Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Synthesis of MH
2.3. Measurements
3. Results and Discussion
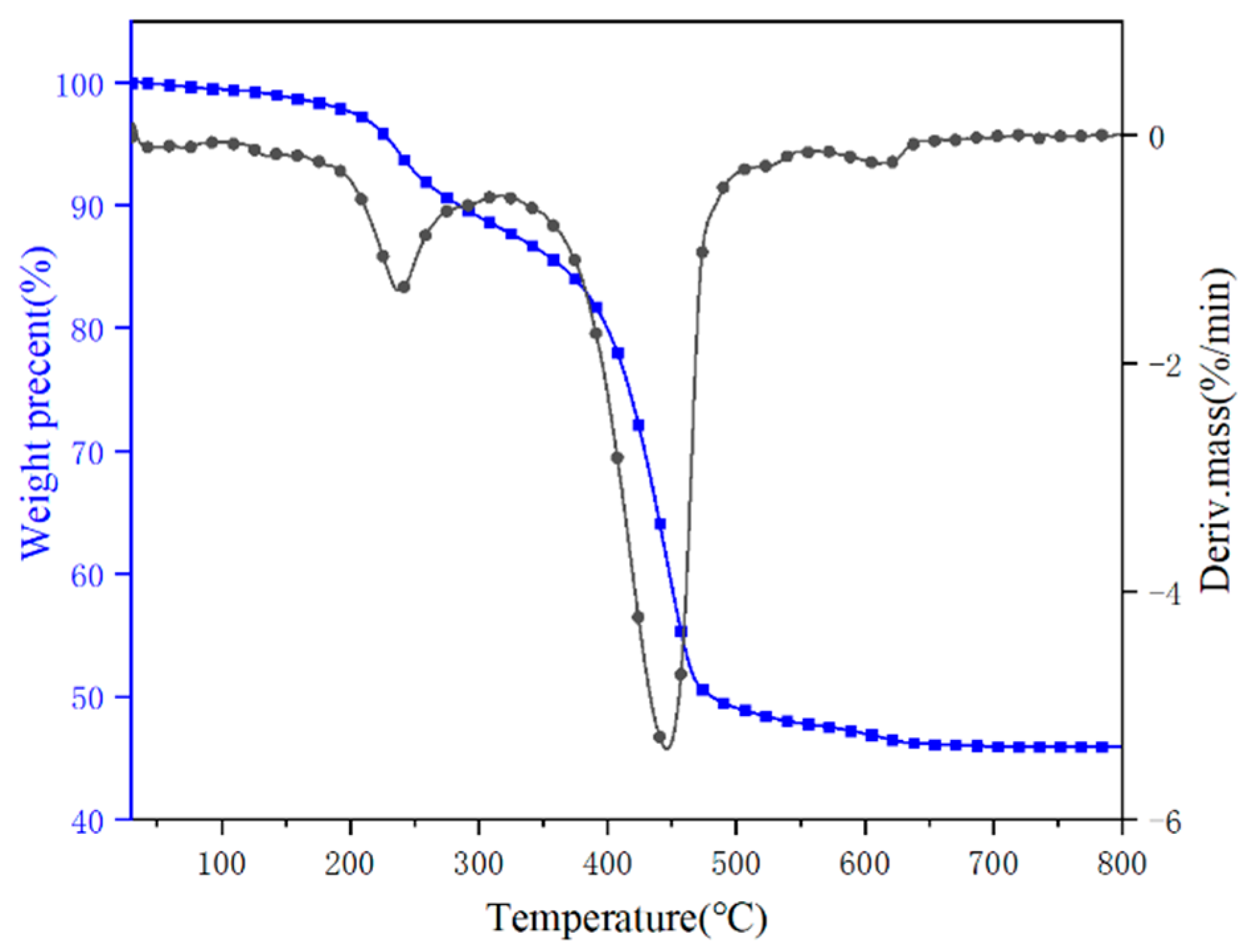
3.1. Thermal Decomposition Behavior of Hydromagnesite
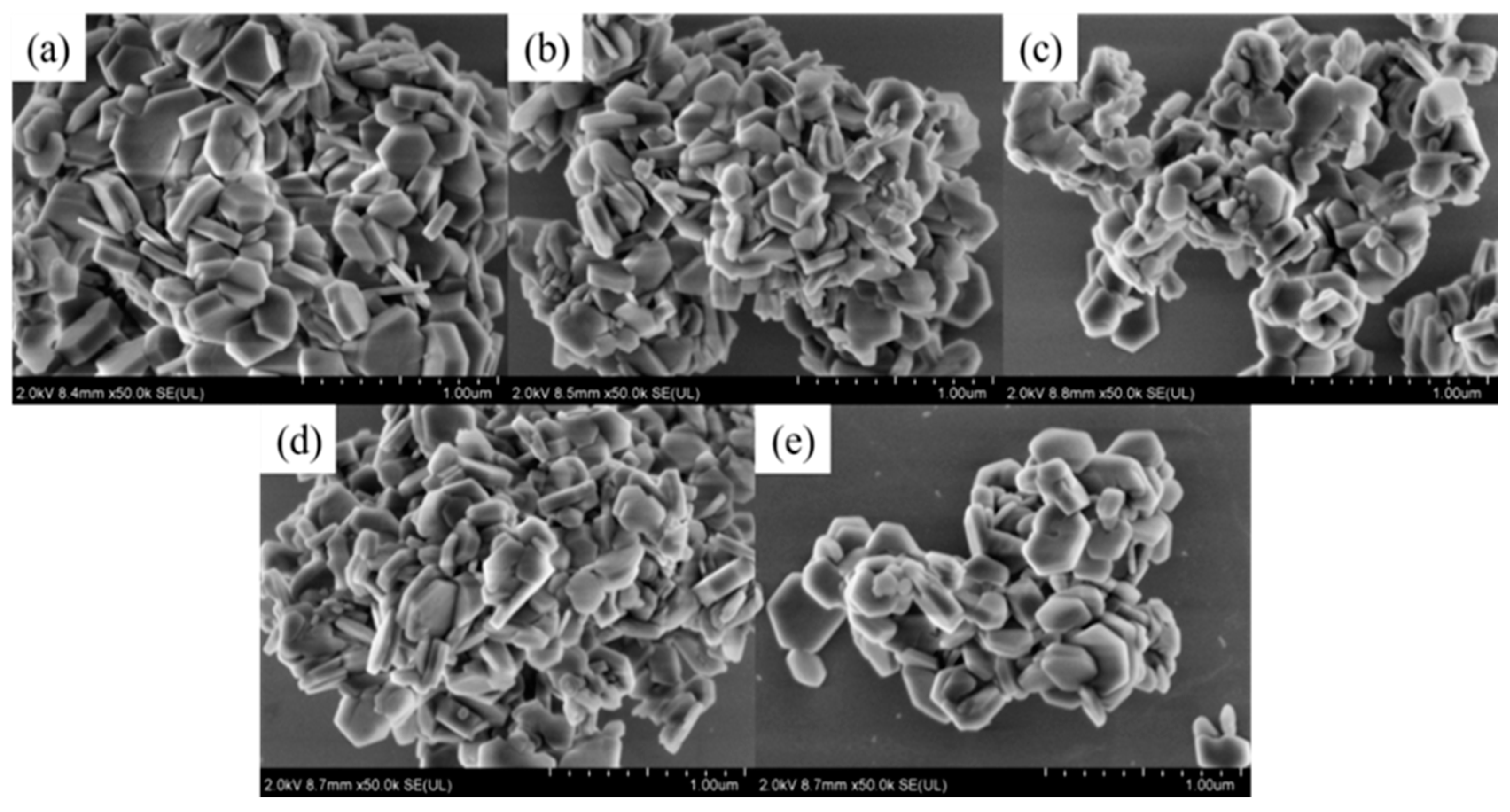
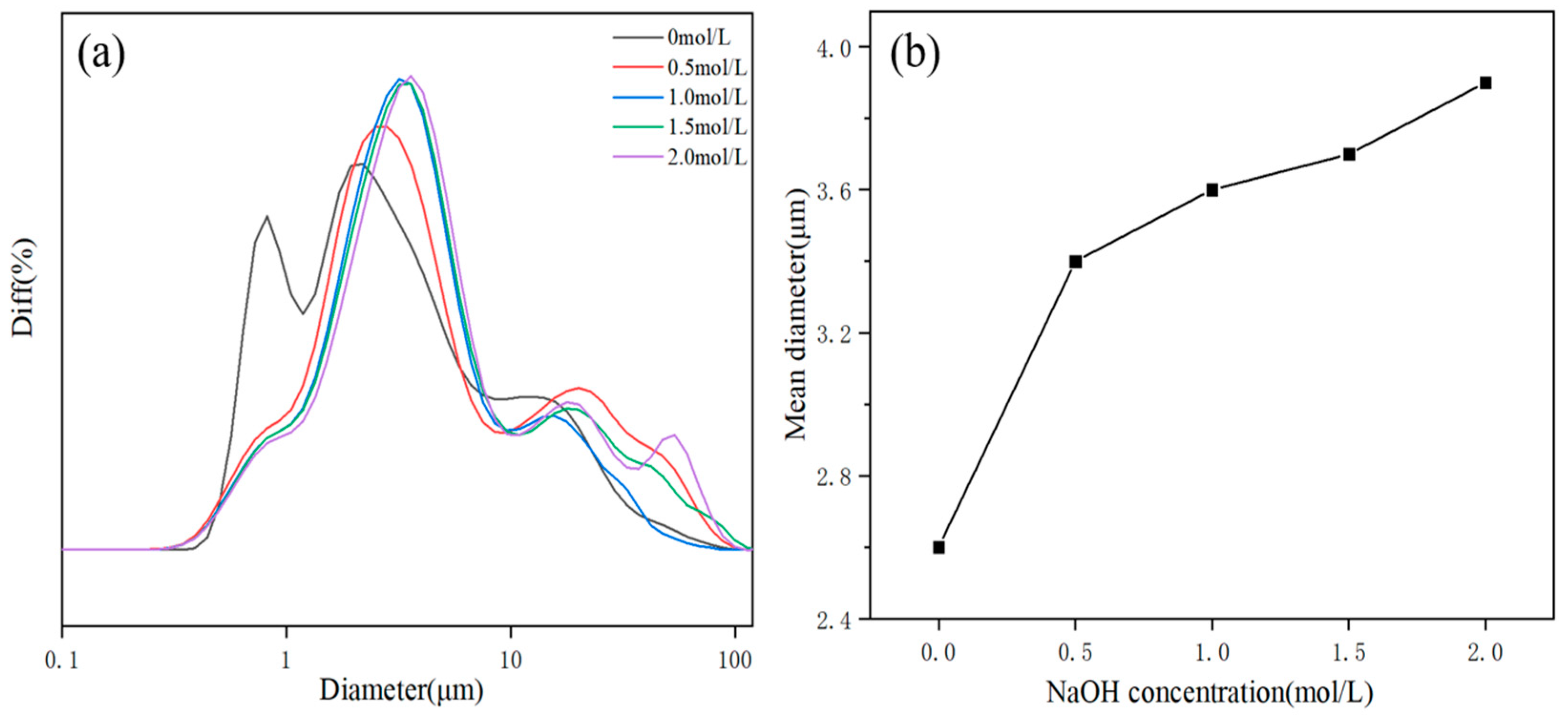
3.2. Morphological Characterization of MH
3.3. Thermal Stability
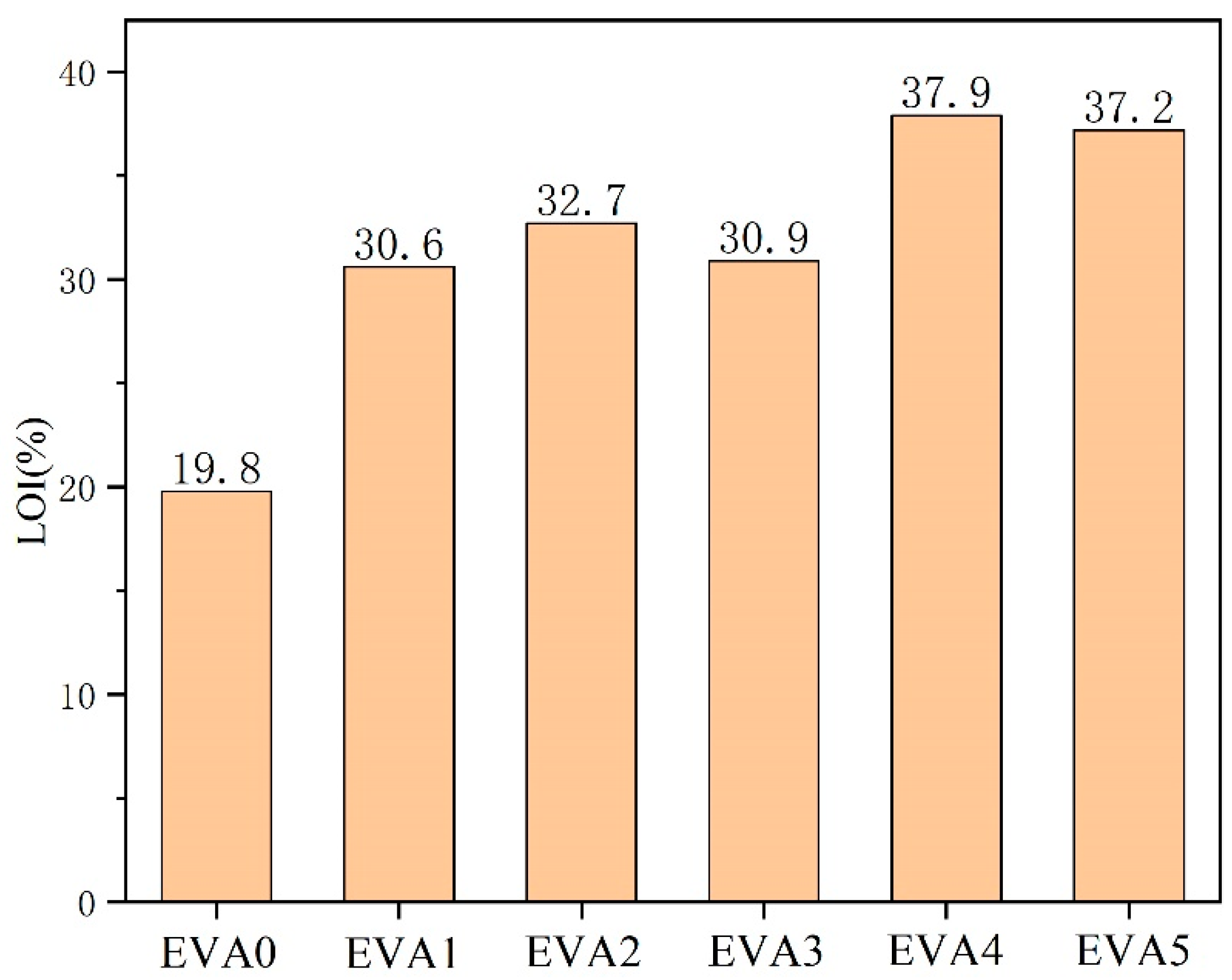
3.4. Flammability
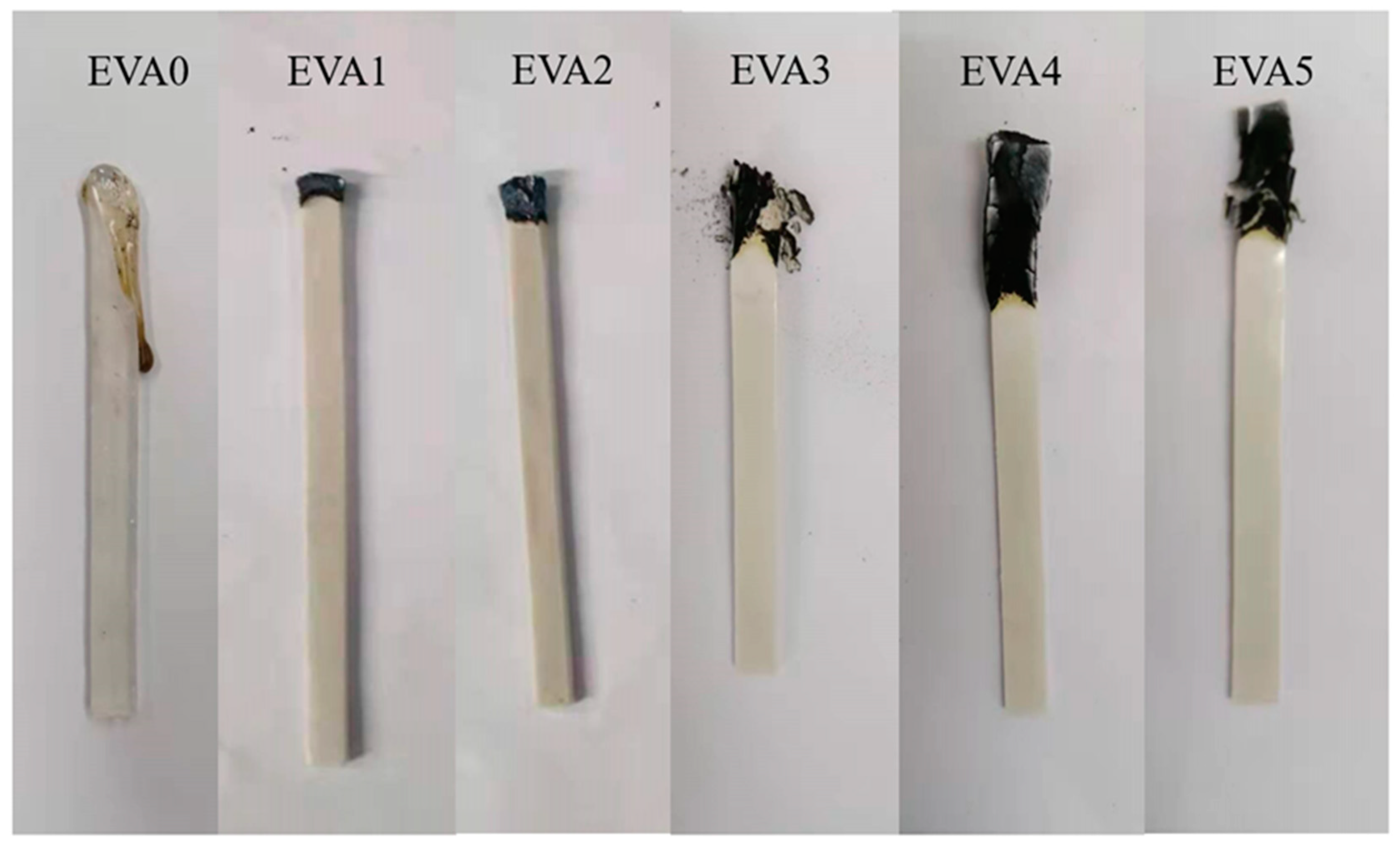
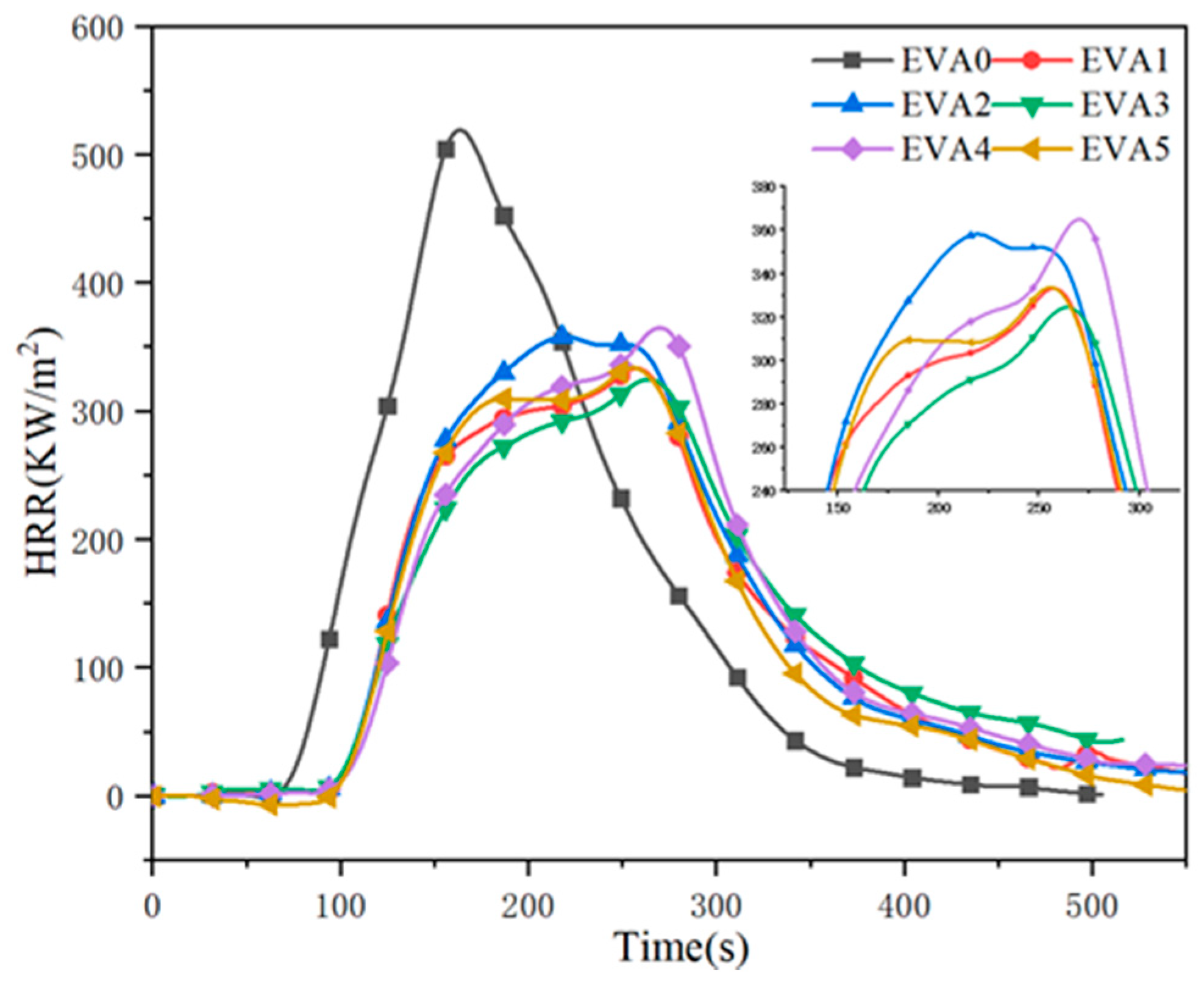
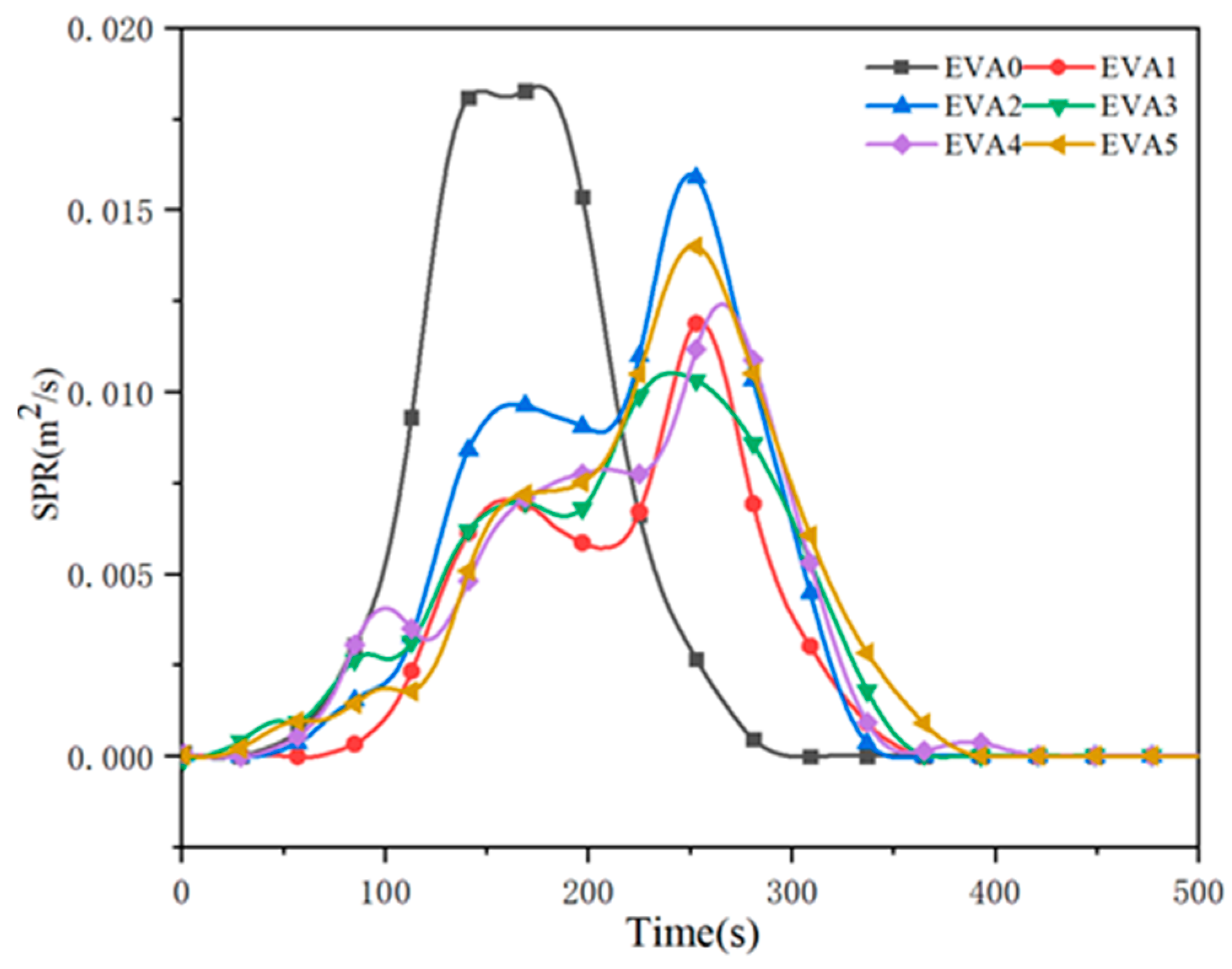
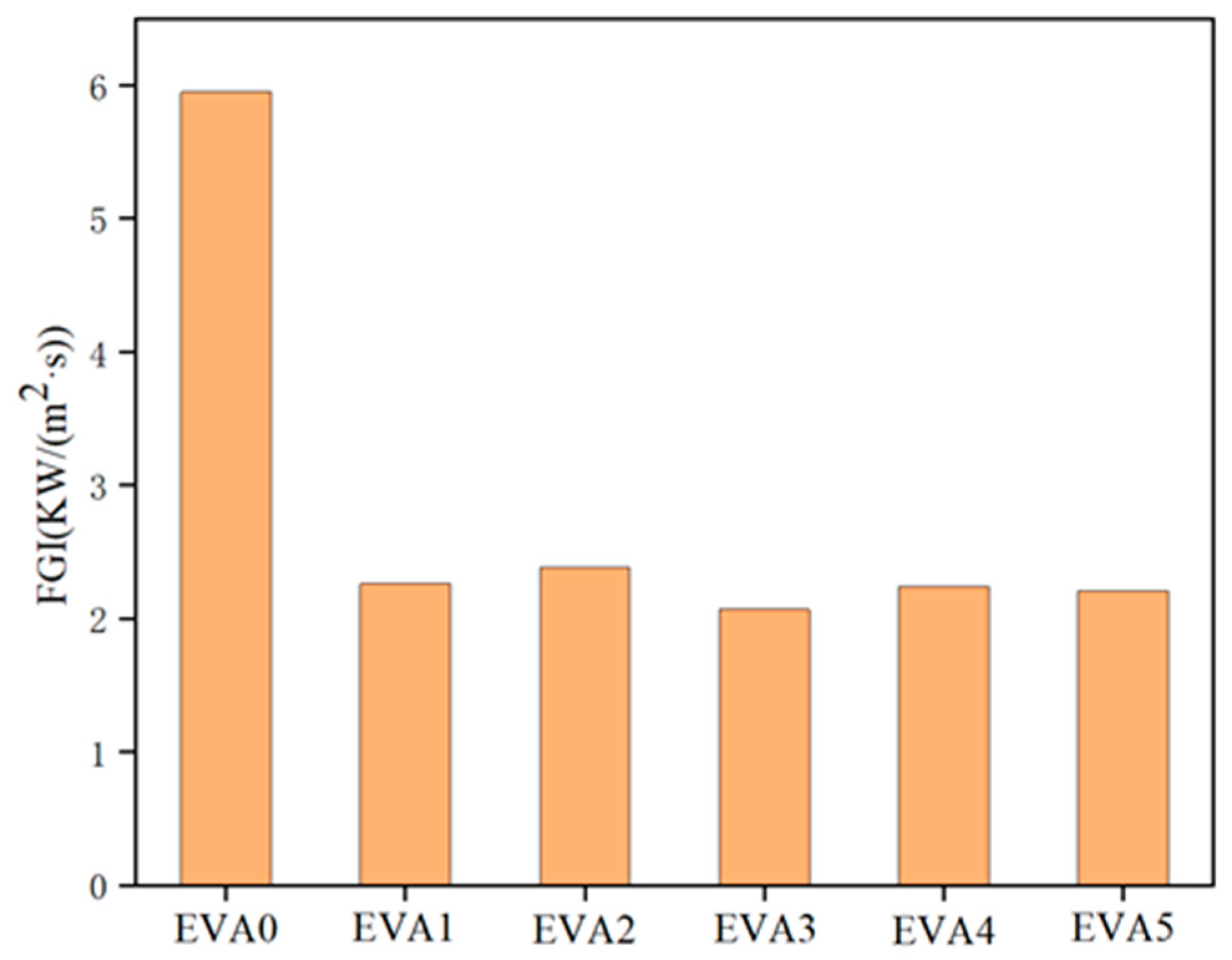
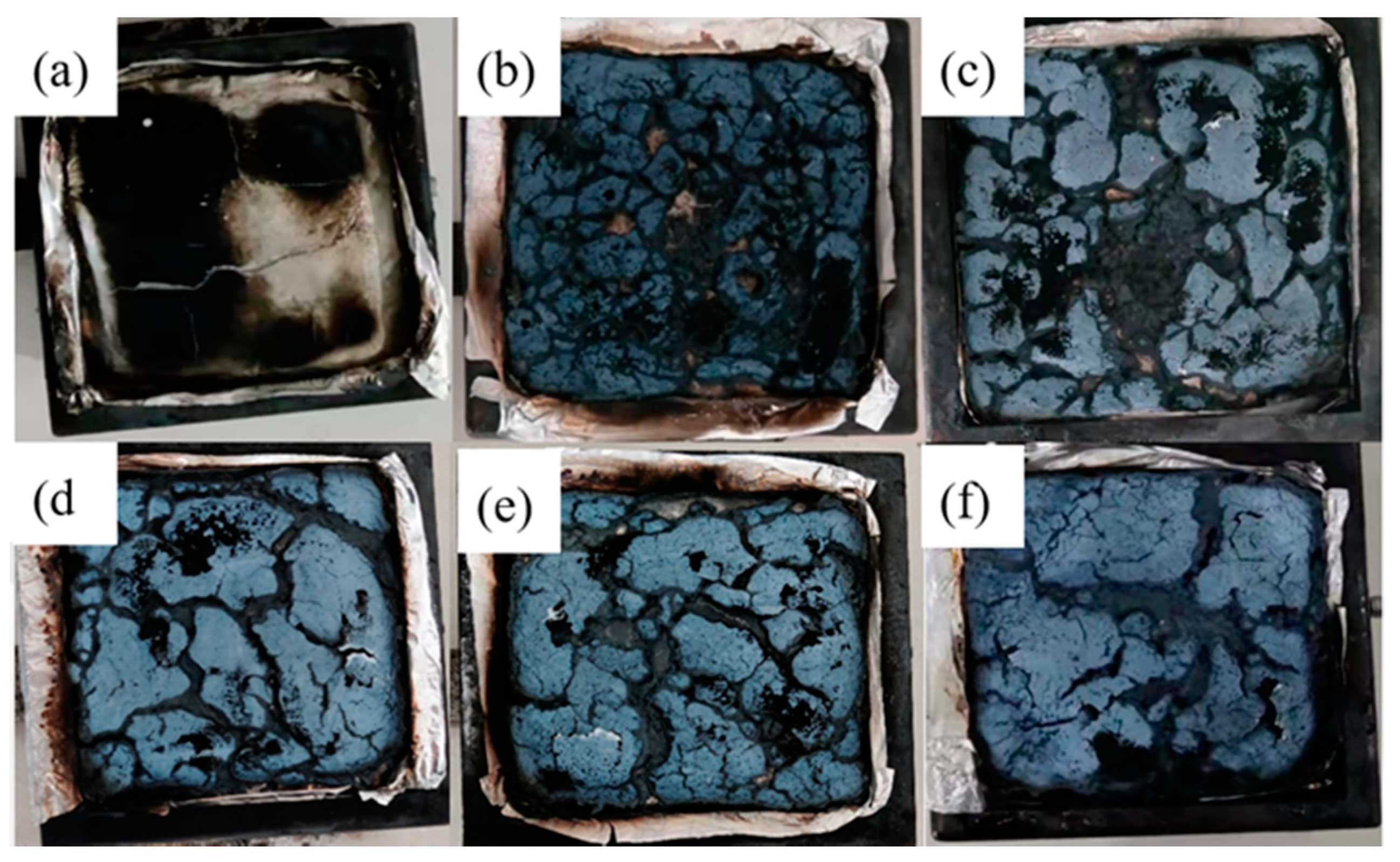
3.5. Combustion Behavior
3.6. Carbon Residue Analysis
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, L.; Miao, Y.; Yan, H.; Li, Z.; Zhou, Y.; Liu, J.; Liu, H. The synergistic effects of boroxo siloxanes with magnesium hydroxide in halogen-free flame retardant EVA/MH blends. Polym. Degrad. Stab. 2013, 98, 868–874. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wilkie, C.A. Thermal stability and flammability characteristics of ethylene vinyl acetate (EVA) composites blended with a phenyl phosphonate-intercalated layered double hydroxide (LDH), melamine polyphosphate and/or boric acid. Polym. Degrad. Stab. 2009, 94, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tang, W.; Sun, J.; Jiang, Y.; Bu, X.; Gu, X. Synergistic effects of modified hydrotalcite on improving the fire resistance of ethylene vinyl acetate containing intumescent flame retardants. Polym. Compos. 2018, 39, 522–528. [Google Scholar] [CrossRef]
- Shen, L.; Shao, C.; Li, R.; Xu, Y. Preparation and characterization of ethylene–vinyl acetate copolymer (EVA)–magnesium hydroxide (MH)–hexaphenoxycyclotriphosphazene (HPCTP) composite flame-retardant materials. Polym. Bull. 2018, 76, 2399–2410. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, K.; Tang, G.; Wang, B.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Synthesis of Co3 (HPO4)2 (OH)2 nanosheets and its synergistic effect with intumescent flame retardants in ethylene-vinyl acetate copolymer. Polym. Compos. 2016, 39, 238–246. [Google Scholar] [CrossRef]
- Oliveira, M.C.C.D.; Diniz Cardoso, A.S.A.; Viana, M.M.; Lins, V.d.F.C. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Chen, Q.; Ling, Q. Synthesis and characterization of two-component acidic ion intercalated layered double hydroxide and its use as a nanoflame-retardant in ethylene vinyl acetate copolymer (EVA). RSC Adv. 2017, 7, 53064–53075. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, A.; Hollingbery, L.; Hull, T.R. Fire Retardancy of Mineral Fillers in EVA Copolymers. In Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science; ACS Publications: Washington, DC, USA, 2012; pp. 97–111. [Google Scholar]
- Wang, Q.; Dai, S.; Xi, Y. Preparation of Magnesia from Hydromagnesite Ore by Calcination. JOM 2021, 73, 856–861. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Shi, T.; Yang, B.; Li, C.; Xu, H.; Yin, W. Preparation, properties and phase transition of mesoporous hydromagnesite with various morphologies from natural magnesite. Powder Technol. 2020, 364, 822–830. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The fire retardant effects of huntite in natural mixtures with hydromagnesite. Polym. Degrad. Stab. 2012, 97, 504–512. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The thermal decomposition of huntite and hydromagnesite—A review. Thermochim. Acta 2010, 509, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.; Yao, D.; Yin, G.-Z.; You, J.; Liu, X.-Q.; Wang, N.; Wang, D.-Y. Surface engineering of magnesium hydroxide via bioinspired iron-loaded polydopamine as green and efficient strategy to epoxy composites with improved flame retardancy and reduced smoke release. React. Funct. Polym. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Ren, M.; Yang, M.; Li, S.; Chen, G.; Yuan, Q. High throughput preparation of magnesium hydroxide flame retardant via microreaction technology. RSC Adv. 2016, 6, 92670–92681. [Google Scholar] [CrossRef]
- Yao, D.; Yin, G.-Z.; Bi, Q.; Yin, X.; Wang, N.; Wang, D.-Y. Basalt Fiber Modified Ethylene Vinyl Acetate/Magnesium Hydroxide Composites with Balanced Flame Retardancy and Improved Mechanical Properties. Polymers 2020, 12, 2107. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhang, X.; Wei, Z.; Chen, J.; Dong, D.; Li, X.; Zhang, L. Effect of particle size and content of magnesium hydroxide on flame retardant properties of asphalt. J. Appl. Polym. Sci. 2013, 129, 2261–2272. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, X.-F.; Chen, J.-Y.; Wang, J.-X.; Zhang, L.-L.; Chen, J.-F. Magnesium hydroxide nanodispersion for polypropylene nanocomposites with high transparency and excellent fire-retardant properties. Polym. Degrad. Stab. 2017, 146, 327–333. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Xu, M.; Wang, L. Highly Efficient Composite Flame Retardants for Improving the Flame Retardancy, Thermal Stability, Smoke Suppression, and Mechanical Properties of EVA. Materials 2020, 13, 1251. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Shi, H.; Zhu, P.; Wei, Y.; Wei, P.; Hao, J. Effect of natural basalt fiber for EVA composites with nickel alginate-brucite based flame retardant on improving fire safety and mechanical properties. Polym. Adv. Technol. 2020, 31, 713–721. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, M.; Liu, N.; Dang, P.; Xu, Y.; Chen, X.; Wang, Z.; He, J. Combustion characteristics and thermal properties of high-density polyethylene/ethylene vinyl-acetate copolymer blends containing magnesium hydroxide. J. Thermoplast. Compos. Mater. 2016, 30, 1393–1413. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Liu, B.-W.; Wang, X.-L.; Chen, L.; Wang, X.-L.; Wang, Y.-Z. A flame-retardant-free and thermo-cross-linkable copolyester: Flame-retardant and anti-dripping mode of action. Polymer 2014, 55, 2394–2403. [Google Scholar] [CrossRef]
- Gui, H.; Zhang, X.; Dong, W.; Wang, Q.; Gao, J.; Song, Z.; Lai, J.; Liu, Y.; Huang, F.; Qiao, J. Flame retardant synergism of rubber and Mg(OH)2 in EVA composites. Polymer 2007, 48, 2537–2541. [Google Scholar] [CrossRef]
- Ai, L.; Chen, S.; Zeng, J.; Yang, L.; Liu, P. Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide. ACS Omega 2019, 4, 3314–3321. [Google Scholar] [CrossRef]
- Piscitelli, F.; Scamardella, A.M.; Romeo, V.; Lavorgna, M.; Barra, G.; Amendola, E. Epoxy composites based on amino-silylated MMT: The role of interfaces and clay morphology. J. Appl. Polym. Sci. 2012, 124, 616–628. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Zhang, P.; Song, L.; Yang, H.; Hu, Y. Covalent functionalization of graphene with organosilane and its use as a reinforcement in epoxy composites. Compos. Sci. Technol. 2012, 72, 737–743. [Google Scholar] [CrossRef]
- Yang, H.; Ye, L.; Gong, J.; Li, M.; Jiang, Z.; Wen, X.; Chen, H.; Tian, N.; Tang, T. Simultaneously improving the mechanical properties and flame retardancy of polypropylene using functionalized carbon nanotubes by covalently wrapping flame retardants followed by linking polypropylene. Mater. Chem. Front. 2017, 1, 716–726. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.; Song, L.; Zhan, J.; He, Q. Mechanical properties, fire performance and thermal stability of magnesium hydroxide sulfate hydrate whiskers flame retardant silicone rubber. J. Mater. Sci. 2008, 43, 1057–1062. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, X.; Li, H.; Gao, J.; Zeng, X.; Liao, X. Facile fabrication of a novel polyborosiloxane-decorated layered double hydroxide for remarkably reducing fire hazard of silicone rubber. Compos. Part B Eng. 2019, 175, 107068. [Google Scholar] [CrossRef]
- Lou, F.; Wu, K.; Wang, Q.; Qian, Z.; Li, S.; Guo, W. Improved Flame-Retardant and Ceramifiable Properties of EVA Composites by Combination of Ammonium Polyphosphate and Aluminum Hydroxide. Polymers 2019, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yu, J.; Guo, S. Thermal oxidative degradation kinetics of PP and PP/mg (OH)2 flame-retardant composites. J. Appl. Polym. Sci. 2006, 103, 1978–1984. [Google Scholar] [CrossRef]
- Zhang, S.; Bu, X.; Gu, X.; Sun, J.; Li, H.; Tang, W. Improving the mechanical properties and flame retardancy of ethylene-vinyl acetate copolymer by introducing bis [3-(triethoxysilyl) propyl] tetrasulfide modified magnesium hydroxide. Surf. Interface Anal. 2017, 49, 607–614. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Sun, J.; Gu, X.; Li, H.; Zhang, S. Improving flame retardant and mechanical properties of ethylene–vinyl acetate by cured compound silicone decorated magnesium hydroxide. J. Mater. Sci. 2022, 57, 2243–2256. [Google Scholar] [CrossRef]





| Sample | EVA (wt%) | MH (wt%) | NaOH Concentration (mol/L) |
|---|---|---|---|
| EVA0 | 100 | 0 | - |
| EVA1 | 50 | 50 | 0 |
| EVA2 | 50 | 50 | 0.5 |
| EVA3 | 50 | 50 | 1.0 |
| EVA4 | 50 | 50 | 1.5 |
| EVA5 | 50 | 50 | 2.0 |
| Sample | T−5% (°C) | T−50% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Residue at 800 °C (wt%) |
|---|---|---|---|---|---|---|
| Hydromagnesite | 233 | 481 | 240 | 450 | 618 | 52.82 |
| NaOH Concentration/mol·L−1 | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
|---|---|---|---|---|---|
| I(001)/I(101) | 1.05 | 1.08 | 1.21 | 1.32 | 1.18 |
| Mean particle sizes/μm | 2.60 | 3.45 | 3.66 | 3.72 | 3.93 |
| Samples | T−5% a (°C) | Tmax1 b (°C) | Tmax2 c (°C) | Residue d (wt%) |
|---|---|---|---|---|
| EVA0 | 331.8 | 348.9 | 459.9 | 0 |
| EVA1 | 336.1 | 353.5 | 460.7 | 35.0 |
| EVA2 | 333.7 | 348.0 | 462.1 | 35.4 |
| EVA3 | 333.2 | 352.1 | 462.4 | 35.5 |
| EVA4 | 333.4 | 349.7 | 460.2 | 35.8 |
| EVA5 | 334.2 | 354.7 | 462.8 | 35.6 |
| Samples | TTI a (s) | pHRR b (kW/m2) | TpHRR c (s) | THR d (MJ/m2) | TSP e (m2) | FGI f (KW/(m2·s)) | Residue g (wt%) |
|---|---|---|---|---|---|---|---|
| EVA0 | 50 | 559 | 100 | 82 | 4.9 | 5.95 | 5.3 |
| EVA1 | 83 | 381 | 169 | 65 | 1.4 | 2.26 | 32.3 |
| EVA2 | 82 | 374 | 157 | 74 | 5.3 | 2.38 | 34.6 |
| EVA3 | 77 | 349 | 168 | 65 | 2.9 | 2.07 | 37.0 |
| EVA4 | 94 | 378 | 169 | 68 | 6.7 | 2.24 | 35.4 |
| EVA5 | 76 | 359 | 169 | 70 | 8.5 | 2.21 | 37.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, L.-L.; Zhao, P.-C.; Liu, Z.-Q.; Wu, Q.-S.; Yan, D.-Q.; Li, Y.-L.; Chen, Y.-N.; Li, J.-S. Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA. Polymers 2022, 14, 1567. https://doi.org/10.3390/polym14081567
Jiao L-L, Zhao P-C, Liu Z-Q, Wu Q-S, Yan D-Q, Li Y-L, Chen Y-N, Li J-S. Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA. Polymers. 2022; 14(8):1567. https://doi.org/10.3390/polym14081567
Chicago/Turabian StyleJiao, Ling-Li, Peng-Cheng Zhao, Zhi-Qi Liu, Qing-Shan Wu, Dong-Qiang Yan, Yi-Lan Li, Yu-Nan Chen, and Ji-Sheng Li. 2022. "Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA" Polymers 14, no. 8: 1567. https://doi.org/10.3390/polym14081567
APA StyleJiao, L.-L., Zhao, P.-C., Liu, Z.-Q., Wu, Q.-S., Yan, D.-Q., Li, Y.-L., Chen, Y.-N., & Li, J.-S. (2022). Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA. Polymers, 14(8), 1567. https://doi.org/10.3390/polym14081567






