Facile Synthesis of Hydrogel-Based Ion-Exchange Resins for Nitrite/Nitrate Removal and Studies of Adsorption Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
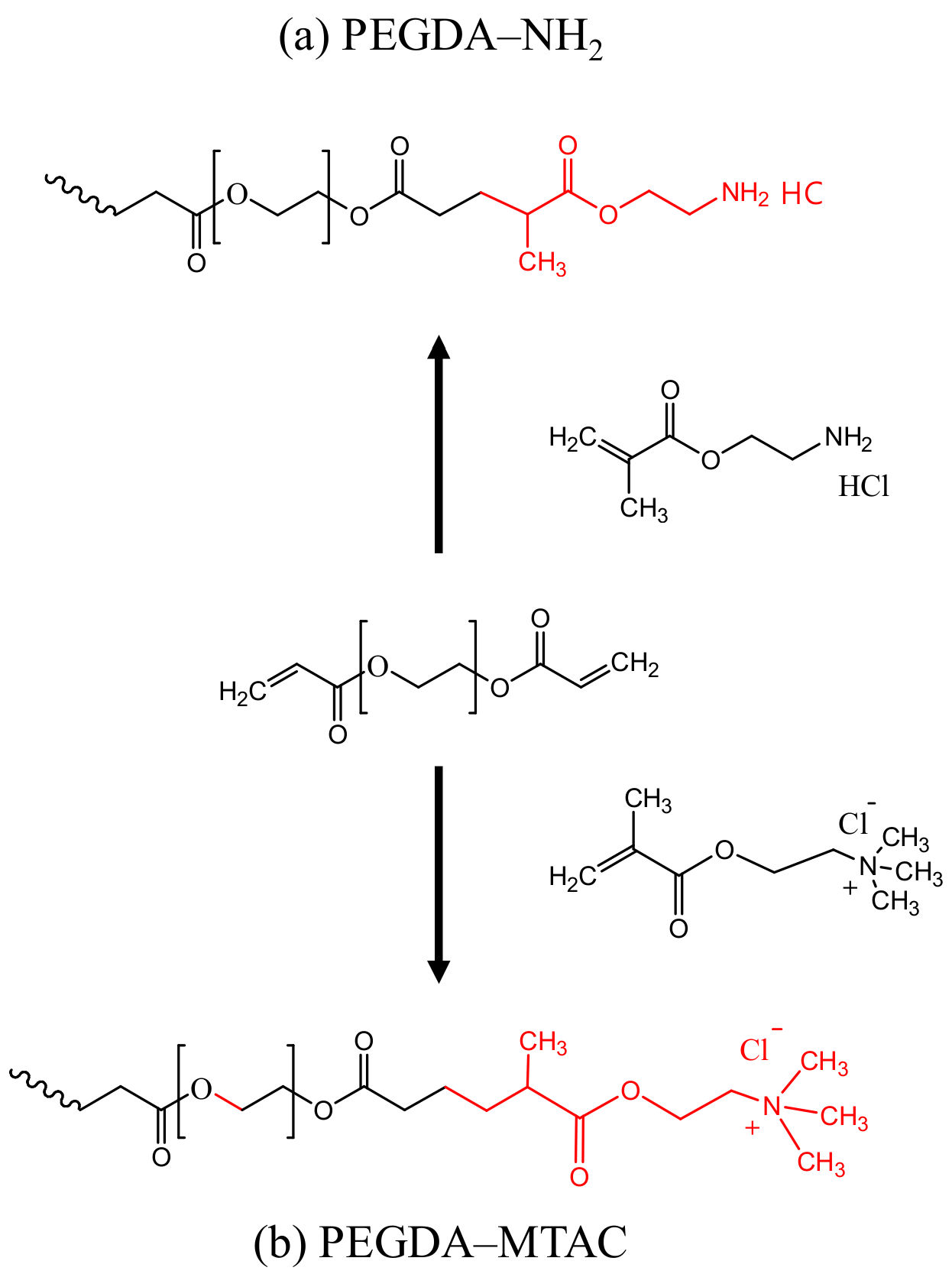
2.2. Preparation of PEGDA/MTAC and PEGDA/AMHC Composites
2.3. Characteristics Analysis
2.4. Batch Experiments
2.4.1. Batch Experiment I
2.4.2. Batch Experiment II
2.5. Column Experiments
3. Results and Discussions
3.1. Characteristics Analysis
3.1.1. Zeta Potential Analysis
3.1.2. FT-IR Spectra Analysis
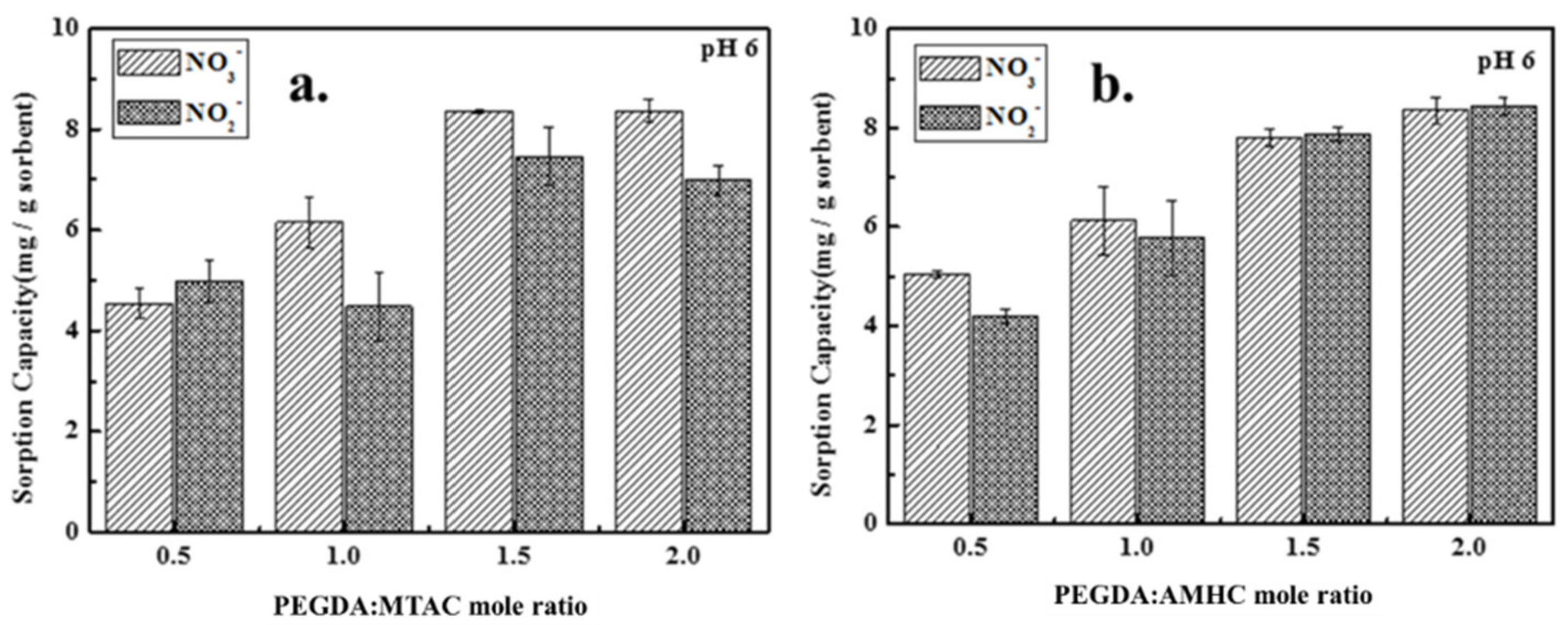
3.1.3. Effect of Modifier Dose
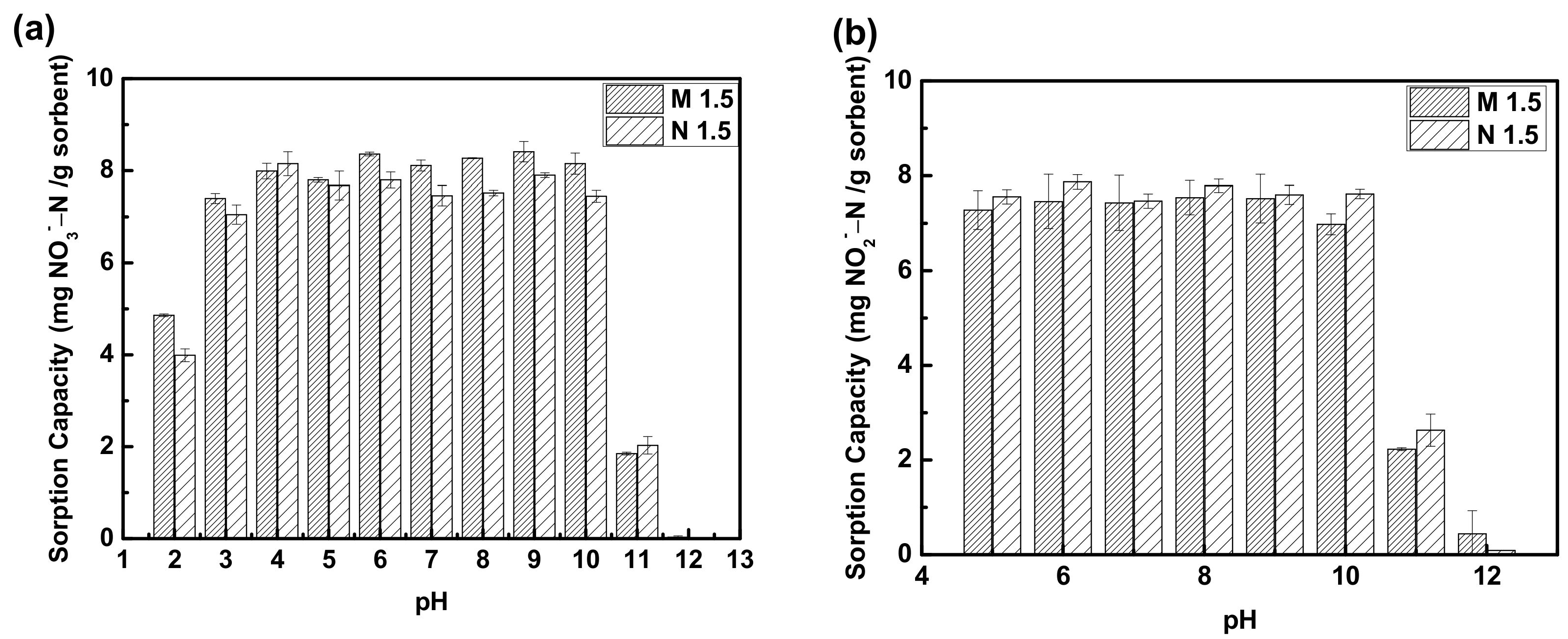
3.1.4. Effect of pH
3.1.5. Effect of Temperature
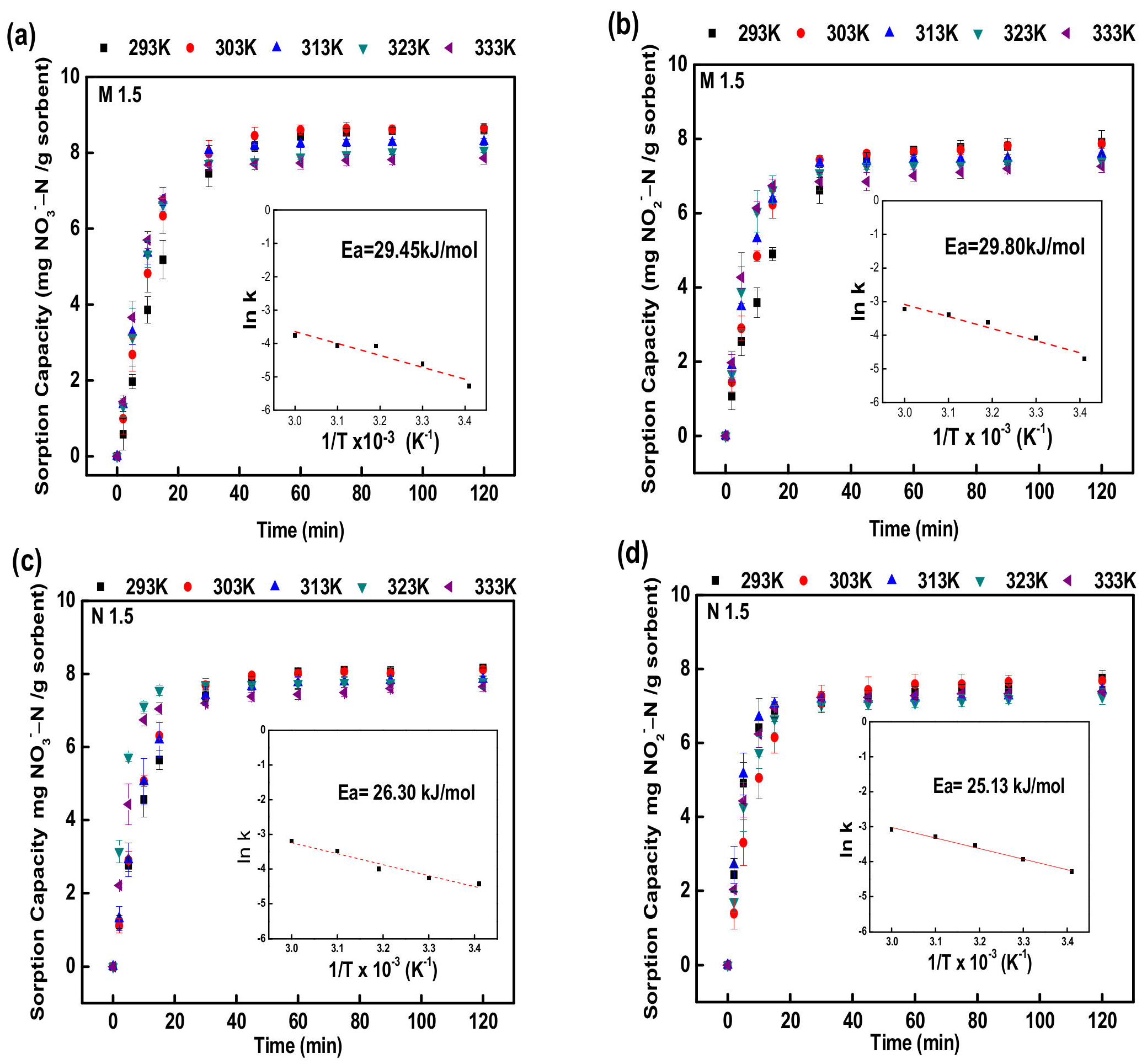
3.1.6. Adsorption Kinetics and Thermodynamics
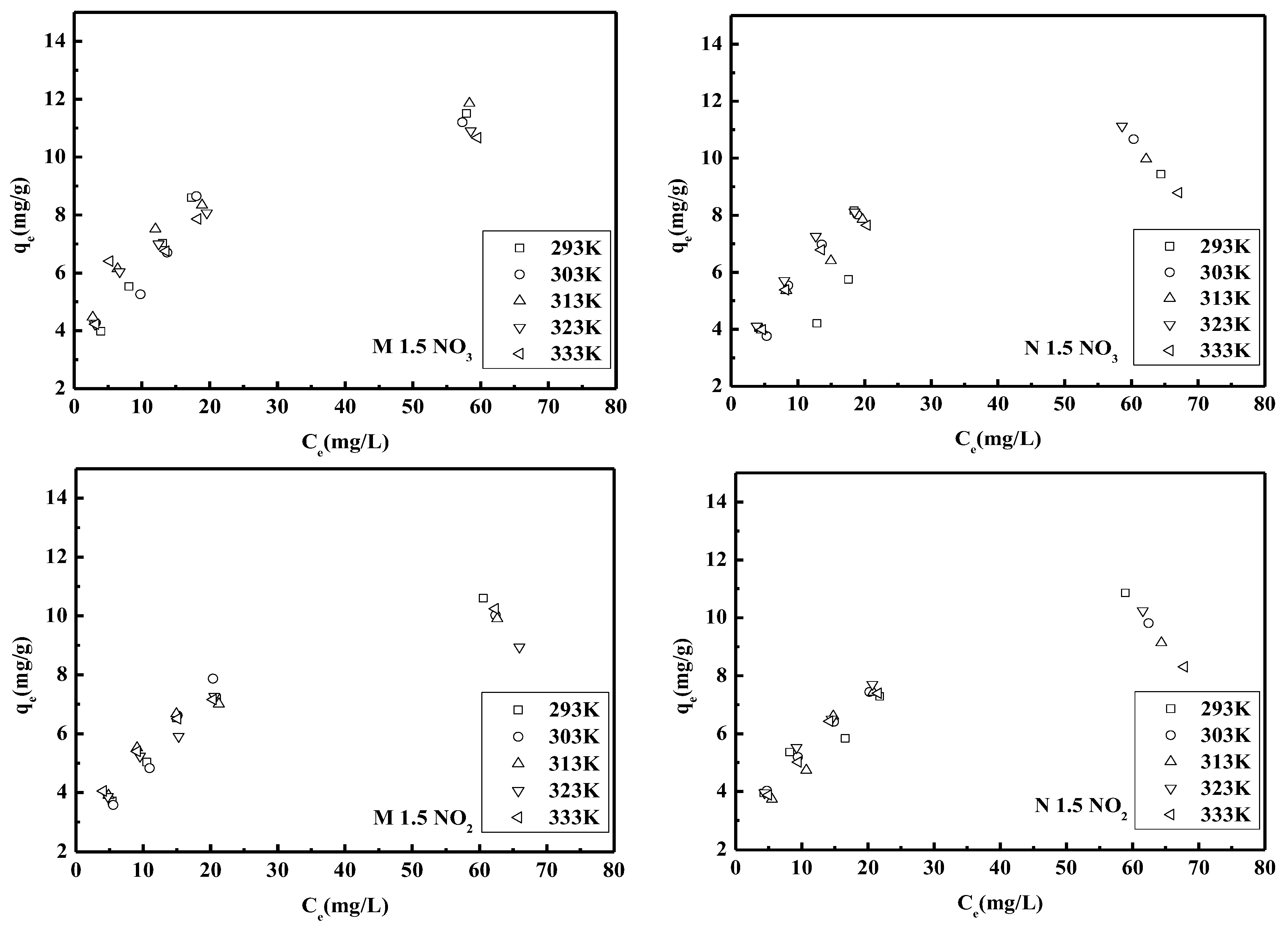
3.1.7. Adsorption Isotherms
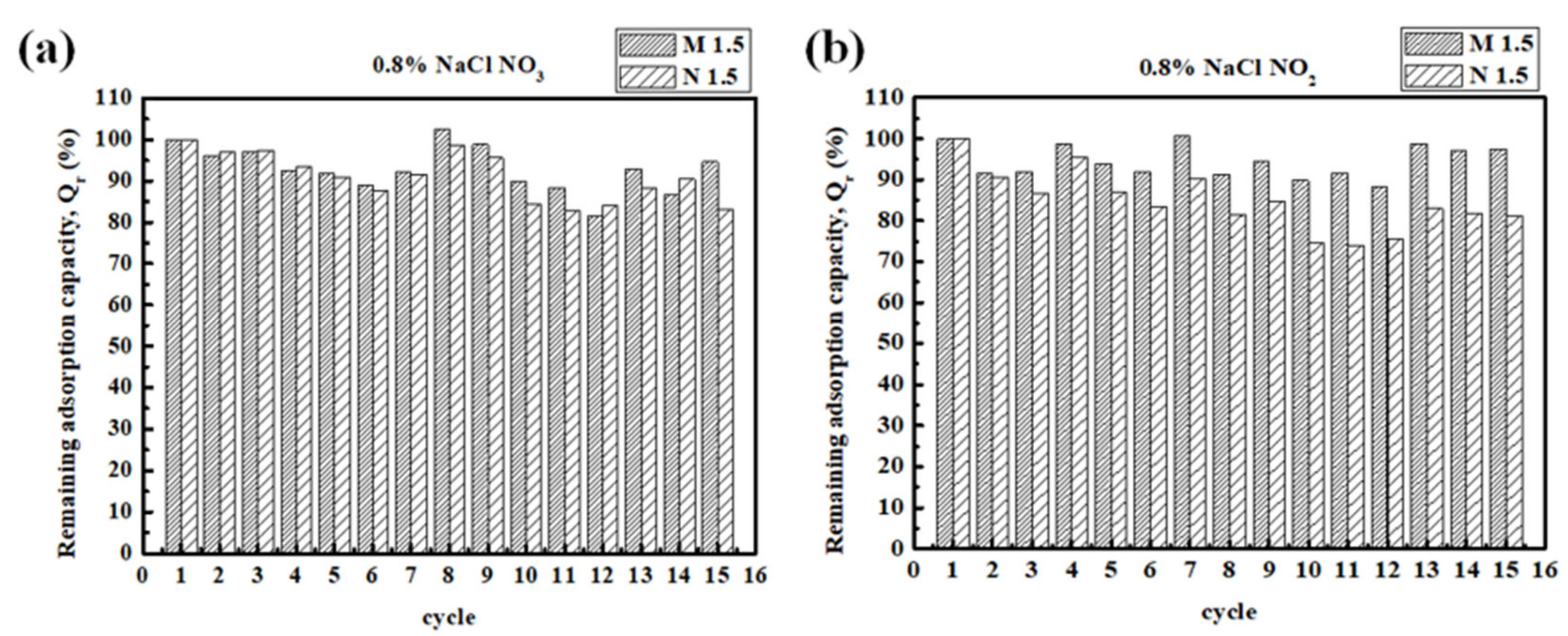
3.2. Reuse of the Adsorbent
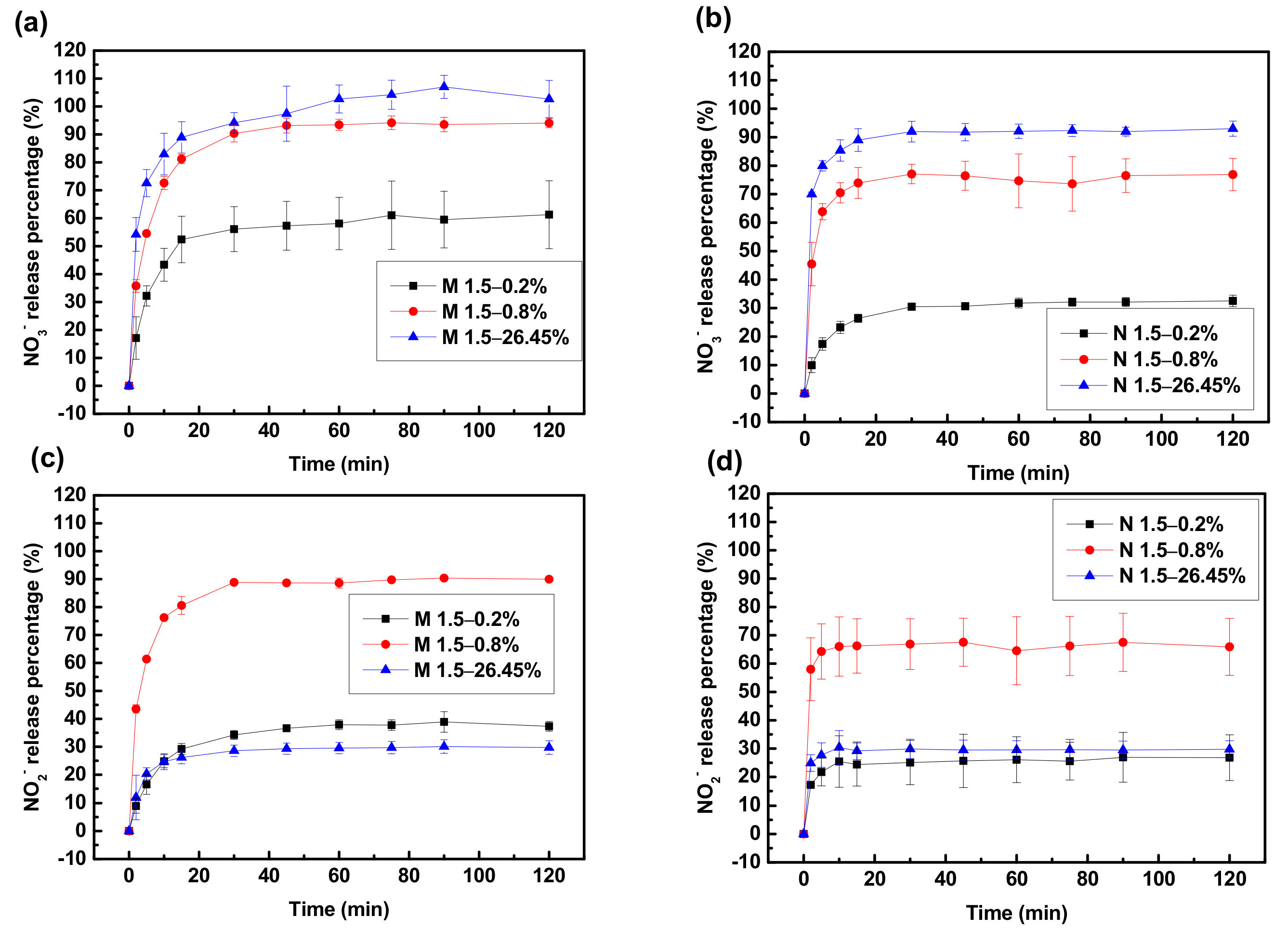
3.3. Column Experiments
3.3.1. Fixed Bed Adsorption/Desorption
3.3.2. Column Dynamic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajeswari, A.; Amalraj, A.; Pius, A. Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. J. Water Process Eng. 2016, 9, 123–134. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Thompson, T.S. Nitrate concentrations in private rural drinking water supplies in Saskatchewan, Canada. Bull. Environ. Contam. Toxicol. 2001, 66, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Parvizishad, M.; Dalvand, A.; Mahvi, A.H.; Goodarzi, F. A Review of Adverse Effects and Benefits of Nitrate and Nitrite in Drinking Water and Food on Human Health. Health Scope, 2017; in press. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, D.; Gupta, N. Nitrate pollution of groundwater and associated human health disorders. Indian J. Environ. Health 2000, 42, 28–39. [Google Scholar]
- Tate, C.H.; Arnold, K.F. Health and Aesthetic Aspects of Water Quality. In Water Quality and Treatment; McGraw-Hill Inc.: New York, NY, USA, 1990; pp. 63–156. [Google Scholar]
- Hekmatzadeh, A.A.; Karimi-Jashani, A.; Talebbeydokhti, N.; Kløve, B.J.D. Modeling of nitrate removal for ion exchange resin in batch and fixed bed experiments. Desalination 2012, 284, 22–31. [Google Scholar] [CrossRef]
- Fewtrell, L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: A discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.C.; Patel, R.K. Use of agricultural waste for the removal of nitrate-nitrogen from aqueous medium. J. Environ. Manag. 2009, 90, 519–522. [Google Scholar] [CrossRef]
- Chathuranga, R.A.J.; Liyandeniya, A.B.; Dharmapriya, T.N.; Deeyamulla, M.P.; Priyantha, N. Risk assessment and source apportionment of wet bulk deposition in three typical sites of Gampaha District, Sri Lanka. SN Appl. Sci. 2020, 2, 1394. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Nitrate removal from drinking water. J. Environ. Eng. 1997, 123, 371–380. [Google Scholar] [CrossRef]
- Khan, M.R.; Samdani, M.S.; Azam, M.; Ouladsmane, M. UPLC-ESI/MS analysis of disinfection by-products (perchlorate, bromate, nitrate, nitrite and sulfite) in micro-filtered drinking water obtained from spring, well and tap water (desalinated) sources. J. King Saud Univ. Sci. 2021, 33, 101408. [Google Scholar] [CrossRef]
- Maleki, G.; Khatibi, M.; Khamnian, Z.; Jalali, Z.; Dastgiri, S.; Aroogh, H. Association between nitrate concentration in drinking water and rate of colorectal cancer: A case study in northwestern Iran. Int. J. Environ. Health Res. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Madrakian, T.; Karimi, Z. The effect of acid treatment of carbon cloth on the adsorption of nitrite and nitrate ions. J. Hazard. Mater. 2007, 144, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Preparation, characterization of surfactants modified clay minerals and nitrate adsorption. Appl. Clay Sci. 2010, 48, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Tezuka, S.; Chitrakar, R.; Sonoda, A.; Ooi, K.; Tomida, T. Studies on selective adsorbents for oxo-anions. Nitrate ion-exchange properties of layered double hydroxides with different metal atoms. Green Chem. 2004, 6, 104–109. [Google Scholar] [CrossRef]
- Socías-Viciana, M.M.; Ureña-Amate, M.D.; González-Pradas, E.; García-Cortés, M.J.; López-Teruel, C. Nitrate Removal by Calcined Hydrotalcite-Type Compounds. Clays Clay Miner. 2008, 56, 2–9. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate. J. Hazard. Mater. 2009, 166, 508–513. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.-Y.; Yue, W.-W.; Yue, Q.-Y. Adsorption kinetics of nitrate from aqueous solutions onto modified wheat residue. Colloids Surf. A Physicochem. Eng. Asp. 2007, 308, 1–5. [Google Scholar] [CrossRef]
- Schoeman, J.; Steyn, A.J.D. Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 2003, 155, 15–26. [Google Scholar] [CrossRef]
- Dore, M.; Simon, P.; Deguin, A.; Victot, J. Removal of nitrate in drinking-water by ion-exchange impact on the chemical-quality of treated water. Water Res. 1986, 20, 221–232. [Google Scholar] [CrossRef]
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]
- Cheng, I.F.; Muftikian, R.; Fernando, Q.; Korte, N. Reduction of nitrate to ammonia by zero-valent iron. Chemosphere 1997, 35, 2689–2695. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, T.C. Effects of low pH on nitrate reduction by iron powder. Water Res. 2004, 38, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Li, C.W.; Chen, S.S. Fluidized zero valent iron bed reactor for nitrate removal. Chemosphere 2005, 59, 753–759. [Google Scholar] [CrossRef]
- Liou, Y.H.; Lo, S.L.; Lin, C.J.; Hu, C.Y.; Kuan, W.H.; Weng, S.C. Methods for accelerating nitrate reduction using zerovalent iron at near-neutral pH: Effects of H2-reducing pretreatment and copper deposition. Environ. Sci. Technol. 2005, 39, 9643–9648. [Google Scholar] [CrossRef]
- Ahn, S.C.; Oh, S.Y.; Cha, D.K. Enhanced reduction of nitrate by zero-valent iron at elevated temperatures. J. Hazard. Mater. 2008, 156, 17–22. [Google Scholar] [CrossRef]
- Kumar, M.; Chakraborty, S. Chemical denitrification of water by zero-valent magnesium powder. J. Hazard. Mater. 2006, 135, 112–121. [Google Scholar] [CrossRef]
- Hell, F.; Lahnsteiner, J.; Frischherz, H.; Baumgartner, G. Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 1998, 117, 173–180. [Google Scholar] [CrossRef]
- Pintar, A.; Batista, J.; Levec, J. Catalytic denitrification: Direct and indirect removal of nitrates from potable water. Catal. Today 2001, 66, 503–510. [Google Scholar] [CrossRef]
- Soares, M.I.M. Biological denitrification of groundwater. Water Air Soil Pollut. 2000, 123, 183–193. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Lee, D.Y.; Huang, P.J. Novel reusable hydrogel adsorbents for precious metal recycle. Sci. Rep. 2021, 11, 19577. [Google Scholar] [CrossRef] [PubMed]
- Dharmapriya, T.N.; Li, D.; Chung, Y.-C.; Huang, P.-J. Green Synthesis of Reusable Adsorbents for the Removal of Heavy Metal Ions. ACS Omega 2021, 6, 30478–30487. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Ji, M.; Choi, Y.; Jung, W.; Lee, S.; Kim, S.; Lee, G.; Suk, H.; Kim, H.; Min, B.; et al. Removal of nitrate from water by adsorption onto zinc chloride treated activated carbon. Sep. Sci. Technol. 2008, 43, 886–907. [Google Scholar] [CrossRef]
- Mena-Duran, C.; Kou, M.S.; Lopez, T.; Azamar-Barrios, J.; Aguilar, D.; Domínguez, M.; Odriozola, J.; Quintana, P. Nitrate removal using natural clays modified by acid thermoactivation. Appl. Surf. Sci. 2007, 253, 5762–5766. [Google Scholar] [CrossRef]
- Orlando, U.; Baes, A.; Nishijima, W.; Okada, M. A new procedure to produce lignocellulosic anion exchangers from agricultural waste materials. Bioresour. Technol. 2002, 83, 195–198. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.-Y.; Yue, W.-W.; Yue, Q.-Y. Preparation and utilization of wheat straw anionic sorbent for the removal of nitrate from aqueous solution. J. Environ. Sci. 2007, 19, 1305–1310. [Google Scholar] [CrossRef]
- Cengeloglu, Y.; Tor, A.; Ersoz, M.; Arslan, G. Removal of nitrate from aqueous solution by using red mud. Sep. Purif. Technol. 2006, 51, 374–378. [Google Scholar] [CrossRef]
- Öztürk, N.; Bektaş, T. Nitrate removal from aqueous solution by adsorption onto various materials. J. Hazard. Mater. 2004, 112, 155–162. [Google Scholar] [CrossRef]
- Özcan, A.; Şahin, M.; Özcan, A.S. Adsorption of nitrate ions onto sepiolite and surfactant-modified sepiolite. Adsorpt. Sci. Technol. 2005, 23, 323–334. [Google Scholar] [CrossRef]
- Namasivayam, C.; Höll, W.H. Quaternized biomass as an anion exchanger for the removal of nitrate and other anions from water. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 164–168. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef] [PubMed]
- Boumediene, M.; Achour, D. Denitrification of the underground waters by specific resin exchange of ion. Desalination 2004, 168, 187–194. [Google Scholar] [CrossRef]
- Burge, S.; Halden, R. Report on Environmental Restoration Division, Lawrence Livermore National Laboratory; UCRL-ID-135639; University of California: Livermore, CA, USA, 1999. [Google Scholar]
- Xu, X.; Gao, B.; Zhao, Y.; Chen, S.; Tan, X.; Yue, Q.; Lin, J.; Wang, Y. Nitrate removal from aqueous solution by Arundo donax L. reed based anion exchange resin. J. Hazard. Mater. 2012, 203, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, R.; Ray, S.K. Removal of congo red and methyl violet from water using nano clay filled composite hydrogels of poly acrylic acid and polyethylene glycol. Chem. Eng. J. 2015, 260, 269–283. [Google Scholar] [CrossRef]
- Kwak, N.-S.; Yang, J.R.; Hwang, C.W.; Hwang, T.S. The effect of a molecular weight and an amount of PEGDA (poly(ethylene glycol)diacrylate) on a preparation of sodium methallyl sulfonate-co-PEGDA microspheres and sorption behavior of Co(II). Chem. Eng. J. 2013, 223, 216–223. [Google Scholar] [CrossRef]
- Zhong, C.; Wu, J.; Reinhart-King, C.; Chu, C. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan–polyethylene glycol diacrylate hybrid hydrogels. Acta Biomater. 2010, 6, 3908–3918. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.; Ding, X.; Singh, R.P. Selective removal of nitrate using a novel asymmetric amine based strongly basic anion exchange resin. Adsorpt. Sci. Technol. 2020, 38, 271–285. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, W.; Cheng, J.; Liu, C.; Zheng, J. Determination of reactivity ratios of copolymerization of acrylamide (AM) and methacryloxyethyltrimethyl ammonium chloride (DMC) with ultraviolet initiation, and their sequence length distribution. Polym. Polym. Compos. 2016, 24, 307–314. [Google Scholar] [CrossRef]
- Xiao, Z.; Tan, Y.; Ma, J.; Wang, D.; Yu, L.; Yue, F.; Xu, S.; Huang, J. Fast swelling behaviors of thermosensitive poly(N-isopropylacrylamide-co-methacryloxyethyltrimethyl ammonium chloride)/Na2WO4 cationic composite hydrogels. J. Appl. Polym. Sci. 2018, 135, 46375. [Google Scholar] [CrossRef]
- Wypych, F.; Arízaga GG, C.; da Costa Gardolinski, J.E.F. Intercalation and functionalization of zinc hydroxide nitrate with mono- and dicarboxylic acids. J. Colloid Interface Sci. 2005, 283, 130–138. [Google Scholar] [CrossRef]
- Elmelouky, A.; Mortadi, A.; Chahid, E.; Elmoznine, R. Impedance spectroscopy as a tool to monitor the adsorption and removal of nitrate ions from aqueous solution using zinc aluminum chloride anionic clay. Heliyon 2018, 4, e00536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Halder, G. A review on the sorptive elimination of fluoride from contaminated wastewater. J. Environ. Chem. Eng. 2018, 6, 1257–1270. [Google Scholar] [CrossRef]
- Singha, N.R.; Chattopadhyay, P.K.; Dutta, A.; Mahapatra, M.; Deb, M. Review on additives-based structure-property alterations in dyeing of collagenic matrices. J. Mol. Liq. 2019, 293, 111470. [Google Scholar] [CrossRef]
- Lin, G.; Wang, S.; Zhang, L.; Hu, T.; Peng, J.; Cheng, S.; Fu, L. Selective adsorption of Ag+ on a new cyanuric-thiosemicarbazide chelating resin with high capacity from acid solutions. Polymers 2017, 9, 568. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-Second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wan, D.; Liu, H.; Liu, R.; Qu, J.; Li, S.; Zhang, J. Adsorption of nitrate and nitrite from aqueous solution onto calcined (Mg–Al) hydrotalcite of different Mg/Al ratio. Chem. Eng. J. 2012, 195, 241–247. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- McKay, G.; Poots, V.J.P. Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. J. Chem. Technol. Biotechnol. 1980, 30, 279–292. [Google Scholar] [CrossRef]
- Yakout, S.M.; Elsherif, E. Carbon—Science and Technology; Applied Science Innovations Pvt. Ltd.: Maharashtra, India, 2010; Volume 1, pp. 144–153. [Google Scholar]
- Zhang, D.; Zhang, C.; Zhou, P. Preparation of porous nano-calcium titanate microspheres and its adsorption behavior for heavy metal ion in water. J. Hazard. Mater. 2011, 186, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Tang, Y.P.; Tao, S.R. Kinetics, equilibrium and thermodynamic study on removal of Cr (VI) from aqueous solutions using low-cost adsorbent Alligator weed. Chem. Eng. J. 2009, 148, 217–225. [Google Scholar] [CrossRef]
- Sowmya, A.; Meenakshi, S. A novel quaternized chitosan–melamine–glutaraldehyde resin for the removal of nitrate and phosphate anions. Int. J. Biol. Macromol. 2014, 64, 224–232. [Google Scholar] [CrossRef] [PubMed]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Sánchez-Duarte, R.G.; Correa-Murrieta, M.A. Study of a fixed-bed column in the adsorption of an azo dye from an aqueous medium using a chitosan–glutaraldehyde biosorbent. Adsorpt. Sci. Technol. 2018, 36, 215–232. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Rashid, A.K.; Rafique, R.F.; Khalid, K. Breakthrough curve analysis for column dynamics sorption of Mn (II) ions from wastewater by using Mangostana garcinia peel-based granular-activated carbon. J. Chem. 2013, 2013, 959761. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Chen, P.; Luo, S.; Tu, X.; Cao, Q.; Shu, M. Synthesis of novel nanocomposite Fe3O4/ZrO2/chitosan and its application for removal of nitrate and phosphate. Appl. Surf. Sci. 2013, 284, 942–949. [Google Scholar] [CrossRef]

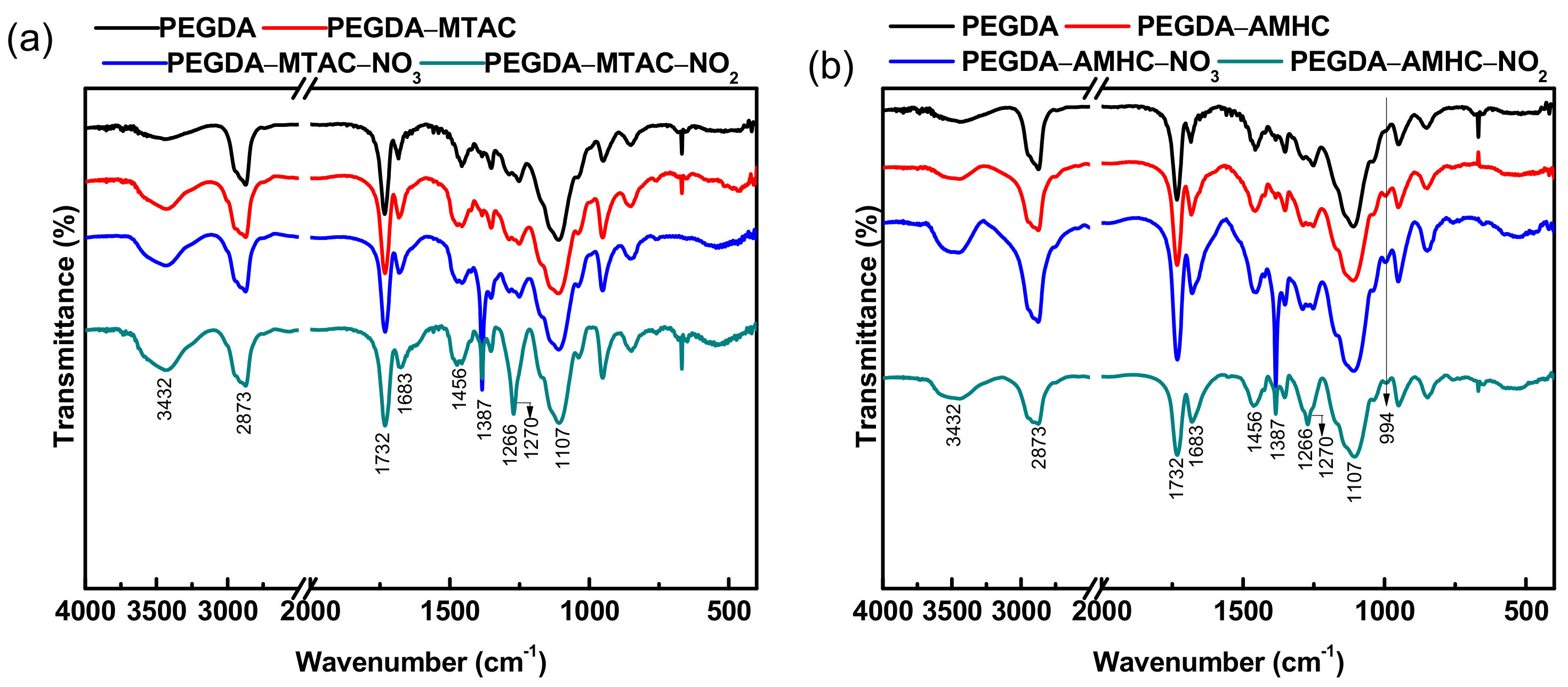

| Wave Numbers/cm−1 | Group | Compound |
|---|---|---|
| 3432 | O–H stretching | Internal water |
| 2873 | C–H stretching | PEGDA, PEGDA-MTAC/AMHC |
| 1732, 1683 | C=O stretching | PEGDA, PEGDA-MTAC/AMHC |
| 1107 | C–O stretching | PEGDA, PEGDA-MTAC/AMHC |
| 1466 | C–H bending | PEGDA-MTAC/AMHC |
| 1266 | C–N stretching | PEGDA-MTAC/AMHC |
| 1387 | N–O stretching | PEGDA-MTAC/AMHC |
| Parameters | PEGDA-MTAC 1:1.5 | PEGDA-AMHC 1:1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | 333 K | 293 K | 303 K | 313 K | 323 K | 333 K | |
| Pseudo-first-order | ||||||||||
| qe (mg/g) | 9.1798 | 7.4231 | 5.2788 | 5.0113 | 4.0084 | 6.1159 | 5.0496 | 5.3431 | 1.9422 | 3.0606 |
| k1 (min−1) | 0.1391 | 0.1734 | 0.1621 | 0.1278 | 0.1340 | 0.1313 | 0.1317 | 0.1435 | 0.1361 | 0.1066 |
| R2 | 0.9721 | 0.9489 | 0.9462 | 0.9388 | 0.8964 | 0.9389 | 0.8956 | 0.9642 | 0.8001 | 0.8561 |
| Pseudo-second-order | ||||||||||
| qe (mg/g) | 9.9108 | 9.6993 | 8.9206 | 8.6505 | 8.3056 | 8.9847 | 8.8417 | 8.4746 | 7.9114 | 7.8493 |
| k2 (g/mg·min) | 0.0051 | 0.0099 | 0.0170 | 0.0171 | 0.0234 | 0.0119 | 0.0141 | 0.0166 | 0.0307 | 0.0410 |
| R2 | 0.9889 | 0.9917 | 0.9962 | 0.9969 | 0.9974 | 0.9968 | 0.9948 | 0.9969 | 0.9997 | 0.9994 |
| Intraparticle diffusion | ||||||||||
| k (mg/g·min0.5) | 0.8558 | 0.8490 | 0.7626 | 0.7334 | 0.6851 | 0.7836 | 0.7632 | 0.7257 | 0.5404 | 0.5876 |
| R2 | 0.9077 | 0.8286 | 0.7786 | 0.7781 | 0.7368 | 0.8471 | 0.7978 | 0.7965 | 0.5761 | 0.6555 |
| Parameters | PEGDA-MTAC 1:1.5 | PEGDA-AMHC 1:1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | 333 K | 293 K | 303 K | 313 K | 323 K | 333 K | |
| Pseudo-first-order | ||||||||||
| qe (mg/g) | 6.6559 | 5.0455 | 3.6433 | 3.2275 | 2.9928 | 3.2847 | 4.9833 | 2.0333 | 2.9503 | 2.6018 |
| k1 (min−1) | 0.1172 | 0.1218 | 0.1105 | 0.1195 | 0.1011 | 0.1690 | 0.1382 | 0.0815 | 0.1117 | 0.1177 |
| R2 | 0.9782 | 0.9355 | 0.8396 | 0.8742 | 0.8461 | 0.9885 | 0.9587 | 0.6610 | 0.8445 | 0.8187 |
| Pseudo-second-order | ||||||||||
| qe (mg/g) | 8.9206 | 8.4459 | 7.9365 | 7.6923 | 7.4571 | 8.3963 | 8.2034 | 7.5019 | 7.4963 | 7.5988 |
| k2 (g/mg·min) | 0.0091 | 0.0168 | 0.0269 | 0.0335 | 0.0398 | 0.0137 | 0.0195 | 0.0289 | 0.0375 | 0.0460 |
| R2 | 0.9969 | 0.9977 | 0.9989 | 0.9986 | 0.9993 | 0.9958 | 0.9980 | 0.9995 | 0.9988 | 0.9991 |
| Intraparticle diffusion | ||||||||||
| k (mg/g·min0.5) | 0.7822 | 0.7214 | 0.6478 | 0.6077 | 0.5608 | 0.7311 | 0.6911 | 0.5222 | 0.5812 | 0.5750 |
| R2 | 0.8905 | 0.8017 | 0.7562 | 0.6940 | 0.6635 | 0.8244 | 0.7871 | 0.5953 | 0.6805 | 0.6527 |
| Parameters | PEGDA-MTAC 1:1.5 | PEGDA-AMHC 1:1.5 | ||
|---|---|---|---|---|
| NO3− | NO2− | NO3− | NO2− | |
| Ea (kJ/mol) | 29.4507 | 29.8090 | 26.2979 | 25.1312 |
| A (g/mg·min) | 1077.0704 | 2151.6709 | 517.2412 | 564.8160 |
| R2 | 0.9148 | 0.9512 | 0.9405 | 0.9879 |
| Parameters | PEGDA-MTAC 1:1.5 | PEGDA-AMHC 1:1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | 333 K | 293 K | 303 K | 313 K | 323 K | 333 K | |
| Langmuir | ||||||||||
| qm (mg/g) | 13.6612 | 12.3916 | 13.2802 | 12.1951 | 11.7371 | 11.2486 | 12.7714 | 11.5075 | 12.8866 | 9.6154 |
| KL (L/mg) | 0.0912 | 0.1039 | 0.1242 | 0.1290 | 0.1433 | 0.0769 | 0.0856 | 0.1023 | 0.1036 | 0.1659 |
| RL | 0.0988 | 0.0878 | 0.0745 | 0.0719 | 0.0652 | 0.1151 | 0.1046 | 0.0890 | 0.0880 | 0.0569 |
| R2 | 0.9973 | 0.9818 | 0.9904 | 0.9937 | 0.9891 | 0.9348 | 0.9990 | 0.9972 | 0.9980 | 0.9995 |
| Thermodynamics | ||||||||||
| ΔG° (kJ/mol) | 29.0577 | 29.7368 | 30.4159 | 31.0949 | 31.7740 | 29.6692 | 30.2033 | 30.7374 | 31.2716 | 31.8057 |
| ΔH° (kJ/mol) | 9.1612 | 14.0191 | ||||||||
| ΔS° (kJ/K·mol) | −0.0679 | −0.0534 | ||||||||
| R2 | 0.9755 | 0.8541 | ||||||||
| Parameters | PEGDA-MTAC 1:1.5 | PEGDA-AMHC 1:1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | 333 K | 293 K | 303 K | 313 K | 323 K | 333 K | |
| Langmuir | ||||||||||
| qm (mg/g) | 13.2979 | 12.3305 | 11.4811 | 10.1215 | 11.8624 | 13.1234 | 11.4025 | 10.6838 | 11.9332 | 9.1491 |
| KL (L/mg) | 0.0627 | 0.0720 | 0.0935 | 0.1131 | 0.0920 | 0.0691 | 0.0953 | 0.0948 | 0.0938 | 0.1535 |
| RL | 0.1376 | 0.1220 | 0.0966 | 0.0812 | 0.0980 | 0.1264 | 0.0950 | 0.0954 | 0.0963 | 0.0612 |
| R2 | 0.9949 | 0.9921 | 0.9949 | 0.9975 | 0.9913 | 0.9599 | 0.9975 | 0.9945 | 0.9970 | 0.9985 |
| Thermodynamics | ||||||||||
| ΔG° (kJ/mol) | 29.8930 | 30.5705 | 31.2480 | 31.9255 | 32.6029 | 29.7188 | 30.2944 | 30.8700 | 31.4457 | 32.0213 |
| ΔH° (kJ/mol) | 10.0425 | 12.8526 | ||||||||
| ΔS° (kJ/K·mol) | −0.0678 | −0.0576 | ||||||||
| R2 | 0.7002 | 0.7620 | ||||||||
| Adsorbent/Ion Exchange Resin | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| ZnCl2 treated coconut granular activated carbon | 10.2 | [35] |
| Modified lignite granular activated carbon | 10 | [68] |
| Polyvinyl alcohol/chitosan | 35 | [1] |
| Calcined (Mg–Al) hydrotalcite | 34.36 | [61] |
| Untreated coconut granular activated carbon | 1.7 | [35] |
| HDTMA modified QLD-bentonite | 12.83–14.76 | [15] |
| Cross-linked and quaternized Chinese reed | 7.55 | [42] |
| Wheat straw charcoal | 1.10 | [9] |
| PEGDA-MTAC/AMHC | 13.51, 13.16 | This study |
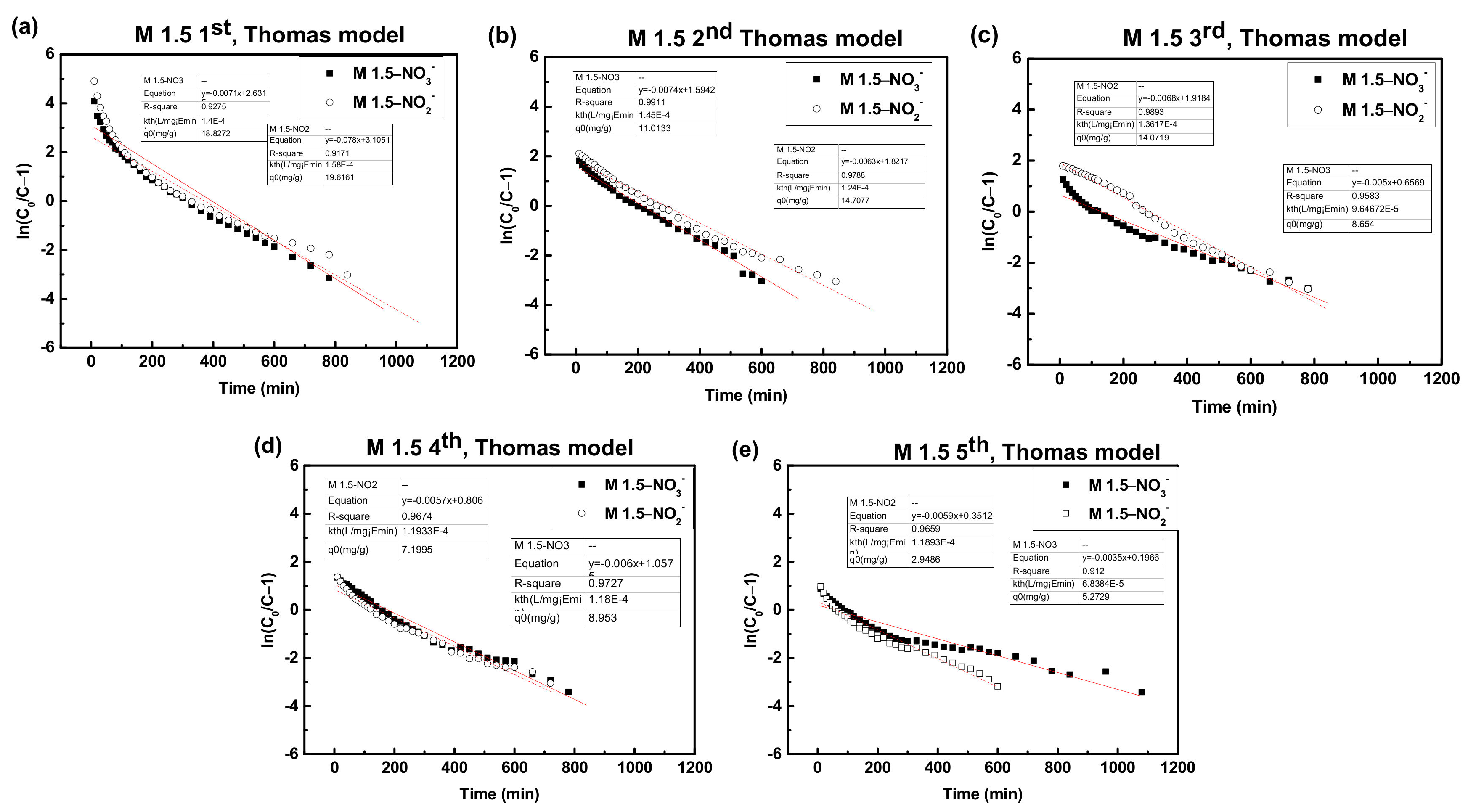
| Cycle | PEGDA-MTAC 1:1.5 | ||||||
|---|---|---|---|---|---|---|---|
| Breakthrough Point Time (min) | Operating Limit Time (min) | qe(exp) (mg/g) | Removal (%) | Thomas Model | |||
| kth (L/mg·min) | q0 (mg/g) | R2 | |||||
| 1 | 40 | 780 | 16.49 | 42.34 | 1.63 10−4 | 17.78 | 0.9602 |
| 2 | - | 600 | 11.28 | 37.64 | 1.48 10−4 | 11.00 | 0.9914 |
| 3 | - | 780 | 9.87 | 25.33 | 1.00 10−4 | 6.91 | 0.9642 |
| 4 | - | 780 | 10.44 | 26.80 | 1.18 10−4 | 8.92 | 0.9676 |
| 5 | - | 1080 | 10.52 | 19.50 | 6.84 10−5 | 2.87 | 0.9120 |
| Cycle | PEGDA-MTAC 1:1.5 | ||||||
|---|---|---|---|---|---|---|---|
| Breakthrough Point Time (min) | Operating Limit Time (min) | qe(exp) (mg/g) | Removal (%) | Thomas Model | |||
| kth (L/mg·min) | q0 (mg/g) | R2 | |||||
| 1 | 60 | 840 | 17.54 | 41.75 | 1.62 10−4 | 19.36 | 0.9070 |
| 2 | - | 840 | 14.46 | 34.46 | 1.28 10−4 | 14.59 | 0.9808 |
| 3 | - | 780 | 14.06 | 35.83 | 1.38 10−4 | 13.97 | 0.9882 |
| 4 | - | 720 | 9.37 | 26.07 | 1.16 10−4 | 7.25 | 0.9620 |
| 5 | - | 600 | 6.61 | 22.05 | 1.19 10−4 | 2.95 | 0.9533 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmapriya, T.N.; Shih, H.-Y.; Huang, P.-J. Facile Synthesis of Hydrogel-Based Ion-Exchange Resins for Nitrite/Nitrate Removal and Studies of Adsorption Behavior. Polymers 2022, 14, 1442. https://doi.org/10.3390/polym14071442
Dharmapriya TN, Shih H-Y, Huang P-J. Facile Synthesis of Hydrogel-Based Ion-Exchange Resins for Nitrite/Nitrate Removal and Studies of Adsorption Behavior. Polymers. 2022; 14(7):1442. https://doi.org/10.3390/polym14071442
Chicago/Turabian StyleDharmapriya, Thakshila Nadeeshani, Hsin-Yin Shih, and Po-Jung Huang. 2022. "Facile Synthesis of Hydrogel-Based Ion-Exchange Resins for Nitrite/Nitrate Removal and Studies of Adsorption Behavior" Polymers 14, no. 7: 1442. https://doi.org/10.3390/polym14071442
APA StyleDharmapriya, T. N., Shih, H.-Y., & Huang, P.-J. (2022). Facile Synthesis of Hydrogel-Based Ion-Exchange Resins for Nitrite/Nitrate Removal and Studies of Adsorption Behavior. Polymers, 14(7), 1442. https://doi.org/10.3390/polym14071442








