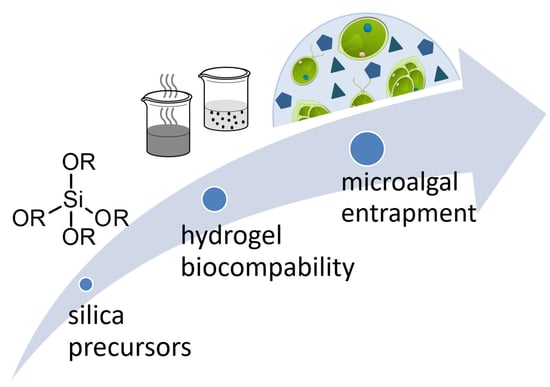
Silica Hydrogels as Entrapment Material for Microalgae
Abstract
1. Introduction
2. Sol–Gel Methods for the Production of Silica Hydrogels
2.1. Sol Synthesis with Alkoxysilanes
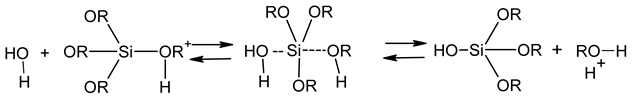
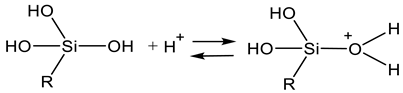
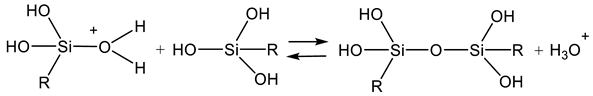
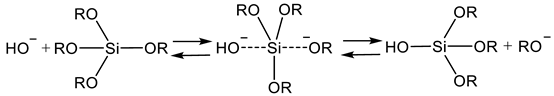
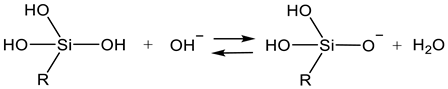

2.2. Sol Synthesis with Aqueous Silicates
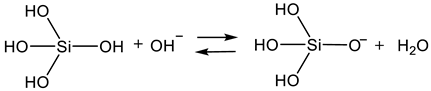



2.3. Sol Synthesis with Aminosilanes
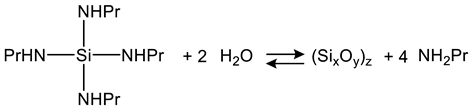

2.4. Gel Synthesis
3. Biocompatibility
3.1. Conventional Sol–Gel Method for the Entrapment of Insensitive Biological Material
3.2. Reduction of Released By-Products and Avoidance of Increased Ion Concentrations
3.3. Facilitation of Cell Proliferation by Reducing the Stiffness
4. Entrapment of Microalgae in Silica Hydrogels
4.1. Entrapment of Microalgae in Alkoxysilanes
4.2. Entrapment of Microalgae in Aqueous Silicates
4.3. Entrapment of Microalgae in Aqueous Silicates with Metal Ion Removal
4.4. Entrapment of Microalgae in Aminosilane-Based Silica Hydrogels
4.5. Core-Shell and Two-Step Entrapment of Microalgae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Moheimani, N.R. Sustainable biofuels from algae. Mitig. Adapt. Strateg. Glob. Change 2013, 18, 13–25. [Google Scholar]
- Fresewinkel, M.; Rosello, R.; Wilhelm, C.; Kruse, O.; Hankamer, B.; Posten, C. Integration in microalgal bioprocess development: Design of efficient, sustainable, and economic processes. Eng. Life Sci. 2014, 14, 560–573. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Kholssi, R.; Ramos, P.V.; Marks, E.A.; Montero, O.; Rad, C. 2 Biotechnological uses of microalgae: A review on the state of the art and challenges for the circular economy. Biocatal. Agric. Biotechnol. 2021, 36, 102114. [Google Scholar] [CrossRef]
- Morais, W.G., Jr.; Gorgich, M.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 2020, 528, 735562. [Google Scholar] [CrossRef]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A realistic scenario on microalgae based biodiesel production: Third generation biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiumparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Harder, R.; Von Witsch, H. Über Massenkultur von Diatomeen. Ber. Dtsch. Bot. Gesellschaft. 1942, 60, 146–152. [Google Scholar] [CrossRef]
- Tamiya, H. Mass Culture of Algae. Annu. Rev. Plant Physiol. 1957, 8, 309–334. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I. Microalgal Immobilization Methods. In Immobilization of Enzymes and Cells. Methods in Molecular Biology (Methods and Protocols); Guisan, J., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1051, pp. 327–347. [Google Scholar] [CrossRef]
- Polakovic, M.; Švitel, J.; Bučko, M.; Filip, J.; Nedela, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N. Immobilization of Microalgae. In Immobilization of Enzymes and Cells: Methods and Protocols; Guisan, J.M., Ed.; Springer: New York, NY, USA, 2020; pp. 453–471. [Google Scholar]
- Caldwell, G.S.; In-Na, P.; Hart, R.; Sharp, E.; Stefanova, A.; Pickersgill, M.; Walker, M.; Unthank, M.; Perry, J.; Lee, J. Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing. Energies 2021, 14, 2566. [Google Scholar] [CrossRef]
- Vasilieva, S.; Lobakova, E.; Solovchenko, A. Biotechnological Applications of Immobilized Microalgae. In Environmental Biotechnology; Gothandam, K.M., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 3, pp. 193–220. [Google Scholar] [CrossRef]
- Rösch, C.; Posten, C. Challenges and Perspectives of Microalgae Production. TATuP—Z. Für Tech. Theor. Und Prax. 2012, 21, 5–16. [Google Scholar] [CrossRef][Green Version]
- Lauersen, K.J.; Vanderveer, T.L.; Berger, H.; Kaluza, I.; Mussgnug, J.H.; Walker, V.K.; Kruse, O. Ice recrystallization inhibition mediated by a nuclear-expressed and -secreted recombinant ice-binding protein in the microalga Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2013, 97, 9763–9772. [Google Scholar] [CrossRef]
- Lauersen, K.J.; Berger, H.; Mussgnug, J.H.; Kruse, O. Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii. J. Biotechnol. 2013, 167, 101–110. [Google Scholar] [CrossRef]
- Gerbsch, N.; Buchholz, R. New processes and actual trends in biotechnology. FEMS Microbiol. Rev. 1995, 16, 259–269. [Google Scholar] [CrossRef]
- Park, R.B.; Kelly, J.; Drury, S.; Sauer, K. The Hill reaction of chloroplasts isolated from glutaraldehyde-fixed spinach leaves. Proc. Natl. Acad. Sci. USA 1966, 55, 1056–1062. [Google Scholar] [CrossRef]
- Lebeau, T.; Robert, J.-M. Biotechnology of immobilized microalgae: A culture technique for the future. In Algal Cultures, Analogues of Blooms and Applications; Science Publishers: Enfield, NH, USA, 2006; pp. 801–837. [Google Scholar]
- Gotovtsev, P.M.; Yuzbasheva, E.Y.; Gorin, K.V.; Butylin, V.V.; Badranova, G.U.; Perkovskaya, N.I.; Mostova, E.B.; Namsaraev, Z.B.; Rudneva, N.I.; Komova, A.V.; et al. Immobilization of microbial cells for biotechnological production: Modern solutions and promising technologies. Appl. Biochem. Microbiol. 2015, 51, 792–803. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Tian, G.; Jiang, N.; Su, B.-L. Immobilization technology: A sustainable solution for biofuel cell design. Energy Environ. Sci. 2012, 5, 5540–5563. [Google Scholar] [CrossRef]
- Gallo, M.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Novel Microbial Immobilization Techniques. In Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 35–55. [Google Scholar]
- Vemmer, M.; Patel, A. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Willaert, R. Cell Immobilization and Its Applications in Biotechnology: Current Trends and Future Prospects. In Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 315–390. [Google Scholar] [CrossRef]
- Leong, J.-Y.; Lam, W.-H.; Ho, K.-W.; Voo, W.-P.; Lee, M.F.-X.; Lim, H.P.; Lim, S.-L.; Tey, B.T.; Poncelet, D.; Chan, E.-S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Borin, G.P.; de Melo, R.R.; Crespim, E.; Sato, H.H.; Contesini, F.J. An Overview on Polymer Gels Applied to Enzyme and Cell Immobilization. In Polymer Gels: Science and Fundamentals; Thakur, V.K., Thakur, M.K., Eds.; Springer: Singapore, 2018; pp. 63–86. [Google Scholar]
- Zajkoska, P.; Rebroš, M.; Rosenberg, M. Biocatalysis with immobilized Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Es, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Livage, J.; Coradin, T.; Roux, C. Encapsulation of biomolecules in silica gels. J. Phys. Condens. Matter 2001, 13, R673–R691. [Google Scholar] [CrossRef]
- Holzmeister, I.; Schamel, M.; Groll, J.; Gbureck, U.; Vorndran, E. Artificial inorganic biohybrids: The functional combination of microorganisms and cells with inorganic materials. Acta Biomater. 2018, 74, 17–35. [Google Scholar] [CrossRef]
- Carturan, G.; Campostrini, R.; Diré, S.; Scardi, V.; De Alteriis, E. Inorganic gels for immobilization of biocatalysts: Inclusion of invertase-active whole cells of yeast (saccharomyces cerevisiae) into thin layers of SiO2 gel deposited on glass sheets. J. Mol. Catal. 1989, 57, L13–L16. [Google Scholar] [CrossRef]
- Coradin, T.; Livage, J. Aqueous Silicates in Biological Sol–Gel Applications: New Perspectives for Old Precursors. Acc. Chem. Res. 2007, 40, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Nassif, N.; Bouvet, O.; Rager, M.N.; Roux, C.; Coradin, T.; Livage, J. Living bacteria in silica gels. Nat. Mater. 2002, 1, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.C.; Léonard, A.; Su, B.-L. Targeting photobioreactors: Immobilisation of cyanobacteria within porous silica gel using biocompatible methods. J. Mater. Chem. 2008, 18, 1333–1341. [Google Scholar] [CrossRef]
- Rooke, J.C.; Léonard, A.; Meunier, C.F.; Sarmento, H.; Descy, J.-P.; Su, B.-L. Hybrid photosynthetic materials derived from microalgae Cyanidium caldarium encapsulated within silica gel. J. Colloid Interface Sci. 2010, 344, 348–352. [Google Scholar] [CrossRef]
- Rooke, J.C.; Léonard, A.; Sarmento, H.; Meunier, C.F.; Descy, J.-P.; Su, B.-L. Novel photosynthetic CO2 bioconvertor based on green algae entrapped in low-sodium silica gels. J. Mater. Chem. 2011, 21, 951–959. [Google Scholar] [CrossRef]
- Rooke, J.C.; Vandoorne, B.; Léonard, A.; Meunier, C.F.; Cambier, P.; Sarmento, H.; Descy, J.-P.; Su, B.-L. Prolonging the lifetime and activity of silica immobilised Cyanidium caldarium. J. Colloid Interface Sci. 2011, 356, 159–164. [Google Scholar] [CrossRef]
- Branyik, T.; Kuncová, G.; Páca, J.; Demnerová, K. Encapsulation of Microbial Cells into Silica Gel. J. Sol-Gel Sci. Technol. 1998, 13, 283–287. [Google Scholar] [CrossRef]
- Dickson, D.; Page, C.; Ely, R. Photobiological hydrogen production from Synechocystis sp. PCC 6803 encapsulated in silica sol–gel. Int. J. Hydrog. Energy 2009, 34, 204–215. [Google Scholar] [CrossRef]
- Premkumar, J.R.; Sagi, E.; Rozen, R.; Belkin, S.; Modestov, A.D.; Lev, O. Fluorescent Bacteria Encapsulated in Sol−Gel Derived Silicate Films. Chem. Mater. 2002, 14, 2676–2686. [Google Scholar] [CrossRef]
- Premkumar, J.R.; Rosen, R.; Belkin, S.; Lev, O. Sol–gel luminescence biosensors: Encapsulation of recombinant E. coli reporters in thick silicate films. Anal. Chim. Acta 2002, 462, 11–23. [Google Scholar] [CrossRef]
- Livage, J.; Coradin, T. Living cells in oxide glasses. Rev. Mineral. Geochem. 2006, 64, 315–332. [Google Scholar] [CrossRef]
- Nassif, N.; Livage, J. From diatoms to silica-based biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef]
- Nassif, N.; Coradin, T.; Bouvet, O.M.M.; Livage, J. Bacteria quorum sensing in silica matrices. J. Mater. Chem. 2004, 14, 2264–2268. [Google Scholar] [CrossRef]
- Homburg, S.V.; Kruse, O.; Patel, A.V. Growth and photosynthetic activity of Chlamydomonas reinhardtii entrapped in lens-shaped silica hydrogels. J. Biotechnol. 2019, 302, 58–66. [Google Scholar] [CrossRef]
- Homburg, S.V.; Venkanna, D.; Kraushaar, K.; Kruse, O.; Kroke, E.; Patel, A.V. Entrapment and growth of Chlamydomonas reinhardtii in biocompatible silica hydrogels. Colloids Surf. B Biointerfaces 2018, 173, 233–241. [Google Scholar] [CrossRef]
- Carturan, G.; Toso, R.D.; Boninsegna, S.; Monte, R.D. Encapsulation of functional cells by sol–gel silica: Actual progress and perspectives for cell therapy. J. Mater. Chem. 2004, 14, 2087–2098. [Google Scholar] [CrossRef]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol–gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Coradin, T.; Allouche, J.; Boissiere, M.; Livage, J. Sol-Gel Biopolymer/Silica Nanocomposites in Biotechnology. Curr. Nanosci. 2006, 2, 219–230. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Keefer, K.D. Fractal Geometry of Silica Condensation Polymers. Phys. Rev. Lett. 1984, 53, 1383. [Google Scholar] [CrossRef]
- Schaefer, D.W. Fractal Models and the Structure of Materials. MRS Bull. 1988, 13, 22–27. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-gel glass: I. Gelation and gel structure. J. Non-Cryst. Solids 1985, 70, 301–322. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Schaefer, D.W. Polymers, fractals, and ceramic materials. Science 1989, 243, 1023–1027. [Google Scholar] [CrossRef]
- Iler, K.R. The Chemistry of Silica. Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley: Hoboken, NJ, USA, 1979. [Google Scholar]
- Iler, R.K. Isolation and characterization of particle nuclei during the polymerization of silicic acid to colloidal silica. J. Colloid Interface Sci. 1980, 75, 138–148. [Google Scholar] [CrossRef]
- Kraushaar, K.; Wiltzsch, C.; Wagler, J.; Böhme, U.; Schwarzer, A.; Roewer, G.; Kroke, E. From CO2 to Polysiloxanes: Di(carbamoyloxy)silanes Me2Si[(OCO)NRR′]2 as Precursors for PDMS. Organometallics 2012, 31, 4779–4785. [Google Scholar] [CrossRef]
- Wiltzsch, C.; Wagler, J.; Roewera, G.; Kroke, E. Sol-gel analogous aminolysis-ammonolysis of chlorosilanes to chlorine-free Si/(C)/N-materials. Dalton Trans. 2009, 5474–5477. [Google Scholar] [CrossRef]
- Müller, C. Aminosilan-basierende Sol-Gel-Synthese zur Herstellung transparenter Hydro- und Xerogele zur Einschlussimmobilisierung von Kobalt-Nanopartikeln und biologischem Material. In Fakultät für Chemie und Physik; Technische Universität Bergakademie Freiberg: Freiberg, Germany, 2014. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 94th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Müller, C.; Kraushaar, K.; Doebbe, A.; Mussgnug, J.H.; Kruse, O.; Kroke, E.; Patel, A.V. Synthesis of transparent aminosilane-derived silica based networks for entrapment of sensitive materials. Chem. Commun. 2013, 49, 10163–10165. [Google Scholar] [CrossRef]
- Brinker, C.J. Sol—Gel Processing of Silica. In The Colloid Chemistry of Silica; American Chemical Society: Washington, DC, USA, 1994; pp. 361–401. [Google Scholar]
- Pope, E.J.A.; Mackenzie, J.D. Sol-gel processing of silica. J. Non-Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Young, S.K. Overview of Sol-Gel Science and Technology; Army Research Lab.: Adelphi, MD, USA, 2002. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Burgos-Asperilla, L.; Llobera, A.; Cadarso, V.J.; Fernández-Sánchez, C.; Ruiz-Hitzky, E. Algae–silica systems as functional hybrid materials. J. Mater. Chem. 2010, 20, 9362–9369. [Google Scholar] [CrossRef]
- Fiedler, D.; Hager, U.; Franke, H.; Soltmann, U.; Böttcher, H. Algae biocers: Astaxanthin formation in sol–gel immobilised living microalgae. J. Mater. Chem. 2007, 17, 261–266. [Google Scholar] [CrossRef]
- Dickson, D.J.; Ely, R.L. Evaluation of encapsulation stress and the effect of additives on viability and photosynthetic activity of Synechocystis sp. PCC 6803 encapsulated in silica gel. Appl. Microbiol. Biotechnol. 2011, 91, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Rappoport, S.; Zusman, R.; Avnir, D.; Ottolenghi, M. Biochemically active sol-gel glasses: The trapping of enzymes. Mater. Lett. 1990, 10, 1–5. [Google Scholar] [CrossRef]
- Dave, B.C.; Dunn, B.; Valentine, J.S.; Zink, J.I. Sol-gel encapsulation methods for biosensors. Anal. Chem. 1994, 66, 1120A–1127A. [Google Scholar] [CrossRef]
- Conroy, J.F.; Power, M.E.; Martin, J.; Earp, B.; Hosticka, B.; Daitch, C.E.; Norris, P.M. Cells in Sol-Gels I: A Cytocompatible Route for the Production of Macroporous Silica Gels. J. Sol-Gel Sci. Technol. 2000, 18, 269–283. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A. Encapsulation of biologicals within silicate, siloxane, and hybrid sol-gel polymers: An efficient and generic approach. J. Am. Chem. Soc. 1998, 120, 8587–8598. [Google Scholar] [CrossRef]
- Carturan, G.; Monte, R.D.; Pressi, G.; Secondin, S.; Verza, P. Production of Valuable Drugs from Plant Cells Immobilized by Hybrid Sol-Gel SiO2. J. Sol-Gel Sci. Technol. 1998, 13, 273–276. [Google Scholar] [CrossRef]
- Pressi, G.; Toso, R.D.; Monte, R.D.; Carturan, G. Production of Enzymes by Plant Cells Immobilized by Sol-Gel Silica. J. Sol-Gel Sci. Technol. 2003, 26, 1189–1193. [Google Scholar] [CrossRef]
- Muraca, M.; Vilei, M.T.; Zanusso, G.E.; Ferraresso, C.; Boninsegna, S.; Monte, R.D.; Carraro, P.; Carturan, G. SiO2 Entrapment of Animal Cells: Liver-Specific Metabolic Activities in Silica-Overlaid Hepatocytes. Artif. Organs 2002, 26, 664–669. [Google Scholar] [CrossRef]
- Carturan, G.; Muraca, M.; Dal Monte, R. Encapsulation of Supported Animal Cells Using Gas-Phase Inorganic Alkoxides. U.S. Patent No. 6,214,593, 10 April 2001. [Google Scholar]
- Matys, S.; Raff, J.; Soltmann, U.; Selenska-Pobell, S.; Böttcher, H.; Pompe, W. Calcium Dipicolinate Induced Germination of Bacillus Spores Embedded in Thin Silica Layers: Novel Perspectives for the Usage of Biocers. Chem. Mater. 2004, 16, 5549–5551. [Google Scholar] [CrossRef]
- Inama, L.; Diré, S.; Carturan, G.; Cavazza, A. Entrapment of viable microorganisms by SiO2 sol-gel layers on glass surfaces: Trapping, catalytic performance and immobilization durability of Saccharomyces cerevisiae. J. Biotechnol. 1993, 30, 197–210. [Google Scholar] [CrossRef]
- Ferrer, M.L.; del Monte, F.; Levy, D. A Novel and Simple Alcohol-Free Sol−Gel Route for Encapsulation of Labile Proteins. Chem. Mater. 2002, 14, 3619–3621. [Google Scholar] [CrossRef]
- Ferrer, M.L.; Yuste, L.; Rojo, F.; del Monte, F. Biocompatible Sol−Gel Route for Encapsulation of Living Bacteria in Organically Modified Silica Matrixes. Chem. Mater. 2003, 15, 3614–3618. [Google Scholar] [CrossRef]
- Coradin, T.; Livage, J. Synthesis and Characterization of Alginate/Silica Biocomposites. J. Sol-Gel Sci. Technol. 2003, 26, 1165–1168. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Chen, Y.; Zhang, Z.; Elowe, N.H.; Brook, M.A.; Brennan, J.D. Ultrasensitive ATP Detection Using Firefly Luciferase Entrapped in Sugar-Modified Sol−Gel-Derived Silica. J. Am. Chem. Soc. 2004, 126, 6878–6879. [Google Scholar] [CrossRef]
- Brasack, I.; Böttcher, H.; Hempel, U. Biocompatibility of Modified Silica-Protein Composite Layers. J. Sol-Gel Sci. Technol. 2000, 19, 479–482. [Google Scholar] [CrossRef]
- Bhatia, R.B.; Brinker, C.J.; Gupta, A.K.; Singh, A.K. Aqueous Sol−Gel Process for Protein Encapsulation. Chem. Mater. 2000, 12, 2434–2441. [Google Scholar] [CrossRef]
- Yu, D.; Volponi, J.; Chhabra, S.; Brinker, C.J.; Mulchandani, A.; Singh, A.K. Aqueous sol–gel encapsulation of genetically engineered Moraxella spp. cells for the detection of organophosphates. Biosens. Bioelectron. 2005, 20, 1433–1437. [Google Scholar] [CrossRef]
- Léonard, A.; Rooke, J.C.; Meunier, C.F.; Sarmento, H.; Descy, J.-P.; Su, B.-L. Cyanobacteria immobilised in porous silica gels: Exploring biocompatible synthesis routes for the development of photobioreactors. Energy Environ. Sci. 2010, 3, 370–377. [Google Scholar] [CrossRef]
- Coradin, T.; Nassif, N.; Livage, J. Silica–alginate composites for microencapsulation. Appl. Microbiol. Biotechnol. 2003, 61, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Desmet, J.; Meunier, C.F.; Danloy, E.P.; Duprez, M.-E.; Hantson, A.-L.; Thomas, D.; Cambier, P.; Rooke, J.C.; Su, B.-L. Green and sustainable production of high value compounds via a microalgae encapsulation technology that relies on CO2 as a principle reactant. J. Mater. Chem. A 2014, 2, 20560–20569. [Google Scholar] [CrossRef]
- Rooke, J.C.; Léonard, A.; Sarmento, H.; Descy, J.-P.; Su, B.-L. Photosynthesis within porous silica gel: Viability and activity of encapsulated cyanobacteria. J. Mater. Chem. 2008, 18, 2833–2841. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, H.; Tran-Minh, C. Sol–gel process for vegetal cell encapsulation. Mater. Sci. Eng. C 2007, 27, 607–611. [Google Scholar] [CrossRef]
- Coiffier, A.; Coradin, T.; Roux, C.; Bouvet, O.M.M.; Livage, J. Sol–gel encapsulation of bacteria: A comparison between alkoxide and aqueous routes. J. Mater. Chem. 2001, 11, 2039–2044. [Google Scholar] [CrossRef]
- Nassif, N.; Coiffier, A.; Coradin, T.; Roux, C.; Livage, J.; Bouvet, O. Viability of Bacteria in Hybrid Aqueous Silica Gels. J. Sol-Gel Sci. Technol. 2003, 26, 1141–1144. [Google Scholar] [CrossRef]
- Nassif, N.; Roux, C.; Coradin, T.; Rager, M.-N.; Bouvet, O.M.M.; Livage, J. A sol–gel matrix to preserve the viability of encapsulated bacteria. J. Mater. Chem. 2003, 13, 203–208. [Google Scholar] [CrossRef]
- Pope, E.J.A. Gel encapsulated microorganisms: Saccharomyces cerevisiae—Silica gel biocomposites. J. Sol-Gel Sci. Technol. 1995, 4, 225–229. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Woolfrey, J.L. Encapsulation of sulfate-reducing bacteria in a silica host. J. Mater. Chem. 2000, 10, 1099–1101. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Xin, J.; Li, S.; Xia, C.; Cui, J. Efficient immobilization of whole cells of Methylomonas sp. strain GYJ3 by sol–gel entrapment. J. Mol. Catal. B: Enzym. 2004, 30, 167–172. [Google Scholar] [CrossRef]
- Brennan, J.D.; Benjamin, D.; DiBattista, E.; Gulcev, M.D. Using Sugar and Amino Acid Additives to Stabilize Enzymes within Sol−Gel Derived Silica. Chem. Mater. 2003, 15, 737–745. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, I.-W. Encapsulation of protein molecules in transparent porous silica matrices via an aqueous colloidal sol–gel process. Acta Mater. 1999, 47, 4535–4544. [Google Scholar] [CrossRef]
- DeSimone, M.F.; De Marzi, M.C.; Copello, G.J.; Fernández, M.M.; Malchiodi, E.L.; Diaz, L.E. Efficient preservation in a silicon oxide matrix of Escherichia coli, producer of recombinant proteins. Appl. Microbiol. Biotechnol. 2005, 68, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Chernev, G.; Samuneva, B.; Djambaski, P.; Kabaivanova, L.; Emanuilova, E.; Salvado, I.M.M.; Fernandes, M.H.; Wu, A. Synthesis and structure of new biomaterials containing silica and chitosan. Phys. Chem. Glasses-Eur. J. Glass Sci. Technol. Part B 2008, 49, 11–14. [Google Scholar]
- Uo, M.; Yamashita, K.; Suzuki, M.; Tamiya, E.; Karube, I.; Makishima, A. Immobilization of Yeast Cells in Porous Silica Carrier with Sol-Gel Process. J. Ceram. Soc. Jpn. 1992, 100, 426–429. [Google Scholar] [CrossRef]
- Bressler, E.; Pines, O.; Goldberg, I.; Braun, S. Conversion of Fumaric Acid to L-Malic by Sol-Gel Immobilized Saccharomyces cerevisiae in a Supported Liquid Membrane Bioreactor. Biotechnol. Prog. 2002, 18, 445–450. [Google Scholar] [CrossRef]
- Perullini, M.; Jobbágy, M.; Moretti, M.B.; García, S.C.; Bilmes, S.A. Optimizing Silica Encapsulation of Living Cells: In Situ Evaluation of Cellular Stress. Chem. Mater. 2008, 20, 3015–3021. [Google Scholar] [CrossRef]
- Rietti-Shati, M.; Ronen, D.; Mandelbaum, R.T. Atrazine degradation by Pseudomonas strain ADP entrapped in sol-gel glass. J. Sol-Gel Sci. Technol. 1996, 7, 77–79. [Google Scholar] [CrossRef]
- Fennouh, S.; Guyon, S.; Jourdat, C.; Livage, J.; Roux, C. Encapsulation of bacteria in silica gels. Comptes Rendus L’académie Sci. —Ser. IIC—Chem. 1999, 2, 625–630. [Google Scholar] [CrossRef]
- Fennouh, S.; Guyon, S.; Livage, J.; Roux, C. Sol-Gel Entrapment of Escherichia coli. J. Sol-Gel Sci. Technol. 2000, 19, 647–649. [Google Scholar] [CrossRef]
- Premkumar, J.R.; Lev, O.; Rosen, R.; Belkin, S. Encapsulation of Luminous Recombinant E. coli in Sol–Gel Silicate Films. Adv. Mater. 2001, 13, 1773–1775. [Google Scholar] [CrossRef]
- Taylor, A.P.; Finnie, K.S.; Bartlett, J.R.; Holden, P.J. Encapsulation of Viable Aerobic Microorganisms in Silica Gels. J. Sol-Gel Sci. Technol. 2004, 32, 223–228. [Google Scholar] [CrossRef]
- Perullini, M.; Amoura, M.; Jobbágy, M.; Roux, C.; Livage, J.; Coradin, T.; Bilmes, S.A. Improving bacteria viability in metal oxide hosts via an alginate-based hybrid approach. J. Mater. Chem. 2011, 21, 8026–8031. [Google Scholar] [CrossRef]
- Perullini, M.; Amoura, M.; Roux, C.; Coradin, T.; Livage, J.; Japas, M.L.; Jobbágy, M.; Bilmes, S.A. Improving silica matrices for encapsulation of Escherichia coli using osmoprotectors. J. Mater. Chem. 2011, 21, 4546–4552. [Google Scholar] [CrossRef]
- Perullini, M.; Jobbágy, M.; Soler-Illia, G.J.A.A.; Bilmes, S.A. Cell Growth at Cavities Created Inside Silica Monoliths Synthesized by Sol−Gel. Chem. Mater. 2005, 17, 3806–3808. [Google Scholar] [CrossRef]
- Ferrer, M.L.; Garcia-Carvajal, Z.Y.; Yuste, L.; Rojo, F.; del Monte, F. Bacteria Viability in Sol−Gel Materials Revisited: Cryo-SEM as a Suitable Tool to Study the Structural Integrity of Encapsulated Bacteria. Chem. Mater. 2006, 18, 1458–1463. [Google Scholar] [CrossRef]
- Baca, H.K.; Ashley, C.; Carnes, E.; Lopez, D.; Flemming, J.; Dunphy, D.; Singh, S.; Chen, Z.; Liu, N.; Fan, H.; et al. Cell-Directed Assembly of Lipid-Silica Nanostructures Providing Extended Cell Viability. Science 2006, 313, 337–341. [Google Scholar] [CrossRef]
- Baca, H.K.; Carnes, E.; Singh, S.; Ashley, C.; Lopez, D.; Brinker, C.J. Cell-Directed Assembly of Bio/Nano Interfaces—A New Scheme for Cell Immobilization. Acc. Chem. Res. 2007, 40, 836–845. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, X.; Wang, Z.; Tao, Y.; Niu, X.; Huang, X.; Liu, L.; Li, Z. Immobilization of Lactobacillus rhamnosus in mesoporous silica-based material: An efficiency continuous cell-recycle fermentation system for lactic acid production. J. Biosci. Bioeng. 2016, 121, 645–651. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, H.; Tran-Minh, C. Fluorescent biosensor using whole cells in an inorganic translucent matrix. Anal. Chim. Acta 2007, 583, 161–165. [Google Scholar] [CrossRef]
- Ferro, Y.; Perullini, M.; Jobbágy, M.; Bilmes, S.A.; Durrieu, C. Development of a Biosensor for Environmental Monitoring Based on Microalgae Immobilized in Silica Hydrogels. Sensors 2012, 12, 16879–16891. [Google Scholar] [CrossRef]
- Sicard, C.; Perullini, M.; Spedalieri, C.; Coradin, T.; Brayner, R.; Livage, J.; Jobbágy, M.; Bilmes, S.A. CeO2 Nanoparticles for the Protection of Photosynthetic Organisms Immobilized in Silica Gels. Chem. Mater. 2011, 23, 1374–1378. [Google Scholar] [CrossRef]
- Perullini, M.; Ferro, Y.; Durrieu, C.; Jobbágy, M.; Bilmes, S.A. Sol–gel silica platforms for microalgae-based optical biosensors. J. Biotechnol. 2014, 179, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Durrieu, C.; Ferro, Y.; Perullini, M.; Gosset, A.; Jobbágy, M.; Bilmes, S.A. Feasibility of using a translucid inorganic hydrogel to build a biosensor using immobilized algal cells. Environ. Sci. Pollut. Res. 2016, 23, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Peña-Vázquez, E.; Maneiro, E.; Pérez-Conde, C.; Moreno-Bondi, M.C.; Costas, E. Microalgae fiber optic biosensors for herbicide monitoring using sol–gel technology. Biosens. Bioelectron. 2009, 24, 3538–3543. [Google Scholar] [CrossRef]
- Ahmed, N.B.; Ronsin, O.; Mouton, L.; Sicard, C.; Yéprémian, C.; Baumberger, T.; Brayner, R.; Coradin, T. The physics and chemistry of silica-in-silicates nanocomposite hydrogels and their phycocompatibility. J. Mater. Chem. B 2017, 5, 2931–2940. [Google Scholar] [CrossRef]
- Pannier, A.; Soltmann, U.; Soltmann, B.; Altenburger, R.; Schmitt-Jansen, M. Alginate/silica hybrid materials for immobilization of green microalgae Chlorella vulgaris for cell-based sensor arrays. J. Mater. Chem. B 2014, 2, 7896–7909. [Google Scholar] [CrossRef]
- Desmet, J.; Meunier, C.; Danloy, E.; Duprez, M.-E.; Lox, F.; Thomas, D.; Hantson, A.-L.; Crine, M.; Toye, D.; Rooke, J.; et al. Highly efficient, long life, reusable and robust photosynthetic hybrid core–shell beads for the sustainable production of high value compounds. J. Colloid Interface Sci. 2015, 448, 79–87. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Wang, L.; Charles, V.; Rooke, J.C.; Su, B.-L. Robust and Biocompatible Hybrid Matrix with Controllable Permeability for Microalgae Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 8939–8946. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A.O. Bioencapsulation within synthetic polymers (Part 1): Sol–gel encapsulated biologicals. Trends Biotechnol. 2000, 18, 282–296. [Google Scholar] [CrossRef]
- Gill, I. Bio-doped nanocomposite polymers: sol−gel bioencapsulates. Chem. Mater. 2001, 13, 3404–3421. [Google Scholar] [CrossRef]
- Trujillo, S.; Pérez-Román, E.; Kyritsis, A.; Ribelles, J.L.G.; Pandis, C. Organic-inorganic bonding in chitosan-silica hybrid networks: Physical properties. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1391–1400. [Google Scholar] [CrossRef]
- Cho, G.; Moon, I.-S.; Lee, J.-S. Preparation and characterization of α-amylase immobilized inorganic/organic hybrid membrane using chitosan as a dispersant in the sol-gel process. Chem. Lett. 1997, 26, 577–578. [Google Scholar] [CrossRef]
- Miao, Y.; Tan, S. Amperometric hydrogen peroxide biosensor with silica sol–gel/chitosan film as immobilization matrix. Anal. Chim. Acta 2001, 437, 87–93. [Google Scholar] [CrossRef]
- Perullini, M.; Rivero, M.M.; Jobbágy, M.; Mentaberry, A.; Bilmes, S.A. Plant cell proliferation inside an inorganic host. J. Biotechnol. 2007, 127, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Coradin, T.; Bah, S.; Livage, J. Gelatine/silicate interactions: From nanoparticles to composite gels. Colloids Surf. B Biointerfaces 2004, 35, 53–58. [Google Scholar] [CrossRef]
- Watzke, H.J.; Dieschbourg, C. Novel silica-biopolymer nanocomposites: The silica sol-gel process in biopolymer organogels. Adv. Colloid Interface Sci. 1994, 50, 1–14. [Google Scholar] [CrossRef]
- Kato, M.; Saruwatari, H.; Sakai-Kato, K.; Toyo’Oka, T. Silica sol–gel/organic hybrid material for protein encapsulated column of capillary electrochromatography. J. Chromatogr. A 2004, 1044, 267–270. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Karpenko, T.Y.Y. Hybrid polysaccharide−silica nanocomposites prepared by the sol−gel technique. Langmuir 2004, 20, 3882–3887. [Google Scholar] [CrossRef]
- Heichal-Segal, O.; Rappoport, S.; Braun, S. Immobilization in Alginate-Silicate Sol-Gel Matrix Protects β-Glucosidase Against Thermal and Chemical Denaturation. Bio/Technology 1995, 13, 798–800. [Google Scholar] [CrossRef]
- Fukushima, Y.; Okamura, K.; Imai, K.; Motai, H. A new immobilization technique of whole cells and enzymes with colloidal silica and alginate. Biotechnol. Bioeng. 1988, 32, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, V.; Schulz, P.C.; de Ferreira, M.E.G.; Morini, M.A. A chitosan-templated monolithic siliceous mesoporous-macroporous material. Colloid Polym. Sci. 2000, 278, 964–971. [Google Scholar] [CrossRef]
- Park, S.-B.; You, J.-O.; Park, H.-Y.; Haam, S.J.; Kim, W.-S. A novel pH-sensitive membrane from chitosan—TEOS IPN; preparation and its drug permeation characteristics. Biomaterials 2001, 22, 323–330. [Google Scholar] [CrossRef]
- Suzuki, T.; Mizushima, Y. Characteristics of silica-chitosan complex membrane and their relationships to the characteristics of growth and adhesiveness of L-929 cells cultured on the biomembrane. J. Ferment. Bioeng. 1997, 84, 128–132. [Google Scholar] [CrossRef]
- Ayers, M.R.; Hunt, A.J. Synthesis and properties of chitosan–silica hybrid aerogels. J. Non-Cryst. Solids 2001, 285, 123–127. [Google Scholar] [CrossRef]
- Hu, X.; Littrell, K.; Ji, S.; Pickles, D.; Risen, W. Characterization of silica–polymer aerogel composites by small-angle neutron scattering and transmission electron microscopy. J. Non-Cryst. Solids 2001, 288, 184–190. [Google Scholar] [CrossRef]
- Molvinger, K.; Quignard, F.; Brunel, D.; Boissière, M.; Devoisselle, J.-M. Porous Chitosan-Silica Hybrid Microspheres as a Potential Catalyst. Chem. Mater. 2004, 16, 3367–3372. [Google Scholar] [CrossRef]
- Rashidova, S.; Shakarova, D.; Ruzimuradov, O.; Satubaldieva, D.; Zalyalieva, S.; Shpigun, O.; Varlamov, V.; Kabulov, B. Bionanocompositional chitosan-silica sorbent for liquid chromatography. J. Chromatogr. B 2004, 800, 49–53. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.W.; Tan, R. Immobilization of glucose oxidase on chitosan–SiO2 gel. Enzym. Microb. Technol. 2004, 34, 126–131. [Google Scholar] [CrossRef]
- Chen, X.; Jia, J.; Dong, S. Organically Modified Sol-Gel/Chitosan Composite Based Glucose Biosensor. Electroanalysis 2003, 15, 608–612. [Google Scholar] [CrossRef]
- Airoldi, C.; Monteiro, O.A.C. Chitosan–organosilane hybrids—Syntheses, characterization, copper adsorption, and enzyme immobilization. J. Appl. Polym. Sci. 2000, 77, 797–804. [Google Scholar] [CrossRef]
- Suzuki, T.; Mizushima, Y.; Umeda, T.; Ohashi, R. Further biocompatibility testing of silica-chitosan complex membrane in the production of tissue plasminogen activator by epithelial and fibroblast cells. J. Biosci. Bioeng. 1999, 88, 194–199. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Pylypchuk, I.V.; Tertykh, V.A.; Yanovska, E.S.; Kołodyńska, D. Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res. Lett. 2015, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Kong, Z.-L.; Hwang, D.-F.; Chang, K.L.B. Chitosan-Catalyzed Aggregation during the Biomimetic Synthesis of Silica Nanoparticles. Chem. Mater. 2005, 18, 702–707. [Google Scholar] [CrossRef]
- Witoon, T.; Chareonpanich, M.; Limtrakul, J. Effect of acidity on the formation of silica–chitosan hybrid materials and thermal conductive property. J. Sol-Gel Sci. Technol. 2009, 51, 146–152. [Google Scholar] [CrossRef]
- Witoon, T.; Chareonpanich, M.; Limtrakul, J. Size control of nanostructured silica using chitosan template and fractal geometry: Effect of chitosan/silica ratio and aging temperature. J. Sol-Gel Sci. Technol. 2010, 56, 270–277. [Google Scholar] [CrossRef]
- Witoon, T.; Tepsarn, S.; Kittipokin, P.; Embley, B.; Chareonpanich, M. Effect of pH and chitosan concentration on precipitation and morphology of hierarchical porous silica. J. Non-Cryst. Solids 2011, 357, 3513–3519. [Google Scholar] [CrossRef]
- Witoon, T.; Chareonpanich, M. Interaction of chitosan with tetraethyl orthosilicate on the formation of silica nanoparticles: Effect of pH and chitosan concentration. Ceram. Int. 2012, 38, 5999–6007. [Google Scholar] [CrossRef]
- Voznesenskiy, S.S.; Popik, A.Y.; Gamayunov, E.L.; Orlova, T.Y.; Markina, Z.V.; Postnova, I.V.; Shchipunov, Y.A.; Markina, V.Z.; Shchipunov, A.Y. One-stage immobilization of the microalga Porphyridium purpureum using a biocompatible silica precursor and study of the fluorescence of its pigments. Eur. Biophys. J. 2018, 47, 75–85. [Google Scholar] [CrossRef]
- Rathnayake, I.V.N.; Munagamage, T.; Pathirathne, A.; Megharaj, M. Whole cell microalgal-cyanobacterial array biosensor for monitoring Cd, Cr and Zn in aquatic systems. Water Sci. Technol. 2021, 84, 1579–1593. [Google Scholar] [CrossRef]
- Ben Ahmed, N.; Masse, S.; Laurent, G.; Piquemal, J.-Y.; Yéprémian, C.; Brayner, R.; Coradin, T. Optical microalgal biosensors for aqueous contaminants using organically doped silica as cellular hosts. Anal. Bioanal. Chem. 2018, 410, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.M.; Pfister, K.; Rahire, M.; Togasaki, R.K.; Mets, L.; Rochaix, J.-D. Molecular and Biophysical Analysis of Herbicide-Resistant Mutants of Chlamydomonas reinhardtii: Structure-Function Relationship of the Photosystem II D1 Polypeptide. Plant Cell 1989, 1, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, K.G.; Kluth, J.F.; Andree, R.; Haug, M.; Lindig, M.; Müller, K.H.; Wroblowsky, H.J.; Trebst, A. The herbicide binding niche of photosystem II-a model. Pestic. Sci. 1991, 31, 65–72. [Google Scholar] [CrossRef]
- Heiss, S.; Johanningmeier, U. Analysis of a herbicide resistant mutant obtained by transformation of the Chlamydomonas chloroplast. Photosynth. Res. 1992, 34, 311–317. [Google Scholar] [CrossRef]
- Johanningmeier, U.; Sopp, G.; Brauner, M.; Altenfeld, U.; Orawski, G.; Oettmeier, W. Herbicide Resistance and Supersensitivity in Ala250 or Ala251 Mutants of the D1 Protein in Chlamydomonas reinhardtii. Pestic. Biochem. Physiol. 2000, 66, 9–19. [Google Scholar] [CrossRef]
- Gosset, A.; Oestreicher, V.; Perullini, M.; Bilmes, S.A.; Jobbágy, M.; Dulhoste, S.; Bayard, R.; Durrieu, C. Optimization of sensors based on encapsulated algae for pesticide detection in water. Anal. Methods 2019, 11, 6193–6203. [Google Scholar] [CrossRef]

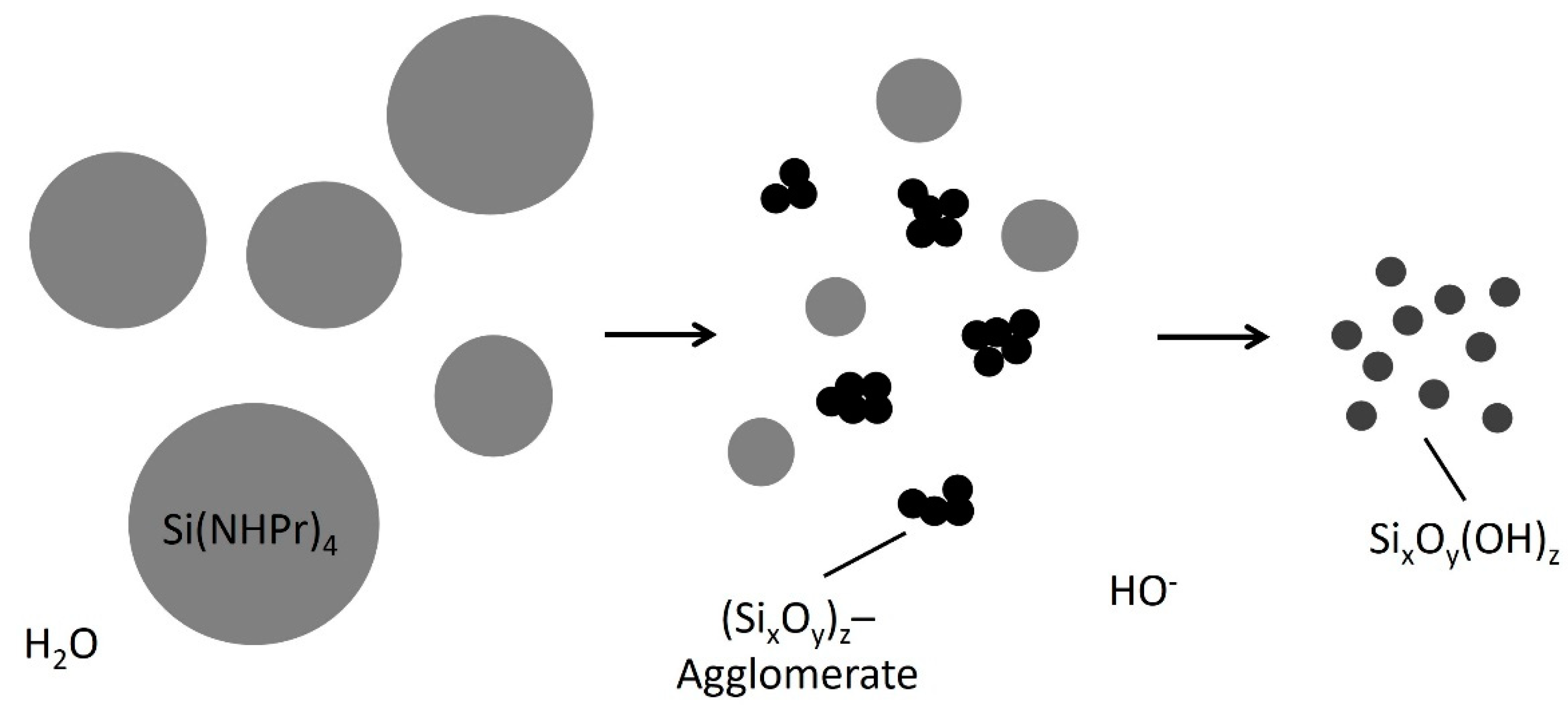

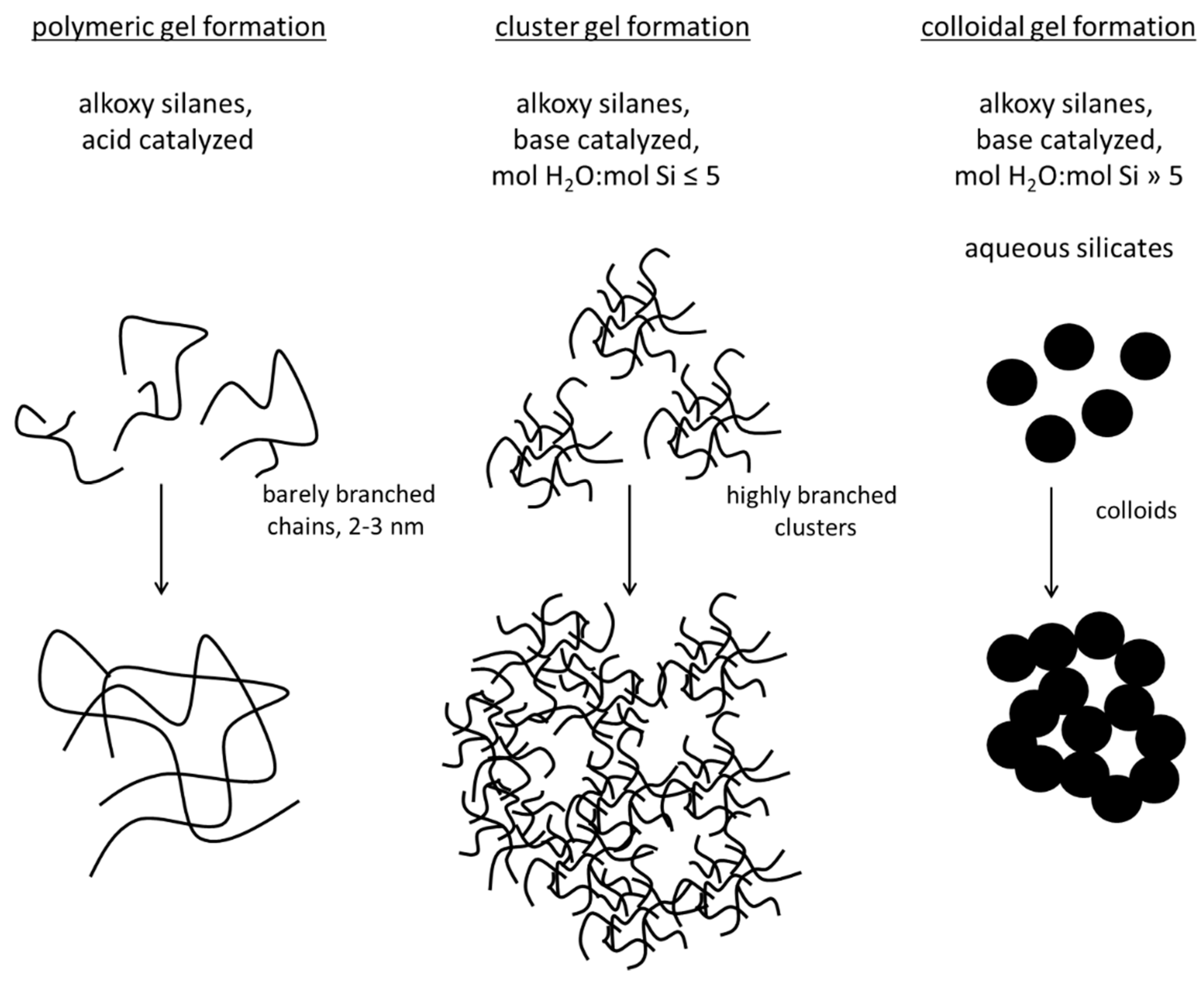
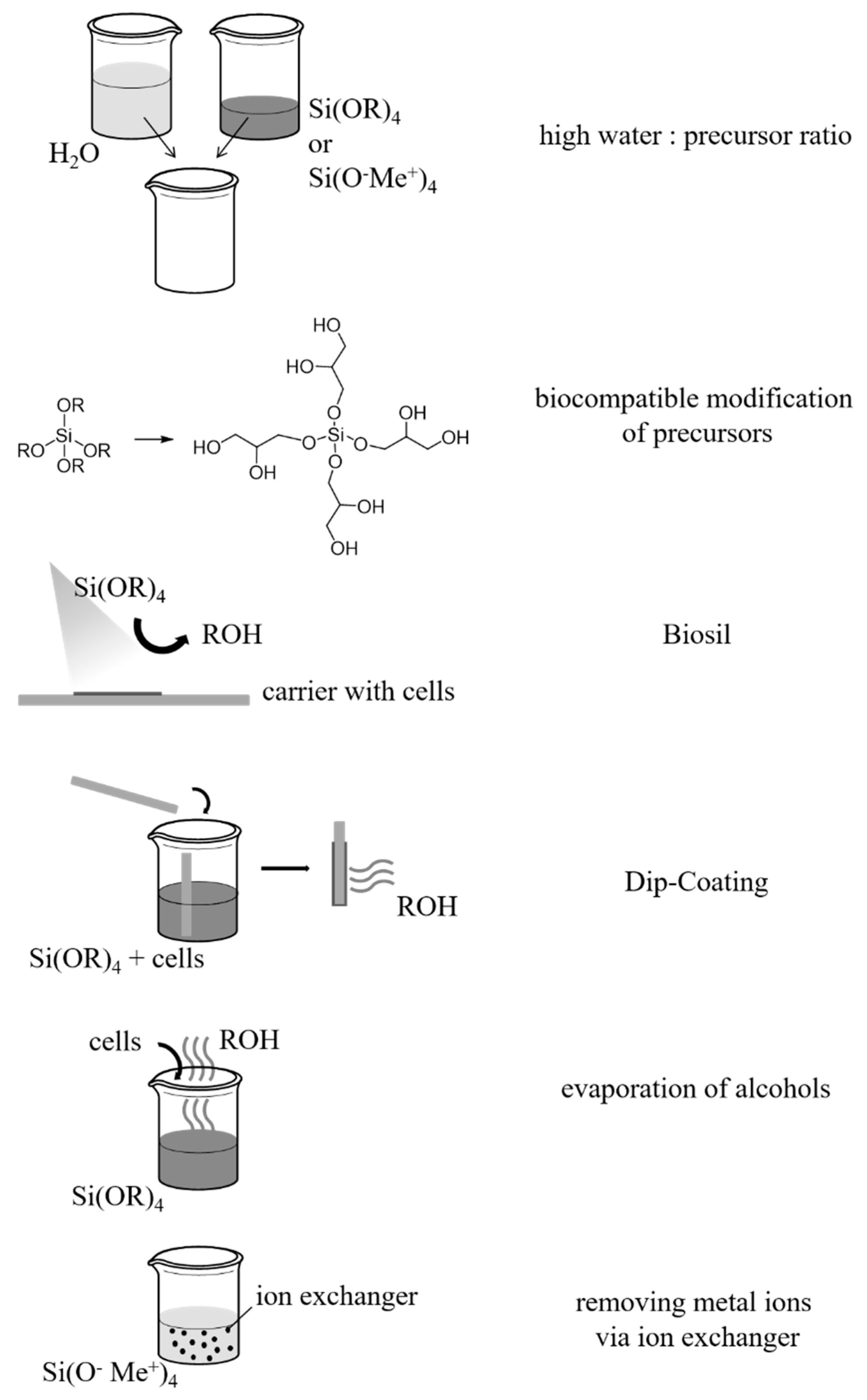
| Silica Precursor and Concentration | Catalysis | pH Adjustment | Additives | Microalgae/ Cyanobacteria | Characteristics/ Viability | Purpose/Aim | Ref. |
|---|---|---|---|---|---|---|---|
| TEOS: 0.45 mol/L/2.27 wt % or 1.79 mol/L/9.25 wt % TEOS/GLYEO: 0.4 mol/L/2.05 wt % | acid catalysis | -- | glycerol, sorbitol, polyether-modified poly-siloxane | Haematococcus pluvialis | entrapped cells viable for more than 40 days | continuous production of the carotinoid dye astaxanthin | [75] |
| TEOS: 1.06–1.70 mol/L/6.46–15.55 wt % TMOS: 1.15–1.97 mol/L/6.87–11.70 wt % MTES: 1.09–1.79 mol/L/6.67–11.14 wt % | acid catalysis with HCl or HNO3 | adjusted to 8 with NaOH or KOH | glycerol or PEG 400 | Synechocystis sp. PCC 6803 wild-type and mutant M55 | H2 production for 5 days similar to free cells | enabling (prolonged) viability and activity for important biotechnological applications, such as biofuels and (secondary) metabolites, here H2 | [46] |
| TEOS: 1.70 mol/L/10.56 wt % | acid catalysis | adjusted by high cell to sol ratio | glycerol or PEG 200 | Synechocystis sp. PCC 6803 | viability, photosynthetic activity over 6 weeks | [76] | |
| MAPTS/TMOS: 2.75 mol/L/17.38 wt %; MTMOS/TMOS/PhTMOS: 2.51 mol/L/15.23 wt % | acid catalysis | -- | -- | Chlorella vulgaris, Anabaena sp. PCC7120 | no viability upon entrapment | electrochemical sensors; bioremediation with non-living tissue | [74] |
| TEOS: 0.23–1.06 mol/L/5–22 wt % | acid catalysis | adjusted to 7.2–7.4 with TRIS | chitosan | Chlamydomonas reinhardtii wildtype cc-124 | photosynthetic activity and growth similar to free cells | continuous production of secondary metabolites (H2) | [52] |
| TEOS: 1.06 mol/L/22 wt % | [53] | ||||||
| Tetrakis(2-hydroxyethyl)orthosilicate: 2.20 mol/L/12.01 wt % | sol synthesis without additional acid or base | gelation at pH 6 | -- | Porphyridium purpureum | immobilization had a stabilizing effect, viability at elevated temperature; pigment fluorescence showed reusability and stability over 2 weeks | whole-cell biosensor for aqueous contaminants | [163] |
| Silica Precursor and Concentration | Catalysis | pH Adjustment | Additives | Microalgae/ Cyanobacteria | Characteristics/ Viability | Purpose/Aim | Ref. |
|---|---|---|---|---|---|---|---|
| Sodium silicate: 0.16 mol/L/1.08 wt % | base catalysis | adjusted to 9 with HCl | -- | Dictyosphaerium chlorelloides, Scenedesmus intermedius, Scenedesmus sp. | chlorophyll fluorescence stable for 3 weeks | whole-cell biosensor for aqueous contaminants | [129] |
| Sodium silicate + LUDOX®: 2.97 mol/L/14.86 wt % | adjusted to 7.5–8.0 with HCl | Mesotaenium sp., Synechococcus sp. | chlorophyll fluorescence; storage time 4 to 8 weeks | [164] | |||
| Sodium silicate + LUDOX®: 3.96 mol/L/18.34 wt % | adjusted to 7 with HCl | glycerol | Chlorella vulgaris CCAP 211/12 | chlorophyll fluorescence, 4 weeks viable | [98] | ||
| activity for 5 weeks | [124] | ||||||
| Sodium silicate + LUDOX®: 0.61 mol/L/3.04 wt % with APTMS: 0.63 mol/L/3.18 wt % with ETES: 0.64 mol/L/ 3.19 wt % | adjusted to 6 with HCl | -- | Anabaena flos-aqua, Chlorella vulgaris, Euglena gracilis | organosilanes enable stable sensitivity to herbicides and metal ions; no investigation of the cells | [165] | ||
| Sodium silicate + LUDOX®: 0.37–2.93 mol/L/ 1.84–14.70 wt % | adjusted to 5–7 with HCl | chlorophyll fluorescence; “best gel” species-specific | biosensors and biotechnological application | [130] | |||
| Sodium silicate + LUDOX®: 5.9 mol/L/27.57 wt % | adjusted to 8 with HCl | glycerol | Synechococcus sp. PCC 6301, PCC 7002, Cyanothece PCC 7418 | viability of cells over 3 months; bioactivity of cells | enabling (prolonged) viability and activity for important biotechnological applications, such as biofuels and (secondary) metabolites | [97] | |
| Sodium silicate + LUDOX®: 3.7 mol/L/8.56 wt % | Synechococcus PCC 6301, PCC 7002, PCC 7418 | chlorophyll intact for several months | [41] | ||||
| Sodium silicate + LUDOX®: 5.9 mol/L/27.57 wt % Sodium silicate: 4.1 mol/L/19 wt % | adjusted to 7–8 with HCl | -- | Cyanidium caldarium SAG 16.91 | proliferation limited; photosynthesis in gels without additives; chlorophyll stable for 4 months | [42] |
| Silica Precursor and Concentration | Catalysis | pH Adjustment | Additives | Microalgae/ Cyanobacteria | Characteristics/ Viability | Purpose/Aim | Ref. |
|---|---|---|---|---|---|---|---|
| Sodium silicate: 4.7 mol/L/21.76 wt %; sodium silicate + SiO2 nanopowder: 5.13 mol/L/25.7 wt % | acid catalysis | adjusted to 6 with KOH | -- | Cyanidium caldarium SAG 16.91 | oxygen production for 75 days | CO2 mitigation, oxygenation of environments, production of secondary metabolites | [44] |
| Chlorella vulgaris SAG 211–11b, Botryococcus braunii SAG 30.81 | viable cells, chlorophyll fluorescence, oxygen production, proliferation limited | [43] | |||||
| Sodium silicate: 0.55 mol/L/4.80 wt % sodium silicate + LUDOX®: 1.02–2.15 mol/L/9.41–23.24 wt % | without LUDOX®: acid catalysis with LUDOX®: base catalysis | adjusted to 7–8 with KOH (without LUDOX®) or HCl (with LUDOX®) | glycerol | Synechococcus sp. PCC 6301 and PCC 7002 | preservation of the photosynthetic pigment of up to 35 weeks; oxygen production for 17 weeks | enabling (prolonged) viability and activity for important biotechnological applications, such as biofuels and (secondary) metabolites | [94] |
| Sodium silicate: 0.3–0.88 mol/L/7–25 wt % | acid catalysis | adjusted to 7.2–7.4 with TRIS | chitosan | Chlamydomonas reinhardtii wildtype cc-124 | photosynthetic activity and growth similar to free cells | continuous production of secondary metabolites (H2) | [52] |
| sodium silicate: 0.88 mol/L/20 wt % | [53] |
| Silica Precursor and Concentration | Catalysis | Reduction of By-Product Concentration | pH Adjustment | Additives | Microalgae/ Cyanobacteria | Characteristics/ Viability | Purpose/Aim | Ref. |
|---|---|---|---|---|---|---|---|---|
| Tetra(n-propylamino)silane: 0.96 mol/L/25 wt % | base catalysis | -- | adjusted to 7 with an unspecified acid | -- | Chlamydomonas reinhardtii wild-type cc-124 | photosynthetic activity drastically reduced over 2 h | entrapment of sensitive material in highly transparent hydrogels | [70] |
| Tetra(n-propylamino)silane: 0.19–0.96 mol/L/5–25 wt % | acid catalysis | removal of propylamine via ion exchanger | adjusted to 7.2–7.4 with TRIS buffer | chitosan | Chlamydomonas reinhardtii wild-type cc-124 | photosynthetic activity and growth of entrapped micro-algae similar to free cells | continuous production of secondary metabolites (H2) | [52] |
| tetra(n-propylamino)silane: 0.96 mol/L/25 wt % | [53] |
| Silica Precursor and Concentration | Catalysis | Reduction of By-Product Concentration | pH Adjustment | Method | Microalgae/ Cyanobacteria | Characteristics/ Viability | Purpose/Aim | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sodium siliate: 0.72 mol/L/ 37.56 wt % | acid catalysis | removal of the sodium ions via ion exchanger | pH adjusted to 5.1 with NaOH | hybrid core-shell beads | Dunaliella tertiolecta | oxygen production and chlorophyll fluorescence show photosynthetic activity for 13 months | enabling (prolonged) viability and activity for important biotechnological applications, like biofuels and (secondary) metabolites | [96] |
| [132] | ||||||||
| Chlamydomonas reinhardtii | viability and cellular functionality for more than 4 months | [133] | ||||||
| TEOS: 1.66 mol/L/ 10.15 wt% | acid catalysis | evaporation of alcohols | adjusted with phosphate buffer pH 7 | two-step entrapment | Chlorella vulgaris | chlorophyll preservation (green intensity) at UV irridation | development of robust silica hydrogels with CeO2 nano- particles that protects encapsulated cells for green energy | [126] |
| adjusted with KOH | Chlorella vulgaris, Pseudokirchneriella subcapitata, Chlamydomonas reinhardtii | cell growth was unaffected by encapsulation | whole-cell biosensor for aqueous contaminants | [127] | ||||
| Sodium silicate + LUDOX®: 7.95 mol/L/ 36.75 wt % | base catalysis | -- | adjusted to 6.5 with HCl | chlorophyll fluorescence; growth in calcium alginate voids was hardly affected | [125] | |||
| chlorophyll fluorescence | [128] | |||||||
| Sodium silicate + LUDOX®: 0.65–2.17 mol/L/3.53–11.04 wt % | Chlorella vulgaris, Pseudokirchneriella subcapitata, | investigation of silica concentration, ratio of precursors, thickness, and cell loading on sensor’s performance | [170] | |||||
| TEOS + diamino-functionalized silane: 0.17 mol/L/3.16 wt % | acid catalysis | evaporation of alcohols | adjusted to 7.5 with HCl | Chlorella vulgaris | activity maintained for 8 weeks; cell growth in alginate voids observed | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homburg, S.V.; Patel, A.V. Silica Hydrogels as Entrapment Material for Microalgae. Polymers 2022, 14, 1391. https://doi.org/10.3390/polym14071391
Homburg SV, Patel AV. Silica Hydrogels as Entrapment Material for Microalgae. Polymers. 2022; 14(7):1391. https://doi.org/10.3390/polym14071391
Chicago/Turabian StyleHomburg, Sarah Vanessa, and Anant V. Patel. 2022. "Silica Hydrogels as Entrapment Material for Microalgae" Polymers 14, no. 7: 1391. https://doi.org/10.3390/polym14071391
APA StyleHomburg, S. V., & Patel, A. V. (2022). Silica Hydrogels as Entrapment Material for Microalgae. Polymers, 14(7), 1391. https://doi.org/10.3390/polym14071391