Development of a Dual Drug-Loaded, Surfactant-Stabilized Contrast Agent Containing Oxygen
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Unloaded SE61O2 Microbubble Fabrication
2.3. Single-Drug-Loaded SE61O2 Microbubble Fabrication
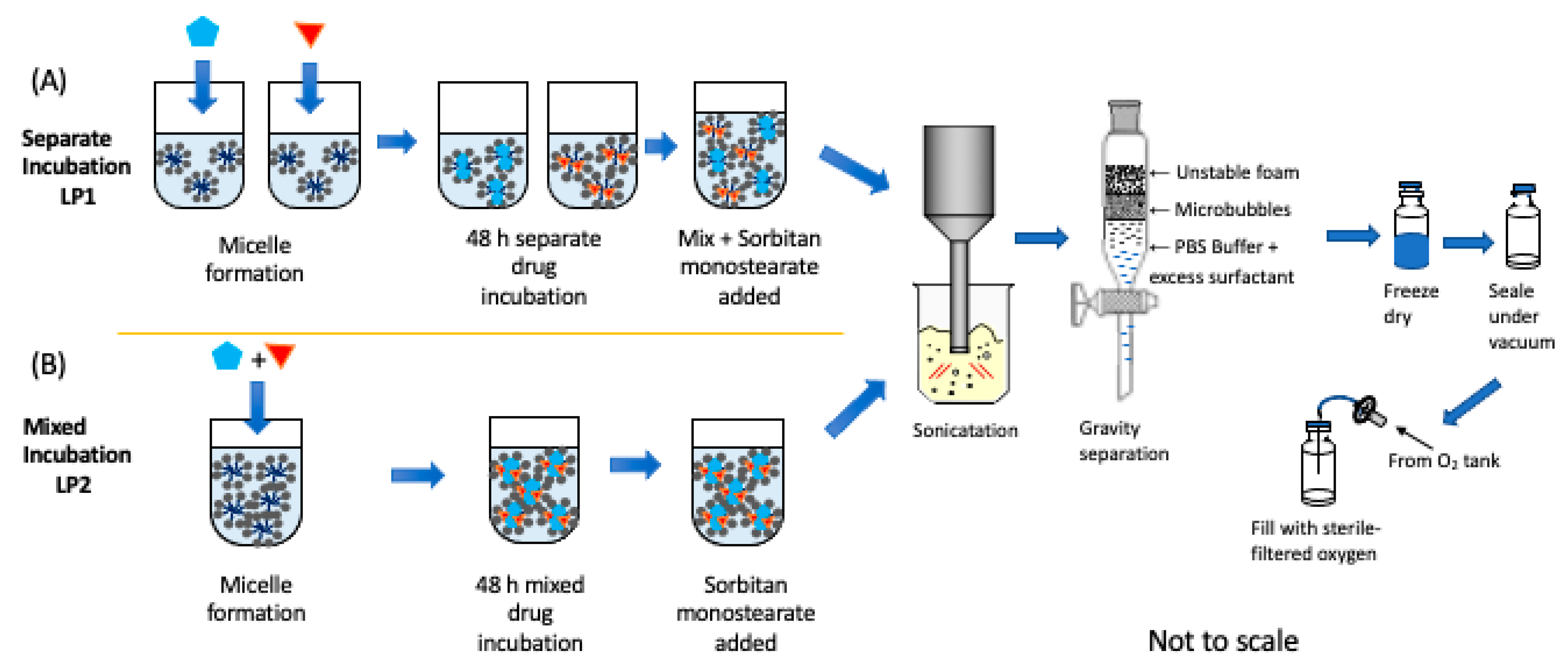
2.4. Dual-Drug-Loaded SE61O2 Microbubble Fabrication
2.5. SE61O2 Microbubble Size Distribution
2.6. Light Microscopy
2.7. In Vitro Acoustic Characterization
2.8. Flow Phantom Analysis
2.8.1. Imaging
2.8.2. Stability Curves
2.9. Drug Loading Quantification
2.10. In Vivo Studies
2.11. Statistical Analysis
3. Results and Discussion
3.1. Incorporation of a Single Drug
3.1.1. SE61O2 Microbubble Size Populations
3.1.2. In Vitro Acoustic Characterization
3.1.3. Drug Loading Quantification
3.2. Dual-Drug LND-and-PTX-Loaded SE61O2
3.2.1. Microbubble Size Populations for Dual Drug Loaded SE61O2
3.2.2. In Vitro Acoustic Characterization
3.2.3. Dual Drug Loading Quantification
Effect of Method on Lonidamine Encapsulation
Effect of the Method on Paclitaxel Encapsulation
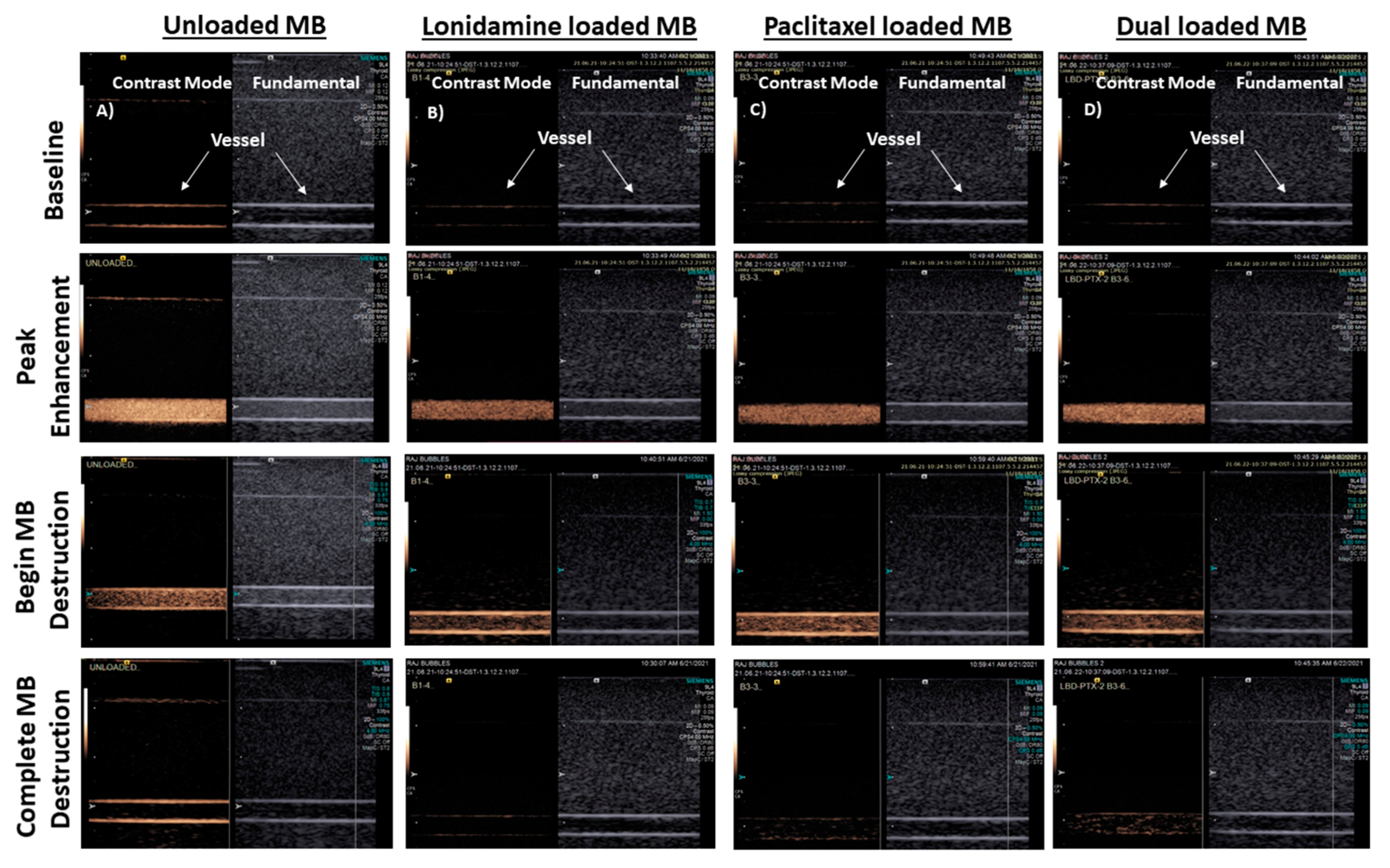
3.3. In Vitro Imaging
3.3.1. Flow Phantom
3.3.2. Stability in the Ultrasound Beam
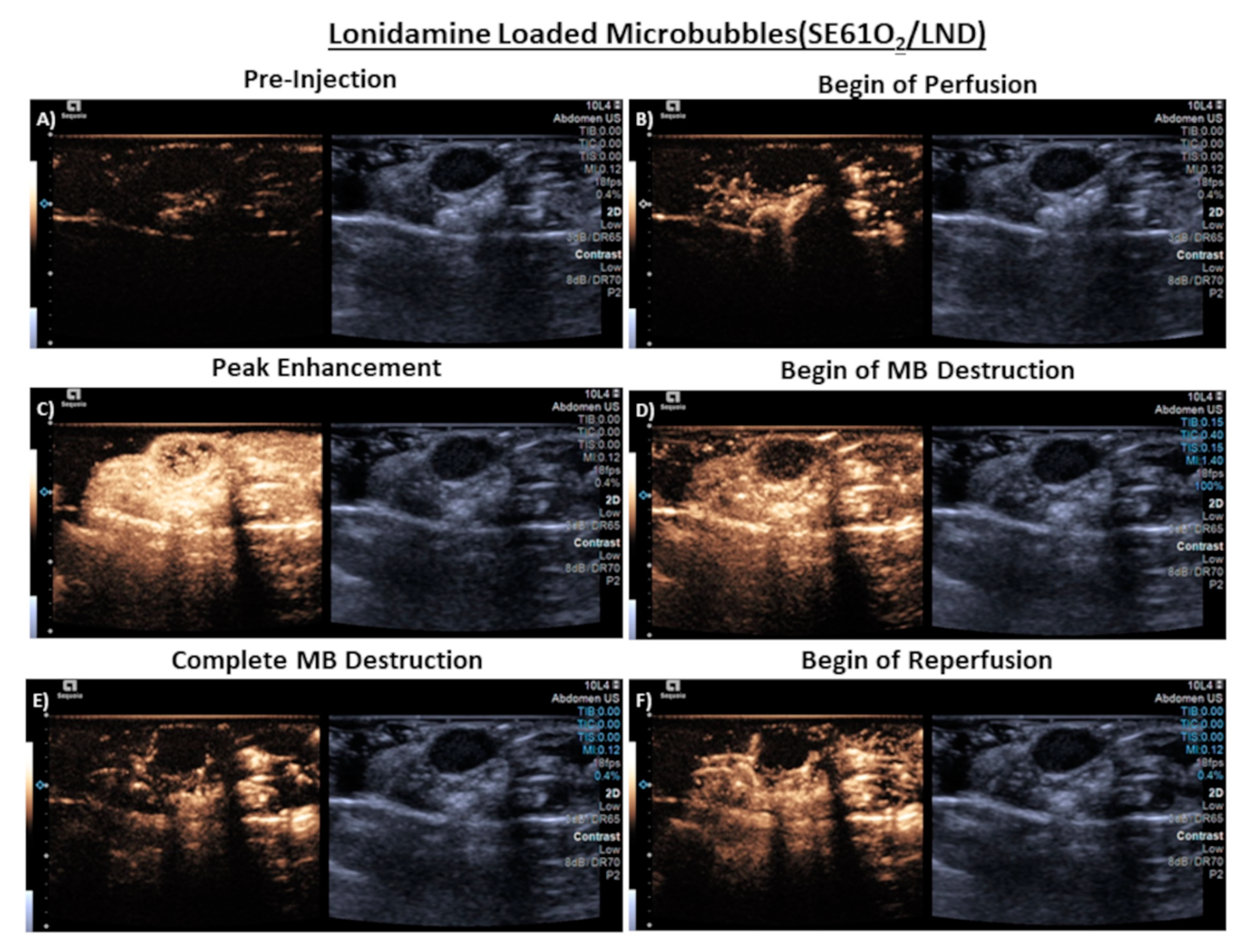
3.4. In Vivo Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rueff, J.; Rodrigues, A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. Methods Mol. Biol. 2016, 1395, 1–18. [Google Scholar]
- Assanhou, A.G.; Li, W.; Zhang, L.; Xue, L.; Kong, L.; Sun, H.; Mo, R.; Zhang, C. Reversal of multidrug resistance by co-delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid dual-functionalized liposome for cancer treatment. Biomaterials 2015, 73, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [PubMed]
- Chen, K.G.; Jaffrézou, J.P.; Fleming, W.H.; Durán, G.E.; Sikic, B.I. Prevalence of multidrug resistance related to activation of the mdr1 gene in human sarcoma mutants derived by single-step doxorubicin selection. Cancer Res. 1994, 54, 4980–4987. [Google Scholar] [PubMed]
- López-Verdín, S.; Lavalle-Carrasco, J.; Carreón-Burciaga, R.G.; Serafín-Higuera, N.; Molina-Frechero, N.; González-González, R.; Bologna-Molina, R. Molecular markers of anticancer drug resistance in head and neck squamous cell carcinoma: A literature review. Cancers 2018, 10, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, I.O.; Lam, K.Y.; Ng, M.; Kwong, D.L.; Sham, J.S. Expression of P-glycoprotein, a multidrug-resistance gene product, is induced by radiotherapy in patients with oral squamous cell carcinoma. Cancer 1998, 83, 851–857. [Google Scholar] [CrossRef]
- Rabkin, D.; Chhieng, D.C.; Miller, M.B.; Jennings, T.; Feustel, P.; Steiniger, J.; Parnes, S.M. P-glycoprotein expression in the squamous cell carcinoma of the tongue base. Laryngoscope 1995, 105, 1294–1299. [Google Scholar] [CrossRef]
- Nath, K.; Guo, L.; Nancolas, B.; Nelson, D.S.; Shestov, A.A.; Lee, S.C.; Roman, J.; Zhou, R.; Leeper, D.B.; Halestrap, A.P.; et al. Mechanism of antineoplastic activity of lonidamine. Biochim. Biophys. Acta 2016, 1866, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The potential of lonidamine in combination with chemotherapy and physical therapy in cancer treatment. Cancers 2020, 12, 3332. [Google Scholar] [CrossRef]
- Milane, L.; Duan, Z.; Amiji, M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int. 2011, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Harriss-Phillips, W.M.; Bezak, E.; Yeoh, E.K. Monte Carlo radiotherapy simulations of accelerated repopulation and reoxygenation for hypoxic head and neck cancer. Br. J. Radiol. 2011, 84, 903–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Briest, S.; Höckel, M. Hypoxia in breast cancer: Pathogenesis, characterization and biological/therapeutic implications. Wien Med. Wochenschr. 2002, 152, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.S.; Taguchi, Y.H. Identification of genes associated with altered gene expression and m6A profiles during hypoxia using tensor decomposition based unsupervised feature extraction. Sci. Rep. 2021, 11, 8909. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xue, B.; Ohulchanskyy, T.Y.; Liu, Y.; Yakovliev, A.; Ziniuk, R.; Xu, M.; Song, J.; Qu, J.; Yuan, Z. Inhibiting tumor oxygen metabolism and simultaneously generating oxygen by intelligent upconversion nanotherapeutics for enhanced photodynamic therapy. Biomaterials 2020, 251, 120088. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.Y.; Cheng, Y.J.; Lei, Q.; Zhang, A.Q.; Zhang, X.Z. Combinational strategy for high-performance cancer chemotherapy. Biomaterials 2018, 171, 178–197. [Google Scholar] [CrossRef]
- Hung, M.S.; Wu, Y.F.; Chen, Y.C. Efficacy of chemoradiotherapy versus radiation alone in patients with inoperable locally advanced non-small-cell lung cancer: A meta-analysis and systematic review. Medicine 2019, 98, e16167. [Google Scholar] [CrossRef]
- Wan, Z.; Mao, H.; Guo, M.; Li, Y.; Zhu, A.; Yang, H.; He, H.; Shen, J.; Zhou, L.; Jiang, Z.; et al. Highly efficient hierarchical micelles integrating photothermal therapy and singlet oxygen-synergized chemotherapy for cancer eradication. Theranostics 2014, 4, 399–411. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef] [PubMed]
- Puiu, R.A.; Balaure, P.C.; Constantinescu, E.; Grumezescu, A.M.; Andronescu, E.; Oprea, O.C.; Vasile, B.S.; Grumezescu, V.; Negut, I.; Nica, I.C.; et al. Anti-Cancer Nanopowders and MAPLE-Fabricated Thin Films Based on SPIONs Surface Modified with Paclitaxel Loaded β-Cyclodextrin. Pharmaceutics 2021, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Malorni, W.; Arancia, G.; De Martino, C.; Nista, A.; Accinni, L.; Masella, R.; Cantafora, A. On the mechanism of action of lonidamine: A study on human erythrocyte membrane. Exp. Mol. Pathol. 1988, 49, 361–372. [Google Scholar] [CrossRef]
- Eisenbrey, J.R.; Albala, L.; Kramer, M.R.; Daroshefski, N.; Brown, D.; Liu, J.B.; Stanczak, M.; O’Kane, P.; Forsberg, F.; Wheatley, M.A. Development of an ultrasound sensitive oxygen carrier for oxygen delivery to hypoxic tissue. Int. J. Pharm. 2015, 478, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, F.; Liu, J.B.; Patel, M.; Liu, L.; Lin, L.; Solis, C.; Fox, T.B.; Wheatley, M.A. Preclinical acute toxicology study of surfactant-stabilized ultrasound contrast agents in adult rats. Int. J. Toxicol. 2010, 29, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Eisenbrey, J.R.; Shraim, R.; Liu, J.B.; Li, J.; Stanczak, M.; Oeffinger, B.; Leeper, D.B.; Keith, S.W.; Jablonowski, L.J.; Forsberg, F.; et al. Sensitization of Hypoxic Tumors to Radiation Therapy Using Ultrasound-Sensitive Oxygen Microbubbles. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 88–96. [Google Scholar] [CrossRef]
- Delaney, L.J.; Ciraku, L.; Oeffinger, B.E.; Wessner, C.E.; Liu, J.B.; Li, J.; Nam, K.; Forsberg, F.; Leeper, D.B.; O’Kane, P. Breast Cancer Brain Metastasis Response to Radiation After Microbubble Oxygen Delivery in a Murine Model. J. Ultrasound Med. 2019, 38, 3221–3228. [Google Scholar] [CrossRef]
- Lacerda, Q.; Oeffinger, B.; Patel, R.; Wheatley, M.A.; Leeper, D.B.; Forsberg, F.; O’Kane, P.; Rochani, A.; Kaushal, G.; Eisenbrey, J.R. Incubation Method for Loading Lonidamine in Oxygen Microbubbles for Targeted Drug Delivery. In Proceedings of the 2020 IEEE International Ultrasonics Symposium (IUS), Las Vegas, NV, USA, 7–11 September 2020. [Google Scholar]
- Fumoto, S.; Nishida, K. Co-delivery Systems of Multiple Drugs Using Nanotechnology for Future Cancer Therapy. Chem. Pharm. Bull. 2020, 68, 603–612. [Google Scholar] [CrossRef]
- Oeffinger, B.E.; Vaidya, P.; Ayaz, I.; Shraim, R.; Eisenbrey, J.R.; Wheatley, M.A. Preserving the Integrity of Surfactant-Stabilized Microbubble Membranes for Localized Oxygen Delivery. Langmuir 2019, 35, 10068–10078. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, R.; Wang, S.; Dong, Z. Paclitaxel: New uses for an old drug. Drug Des. Dev. Ther. 2014, 8, 279–284. [Google Scholar]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Moser, C.C.; Wheatley, M.A. Langmuir trough study of surfactant mixtures used in the production of a new ultrasound contrast agent consisting of stabilized microbubbles. J. Phys. Chem. 1996, 100, 13815–13821. [Google Scholar] [CrossRef]
- Vaidya, P.B.; Oeffinger, B.E.; Patel, R.; Lacerda, Q.; Powell, J.; Eisenbrey, J.R.; Wheatley, M.A. Shaping the synthesis of surfactant-stabilized oxygen microbubbles to accommodate encapsulated drug. Colloids Surf. B Biointerfaces 2021, 208, 112049. [Google Scholar] [CrossRef] [PubMed]
- Cochran, M.C.; Eisenbrey, J.; Ouma, R.O.; Soulen, M.; Wheatley, M.A. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int. J. Pharm. 2011, 414, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Floridi, A.; Paggi, M.G.; Marcante, M.L.; Silvestrini, B.; Caputo, A.; De Martino, C. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J. Natl. Cancer Inst. 1981, 66, 497–499. [Google Scholar]
- Gan, Y.; Wientjes, M.G.; Lu, J.; Au, J.L. Cytostatic and apoptotic effects of paclitaxel in human breast tumors. Cancer Chemother. Pharmacol. 1998, 42, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, L.J.; Alfego, D.; Andorko, J.I.; Eisenbrey, J.R.; Teraphongphom, N.; Wheatley, M.A. Balancing stealth and echogenic properties in an ultrasound contrast agent with drug delivery potential. Biomaterials 2016, 103, 197–206. [Google Scholar] [CrossRef]
- Rochani, A.K.; Wheatley, M.; Oeffinger, B.E.; Eisenbrey, J.R.; Kaushal, G. LC-MS based stability-indicating method for studying the degradation of lonidamine under physical and chemical stress conditions. Res. Pharm. Sci. 2020, 15, 312–322. [Google Scholar]
- Wenk, M.R.; Fahr, A.; Reszka, R.; Seelig, J. Paclitaxel partitioning into lipid bilayers. J. Pharm. Sci. 1996, 85, 228–231. [Google Scholar] [CrossRef]
- Wheatley, M.A.; Forsberg, F.; Oum, K.; Ro, R.; El-Sherif, D. Comparison of in vitro and in vivo acoustic response of a novel 50:50 PLGA contrast agent. Ultrasonics 2006, 44, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Chen, C.C.; Tung, Y.S.; Olumolade, O.O.; Konofagou, E.E. Effects of the microbubble shell physicochemical properties on ultrasound-mediated drug delivery to the brain. J. Control. Release 2015, 212, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; He, Z.; Schulz, A.; Bronich, T.K.; Jordan, R.; Luxenhofer, R.; Kabanov, A.V. Synergistic combinations of multiple chemotherapeutic agents in high capacity poly(2-oxazoline) micelles. Mol. Pharm. 2012, 9, 2302–2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Jordan, R.; Wang, A.; Sokolsky, M.; Kabanov, A.V. Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 2019, 192, 1–14. [Google Scholar] [CrossRef]
- Wan, X.; Min, Y.; Bludau, H.; Keith, A.; Sheiko, S.S.; Jordan, R.; Wang, A.Z.; Sokolsky-Papkov, M.; Kabanov, A.V. Drug combination synergy in worm-like polymeric micelles improves treatment outcome for small cell and non-small cell lung cancer. ACS Nano 2018, 12, 2426–2439. [Google Scholar] [CrossRef] [Green Version]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.M.; Moser, C.C.; Wheatley, M.A. Surfactant-Stabilized Microbubbles as Ultrasound Contrast Agents: Stability Study of Span 60 and Tween 80 Mixtures Using a Langmuir Trough. Langmuir 1993, 9, 2426–2429. [Google Scholar] [CrossRef]
- Wheatley, M.; Singhal, S. Structural studies on stabilized microbubbles: Development of a novel contrast agent for diagnostic ultrasound. React. Polym. 1995, 25, 157–166. [Google Scholar] [CrossRef]
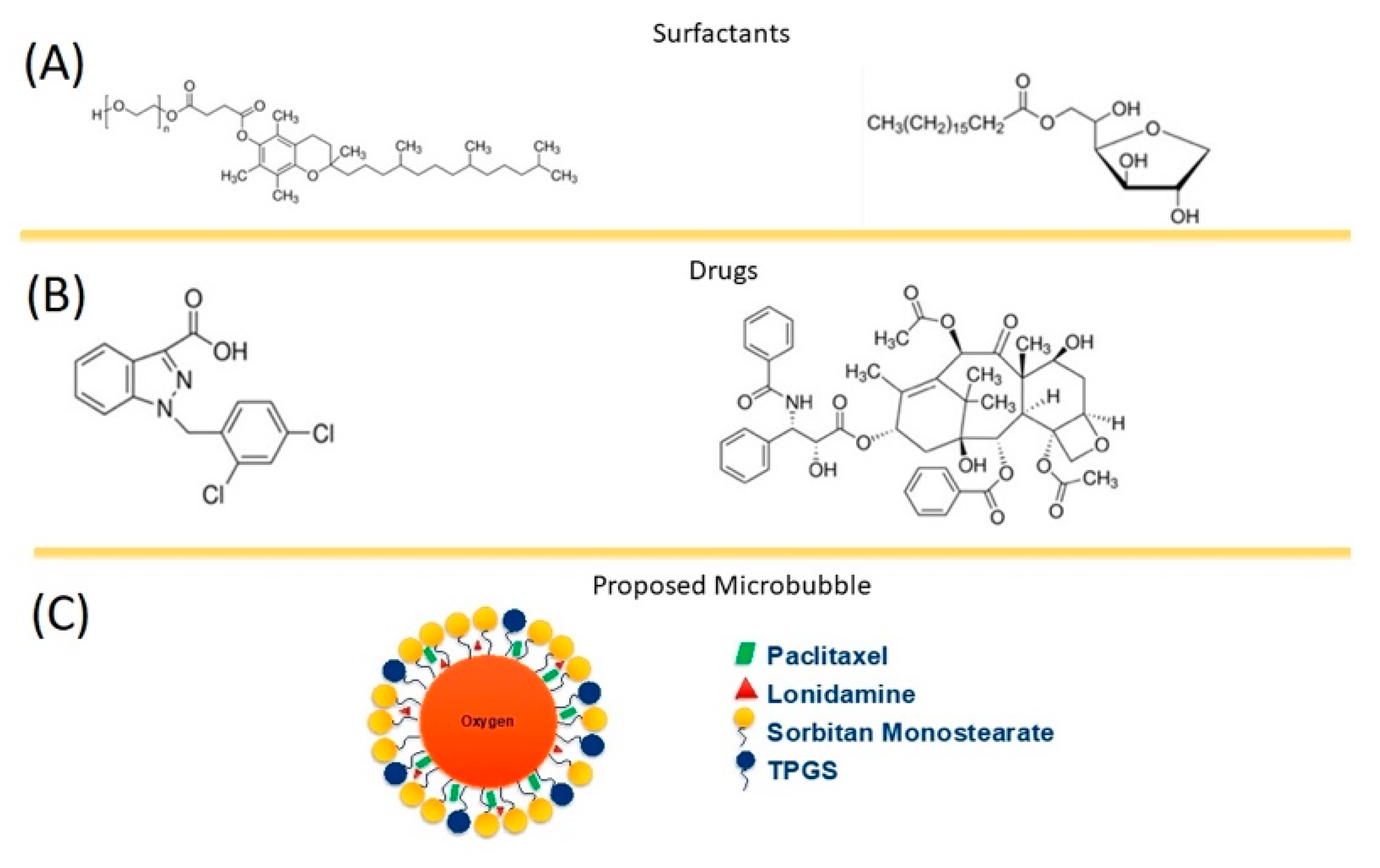
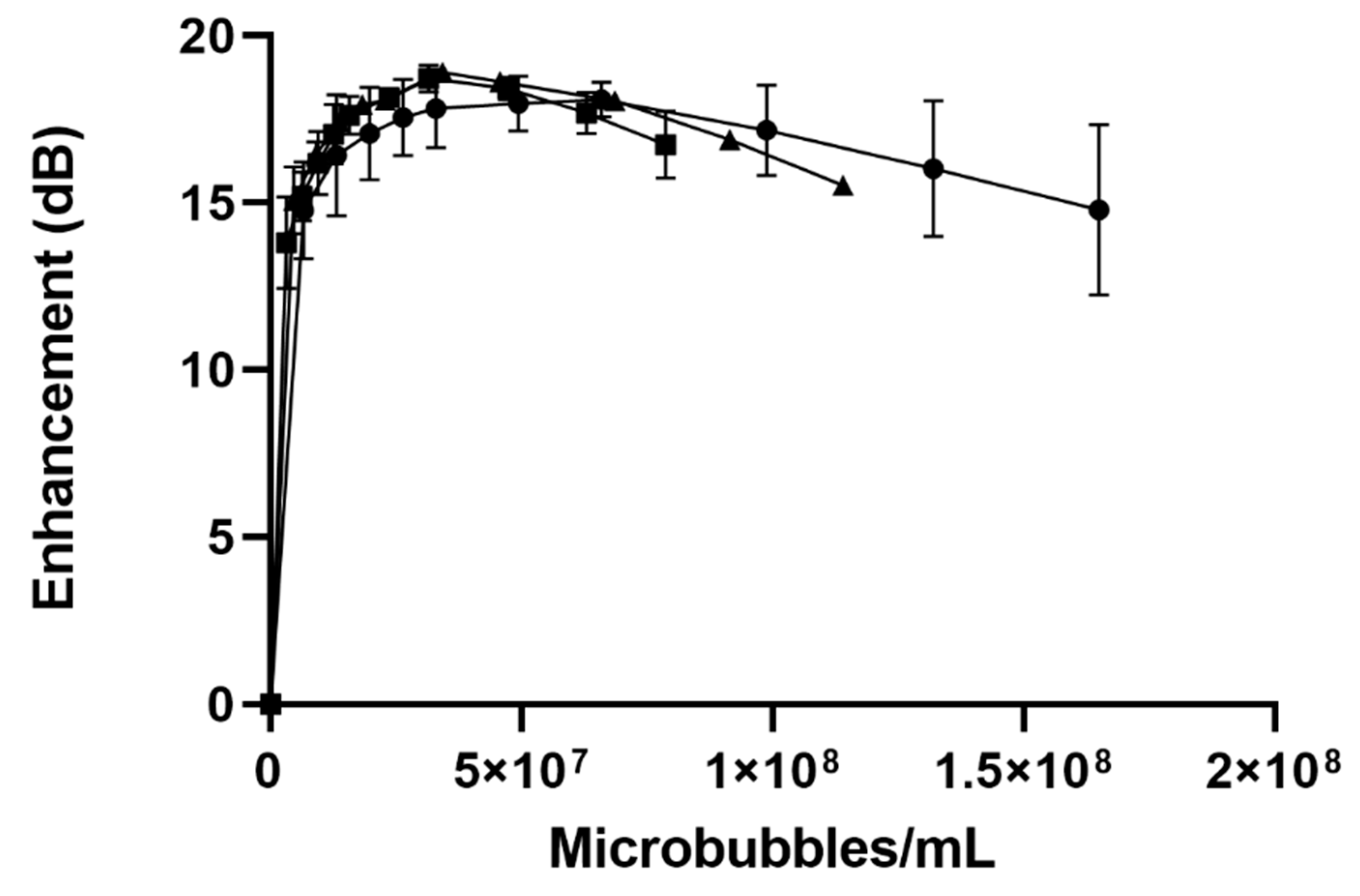
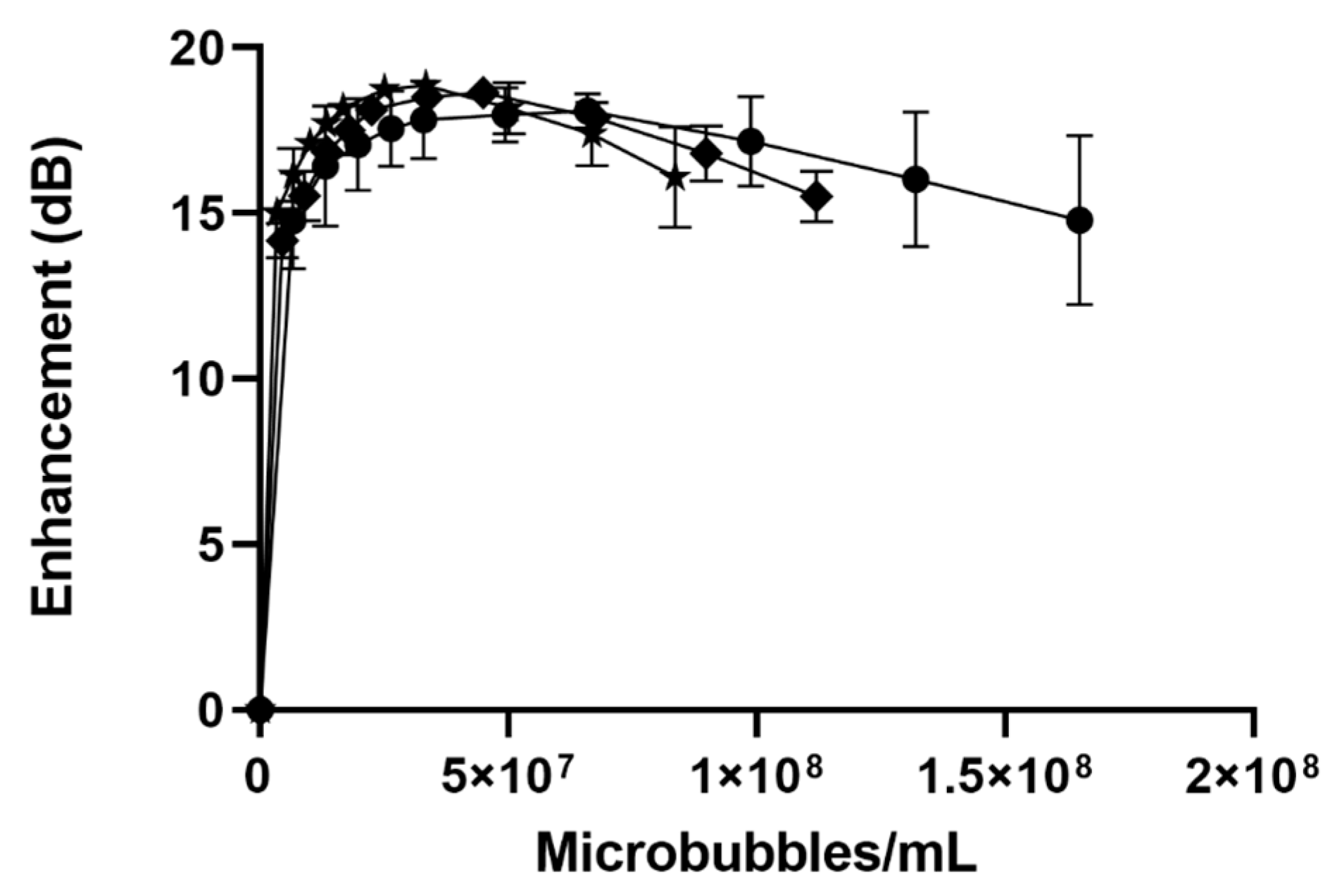
 -), SE61O2 microbubbles.
-), SE61O2 microbubbles.
 -), SE61O2 microbubbles.
-), SE61O2 microbubbles.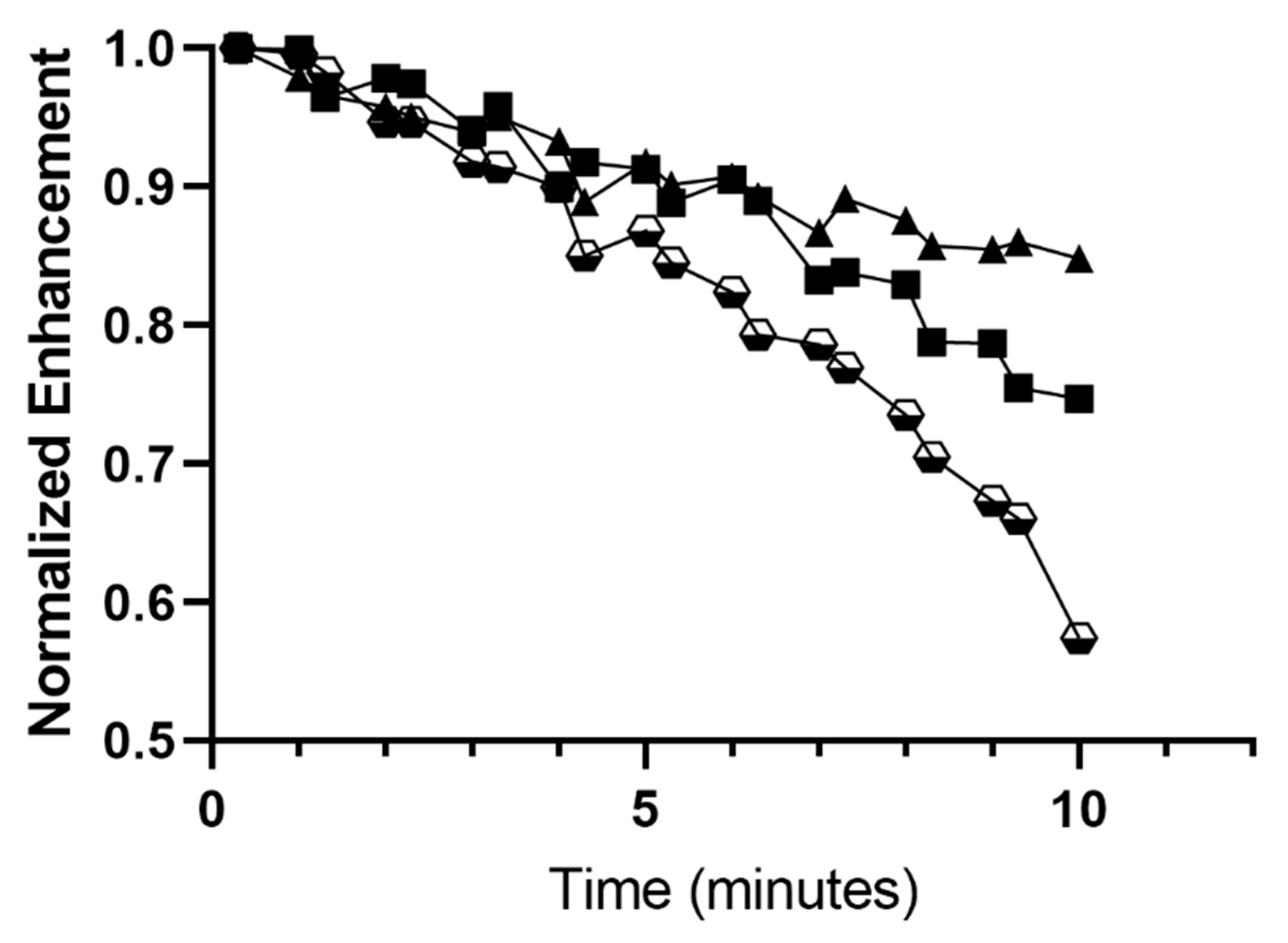
| Diameter (µm) | Concentration × 109 (Microbubbles/mL) | Maximum Enhancement (dB) | Dose at Max (MB/mL × 107) | |
|---|---|---|---|---|
| Unloaded | 1.69 ± 0.10 | 3.30 ± 1.35 | 18.10 ± 0.52 | 4.94 ± 1.65 |
| LND-SE61O2 | 1.74 ± 0.05 | 1.57 ± 0.62 | 18.70 ± 0.40 | 3.67 ± 0.91 |
| PTX-SE61O2 | 1.68 ± 0.08 | 2.29 ± 0.50 | 18.90 ± 0.18 | 3.81 ± 0.66 |
| Added Drug (mg) | μg Drug/mL Microbubble | μg Drug/g Microbubble | µMoles Drug/g Microbubble | Encapsulation Efficiency (%) | |
|---|---|---|---|---|---|
| PTX SE61O2 | 5.27 ± 0.30 | 4.47 ± 0.97 | 84.27 ± 4.39 | 0.0986 | 1.66 |
| LND SE61O2 | 4.07 ± 0.15 | 4.19 ± 0.75 | 64.04 ± 15.62 | 0.0139 | 1.54 |
| Diameter (µm) | Concentration (Microbubbles/mL) | Maximum Enhancement (dB) | Dose at Max (MB/mL × 107) | |
|---|---|---|---|---|
| Separate Micelle incubation (LP1) | 1.74 ± 0.03 | 2.25 ± 1.19 × 109 | 18.63 ± 0.05 | 4.49 ± 0.00 |
| Mixed Micelle incubation (LP2) | 1.64 ± 0.02 | 1.67 ± 0.47 × 109 | 18.85 ± 0.02 | 4.46 ± 0.97 |
| µg LND/mL Microbubbles | µg LND/g Microbubble | Encapsulation Efficiency (%) | |
|---|---|---|---|
| Separate TPGS micelles (LP1) | 5.01 ± 0.99 | 104.85 ± 22.87 | 2.42 |
| Mixed Incubation (LP2) | 4.17 ± 0.43 | 87.54 ± 16.41 | 2.06 |
| LND in SE61O2 | 4.19 ± 0.75 | 64.04 ± 15.62 | 1.54 |
| µg PTX/mL Microbubbles | µg PTX/g Microbubbles | Encapsulation Efficiency (%) | |
|---|---|---|---|
| Separate TPGS micelles (LP1) | 8.99 ± 0.56 | 187.35 ± 8.38 | 3.48 |
| Mixed Incubation (LP2) | 6.50 ± 1.01 | 136.51 ± 30.66 | 2.56 |
| PTX in SE61O2 | 4.47 ± 0.97 | 84.29 ± 4.39 | 1.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Lacerda, Q.; Oeffinger, B.E.; Eisenbrey, J.R.; Rochani, A.K.; Kaushal, G.; Wessner, C.E.; Wheatley, M.A. Development of a Dual Drug-Loaded, Surfactant-Stabilized Contrast Agent Containing Oxygen. Polymers 2022, 14, 1568. https://doi.org/10.3390/polym14081568
Patel R, Lacerda Q, Oeffinger BE, Eisenbrey JR, Rochani AK, Kaushal G, Wessner CE, Wheatley MA. Development of a Dual Drug-Loaded, Surfactant-Stabilized Contrast Agent Containing Oxygen. Polymers. 2022; 14(8):1568. https://doi.org/10.3390/polym14081568
Chicago/Turabian StylePatel, Raj, Quezia Lacerda, Brian E. Oeffinger, John R. Eisenbrey, Ankit K. Rochani, Gagan Kaushal, Corinne E. Wessner, and Margaret A. Wheatley. 2022. "Development of a Dual Drug-Loaded, Surfactant-Stabilized Contrast Agent Containing Oxygen" Polymers 14, no. 8: 1568. https://doi.org/10.3390/polym14081568
APA StylePatel, R., Lacerda, Q., Oeffinger, B. E., Eisenbrey, J. R., Rochani, A. K., Kaushal, G., Wessner, C. E., & Wheatley, M. A. (2022). Development of a Dual Drug-Loaded, Surfactant-Stabilized Contrast Agent Containing Oxygen. Polymers, 14(8), 1568. https://doi.org/10.3390/polym14081568









