1. Introduction
A variety of reports have emerged in recent decades documenting the use of sterically stabilized colloidal dispersions of conductive polymers, which are much more processable than traditional ones [
1]. In the case of dispersion polymerization, and when the reaction medium contains a soluble stabilizer, the macroscopic aggregation and precipitation of the resulting conductive polymers can be avoided, leading to submicrometer well-dispersed particles [
2]. In this context, several water-soluble polymers, such as cellulosic derivatives [
3,
4], poly(vinyl alcohol) [
5], poly(ethylene oxide) [
6], and poly(methyl vinyl ether) [
7], were used as steric stabilizers to overcome the processing hardness of conductive polymers, which is related to their very low solubility in common solvents and/or infusibility, limiting their applications.
Polyaniline (PANI) is among the most studied and developed organic conductive polymers. Its large-scale applications stem from its good redox exchange properties, its simple and cheap preparation methods as well as its great environmental stability [
8,
9]. However, as with other conductive polymers, polyaniline is very difficult to be processed and the preparation of colloidal PANI-based dispersions seems to be a promising way to overcome such a problem [
10]. Several workers have taken advantage of this approach to produce processable dispersions including PANI/poly(methyl methacrylate) particles stabilized in hydrocarbons [
11], PANI stabilized with partially hydrolyzed poly(vinyl alcohol) [
12] or with poly(N-vinylpyrrolidone) [
13], and PANI/hydroxypropyl cellulose [
14].
Numerous studies were also conducted to exploit biopolymers issued from renewable natural and agricultural resources, to develop novel bio-composites with conductive polymers, thus leading to entirely new directions in the research of advanced functional materials for a sustainable future [
14,
15]. In such a case, the water-soluble or water-dispersible biopolymers bind PANI chains and lead to stabilized colloids [
16,
17,
18]. In this context, the “intelligent behavior” of PANI colloids prepared with chitosan or poly(N-isopropyl acrylamide) [
19,
20], cellulose derivatives [
21], nanocellulose [
22] and pectin [
23,
24], as steric stabilizers, was reported.
The resulting biocomposites could not only improve the processability of PANI, but also enhance its biocompatibility [
25,
26,
27]. For example, PANI-based materials have been used for the detection and analysis of bio-molecules (enzymes, antibodies, DNA, proteins, etc.), making them suitable candidates for biomedical applications [
25,
27,
28]. On the other hand, even if in vivo studies have shown that the emeraldine base form of PANI with electroactive properties [
29] did not provoke inflammatory responses in a rodent model, suggesting good tolerance and bio-/histo-compatibility [
30], risks may arise from the presence of polymerization by-products (benzidine, aniline dimers, and oligomers). Such by-products, indeed, can be cytotoxic, also with carcinogenic effects. All in all, although their presence can be avoided by suitable washing steps [
31], the use of PANI-based materials still deserves many precautions.
In our work, we have used starch, which is the most abundant polysaccharide produced by higher plants, and it is extracted intracellularly in the form of granules of 2–100 µm in diameter [
32]. In general, starches are semi-crystalline polymers made of a mixture of amylose and amylopectin chains, with a crystallinity of about 20–45% [
33].
Starch was used by several authors to develop stabilized latexes [
34,
35,
36]. In the work of Pradeep et al. [
34], in particular, flexible semiconducting thin films from pure vulcanized natural rubber latex with enhanced electrical conductivity were produced. Other starch-stabilized colloids were also developed with bimetallic nanoparticles to improve the stability and reactivity of nanoparticles [
37,
38,
39], PLGA (polylactic-co-glycolic acid) nanospheres [
40], and PMMA (polymethylmethacrylate) lattices [
41].
The usage of starch as a non-toxic and biodegradable biopolymer to elaborate PANI-based composites has led to the formation of advanced functional materials for various applications (water remediation, energy generation, and storage, electrochemical, medical and biomedical applications, etc.) [
15,
42,
43,
44,
45,
46]. In the same framework, we have attempted to elaborate water dispersible and processable polyaniline using starch as a steric stabilizer. The oxidative chemical polymerization of polyaniline was performed in an aqueous solution containing various ratios of aniline to starch (
w/
w). The obtained PANI/starch biocomposites were characterized by spectroscopic, microscopic, thermal, and electrochemical techniques, in order to assess the most promising PANI: starch ratio. Moreover, computational calculations based on DFT were carried out to find missing information about the interactions between starch–aniline and starch–polyaniline during the synthesis and in the final form of the biocomposites.
2. Materials and Methods
2.1. Synthesis of PANI/Starch Biocomposites
Polyaniline/starch materials (
Table 1) were prepared by in situ polymerization of aniline in the presence of aqueous starch solutions under magnetic stirring. In a typical procedure [
47], in a three-necked flask, aniline was added to the aqueous dispersions of starch at various ratios of aniline: starch (
w/
w) at room temperature (20 °C) and stirred for 4 h. The chemical polymerization of aniline was carried out at low temperatures in an ice bath (T~0–4 °C) using an APS (ammonium persulfate) oxidative solution. The mixture was maintained under magnetic stirring for 3 h, then left without stirring for 48 h to allow the majority of aniline to react, after which the dark powder obtained was filtered, washed with water and acetone to remove low-molecular-weight reaction by-products, i.e., aniline dimers and oligomers, then dried at 40 °C for 48 h.
2.2. Characterization
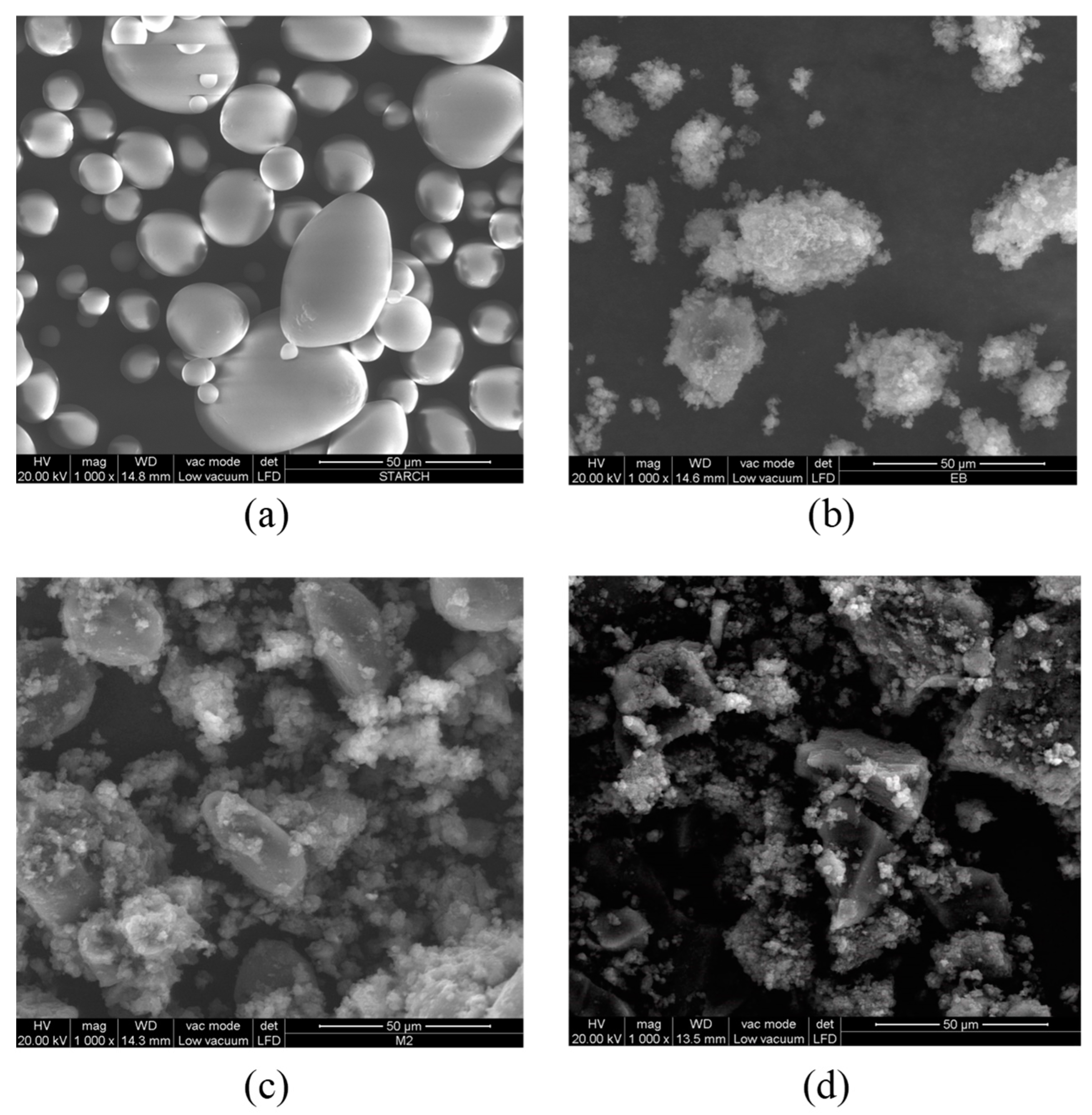
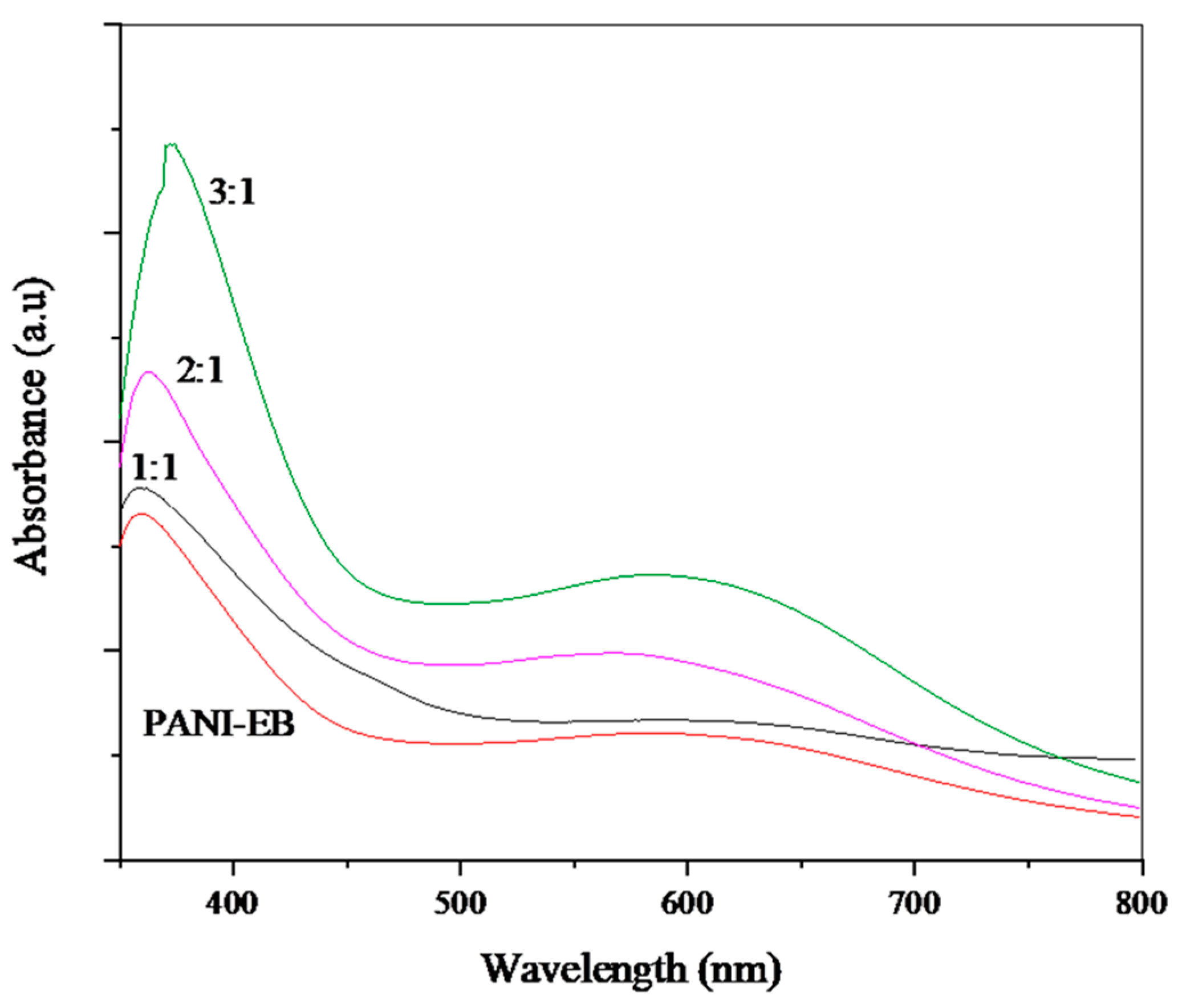
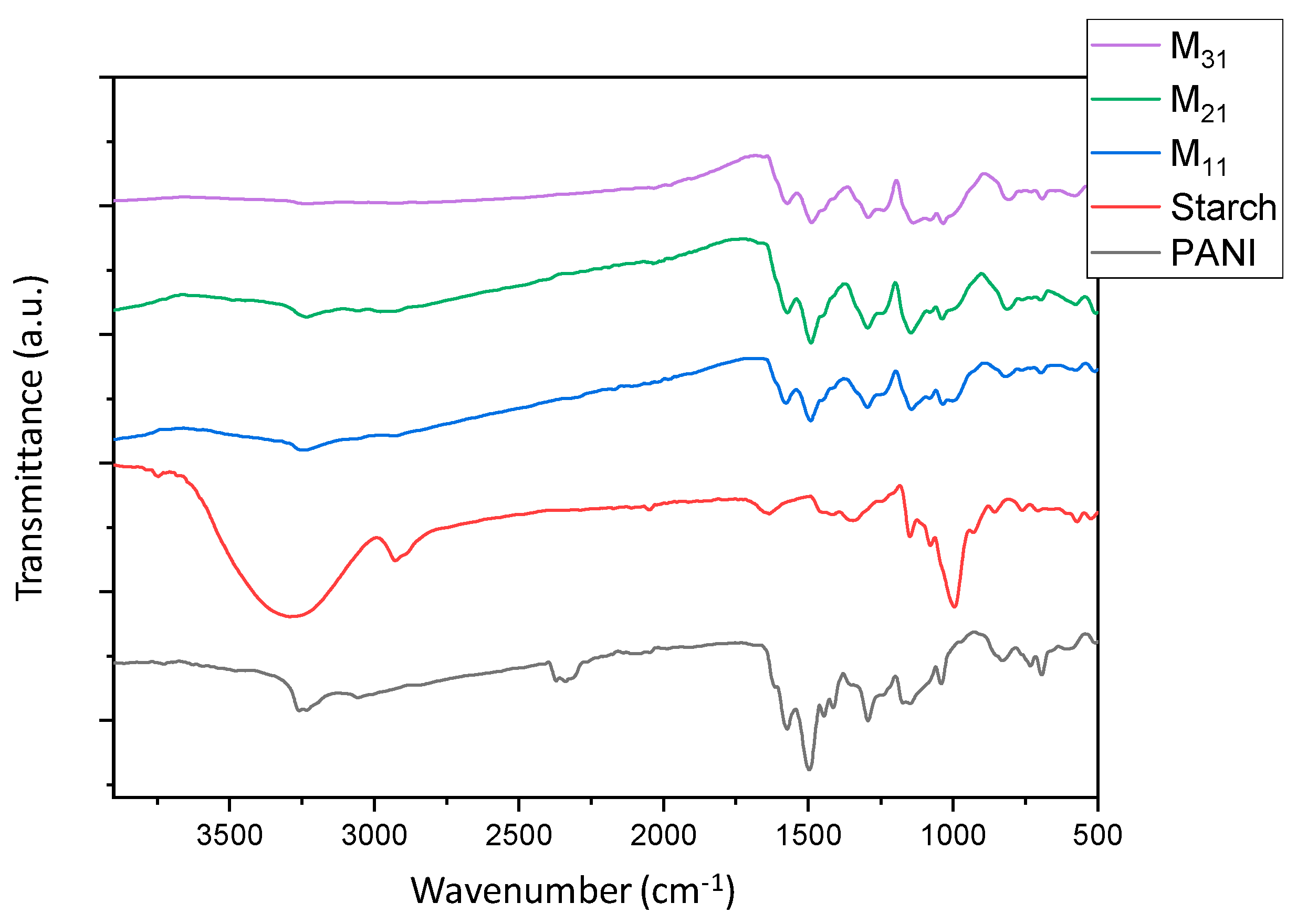
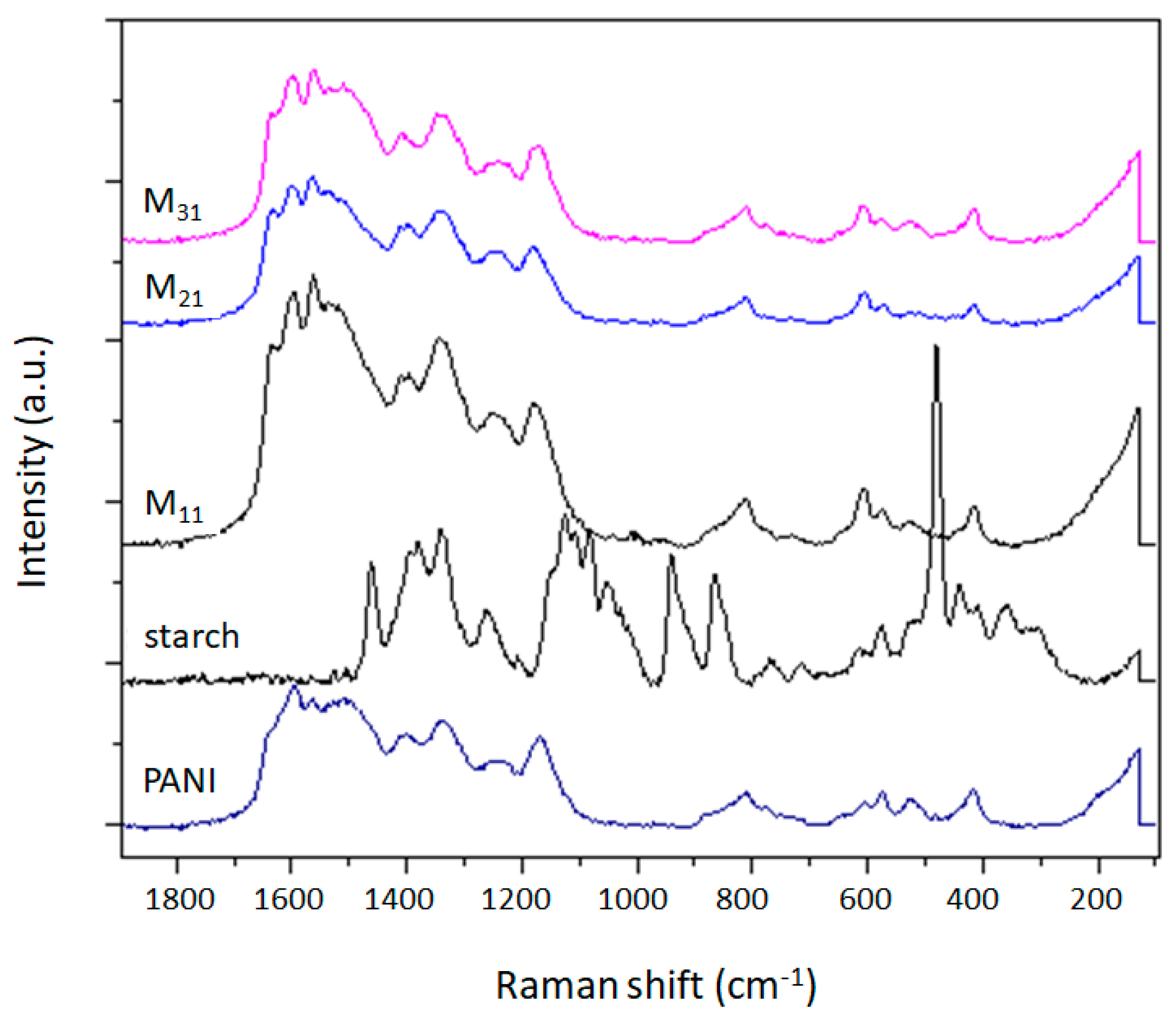
Structural characterization of samples was carried out by FTIR spectroscopy using a Nicolet 5700 spectrometer (Thermo Fisher, Waltham, MA, USA), equipped with a diamond ATR sampling accessory, on the powdered material, UV-Visible spectroscopy using a Cary 5000 UV−vis−NIR spectrometer (Varian, Palo Alto, CA, USA) on solutions prepared by dissolving 2 mg in 10 mL of DMF, and RAMAN spectroscopy using a DXR2 Raman spectrometer (Thermo Scientific, Madison, WI, USA), operating at 532 nm. The solubility tests were performed at different temperatures (20, 40, 50, 60, 80 °C) by dispersing the same weight of all materials in 10 mL of solvents, to obtain a dilute concentration (0.2% w/v). The solvents used are water, acetone, methanol, ethanol, glycerol, ethyl acetate, chloroform, toluene, hexane, cyclohexane, DMSO, and DMF. While colorless supernatants were obtained with insoluble mixtures, the presence of residual solids, in the case of partially soluble mixtures, was observed by a lens.
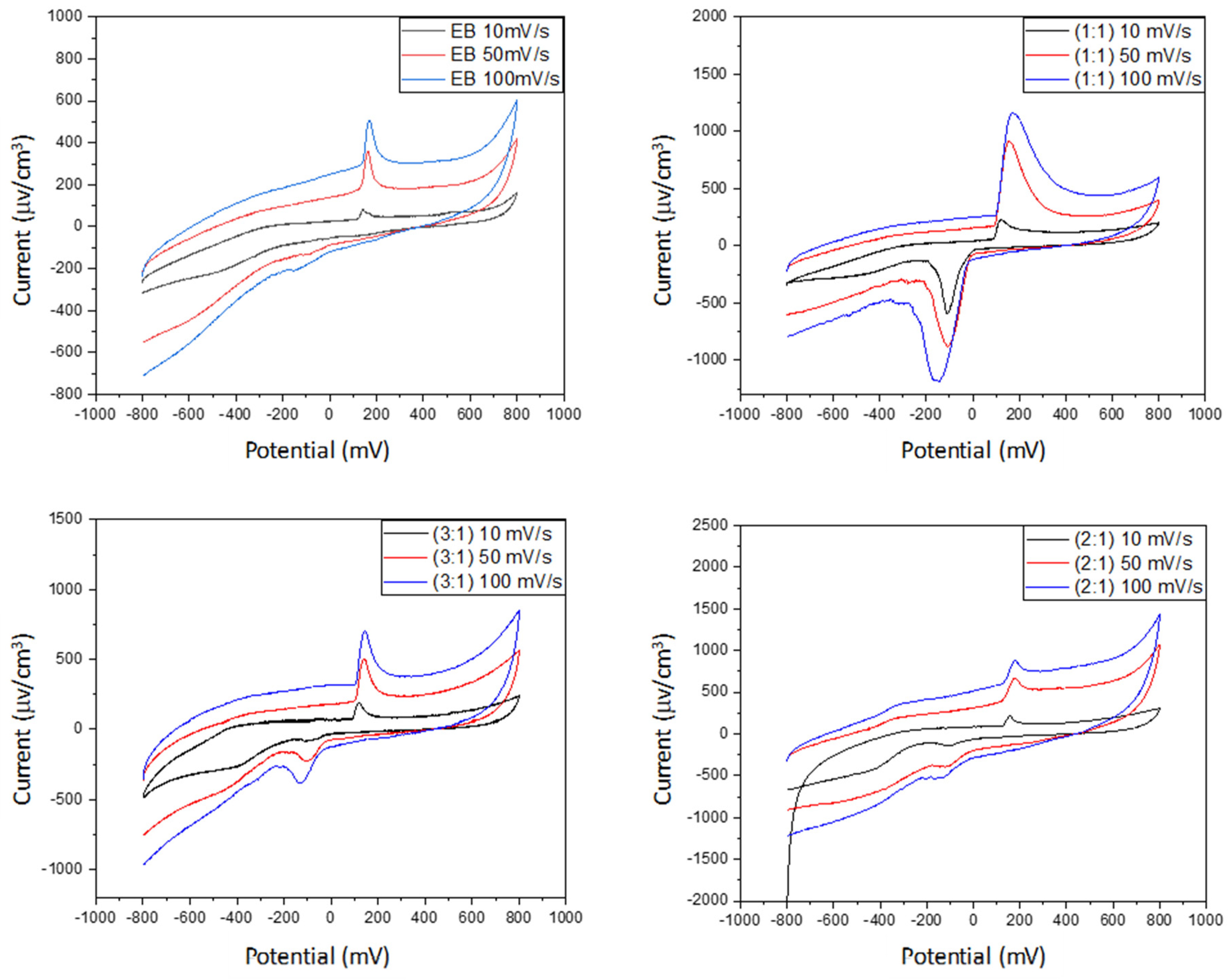
Homopolymers and biocomposites were subjected to laser granulometry analysis by using a CILAS 1190 analyzer (CPS Us, Inc., Madison, WI, USA). Thermal gravimetric analyses (with a TGA Q5000 instrument, TA Instruments, Hüllhorst, Germany) were performed until 700 °C (10 °C/min), while DSC (with a TA instrument Q20) was set from room temperature to 300 °C at 10 °C/min, under a nitrogen atmosphere; thermodynamic parameters were calculated by using the software TA Universal Analysis. X-ray diffraction analysis was performed using CuKα radiation (λ = 1.54 Å) (X’Pert3 Powder apparatus) from 2θ = 10° to 60°, at a scan rate of 0.02 s−1; the peak crystalline areas were determined by using the X’pert HighScore software. SEM images of the composites were obtained after metallization with sputter quorum Q 150R E settings using Quanta 200 apparatus (FEI, Hillsboro, OR, USA). Cyclic voltammetry tests (with PGZ 301 potentiometer, Radiometer Analytical SAS, Lyon, France) were examined in a three-electrode system constituted of a reference electrode (Ag/AgCl), an auxiliary platinum electrode, and a carbon electrode as the working electrode. The electrolyte was a KCl solution (1 M) and the voltammograms were recorded in a voltage window between −0.8 and +0.8 mV at various scan rates (10, 50, and 100 mV s−1).
3. Computational Study
Geometric optimization of the molecules was performed with the Dmol3 module [
48] utilizing a modeling package created by Accelrys Incorporation (Cambridge, UK): Materials Studio 2017TM [
49], B3LYP functional [
50], and DNP basis set (4.4 basis file) [
51].
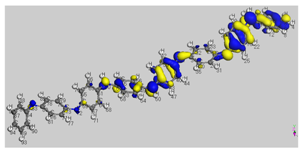
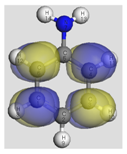
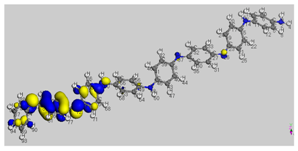
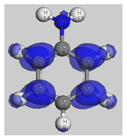
The HOMO (Highest Occupied Molecular Orbital) and LUMO (Lowest Unoccupied Molecular Orbital) frontier molecular orbitals (FMOs) were investigated [
52]. The high value of the energy ξ
HOMO justifies the inclination to transfer electrons to a suitable acceptor molecule. In contrast, the low value of the energy ξ
LUMO justifies the molecule’s capacity to accept electrons from donor molecules [
53].
The electronic chemical potential (μ) and global hardness (η) were determined using the ξ
HOMO and ξ
LUMO energies [
54,
55,
56]:
The chemical potential µ is related to the electrophilicity index ω by the following relationship [
57]:
ω indicates an electrophile’s capacity to gain an extra electrical charge [
58].
The optimal fraction of transferred electrons (ΔN) is given by [
59]:
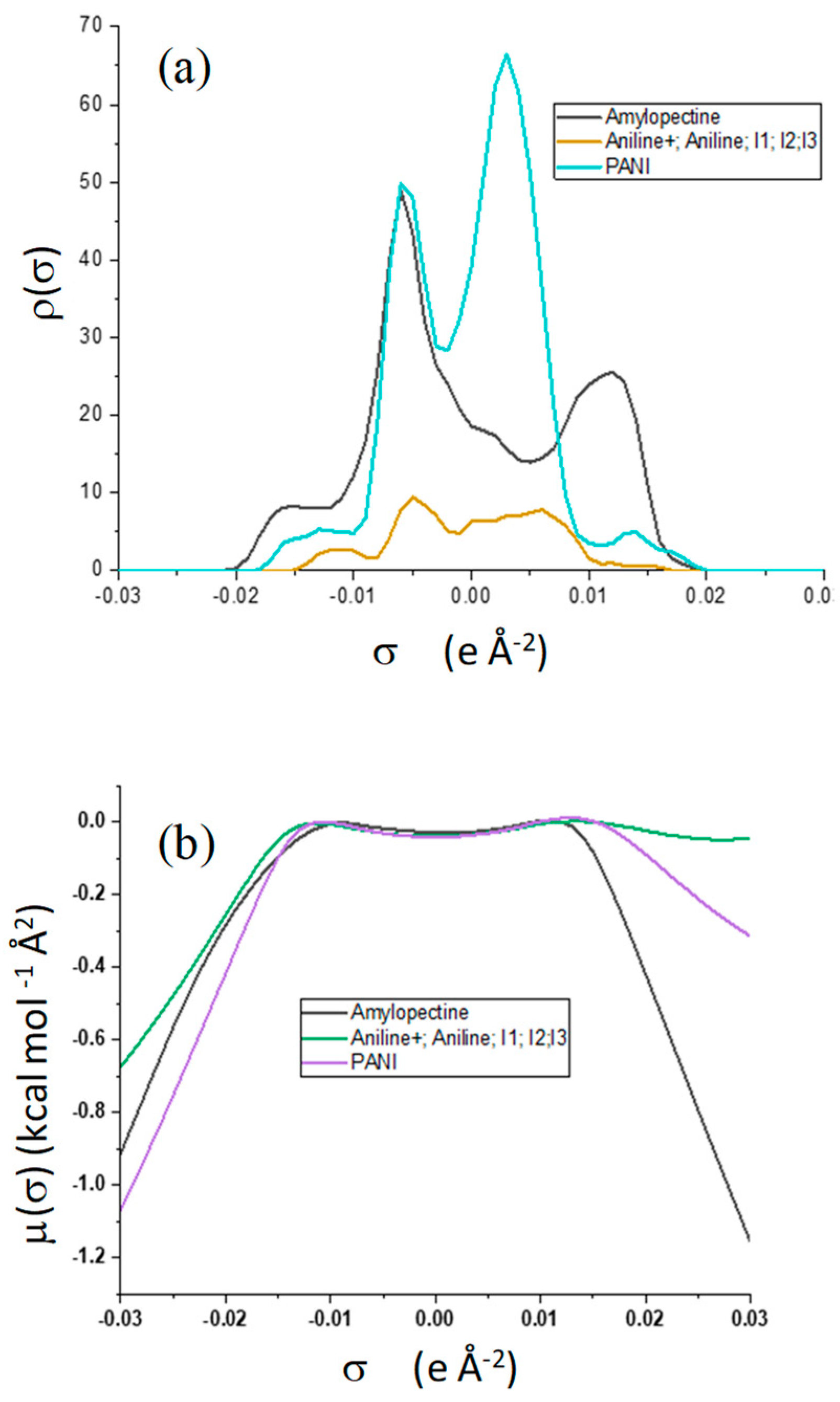
COSMO-RS (Conductor-like Screening Model for Real Solvents) is a thermodynamic-based quantum chemistry technique for determining chemical potentials in solutions [
60]. For each species in the solution, this technique can estimate sigma charge densities and chemical potentials. The computation is divided into two parts: first, the molecule was geometrically optimized; then, using the COSMOtherm program [
61] (p. 2), sigma profiles and sigma potentials were computed using the acquired cosmo-files.
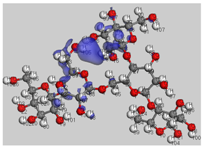
Intermolecular interactions may be calculated using the Atoms in Molecule (AIM) method. The main goal of AIM is to examine the character and strength of the bonding interaction in molecular systems by using the electron density ρ(r) of the molecules as a tool. Based on the second derivative, ∇^2 ρ(r) sign [
62,
63], the kind of chemical bonds may be identified at the bond critical point (BCP) where it is minimum. As a result, if the ρ value is large and ∇^2 ρ(r) is negative, the bond is covalent (polar). On the other hand, positive ∇^2 ρ(r) indicates that the kinetic energy G(r) is greater than the potential energy V(r) [
64]. The |V|/G ratio indicates that the system’s ability to group electrons, V(r), and its ability to dilute them through electronic mobility, G(r), compete within the system. As a result, the ratio |V|/G > 1 indicates that an excess of electric charges is utilized to generate the interaction, whereas |V|/G < 1 indicates the opposite. The total energy density, H(r) = V(r) + G(r), in the first situation, has a negative value, whereas in the second case, it has a positive value. Thus, the categorization of interactions is based on the values of that indicator: (i) pure interactions with closed layers, |V|/G < 1; (ii) interactions with closed layers, 1 < |V|/G < 2; and (iii) interactions with the shared layer, |V|/G > 2. The first two forms of interaction are ascribed to hydrogen bonding, whereas the third type is a covalent interaction. Furthermore, positive, ∇^2 ρ(r), and H values indicate electrostatic contact, whereas negative values indicate a covalent bond. A partly covalent bond is defined as a positive ∇^2 ρ(r) combined with a negative H value [
65].
The Amsterdam Density Functional (ADF) program [
66,
67] was used to perform AIM calculations. For molecular structure optimization, the DFT method was employed. The AIM research [
67,
68] used the def-TZVP basis set to describe exchange correlation effects between these variables (Becke 3-Parameter, Lee, Yang, and Parr).
5. Conclusions
The results of this work demonstrate a facile method for the synthesis of polyaniline colloids. In particular, potato starch was employed as a steric stabilizer to obtain stable dispersions of polyaniline (emeraldine base). Polyaniline/starch biocomposites with various aniline/starch ratios were thus synthesized via an oxidative polymerization and characterized. To evaluate the effect of aniline/starch ratios variation, several tests (UV-vis, FTIR-ATR, Raman, XRD, TGA, DSC), as well as computational calculation (DFT, COSMO-RS, AIM), were performed. This study demonstrates the possibility of using starch as a steric stabilizer, and so to empower the processability of the polyaniline by improving the solubility in DMSO and DMF and the dispersion behavior in water, as well as in glycerol and chloroform, at higher temperatures.
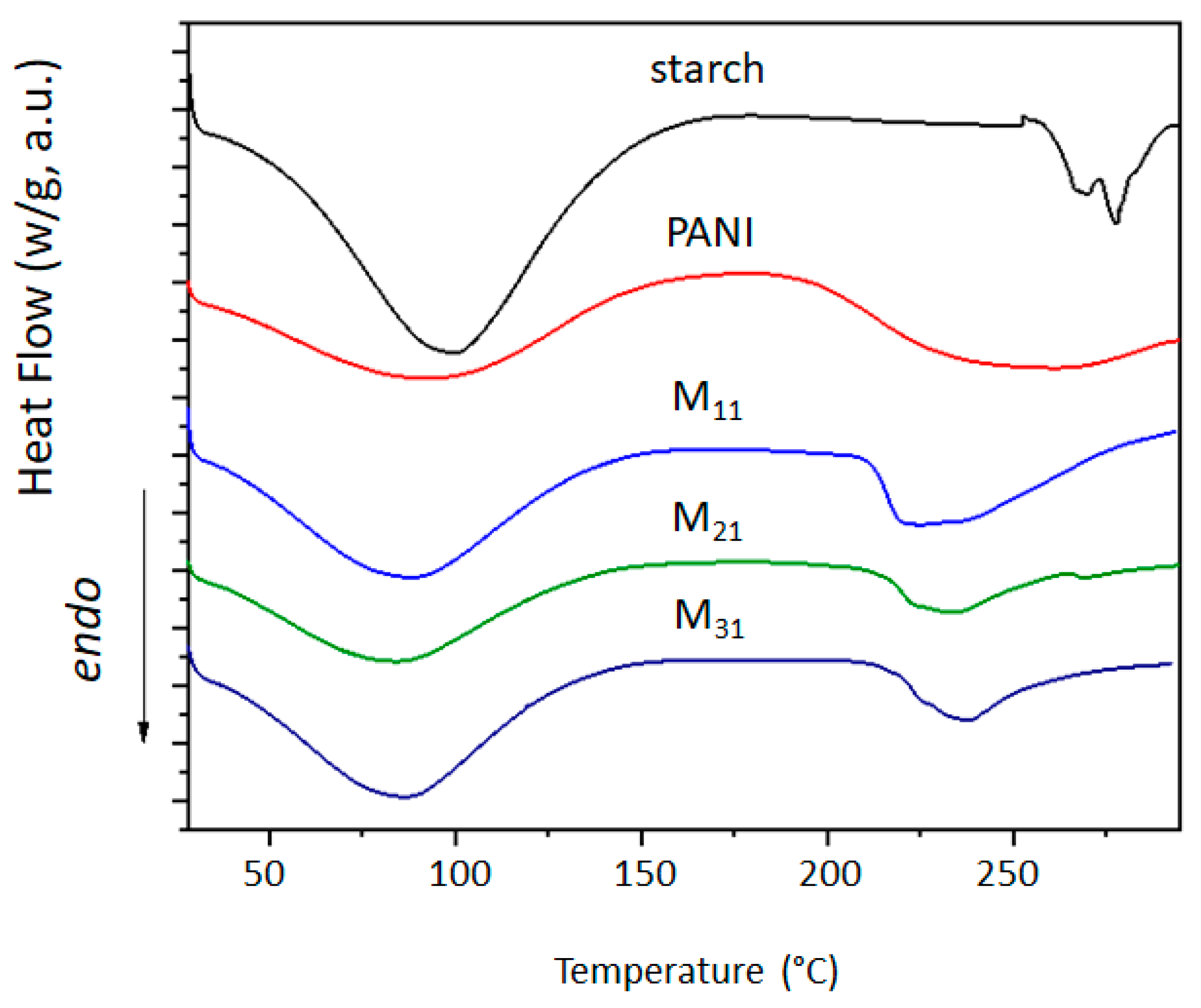
The growth of polyaniline is observed on the starch particles’ surfaces. The particle dimension and the degree of crystallinity appear to be strongly affected in the composite material, as demonstrated by the formation of smaller particles and the loss of the original starch crystalline lattice. Although less thermally stable, M11 appears to be the best in terms of morphology and retention of crystallinity degree. The cyclic voltammetry test shows better electro-activity in terms of the electron exchange and capacitive behavior of the composite materials, with the ratio of 1:1 being the best composition among these materials. Finally, the strong interactions between the PANI and amylopectin (the main starch component) were investigated by the DFT, COSMO-RS, and AIM methods, demonstrating their purely physical nature.
These results attest that the prepared composites as promising versatile materials, which are expected to broaden the electrochemical applications of PANI.