Exploring the Impact of Chitosan Composites as Artificial Organs
Abstract
:1. Introduction
2. Snapshot of Biomedical Achievements of Chitosan
3. Development of Chitosan-Based Materials for Biomedical Applications
3.1. Solvent-Casting/Solvent-Evaporation/Tape-Casting Method
3.2. Compression-Molding Method
3.3. Freeze-Drying Method
3.4. Electrospinning Method
3.5. Microwave-Assisted Method
3.6. Dense-Gas-Foaming Method
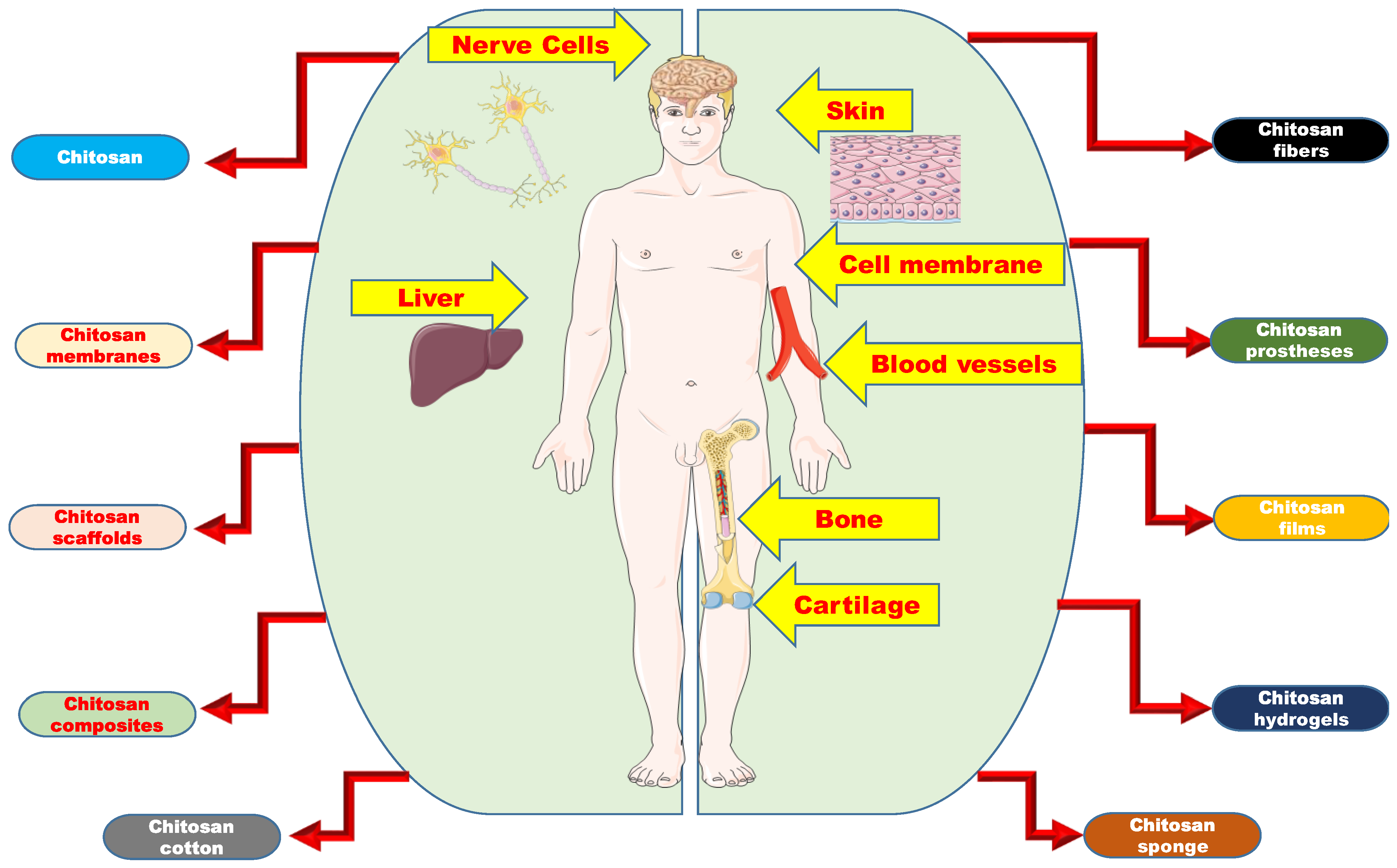
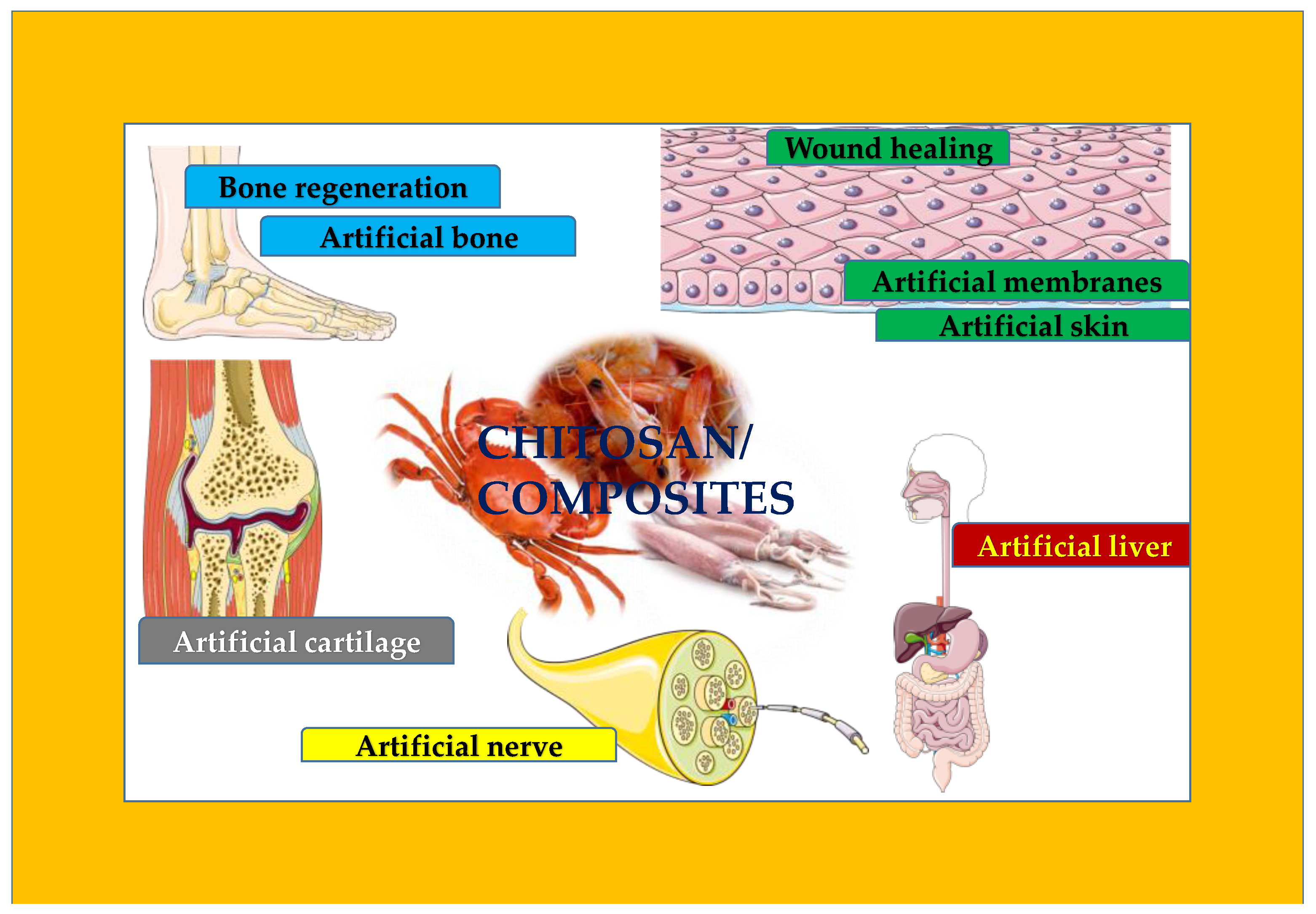
4. Chitosan-Based Artificial Organs
4.1. Chitosan as Artificial Membranes
4.2. Chitosan as Artificial Skin
4.3. Chitosan as Artificial Bone
4.4. Chitosan as Artificial Cartilage
4.5. Chitosan as Artificial Liver
4.6. Chitosan as Artificial Nerve
4.7. Chitosan as Artificial Blood Vessel
5. Chitosan Composites in Organ 3D Bioprinting
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Acosta, N.; Jimenez, C.; Borau, V.; Heras, A. Extraction and characterization of chitin from crustaceans. Biomass Bioenergy 1993, 5, 145–153. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials. A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Nwe, N.; Furuike, T.; Tamura, H. The mechanical and biological properties of Chitosan scaffolds for tissue regeneration templates are significantly enhanced by Chitosan from Gongronella butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef] [Green Version]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Gemma, G.; Angeles, H. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources, Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S. Competitive biological activities of Chitosan and its derivatives: Antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- El-hefian, E.; Nasef, M.; Yahaya, A. Chitosan physical forms: A shortreview. Aust. J. Basic Appl. Sci. 2011, 5, 670–677. [Google Scholar]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in medicalimplants: A brief review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Jung, W.K.; Ahn, C.B.; Kim, S.K. Anticoagulant activity of heterochitosans and their oligosaccharide sulfates. Eur. Food Res. Technol. 2004, 219, 529–533. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies onits safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect ofchitosan (poly-N-acetyl glucosamine) on lingual hemostasis inheparinized rabbits. J. Oral Maxillofac. Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special reporton the chitosan-based hemostatic dressing: Experience in currentcombat operations. J. Trauma Acute Care Surg. 2006, 60, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree ofacetylation on some biological properties of chitosan films. Biomaterials 2000, 22, 261–268. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 12, 487. [Google Scholar] [CrossRef] [Green Version]
- Ramya, R.; Venkatesan, J.; Kim, S.K.; Sudha, P.N. Biomedical Applications of Chitosan: An Overvie. J. Biomater. Tissue Eng. 2012, 2, 100–111. [Google Scholar] [CrossRef]
- Mohammad, R.H.; Abul, K.M.; Mohammed, M.R. Chapter 7—Fundamentals of chitosan for biomedical applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–230. [Google Scholar]
- Nafee, N.; Schneider, M.; Schaefer, U.F.; Lehra, C. Relevance of the colloidal stability of chitosan/PLGA nanoparticles on their cytotoxicity profile. Int. J. Pharm. 2009, 381, 130–139. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, L.; Zhu, C. Genipin crosslinked ethyl cellulose-chitosan complex microspheres for anti-tuberculosis delivery. Colloids Surf. B Biointerfaces 2013, 103, 530–537. [Google Scholar] [CrossRef]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Jayasree, R.S.; Rathinam, K.; Sharma, C.P. Development of artificial skin (Template) and influence of different types of sterilization procedures on wound healing pattern in rabbits and guinea pigs. J. Biomater. Appl. 1995, 10, 144–162. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Mattioli-Belmonte, M.; Pugnaloni, A.; Biagini, G. Biochemistry, histology and clinical uses of chitins and chitosans in wound healing. EXS 1999, 87, 251–264. [Google Scholar]
- Sankar, P.C.K.; Rajmohan, G.; Rosemary, M.J. Physico-chemical characterisation and biological evaluation of freeze dried chitosan sponge for wound care. Mater. Lett. 2017, 208, 130–132. [Google Scholar] [CrossRef]
- Lee, J.Y.; Seol, Y.J.; Kim, K.H.; Lee, Y.M.; Park, Y.J.; Rhyu, I.C.; Chung, C.P.; Lee, S.J. Transforming growth factor (TGF)-β1releasing tricalcium phosphate/chitosan microgranules as bone substitutes. Pharm. Res. 2004, 21, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/O-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef]
- Cavalli, R.; Leone, F.; Minelli, R.; Fantozzi, R.; Dianzani, C. New chitosan nanospheres for the delivery of 5-fluorouracil: Preparation, characterization and in vitro studies. Curr. Drug Deliv. 2014, 11, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Siegel, A.R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer Statistics. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar]
- Yang, R.; Shim, W.S.; Cui, F.D.; Cheng, G.; Han, X.; Jin, Q.R.; Kim, D.D.; Chung, S.J.; Shim, C.K. Enhanced electrostatic interaction between chitosan-modified PLGA nanoparticle and tumor. Int. J. Pharm. 2009, 371, 142–147. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, K.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Zhou, Q.; Ma, G.; Wan, Y.; Su, Z. Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf. B Biointerfaces 2006, 50, 126–135. [Google Scholar] [CrossRef]
- Jing, Z.W.; Ma, Z.W.; Li, C.; Jia, Y.Y.; Luo, M.; Ma, X.X.; Zhou, S.Y.; Zhang, B.L. Chitosan cross-linked with poly (ethylene glycol) dialdehyde via reductive amination as effective controlled release carriers for oral protein drug delivery. Bioorg. Med. Chem. Lett. 2017, 27, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Harde, H.; Agrawal, A.K.; Jain, S. Development of stabilized glucomannosylated chitosan nanoparticles using tandem crosslinking method for oral vaccine delivery. Nanomedicine 2014, 9, 2511–2529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Benson, H.A.; Mukkur, T.K.; Rigby, P.; Chen, Y. Preliminary studies on the development of IgA-loaded chitosan-dextran sulphate nanoparticles as a potential nasal delivery system for protein antigens. J. Microencapsul. 2013, 30, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, S.; Li, W.; Yu, L.; Duan, X.; Han, J.; Wang, X.; Jin, Z. Quaternized chitosan nanoparticles loaded with the combined attenuated live vaccine against newcastle disease and infectious bronchitis elicit immune response in chicken after intranasal administration. Drug Deliv. 2017, 24, 1574–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Ziemer, K.S.; Gill, H.S. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza a virus. Nanomedicine 2013, 9, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Guliyeva, U.; Oner, F.; Ozsoy, S.; Haziroglu, R. Chitosan microparticles containing plasmid DNA as potential oral gene delivery system. Eur. J. Pharm. Biopharm. 2006, 62, 17–25. [Google Scholar] [CrossRef]
- Akbuga, J.; Özbas-Turan, S.; Erdogan, N. Plasmid-DNA loaded chitosan microspheres for in vitro IL-2 expression. Eur. J. Pharm. Biopharm. 2004, 58, 501–507. [Google Scholar] [CrossRef]
- Ozbas-Turan, S.; Aral, C.; Kabasakal, L.; Keyer-Uysal, M.; Akbuga, J. Co-encapsulation of two plasmids in chitosan microspheres as a non-viral gene delivery vehicle. J. Pharm. Pharm. Sci. 2003, 6, 27–32. [Google Scholar]
- Rafique, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Rehman, S. Chitosan functionalized poly(vinyl alcohol) for prospects biomedical and industrial applications: A review. Int. J. Biol. Macromol. 2016, 87, 141–154. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Silva, D.J.B.; Zuluaga, F.; Carlos, H. Evaluation of Biocompatibility of Chitosan Films from the Mycelium of Aspergillus niger in Connective Tissue of Rattus norvegicus. J. Mol. Genet. Med. 2015, 9, 1–8. [Google Scholar]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; EncinasEncinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Rosas-Burgos, E.C.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohydr. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.G.; Liu, S.; Kan, J.; Jin, C.H. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, S.; Oh, D.H. Chitosan grafted monomethyl fumaric acid as a potential food preservative. Carbohydr. Polym. 2016, 152, 87–96. [Google Scholar] [CrossRef]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Käch, A.; Maake, C.; Patzke, G.R. Chitosanthioglycolic acid as a 117 versatile antimicrobial agent. Biomacromolecules 2013, 14, 1010–1017. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, X.; Han, J.; Song, G.; Nie, J. Photo-polymeriable chitosan derivative prepared by Michael reaction of chitosan and polyethylene glycol diacrylate (PEGDA). Int. J. Biol. Macromol. 2009, 45, 499–503. [Google Scholar] [CrossRef]
- Ahmed, M.; Rozina, K.; Naveera, N.; Fazli, W. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.N.; Mei, L.H.I.; Santos, A.R. Sorbitol-plasticized and neutralized chitosan membranes as skin substitutes. Mater. Res. 2015, 18, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Madni, A.; Khan, R.; Ikram, M.; Naz, S.S.; Khan, T.; Wahid, F. Fabrication and characterization of chitosan-vitamin C-lactic acid composite membrane for potential skin tissue engineering. Int. J. Polym. Sci. 2019, 2019, 4362395. [Google Scholar] [CrossRef]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Sousa, A.M.M.; Hilliou, L.; Gonçalves, M.P.; Souza, H.K.S. Thermo-compression molding of chitosan with a deep eutectic mixture forbiofilms development. Green Chem. 2016, 18, 1571–1580. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, W.I. Compression Molding in Polymer Matrix composites. Manufacturing Techniques for Polymer Matrix Composites (PMCs). In Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2012; pp. 47–94. [Google Scholar]
- Garg, T.; Chanana, A.; Joshi, R. Preparation of chitosan scaffolds for tissue engineering using freeze drying technology. IOSR J. Pharm. 2012, 2, 72–73. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 2566262. [Google Scholar] [CrossRef] [Green Version]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.J.; Dean, D.R.; Jun, H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Prasad, T.; Shabeena, E.A.; Vinod, D.; Kumary, T.V.; Kumar, P.R.A. Characterization and in vitro evaluation of electrospun chitosan/polycaprolactone blend fibrous mat for skin tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 5352–5358. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Yuan, X.Y.; Zhu, Y.; Zhang, Y.Y.; Li, X.L.; Zhang, Y.; De Yao, K. A nanofibrous composite membrane of PLGA-chitosan/PVA prepared by electrospinning. Eur. Polym. J. 2006, 42, 2013–2022. [Google Scholar] [CrossRef]
- Hiep, N.T.; Khon, H.C.; Niem, V.V.T.; Toi, V.V.; Ngoc, Q.T.; Hai, N.D.; Ngoc, T.A.M. Microwave-assisted synthesis of chitosan/polyvinyl alcohol silver nanoparticles gel for wound dressing applications. Int. J. Polym. Sci. 2016, 2016, 1584046. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.P.; Goodall, G.W.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Microwave-assisted hydrogel synthesis: A new method for crosslinking polymers inaqueous solutions. Macromol. Rapid Commun. 2012, 33, 332–336. [Google Scholar] [CrossRef]
- Visentin, A.F.; Dong, T.Y.; Poli, J.; Panzer, M.J. Rapid, microwave-assisted thermal polymerization of poly (ethylene glycol) diacrylate-supported ionogels. J. Mater. Chem. A 2014, 2, 7723–7726. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. The fabrication of elastin-based hydrogels using high pressure CO2. Biomaterials 2009, 30, 1–7. [Google Scholar] [CrossRef]
- Pini, R.; Storti, G.; Mazzotti, M.; Tai, H.Y.; Shakesheff, K.M.; Howdle, S.M. Sorption and Swelling of Poly(D, l-lactic acid) and Poly(lactic-co-glycolic acid) in Supercritical CO2. Macromol. Symp. 2007, 259, 197–202. [Google Scholar] [CrossRef]
- Hirano, S. A facile methods for the preparation of novel membranes from N-acyl-and N-arylidene chitosan gels. Agric. Biol. Chem. 1978, 42, 1938–1940. [Google Scholar] [CrossRef]
- Hirano, S.; Tobetto, K.; Hasegawa, M.; Matsuda, N. Permeability properties of gels and membranes derived from chitosan. J. Biomed. Mater. Res. 1980, 14, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chandy, T. Protein blended Chitosan membranes for an improved hemodialysis. Trans. Soc. Biomater. 1987, 10, 31. [Google Scholar]
- Chandy, T.; Sharma, C.P. Chitosan as a biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Noishiki, Y. The blood compatibility of Chitosan and N-acylchitosans. J. Biomed. Mater. Res. 1985, 19, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Chandy, T.; Shanna, C.P. Bioactive molecules immobilized to liposome modified albumin-blended chitosan membranes—Antithrombotic and permeability properties. J. Colloid. Interface Sci. 1989, 130, 131–140. [Google Scholar] [CrossRef]
- Claramma, C.V.; ChtlilCiy, T.; Sharma, C.P. Phosphoryl choline bilayer imlwbilized on albumin blended chitosan membrane-permeability properties. Trans. Soc. Biomater. 1988, 14, 477. [Google Scholar]
- Kim, K.Y.; Min, D.S. Wound covering materials from polyelectrolyte complexes of Chitasan with sulfonated chitosan. Trans. Soc. Biomater. 1988, 11, 558. [Google Scholar]
- Taravel, M.N.; Domard, A. Collagen and its interaction with chitosan: II. Influence of the physicochemical characteristics of collagen. Biomaterials 1995, 16, 865–871. [Google Scholar] [CrossRef]
- Taravel, M.N.; Domard, A. Collagen and its interaction with chitosan: III. Some biological and mechanical properties. Biomaterials 1996, 17, 451–455. [Google Scholar] [CrossRef]
- Cho, T.W.; Cho, Y.N.; Chung, S.H.; Yoo, G.; Ko, S.W. Water-soluble chitin as a wound healing accelerator. Biomaterials 1999, 20, 2139–2145. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; He, B.; Chen, J. A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human fetal dermal fibroblasts. Biomaterials 2001, 22, 331–336. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Mizuno, K.; Yamamura, K.; Yano, K.; Osada, T.; Saeki, S.; Takimoto, N.; Sakurai, T.; Nimura, Y. Effect of chitosan film containing basic fibroblast growth factor on wound healing in genetically diabetic mice. J. Biomed. Mater. Res. 2003, 64, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Howling, G.I.; Dettmar, P.W.; Goddard, P.A.; Hampson, F.C.; Dornish, M.; Wood, E.J. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 2001, 22, 2959–2966. [Google Scholar] [CrossRef]
- Yan, X.L.; Khor, E.; Lim, L.Y. PEC films prepared from chitosan alginate coacervates. Chem. Pharm. Bull. 2000, 48, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.S.; Khor, E.; Wee, A.; Lim, L.Y. Chitosan–alginate PEC membrane as a wound dressing: Assessment of incisional wound dressing. J. Biomed. Mater. Res. 2002, 63, 610–618. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. Surface-active biomaterials. Science 1984, 226, 630–636. [Google Scholar] [CrossRef]
- Martino, A.D.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, M.; Zhang, M.; Ratner, B. Calcium phosphate chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003, 9, 337–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 2002, 62, 378–786. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Grande, D.A.; Halberstadt, C.; Naughton, G.; Schwartz, R.; Ryhana, M. Evaluation of matrix scaffolds for the tissue engineering of articular cartilage grafts. J. Biomed. Mater. Res. 1997, 34, 211–220. [Google Scholar] [CrossRef]
- Kosher, R.A.; Church, R.L. Stimulation of in vitro somite chondrogenesis by procollagen and collagen. Nature 1975, 258, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Kosher, R.A.; Lash, J.W.; Minor, R.R. Environmental enhancement of invitro chondrogenesis. Stimulation of somite chondrogenesis by exogenous chondromucoprotein. Dev. Biol. 1973, 35, 210–220. [Google Scholar] [CrossRef]
- Lu, J.X.; Prudhommeaux, F.; Meunier, A.; Sedel, L.; Guillemin, G. Effects of chitosan on rat knee cartilages. Biomaterials 1999, 20, 1937–1944. [Google Scholar]
- Mattioli-Belmonte, M.; Gigante, A.; Muzzarelli, R.A.; Politano, R.; DeBenedittis, A.; Specchia, N.; Buffa, A.; Biagini, G.; Greco, F. N,N-dicarboxymethyl chitosan asdelivery agent for bone morphogenetic protein in the repair of articular cartilage. Med. Biol. Eng. Comput. 1999, 37, 130–134. [Google Scholar] [CrossRef]
- Sechriest, V.F.; Miao, Y.J.; Niyibizi, C.; Westerhausen-Larson, A.; Matthew, H.W.; Evans, C.H.; Fu, F.H.; Suh, J.-K. GAG-augmented polysachharide hydrogel: A novel biocompatible and biodegrable material to support chondrogenesis. J. Biomed. Mater. Res. 2000, 49, 534–541. [Google Scholar] [CrossRef]
- Yamane, S.; Iwasaki, N.; Majima, T.; Funakoshi, T.; Masuko, T.; Harada, K.; Minami, A.; Monde, K.; Nishimura, S. Feasibility of chitosan-based hyaluronic acid hybrid biomaterial for a novel scaffold in cartilage tissue engineering. Biomaterials 2005, 26, 611–619. [Google Scholar] [CrossRef]
- Hsu, S.H.; Whu, S.W.; Hsieh, S.C.; Tsai, C.L.; Chen, D.C.; Tan, T.S. Evaluation of chitosan–alginate–hyaluronate complexes modified by an RGD-containing protein as tissue-engineering scaffolds for cartilage regeneration. Artif. Organs 2004, 28, 693–703. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.E.; Kwon, I.C.; Ahn, H.J.; Lee, S.H.; Cho, H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 2004, 25, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Hurtig, M.B.; Rossomacha, E.; Sun, J.; Chevrier, A.; Shive, M.S.; Buschmann, M.D. Chitosane glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J. Bone Jt. Surg. Am. 2005, 87, 2671–2686. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Sun, J.; McKee, M.D.; Chevrier, A.; Rossomacha, E.; Rivard, G.E.; Hurtig, M.; Buschmann, M.D. Chitosane glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthr. Cartil. 2007, 15, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevrier, A.; Hoemann, C.D.; Sun, J.; Buschmann, M.D. Chitosaneglycerolphosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthr. Cartil. 2007, 15, 316–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Jiang, H.L.; Kim, Y.K.; Cho, C.S. Factors affecting liver functions in liver tissue engineering. Tissue Eng. Regen. Med. 2006, 3, 27–33. [Google Scholar]
- Lindahl, U.; Hook, M. Glycosaminoglycans and their binding to biological macromolecules. Annu. Rev. Biochem. 1978, 47, 385–417. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, L.; Guo, X.; Yu, Y. Culture of primary rat hepatocytes within porous chitosan scaffolds. J. Biomed. Mater. Res. A 2003, 67, 938–943. [Google Scholar] [CrossRef]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.V.; Matthew, H.W. Vascular cell responses to polysaccharide materials: In vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Zhang, L.; Yu, Y. Culture of hepatocytes on fructose modified chitosan scaffolds. Biomaterials 2003, 24, 2317–2322. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, D.P.; Wang, W.J.; Feng, Q.L.; Cui, F.Z.; Xu, Y.X.; Song, X.H.; van der Werf, M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials 2003, 24, 3213–3220. [Google Scholar] [CrossRef]
- Park, I.K.; Yang, J.; Jeong, H.J.; Bom, H.S.; Harada, I.; Akaike, T.; Kim, S.; Cho, C. Galactosylated chitosan as a synthetic extracellular matrix for hepatocytes attachment. Biomaterials 2003, 24, 2331–2337. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef]
- Heath, C.A.; Rutkowski, G.E. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998, 16, 163–168. [Google Scholar] [CrossRef]
- Gong, H.; Zhong, Y.; Li, J.; Gong, Y.; Zhao, N.; Zhang, X. Studies on nerve cell affinity of chitosan-derived materials. J. Biomed. Mater. Res. 2000, 52, 285–295. [Google Scholar]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The interaction of Schwanncells with chitosan membranes and fibers in vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Bunge, R.P. The role of the Schwann cell in trophic support and regeneration. J. Neurol. 1994, 242, S19–S21. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Yamaguchi, I.; Suzuki, M.; Ichinose, S.; Takakuda, K.; Kobayashi, H.; Shinomiya, K.; Tanaka, J. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003, 993, 111–123. [Google Scholar] [CrossRef]
- Matsuda, A.; Kobayashi, H.; Itoh, S.; Kataoka, K.; Tanaka, J. Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding. Biomaterials 2005, 26, 2273–2279. [Google Scholar] [CrossRef]
- Kato, K.; Utani, A.; Suzuki, N.; Mochizuki, M.; Yamada, M.; Nishi, N.; Matsuura, H.; Shinkai, A.H.; Nomizu, M. Identification of neurite outgrowth promoting sites on the lamininalpha 3 chain G domain. Biochemistry 2002, 41, 10747–10753. [Google Scholar] [CrossRef]
- Chávez-Delgado, M.E.; Mora-Galindo, J.; Gómez-Pinedo, U.; Feria-Velasco, A.; Castro-Castañeda, S.; López-Dellamary Toral, F.A.; Luquin-De Anda, S.; García-Segura, L.M.; García-Estrada, J. Facial nerve regeneration through progesterone-loaded chitosan prosthesis. A preliminary report. J. Biomed. Mater. Res. B 2003, 67, 702–711. [Google Scholar] [CrossRef]
- Cao, W.; Cheng, M.; Ao, Q.; Gong, Y.; Zhao, N.; Zhang, X. Physical, mechanical and degradation properties, and Schwann cell affinity of cross-linked chitosan films. J. Biomater. Sci. Polym. Ed. 2005, 16, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Gong, K.; Li, J.; Gong, Y.; Zhao, N.; Zhang, X. Surface modification and characterization of chitosan film blended with poly-L-lysine. J. Biomater. Appl. 2004, 19, 59–75. [Google Scholar]
- Cheng, M.; Deng, J.; Yang, F.; Gong, Y.; Zhao, N.; Zhang, X. Study onphysical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials 2003, 2, 2871–2880. [Google Scholar] [CrossRef]
- Freier, T.; Montenegro, R.; Shan Koh, H.; Shoichet, M.S. Chitin-basedtubes for tissue engineering in the nervous system. Biomaterials 2005, 26, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Heart Disease and Stroke Statics—2005 Update; American Heart Association: TX, Dallas, USA, 2004. [Google Scholar]
- Kratz, G.; Arnander, C.; Swedenborg, J.; Back, M.; Falk, C.; Gouda, I.; Larm, O. Heparin-chitosan complexes stimulate wound healing in human skin. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1997, 31, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, M.; Kobayashi, S.; Niwaya, K.; Hamada, Y.; Kitamura, S. Changes in platelet, granulocyte, and complement activation during cardiopulmonary bypass using heparin coated equipment. Artif. Organs 1996, 20, 767–776. [Google Scholar] [CrossRef]
- Bannan, S.; Danby, A.; Cowan, D.; Ashraf, S.; Martin, P.G. Low heparinization with heparin bonded bypass circuits: Is it a safe strategy? Ann. Thorac. Surg. 1997, 63, 663–668. [Google Scholar] [CrossRef]
- Kagisaki, K.; Masai, T.; Kadoba, K.; Sawa, Y.; Nomura, F.; Fukushima, N.; Ichikawa, H.; Ohata, T.; Suzuki, K.; Taketani, S.; et al. Biocompatibility of heparin-coated circuits in pediatric cardiopulmonary bypass. Artif. Organs 1997, 21, 836–840. [Google Scholar] [CrossRef]
- Svenmarker, S.; Sandstrom, E.; Karsson, T.; Jansson, E.; Haggmark, S.; Lindholm, R.; Appelblad, M.; Aberg, T. Clinical effects of the heparin coated surface in cardiopulmonary bypass. Eur. J. Cardio-Thorac. Surg. 1997, 11, 957–964. [Google Scholar] [CrossRef]
- Belboul, A.; Al-Khaja, N. Does heparin coating improve biocompatibility? A study on complement, blood cells and postoperative morbidity during cardiac surgery. Perfusion 1997, 12, 385–391. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, Y.M.; Park, S.N.; Sheen, S.Y.; Chung, C.P.; Lee, S.J. Platelet derived growth factor releasing chitosan sponge for periodontalbone regeneration. Biomaterials 2000, 21, 153–159. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Mattiasson, B.; Kirsebom, H. Cryogelation of chitosan using noble-metal ions: In situ formation of nanoparticles. Biomacromolecules 2014, 15, 2246–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berillo, D.; Cundy, A. 3D-macroporous chitosan-based scaffolds with in situ formed Pd and Pt nanoparticles for nitrophenol reduction. Carbohydr. Polym. 2018, 192, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Berillo, D.; Elowsson, L.; Kirsebom, H. Oxidized dextran as crosslinker for chitosan cryogel scaffolds and formation of polyelectrolyte complexes between chitosan and gelatin. Macromol. Biosci. 2012, 12, 1090–1099. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 2009, 24, 249–265. [Google Scholar]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.Y.; Roh, H.H.; Kim, H.S.; Lee, J.W.; Lee, K.Y. Three-dimensional bioprinting of cell-laden constructs using polysaccharide-based self-healing hydrogels. Biomacromolecules 2019, 20, 1860–1866. [Google Scholar] [CrossRef]
- Demirtas, T.T.; Irmak, G.; Gumusderelioglu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Xi, W.; Kong, F.; Yeo, J.C.; Yu, L.; Sonam, S.; Dao, M.; Gong, X.; Lim, C.T. Soft tubular microfluidics for 2D and 3D applications. Proc. Natl. Acad. Sci. USA 2017, 114, 10590–10595. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Development of a Combined 3D Printer and Its Application in Complex Organ Construction. Master’s Thesis, Tsinghua University, Beijing, China, 2014. [Google Scholar]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for Tissue Repair and Organ Three-Dimensional (3D) Bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestro, I.; Sergi, R.; D’Abusco, A.S.; Mariano, A.; Martinelli, A.; Piozzi, A.; Francolini, I. Chitosan scaffolds with enhanced mechanical strength and elastic response by combination of freeze gelation, photo-crosslinking and freeze-drying. Carbohydr. Polym. 2021, 267, 118156. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]

| Chitosan/Chitosan Derivative | Artificial Organ | Specified Application | Enhanced Properties | References |
|---|---|---|---|---|
| N-acetyl chitosan | Artificial membranes | Dialysis membrane | Improved dialysis membrane, permeability similar to Amicon Diaflo membrane | [85] |
| Chitosan:protein | Artificial membranes | Dialysis membrane | Improved permeability to urea, creatinine, uric acid, and glucose | [86] |
| N-acetyl and N-hexanoyl chitosan membranes | Artificial membranes | Dialysis membrane | Nonthrombogenic membrane properties | [87] |
| Bioactive-complex-immobilized, albumin-blended chitosan membranes | Artificial membranes | Dialysis membrane | Liposome-modified membranes were nonthrombogenic, better permeability, reduced platelet attachment. | [88] |
| Phosphoryl choline bilayer immobilized on albumin-blended chitosan membranes | Artificial membranes | Dialysis membrane | Similar permeability as chitosan membranes and drastic reduction in platelet adhesion offering improved permeability and blood compatibility | [89] |
| Polyelectrolyte complexes of chitosan with sulfonated chitosan | Artificial skin | Wound healing | Regeneration of skin in wound area, promoting wound healing | [90] |
| Chitosan | Artificial skin | Skin tissue engineering | Hemostasis, accelerating the tissue regeneration, and stimulating the fibroblast synthesis of collagen | [91,92,93] |
| Chitosan cotton | Artificial skin | Wound healing | Promotes infiltration of PMN cells at the wound site | [94] |
| Chitosan | Artificial skin | Wound healing | Promotes basic fibroblast growth factor(bFGF), accelerated healing | [95] |
| Highly deacetylated chitosan | Artificial skin | Wound healing | Rapid wound healing | [96] |
| Chitosan–alginate PEC membranes | Controlled release membranes | Wound healing | Accelerated healing of incisional wounds in a rat model | [97,98] |
| Porous chitosan/collagen scaffold by cross-linking with glutaraldehyde | Artificial skin | Wound healing | Good biocompatibility and induces fibroblasts infiltration | [99] |
| Chitosan–calcium phosphates (CP) | Artificial bone | Bone tissue engineering | Minimizes inflammation, biocompatible, biodegradable, moldable, porous allowing osteoconduction | [100,101] |
| CP embedded with chitosan sponge | Artificial bone | Bone reinforcement | Enhanced mechanical properties, matrix reinforcement, preserving osteoblast phenotype | [102,103] |
| Chitosan injected into the rat’s knee articular cavity | Artificial cartilage | Wound healing of articular cartilage | Significant increase in the density of chondrocytes in the knee articular cartilage | [109] |
| Bone morphogenetic protein (BMP)-7/N,N dicarboxymethyl chitosan | Artificial cartilage | Repair | Artificial cartilage lesions in rabbit | [110] |
| GAG-augmented chitosan hydrogel surfaces | Artificial cartilage | Bone tissue engineering | biocompatibility and the chondrogenic characteristics | [111,112,113] |
| Chitosan–alginate–hyaluronan scaffolds | Artificial cartilage | repair | Showed neocartilage formation in vitro | [114] |
| Porous collagen/chitosan/GAG scaffolds with transforming growth factor-β1(TGF-β1) | Artificial cartilage | Bone tissue engineering | Controlled release of TGF-β1 and promoted cartilage regeneration | [115] |
| In situ-solidified chitosan–GP blood implants | Artificial cartilage | Microfractured ovine defect repair | Chitosan is thrombogenic and actively stimulates the wound repair process | [116,117] |
| Chitosan/collagen matrix (CCM) by cross-linking agent EDC | Artificial liver | Liver tissue engineering | Considerable mechanical strength, good hepatocyte compatibility as well as excellent blood compatibility | [123] |
| Chitosan/collagen/heparin matrix | Artificial liver | Aid in implantable bioartificial liver (IBL)applications | Superior blood compatibility | [123] |
| Chitosan modified with galactose | Artificial liver | Liver tissue engineering | Improve hepatocyte attachment and maintain viability | [124] |
| Galactosylated chitosan (GC) | Artificial liver | ECM for hepatocyte attachment | New synthetic ECM | [124] |
| Conjugated fructose onto the porous chitosan scaffold | Artificial liver | Liver tissue engineering | Induces cellular aggregates/enhances liver-specific metabolic activities and cell density | [120,122] |
| Chitosan | Artificial nerve | Neuron tissue culture | Repair of peripheral nervous system | [127,128] |
| Chitosan fibers | Artificial nerve | Regenerating axons to Büngner bands in the nervous system | Supported the adhesion, migration, and proliferation of SCs | [129] |
| Hydroxyapatite coated chitosan tubes loaded with laminin-1 or laminin peptides | Artificial nerve | Nerve regeneration | Peripheral nerve reconstruction | [130] |
| Immobilization of laminin peptide in molecularly aligned chitosan by covalent bonding | Artificial nerve | Nerve regeneration | Regeneration of PNS | [130,131] |
| Peptide conjugate chitosan membranes | Artificial nerve | Nerve repair | Accelerate axonal regeneration | [132] |
| Chitosan prostheses | Artificial nerve | Nerve regenerative response | Better facial response | [133] |
| Hexamethylene diisocyanate (HDI), epichlorohydrin (ECH) and glutaraldehyde (GA) as crosslinked chitosan films | Artificial nerve | Nerve regeneration | Enhanced the spread and proliferation of Schwann cells | [134] |
| Chitosan/poly(L-lysine) composite | Artificial nerve | Nerve regeneration | Improved nerve cell affinity | [135] |
| Chitosan/gelatin composite film | Artificial nerve | Nerve tissue culture | PC12 cells cultured on the composite films differentiated more rapidly and extended longer neurites | [136] |
| Chitin hydrogel tubes | Artificial nerve | Scaffolds in neural tissue engineering | Support nerve cell adhesion and neurite outgrowth | [137] |
| Complexation of GAGs with porous chitosan scaffolds | Artificial blood vessel | Vascular grafts | Growth inhibitory effects on vascular smooth muscle cells and their anticoagulant activity | [121] |
| Chitosan scaffolds, including heparin modified porous tubes | Artificial blood vessel | Blood vessel tissue engineering | Biocompatibility | [5] |
| Heparin–chitosan scaffolds | Artificial blood vessel | Blood vessel tissue engineering | Reduced coagulation, complement and blood cells. stimulates cell proliferation and formation of thick, vascularized granulation layer | [139,140,141,142,143,144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivanesan, I.; Hasan, N.; Muthu, M.; Blessing, G.; Gopal, J.; Chun, S.; Shin, J.; Oh, J.-W. Exploring the Impact of Chitosan Composites as Artificial Organs. Polymers 2022, 14, 1587. https://doi.org/10.3390/polym14081587
Sivanesan I, Hasan N, Muthu M, Blessing G, Gopal J, Chun S, Shin J, Oh J-W. Exploring the Impact of Chitosan Composites as Artificial Organs. Polymers. 2022; 14(8):1587. https://doi.org/10.3390/polym14081587
Chicago/Turabian StyleSivanesan, Iyyakkannu, Nazim Hasan, Manikandan Muthu, Gowsalya Blessing, Judy Gopal, Sechul Chun, Juhyun Shin, and Jae-Wook Oh. 2022. "Exploring the Impact of Chitosan Composites as Artificial Organs" Polymers 14, no. 8: 1587. https://doi.org/10.3390/polym14081587
APA StyleSivanesan, I., Hasan, N., Muthu, M., Blessing, G., Gopal, J., Chun, S., Shin, J., & Oh, J.-W. (2022). Exploring the Impact of Chitosan Composites as Artificial Organs. Polymers, 14(8), 1587. https://doi.org/10.3390/polym14081587









