Design and Evaluation of pH-Sensitive Nanoformulation of Bergenin Isolated from Bergenia ciliata
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Isolation and Extraction of Bergenin
2.2.2. Identification of Bergenin
2.2.3. Preparation of Bergenin-Loaded Nanoparticles
2.2.4. Experimental Design for Optimization of Nanoparticles
2.2.5. Characterization of Prepared NPs
Mean Particle Size, Polydispersity Index and Zeta Potential
Morphological Analysis
Estimation of Percentage Yield, Entrapment Efficiency (EE) and Drug Loading (DL)
Drug–Excipient Interaction
X-ray Diffraction
In Vitro Drug Release Studies
Physical Stability Study
3. Results
3.1. Extraction Efficiency and Bergenin Yield
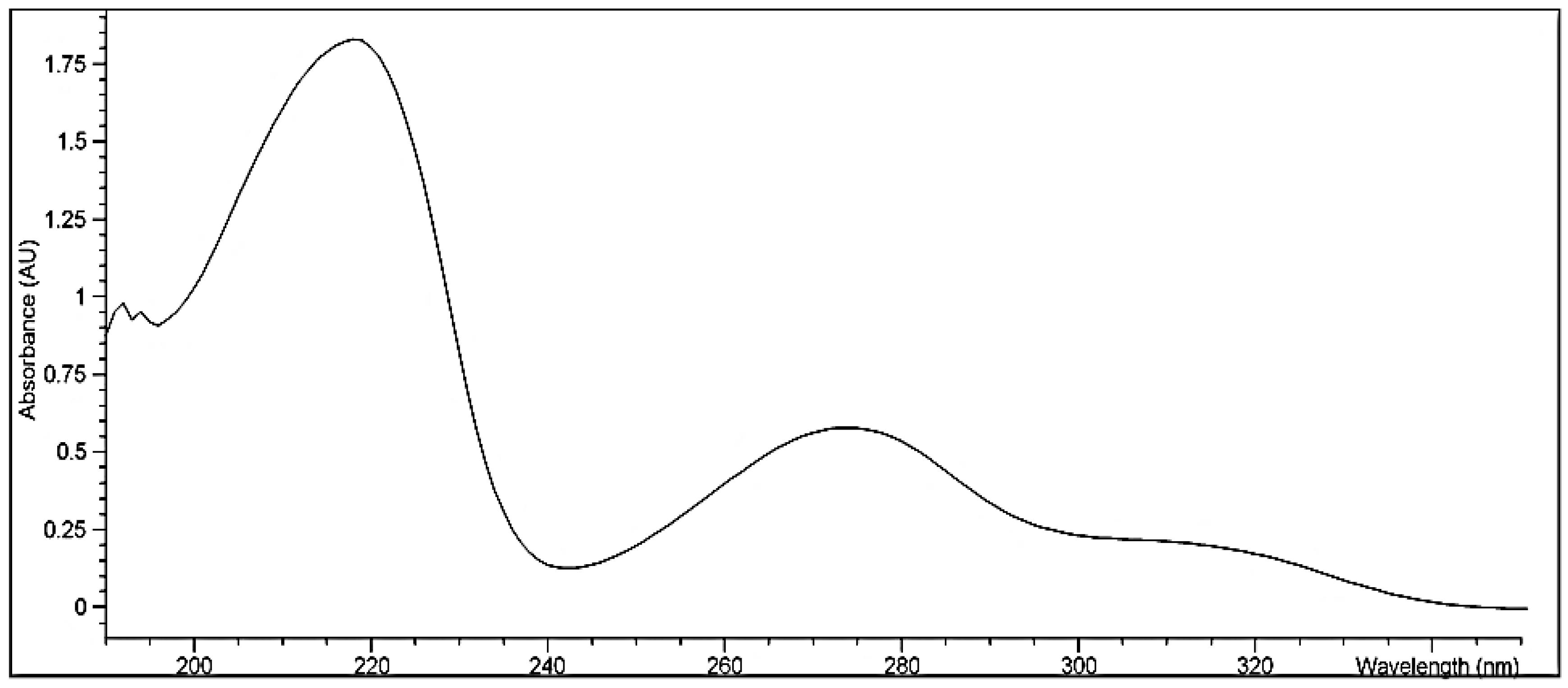
3.1.1. Identification of Bergenin
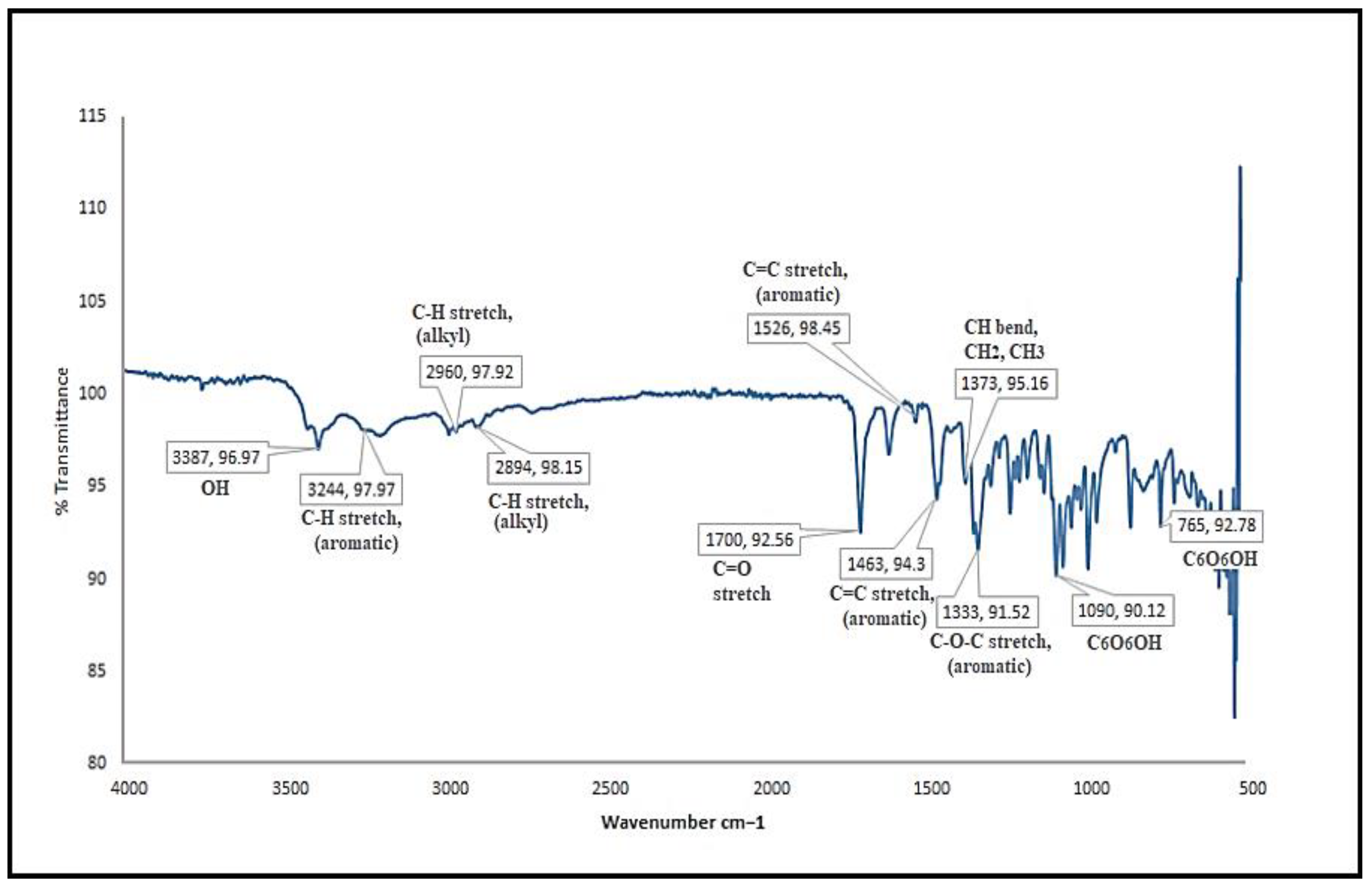
3.1.2. FTIR Spectroscopy of Bergenin
3.2. Preparation of Optimized Formulation Using Factorial Design
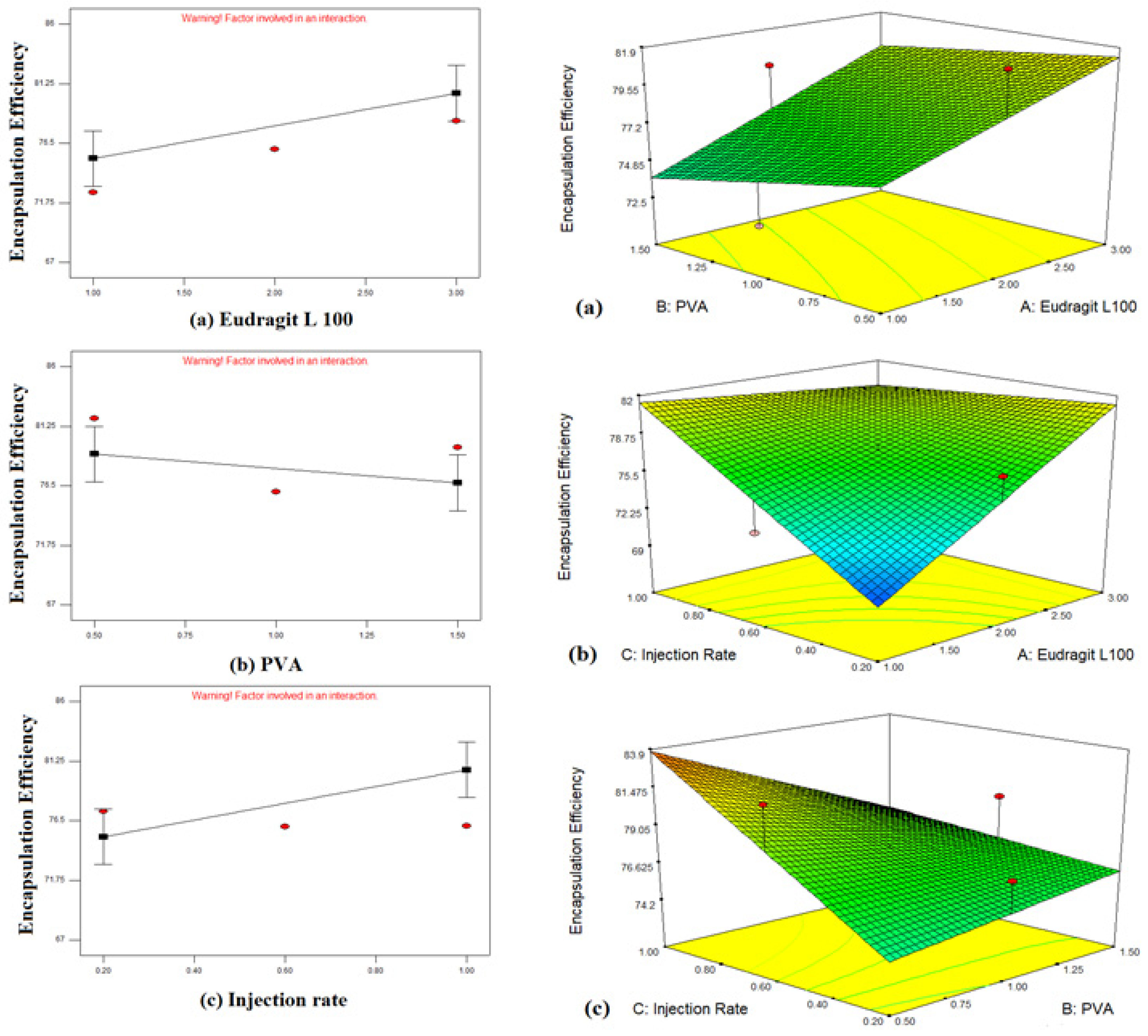
3.2.1. Response Effects of Independent Variables on Particle Size and Entrapment Efficiency
Effect of Independent Variables on Particle Size (Y1)
Effect of Independent Variables on Entrapment Efficiency (Y2)
3.2.2. Selection and Preparation of Optimized Nanoformulation
3.3. Characterization of Optimized Formulation
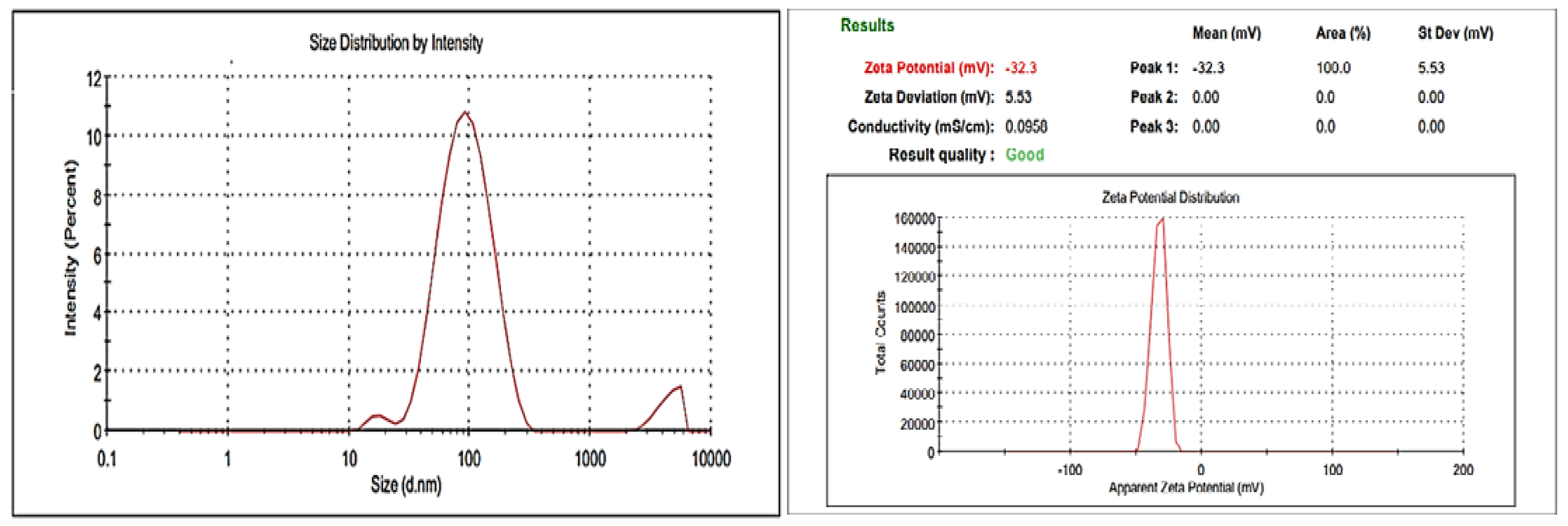
3.3.1. Size Measurement and Zeta Potential
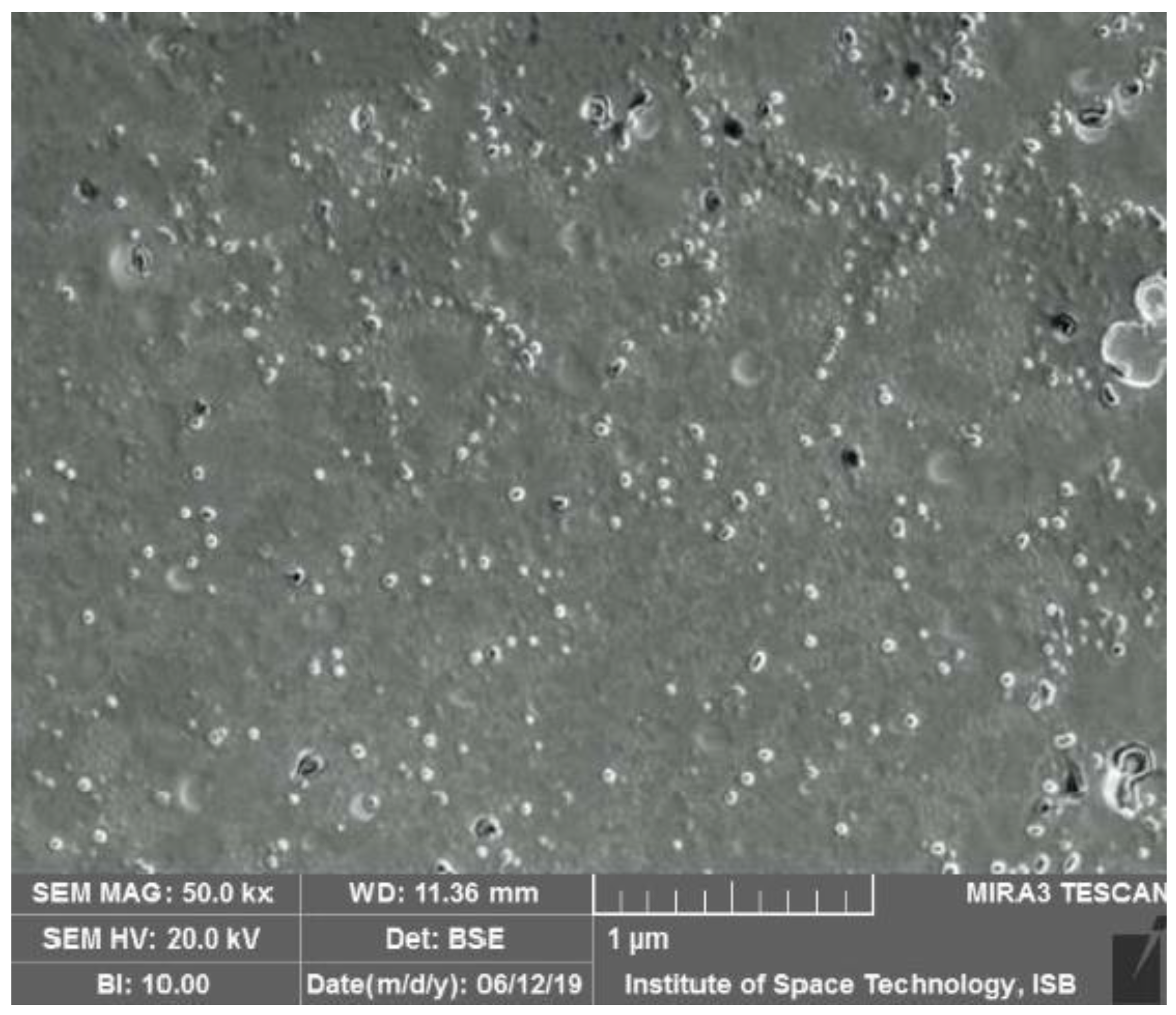
3.3.2. Morphological Analyses
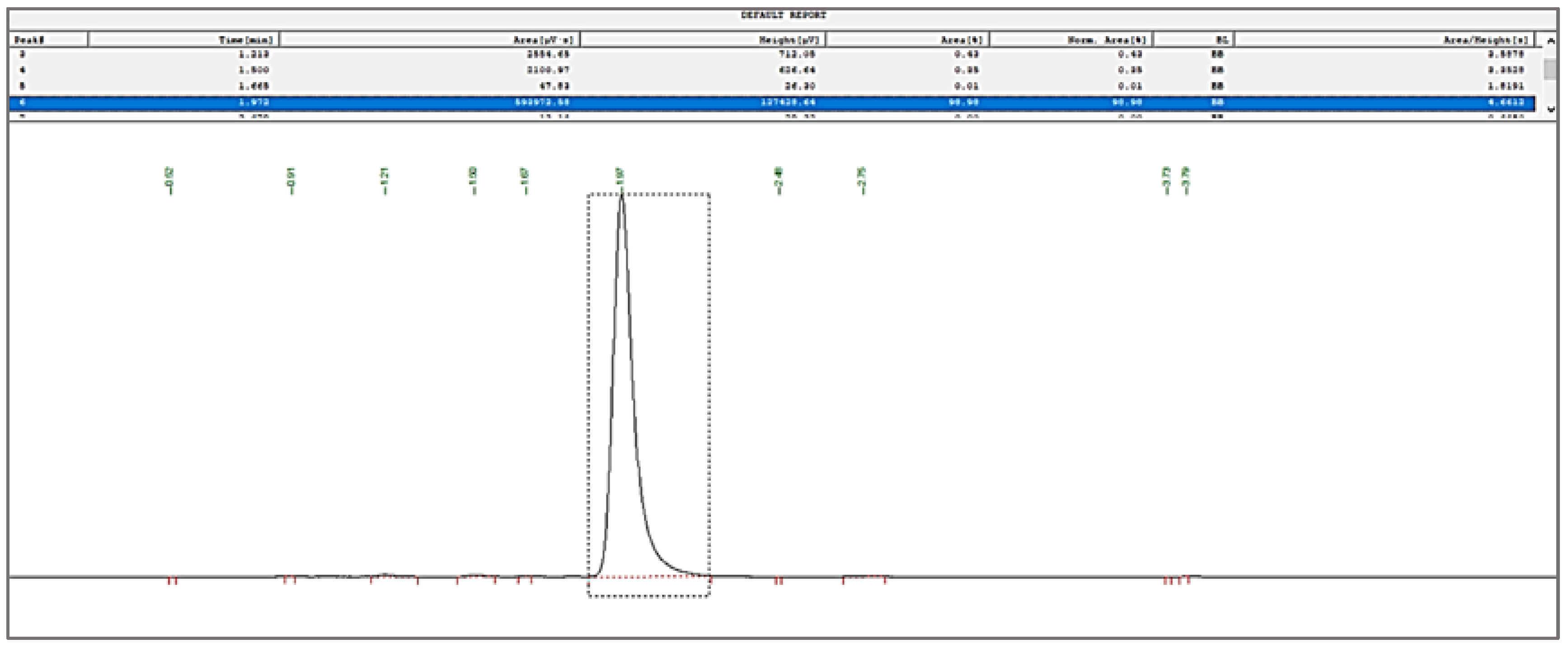
3.3.3. Quantification of Bergenin through RP-HPLC
3.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
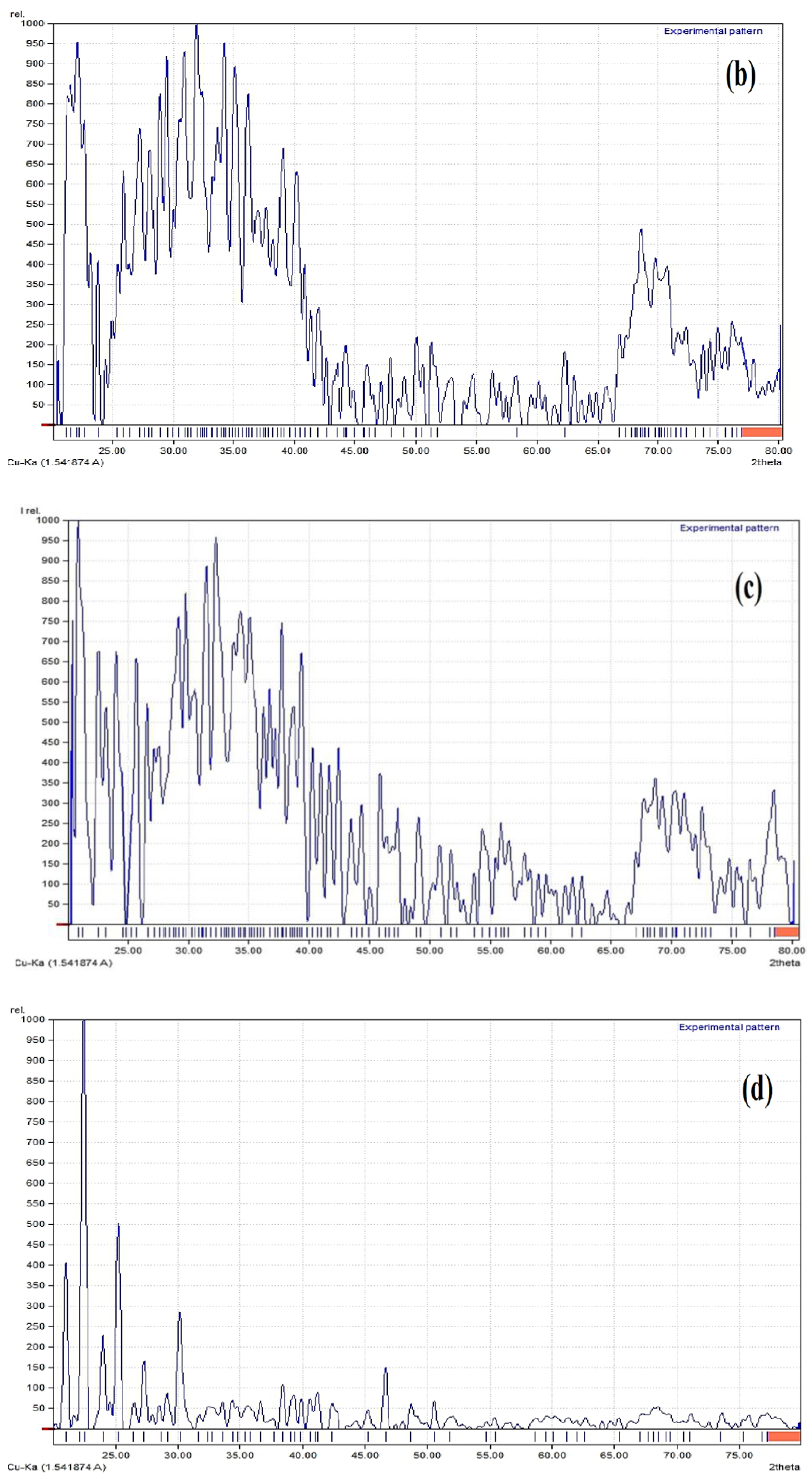
3.3.5. X-ray Diffraction Studies (XRD)
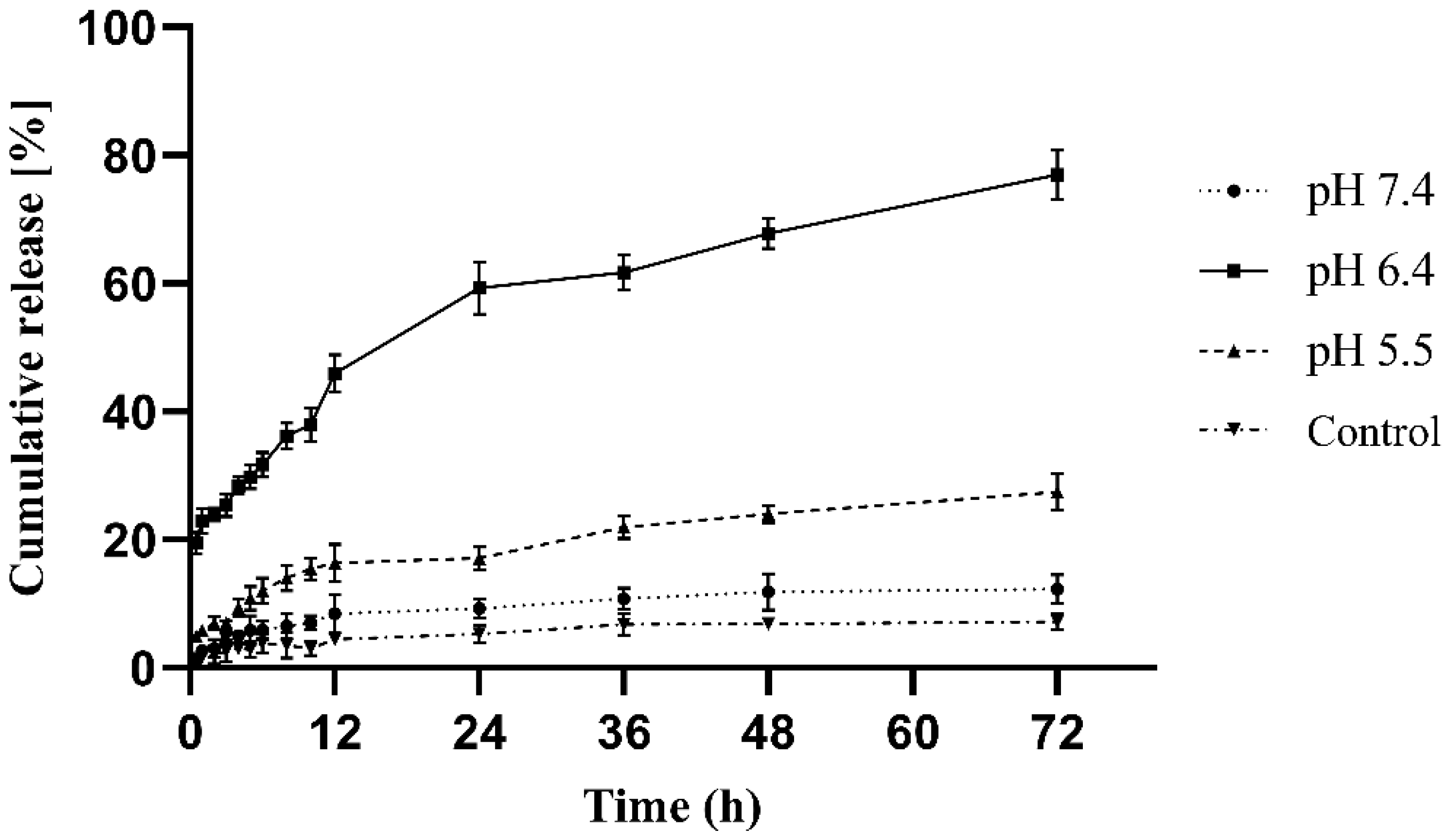
3.3.6. In Vitro Release
3.3.7. Physical Stability Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khan, S. Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement. Med. Ther. 2020, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef]
- Ruby, K.M.; Chauhan, R.; Sharma, S.; Dwivedi, J. Polypharmacological activities of Bergenia species. Int. J. Pharm. Sci. Rev. Res. 2012, 13, 100–110. [Google Scholar]
- Ahmed, M.; Phul, A.R.; Haq, I.-U.; Bibi, G.; Mazhar, K.; Rehman, T.; Zia, M.; Mirza, B. Antioxidant, anticancer and antibacterial potential of Zakhm-e-hayat rhizomes crude extract and fractions. Pak. J. Pharm. Sci. 2016, 29, 895–902. [Google Scholar]
- Chester, K.; Zahiruddin, S.; Ahmad, A.; Khan, W.; Paliwal, S.; Ahmad, S. Bioautography-based Identification of Antioxidant Metabolites of Solanum nigrum L. and Exploration Its Hepatoprotective Potential against D-galactosamine-induced hepatic fibrosis in rats. Pharmacogn. Mag. 2019, 15, 104. [Google Scholar] [CrossRef]
- Jayamohan, N.S. Surveillance of Invitro Antioxidant and Anthelmintic Activity of Methanolic Extract of Syzygium Cumini Bark (Myrtaceae). Int. J. Phytopharm. 2013, 3, 56–62. [Google Scholar] [CrossRef]
- Shal, B.; Khan, A.; Khan, A.; Ullah, R.; Ali, G.; Islam, S.; Haq, I.; Ali, H.; Seo, E.-K.; Khan, S. Alleviation of Memory Deficit by Bergenin via the Regulation of Reelin and Nrf-2/NF-κB Pathway in Transgenic Mouse Model. Int. J. Mol. Sci. 2021, 22, 6603. [Google Scholar] [CrossRef]
- Ahmad, M.; Butt, M.A.; Zhang, G.; Sultana, S.; Tariq, A.; Zafar, M. Bergenia ciliata: A comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. Biomed. Pharmacother. 2018, 97, 708–721. [Google Scholar] [CrossRef]
- Aggarwal, D.; Gautam, D.; Sharma, M.; Singla, S. Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur. J. Pharmacol. 2016, 791, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pandey, N.; Agnihotri, V.; Singh, K.K.; Pandey, A. Antioxidant, antimicrobial activity and bioactive compounds of Bergenia ciliata Sternb.: A valuable medicinal herb of Sikkim Himalaya. J. Tradit. Complement. Med. 2017, 7, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Fitoterapia 2015, 101, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wei, Y.; Xi, L.; Sun, Y.; Zhang, T. Evaluation of Intestinal Absorption and Bioavailability of a Bergenin–Phospholipid Complex Solid Dispersion in Rats. AAPS PharmSciTech 2018, 19, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Roome, T.; Aziz, S.; Razzak, A.; Abbas, G.; Imran, M.; Jabri, T.; Gul, J.; Hussain, M.; Sikandar, B.; et al. Bergenin loaded gum xanthan stabilized silver nanoparticles suppress synovial inflammation through modulation of the immune response and oxidative stress in adjuvant induced arthritic rats. J. Mater. Chem. B 2018, 6, 4486–4501. [Google Scholar] [CrossRef]
- Lin, H.; Wang, P.; Zhang, W.; Yan, H.; Yu, H.; Yan, L.; Chen, H.; Xie, M.; Shan, L. Novel Combined Preparation and Investigation of Bergenin-Loaded Albumin Nanoparticles for the Treatment of Acute Lung Injury: In Vitro and In Vivo Evaluations. Inflammation 2022, 45, 428–444. [Google Scholar] [CrossRef]
- Khan, D.; Qindeel, M.; Ahmed, N.; Asad, M.I.; Shah, K.U.; Ur.Rehman, A. Development of an intelligent, stimuli-responsive transdermal system for efficient delivery of Ibuprofen against rheumatoid arthritis. Int. J. Pharm. 2021, 610, 121242. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Alvarez-Roman, R.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Asad, M.I.; Khan, D.; Rehman, A.; Elaissari, A. Development and In Vitro / In Vivo Evaluation of pH-Sensitive Polymeric Nanoparticles Loaded Hydrogel for the Management of Psoriasis. Nanomaterials 2021, 11, 3433. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Ahmed, N.; Qindeel, M.; Asad, M.I.; Khan, G.M.; Ur.Rehman, A. Development of novel biopolymer-based nanoparticles loaded cream for potential treatment of topical fungal infections. Drug Dev. Ind. Pharm. 2021, 47, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Peeler, D.J.; Sellers, D.L.; Pun, S.H. pH-Sensitive Polymers as Dynamic Mediators of Barriers to Nucleic Acid Delivery. Bioconjugate Chem. 2018, 30, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kumar, R.; Gadewar, M.; Tahilyani, V. Pharmacological and analytical aspects of bergenin: A concise report. Asian Pac. J. Trop. Dis. 2012, 2, 163–167. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Attama, A.A.; Ibezim, E.C.; Nnamani, P.O.; Umeyor, C.E.; Uronnachi, E.M.; Gugu, T.H.; Momoh, M.A.; Ofokansi, K.C.; Akpa, P.A. Surface-modified mucoadhesive microgels as a controlled release system for miconazole nitrate to improve localized treatment of vulvovaginal candidiasis. Eur. J. Pharm. Sci. 2018, 111, 358–375. [Google Scholar] [CrossRef]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Ullah, N.; Khan, A.; Mehwish, S.; Haq, I.-U. Comprehensive investigations of the mechanism of action of Bombax ceiba extracts against Leishmania tropica and its phytochemical investigation. Int. J. Infect. Dis. 2020, 101, 309. [Google Scholar] [CrossRef]
- Khan, I.; Ali, J.S.; Ul-Haq, I.; Zia, M. Biological and Phytochemicals Properties of Monotheca buxifolia: An Unexplored Medicinal Plant. Pharm. Chem. J. 2020, 54, 293–301. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A.; Sharma, A. Antianxiety activity guided isolation and characterization of bergenin from Caesalpinia digyna Rottler roots. J. Ethnopharmacol. 2017, 195, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Kumar, B.; Meena, B.; Srivastava, M.; Mishra, T.; Tiwari, V.; Pal, M.; Nair, N.K.; Upreti, D.K.; Rana, T.S. Major bioactive phenolics in Bergenia species from the Indian Himalayan region: Method development, validation and quantitative estimation using UHPLC-QqQLIT-MS/MS. PLoS ONE 2017, 12, e0180950. [Google Scholar] [CrossRef] [PubMed]
- Dhalwal, K.; Shinde, V.; Biradar, Y.; Mahadik, K. Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin-layer chromatography. J. Food Compos. Anal. 2008, 21, 496–500. [Google Scholar] [CrossRef]
- Sanjeev, S.; Murthy, M.K.; Devi, M.S.; Khushboo, M.; Renthlei, Z.; Ibrahim, K.S.; Kumar, N.S.; Roy, V.; Gurusubramanian, G. Isolation, characterization, and therapeutic activity of bergenin from marlberry (Ardisia colorata Roxb.) leaf on diabetic testicular complications in Wistar albino rats. Environ. Sci. Pollut. Res. 2019, 26, 7082–7101. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; AlAjmi, M.F.; Haq, N.; Siddiqui, N.A.; Alam, P.; Al-Rehaily, A.J. Solubility and thermodynamic function of a bioactive compound bergenin in various pharmaceutically acceptable neat solvents at different temperatures. J. Chem. Thermodyn. 2016, 101, 19–24. [Google Scholar] [CrossRef]
- Sahle, F.F.; Balzus, B.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and in vitro evaluation of polymeric enteric nanoparticles as dermal carriers with pH-dependent targeting potential. Eur. J. Pharm. Sci. 2016, 92, 98–109. [Google Scholar] [CrossRef]
- Tarhini, M.; Benlyamani, I.; Hamdani, S.; Agusti, G.; Fessi, H.; Greige-Gerges, H.; Bentaher, A.; Elaissari, A. Protein-Based Nanoparticle Preparation via Nanoprecipitation Method. Materials 2018, 11, 394. [Google Scholar] [CrossRef]
- Khayata, N.; Abdelwahed, W.; Chehna, M.; Charcosset, C.; Fessi, H. Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: From laboratory scale to large scale using a membrane contactor. Int. J. Pharm. 2012, 423, 419–427. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef]
- Irshad, S.; Siddiqui, B.; Ur.Rehman, A.; Farooq, R.K.; Ahmed, N. Recent trends and development in targeted delivery of therapeutics through enzyme responsive intelligent nanoplatform. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 403–413. [Google Scholar] [CrossRef]
- Feczkó, T.; Tóth, J.; Dósa, G.; Gyenis, J. Influence of process conditions on the mean size of PLGA nanoparticles. Chem. Eng. Process. Process Intensif. 2011, 50, 846–853. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, Y.-T.; Huang, H.-Y.; Yang, C.-S. Surfactant-free formulation of poly(lactic/glycolic) acid nanoparticles encapsulating functional polypeptide: A technical note. AAPS PharmSciTech 2009, 10, 1263–1267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rizvi, S.Z.H.; Shah, F.A.; Khan, N.; Muhammad, I.; Ali, K.H.; Ansari, M.M.; Din, F.U.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; et al. Simvastatin-loaded solid lipid nanoparticles for enhanced anti-hyperlipidemic activity in hyperlipidemia animal model. Int. J. Pharm. 2019, 560, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.P.; Das, M.K. Formulation, optimization, and in vitro/in vivo evaluation of furosemide nanosuspension for enhancement of its oral bioavailability. J. Nanopart. Res. 2014, 16, 2360. [Google Scholar] [CrossRef]




| Independent Variables | Levels | |
|---|---|---|
| −1 | +1 | |
| X1: EL100 w/v (%) | 1.0 | 3.0 |
| X2: PVA w/v (%) | 0.5 | 1.5 |
| X3: Inj. rate (mL/min) | 0.2 | 1 |
| Dependent Variables | Goal | |
| Y1: Mean particle size (nm) | Minimize | |
| Y2: Entrapment efficiency (%) | Maximize | |
| Runs | Independent Variables | Dependent Variables | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| EL100 w/v (%) | PVA w/v (%) | Inj. Rate (mL/min) | Particle Size (nm) (Mean ± SD) | Encapsulation (%) (Mean ± SD) | |
| 1 | 2 | 1 | 0.6 | 65 ± 2.30 | 76 ± 0.13 |
| 2 | 3 | 1.5 | 0.2 | 85 ± 3.20 | 83 ± 0.90 |
| 3 | 1 | 1.5 | 0.2 | 191 ± 2.10 | 68 ± 1.20 |
| 4 | 1 | 1 | 0.6 | 78 ± 1.90 | 73 ± 0.70 |
| 5 | 3 | 0.5 | 0.2 | 188 ± 2.20 | 78 ± 0.50 |
| 6 | 1 | 0.5 | 0.2 | 61 ± 2.50 | 70 ± 1.10 |
| 7 | 2 | 1 | 0.2 | 68 ± 3.30 | 77 ± 0.90 |
| 8 | 2 | 1 | 1 | 94 ± 2.70 | 76 ± 1.20 |
| 9 | 2 | 1.5 | 0.6 | 79 ± 4.00 | 80 ± 1.10 |
| 10 | 2 | 0.5 | 0.6 | 69 ± 2.80 | 82 ± 1.00 |
| 11 | 3 | 1.5 | 1 | 95 ± 3.40 | 76 ± 0.90 |
| 12 | 3 | 1 | 0.6 | 170 ± 2.10 | 78 ± 0.70 |
| 13 | 3 | 0.5 | 1 | 220 ± 1.60 | 86 ± 0.30 |
| 14 | 1 | 0.5 | 1 | 78 ± 2.30 | 84 ± 1.10 |
| 15 | 1 | 1.5 | 1 | 319 ± 3.10 | 81 ± 0.50 |
| Formulation | Storage Temperature Conditions | Initial Particle Size (nm) | Particle Size After 1 Month (nm) | Particle Size After 2 Months (nm) | Particle Size After 3 Months (nm) |
|---|---|---|---|---|---|
| Optimized Formulation | 5 ± 3 °C | 86.17 ± 2.1 | 86.7 ± 2.2 a | 87.6 ± 2.5 a | 88.9 ± 2.8 a |
| 25 ± 2 °C 65 ± 5% RH | 86.5 ± 3.2 a | 88.1 ± 3.5 a | 90.4 ± 3.7 a | ||
| 40 ± 2 °C/ 75 ± 5% RH | 89.5 ± 3.0 a | 93.6 ± 4.2 a | 98.2 ± 4.6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, K.; Khan, M.F.A.; Alhodaib, A.; Ahmed, N.; Naz, I.; Mirza, B.; Tipu, M.K.; Fatima, H. Design and Evaluation of pH-Sensitive Nanoformulation of Bergenin Isolated from Bergenia ciliata. Polymers 2022, 14, 1639. https://doi.org/10.3390/polym14091639
Bashir K, Khan MFA, Alhodaib A, Ahmed N, Naz I, Mirza B, Tipu MK, Fatima H. Design and Evaluation of pH-Sensitive Nanoformulation of Bergenin Isolated from Bergenia ciliata. Polymers. 2022; 14(9):1639. https://doi.org/10.3390/polym14091639
Chicago/Turabian StyleBashir, Kashaf, Muhammad Farhan Ali Khan, Aiyeshah Alhodaib, Naveed Ahmed, Iffat Naz, Bushra Mirza, Muhammad Khalid Tipu, and Humaira Fatima. 2022. "Design and Evaluation of pH-Sensitive Nanoformulation of Bergenin Isolated from Bergenia ciliata" Polymers 14, no. 9: 1639. https://doi.org/10.3390/polym14091639
APA StyleBashir, K., Khan, M. F. A., Alhodaib, A., Ahmed, N., Naz, I., Mirza, B., Tipu, M. K., & Fatima, H. (2022). Design and Evaluation of pH-Sensitive Nanoformulation of Bergenin Isolated from Bergenia ciliata. Polymers, 14(9), 1639. https://doi.org/10.3390/polym14091639







