |
| Combined acid and alkali followed by enzymatic hydrolysis | Glucose | [68] |
| Organosolv processing followed by microwave irradiation | Furfural | [21] |
| Two sequential stages of autohydrolysis followed by enzymatic hydrolysis | Bioethanol, xylooligosaccharides, phenolic compounds, and lignin | [17] |
| Autohydrolysis and delignification followed by enzymatic hydrolysis | Bioethanol and lignin | [69] |
| Steam explosion and enzymatic hydrolysis | Bioethanol | [70] |
| Alkaline hydrolysis and enzymatic hydrolysis | Bioethanol | [47] |
| Alkaline hydrolysis | Biocomposite | [71] |
| UAE * followed by enzymatic hydrolysis | Biogas | [50] |
| Alkaline hydrolysis, bleaching, and acid hydrolysis | Cellulose nanocrystals for nanocomposite materials | [49] |
| - | Wood chips and ashes | [72] |
| Pyrolysis | Ultra-microporous adsorbents | [73] |
| Pyrolysis | Biochar | [74] |
| Alkaline hydrolysis followed by enzymatic hydrolysis | Biobutanol | [75] |
| MAE * | Oligosaccharides, lignin, and cellulose | [76] |
| Alkaline and acidic hydrolysis, followed by enzymatic hydrolysis | L(+)-lactic acid | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, M.; Romaní, A.; Mata, F.; Domingues, L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review. Polymers 2022, 14, 1640. https://doi.org/10.3390/polym14091640
Jesus M, Romaní A, Mata F, Domingues L. Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review. Polymers. 2022; 14(9):1640. https://doi.org/10.3390/polym14091640
Chicago/Turabian StyleJesus, Meirielly, Aloia Romaní, Fernando Mata, and Lucília Domingues. 2022. "Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review" Polymers 14, no. 9: 1640. https://doi.org/10.3390/polym14091640
APA StyleJesus, M., Romaní, A., Mata, F., & Domingues, L. (2022). Current Options in the Valorisation of Vine Pruning Residue for the Production of Biofuels, Biopolymers, Antioxidants, and Bio-Composites following the Concept of Biorefinery: A Review. Polymers, 14(9), 1640. https://doi.org/10.3390/polym14091640