Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chromatographic Analysis of Olaparib
2.3. Preparation of Olaparib Nanocrystals (NCs)
2.4. Particle Characterization
2.5. Scanning Electron Microscopy (SEM)
2.6. Differential Scanning Calorimetry (DSC)
2.7. ATR-FTIR Spectroscopy
2.8. X-ray Diffraction Study
2.9. Solubility of Pure OLA and Its NCs
2.10. In Vitro Release Study
2.11. Stability Study
2.12. In Vitro Cytotoxicity Studies
2.12.1. Maintenance and Growth of Cells
2.12.2. MTT or Cell Proliferation Assay
2.12.3. P53, Caspase-3 and Caspase-9 Activities by ELISA
2.13. Pharmacokinetic Study
2.14. Chromatographic Analysis
Sample Preparation
2.15. Statistical Analysis
3. Results and Discussion
3.1. Chromatographic Analysis of OLA
3.2. Formulation and Characterizations of OLA-Nanocrystals
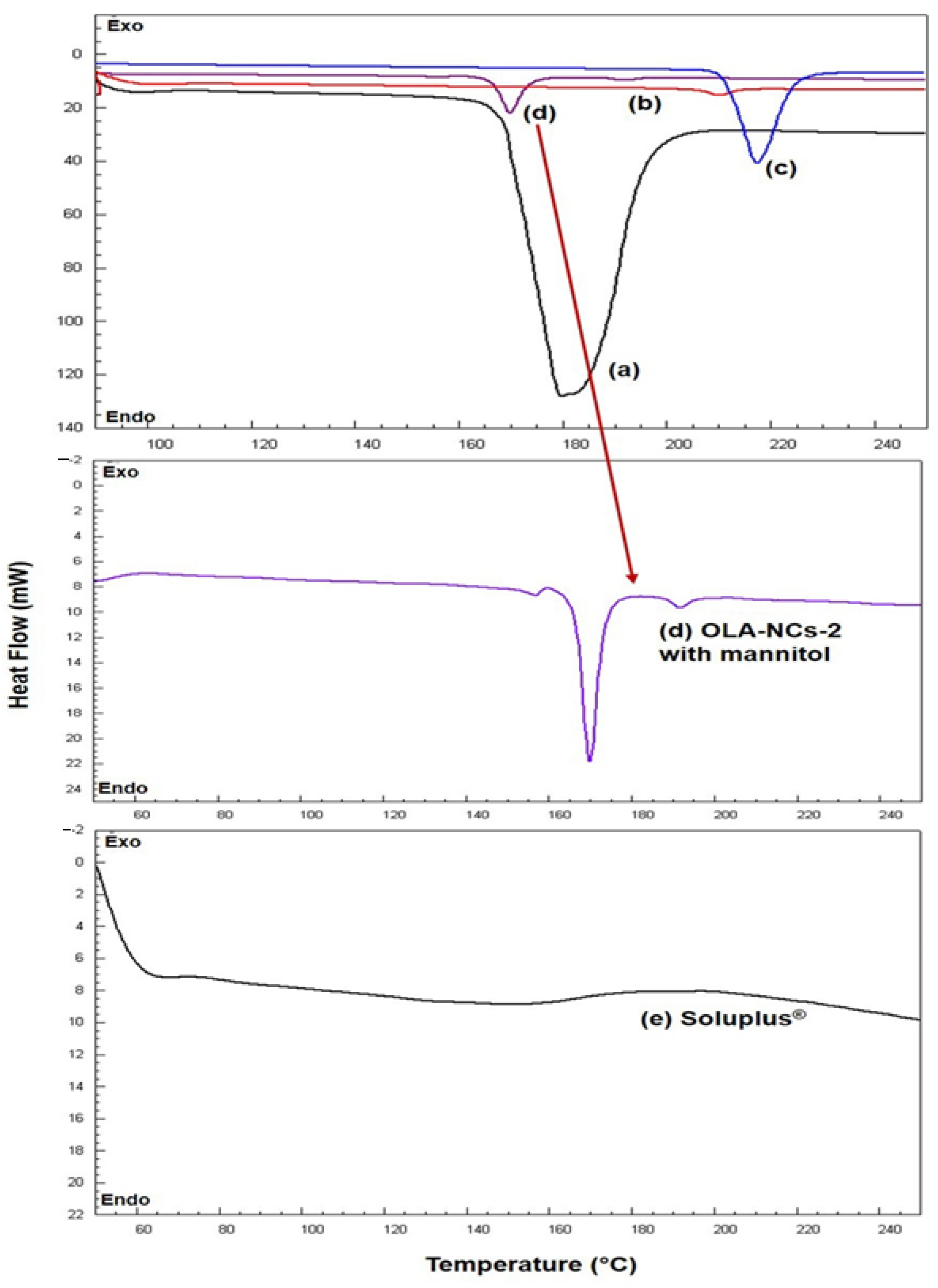
3.3. DSC Analysis
3.4. FTIR Analysis
3.5. XRD Analysis
3.6. Solubility Determination
3.7. In Vitro Drug Release
3.8. Stability Studies
3.9. MTT Assay
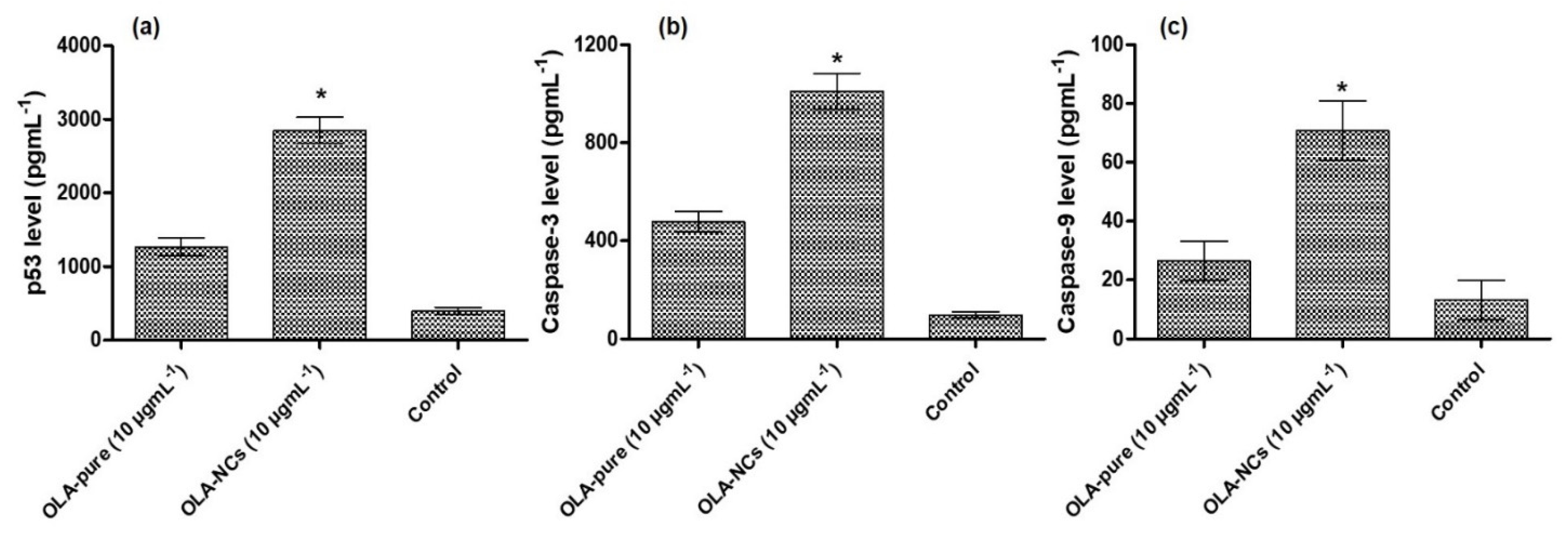
3.10. P53, Caspase-3 and Caspase-9 Assay by ELISA
3.11. In Vivo Pharmacokinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouquet, W.; Ceelen, W.; Fritzinger, B.; Pattyn, P.; Peeters, M.; Remon, J.P.; Vervaet, C. Paclitaxel/β-cyclodextrin complexes for hyperthermic peritoneal perfusion Formulation and stability. Eur. J. Pharm. Biopharm. 2007, 66, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Anwer, K.; Ali, E.A.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Al Saqr, A.; Alalaiwe, A.; Soliman, G.A. Development of Chitosan-Coated PLGA-Based Nanoparticles for Improved Oral Olaparib Delivery: In Vitro Characterization, and In Vivo Pharmacokinetic Studies. Processes 2022, 10, 1329. [Google Scholar] [CrossRef]
- Sun, B.; Yeo, Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr. Opin. Solid State Mater. Sci. 2012, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.W.; Souza, F.E.S.; Bhattacharyya, A.; Khalili, B. Crystalline form of Olaparib. U.S. Patent No. US10662178B2, 1 October 2020. [Google Scholar]
- Alshememry, A.; Alkholief, M.; Kalam, M.A.; Raish, M.; Ali, R.; Alhudaithi, S.S.; Iqbal, M.; Alshamsan, A. Perspectives of Positively Charged Nanocrystals of Tedizolid Phosphate as a Topical Ocular Application in Rabbits. Molecules 2022, 27, 4619. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Colin, P.; Ceelen, W.; Bracke, M.; Van Bocxlaer, J.; Remon, J.P.; Vervaet, C. Development of a Nanocrystalline Paclitaxel Formulation for Hipec Treatment. Pharm. Res. 2012, 29, 2398–2406. [Google Scholar] [CrossRef]
- Pathade, A.D.; Kommineni, N.; Bulbake, U.; Thummar, M.M.; Samanthula, G.; Khan, W. Preparation and Comparison of Oral Bioavailability for Different Nano-formulations of Olaparib. AAPS PharmSciTech 2019, 20, 276. [Google Scholar] [CrossRef]
- Mohammad, I.S.; Hu, H.; Yin, L.; He, W. Drug nanocrystals: Fabrication methods and promising therapeutic applications. Int. J. Pharm. 2019, 562, 187–202. [Google Scholar] [CrossRef]
- Mishra, P.R.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int. J. Pharm. 2009, 371, 182–189. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Sun, W.; Mao, S.; Shi, Y.; Li, L.C.; Fang, L. Nanonization of Itraconazole by High Pressure Homogenization: Stabilizer Optimization and Effect of Particle Size on Oral Absorption. J. Pharm. Sci. 2011, 100, 3365–3373. [Google Scholar] [CrossRef]
- Kassem, M.; Rahman, A.A.; Ghorab, M.M.; Ahmed, M.; Khalil, R.M. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007, 340, 126–133. [Google Scholar] [CrossRef]
- Ganta, S.; Paxton, J.W.; Baguley, B.C.; Garg, S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int. J. Pharm. 2009, 367, 179–186. [Google Scholar] [CrossRef]
- Zhang, H.; Hollis, C.P.; Zhang, Q.; Li, T. Preparation and antitumor study of camptothecin nanocrystals. Int. J. Pharm. 2011, 415, 293–300. [Google Scholar] [CrossRef]
- Singh, S.K.; Srinivasan, K.; Gowthamarajan, K.; Singare, D.S.; Prakash, D.; Gaikwad, N.B. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur. J. Pharm. Biopharm. 2011, 78, 441–446. [Google Scholar] [CrossRef]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef]
- Hardung, H.; Djuric, D.; Ali, S. Combining HME & solubilization: Soluplus®—The solid solution. Drug Deliv. Technol. 2010, 10, 20–27. [Google Scholar]
- Shamma, R.N.; Basha, M. Soluplus®: A novel polymeric solubilizer for optimization of Carvedilol solid dispersions: Formulation design and effect of method of preparation. Powder Technol. 2012, 237, 406–414. [Google Scholar] [CrossRef]
- Daumar, P.; Dufour, R.; Dubois, C.; Penault-Llorca, F.; Bamdad, M.; Mounetou, E. Development and validation of a high-performance liquid chromatography method for the quantitation of intracellular PARP inhibitor Olaparib in cancer cells. J. Pharm. Biomed. Anal. 2018, 152, 74–80. [Google Scholar] [CrossRef]
- Kavitapu, D.; Maruthapillai, A.; Devikala, S.; Selvi, J.A.; Tamilselvi, M.; Mahapatra, S.; Kumar, G.P.; Tyagi, P.K. New Rapid Stability indicating RP-UPLC Method for the Determination of Olaparib, its Related Substances and Degradation Products in Bulk drug and Dosage Form. Mater. Today Proc. 2019, 14, 492–503. [Google Scholar] [CrossRef]
- Nalanda, R.B.; Rao, A.S.; Sankar, D.G. Olaparib Quantification in Human Plasma for Clinical Purposes using High-Performance Liquid Chromatography with UV detector. Anal. Chem. Lett. 2019, 9, 526–534. [Google Scholar] [CrossRef]
- De Waard, H.; Frijlink, H.W.; Hinrichs, W.L. Bottom-Up Preparation Techniques for Nanocrystals of Lipophilic Drugs. Pharm. Res. 2011, 28, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Kong, Y.; Sui, H.; Feng, J.; Zhu, R.; Wang, W. Enhanced oral bioavailability of glycyrrhetinic acid via nanocrystal formulation. Drug Deliv. Transl. Res. 2016, 6, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fu, Y.; Zhang, A.; Wang, X.; Zhao, Z.; Zhang, Y.; Yin, T.; Gou, J.; Wang, Y.; He, H.; et al. Rod-shaped nintedanib nanocrystals improved oral bioavailability through multiple intestinal absorption pathways. Eur. J. Pharm. Sci. 2021, 168, 106047. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Tayade, P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS PharmSciTech 2006, 7, E87–E92. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Sultana, Y.; Ali, A.; Aqil, M.; Mishra, A.K.; Aljuffali, I.A.; Alshamsan, A. Part I: Development and optimization of solid-lipid nanoparticles using Box-Behnken statistical design for ocular delivery of gatifloxacin. J. Biomed. Mater. Res. Part A 2012, 101A, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Alshehri, S.; Alshamsan, A.; Haque, A.; Shakeel, F. Solid liquid equilibrium of an antifungal drug itraconazole in different neat solvents: Determination and correlation. J. Mol. Liq. 2017, 234, 81–87. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In Vitro Dissolution Profile Comparison—Statistics and Analysis of the Similarity Factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Anderson, N.; Bauer, M.; Boussac, N.; Khan-Malek, R.; Munden, P.; Sardaro, M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J. Pharm. Biomed. Anal. 1998, 17, 811–822. [Google Scholar] [CrossRef]
- Samaha, D.; Shehayeb, R.; Kyriacos, S. Modeling and Comparison of Dissolution Profiles of Diltiazem Modified-Release Formulations. Dissolution Technol. 2009, 16, 41–46. [Google Scholar] [CrossRef]
- Kassaye, L.; Genete, G. Evaluation and comparison of in-vitro dissolution profiles for different brands of amoxicillin capsules. Afr. Health Sci. 2013, 13, 369–375. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Kalam, M.A.; Iqbal, M.; Alshememry, A.; Alkholief, M.; Alshamsan, A. Development and Evaluation of Chitosan Nanopar-ticles for Ocular Delivery of Tedizolid Phosphate. Molecules 2022, 27, 2326. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Tomankova, K.; Polakova, K.; Pizova, K.; Binder, S.; Kolarova, M.; Kriegova, E.; Zapletalova, J.; Malina, L.; Horakova, J.; Malohlava, J.; et al. In vitro cytotoxicity analysis of doxorubicin-loaded/superparamagnetic iron oxide colloidal nanoassemblies on MCF7 and NIH3T3 cell lines. Int. J. Nanomed. 2015, 10, 949–961. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Wang, X.; Wang, P.; Zheng, Y.; Yao, D.; Guo, M.; Zhang, L.; Ouyang, L. Crystal structure-based discovery of a novel synthesized PARP1 inhibitor (OL-1) with apoptosis-inducing mechanisms in triple-negative breast cancer. Sci. Rep. 2016, 6, 3. [Google Scholar] [CrossRef]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.-M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.-H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef]
- Shadab; Alhakamy, N.A.; Alharbi, W.S.; Ahmad, J.; Shaik, R.A.; Ibrahim, I.M.; Ali, J. Development and Evaluation of Repurposed Etoricoxib Loaded Nanoemulsion for Improving Anticancer Activities against Lung Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13284. [Google Scholar] [CrossRef]
- Chan, H.-K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, M.V.; Popescu, C. Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 2020, 28, 19–36. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Torino, E. Nanoparticles production by supercritical antisolvent precipitation: A general interpretation. J. Supercrit. Fluids 2007, 43, 126–138. [Google Scholar] [CrossRef]
- Srivalli, K.M.R.; Mishra, B. Drug nanocrystals: A way toward scale-up. Saudi Pharm. J. 2014, 24, 386–404. [Google Scholar] [CrossRef]
- Saengsorn, K.; Jimtaisong, A. Determination of hydrophilic–lipophilic balance value and emulsion properties of sacha inchi oil. Asian Pac. J. Trop. Biomed. 2017, 7, 1092–1096. [Google Scholar] [CrossRef]
- Liu, F.; Park, J.-Y.; Zhang, Y.; Conwell, C.; Liu, Y.; Bathula, S.R.; Huang, L. Targeted Cancer Therapy with Novel High Drug-Loading Nanocrystals. J. Pharm. Sci. 2010, 99, 3542–3551. [Google Scholar] [CrossRef]
- Sharma, M.; Mehta, I. Surface stabilized atorvastatin nanocrystals with improved bioavailability, safety and antihyperlipidemic potential. Sci. Rep. 2019, 9, 16105. [Google Scholar]
- Gombás, Á.; Szabó-Révész, P.; Regdon, G.; Erős, I. Study of thermal behaviour of sugar alcohols. J. Therm. Anal. Calorim. 2003, 73, 615–621. [Google Scholar] [CrossRef]
- Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.K.; Aboudzadeh, M.A. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers 2021, 13, 2272. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Anwer, K.; Ahmed, M.M.; Fatima, F.; Soliman, G.A.; Bhatia, S.; Zafar, A.; Aboudzadeh, M.A. Enhanced Dissolution of Sildenafil Citrate Using Solid Dispersion with Hydrophilic Polymers: Physicochemical Characterization and In Vivo Sexual Behavior Studies in Male Rats. Polymers 2021, 13, 3512. [Google Scholar] [CrossRef]
- Lan, Y.; Ali, S.; Langley, N.J.B.C.; Ingredients, P.; Services, T. Characterization of Soluplus by FTIR and Raman Spectroscopy; BASF: New York, NY, USA, 2010. [Google Scholar]
- Djuris, J.; Nikolakakis, I.; Ibric, S.; Djuric, Z.; Kachrimanis, K. Preparation of carbamazepine–Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug–polymer miscibility by thermodynamic model fitting. Eur. J. Pharm. Biopharm. 2013, 84, 228–237. [Google Scholar] [CrossRef]
- Bayón, R.; Storage, C.-P.C.S.S.U.-T.; Rojas, E. Feasibility study of D-mannitol as phase change material for thermal storage. AIMS Energy 2017, 5, 404–424. [Google Scholar] [CrossRef]
- Krüger, A.; Bürkle, A.; Hauser, K.; Mangerich, A. Real-time monitoring of PARP1-dependent PARylation by ATR-FTIR spectroscopy. Nat. Commun. 2020, 11, 2174. [Google Scholar] [CrossRef]
- Pathi, S.L.; Chennuru, R.; Bollineni, M. Olaparib Co-Crystals and Process of Preparation Thereof. WIPO (PCT) Patent No. WO2021044437A1, 3 November 2021. [Google Scholar]
- Salmani, J.M.M.; Lv, H.; Asghar, S.; Zhou, J. Amorphous solid dispersion with increased gastric solubility in tandem with oral disintegrating tablets: A successful approach to improve the bioavailability of atorvastatin. Pharm. Dev. Technol. 2014, 20, 465–472. [Google Scholar] [CrossRef]
- Hattori, Y.; Haruna, Y.; Otsuka, M. Dissolution process analysis using model-free Noyes–Whitney integral equation. Colloids Surf. B Biointerfaces 2013, 102, 227–231. [Google Scholar] [CrossRef]
- Lawlor, D.; Martin, P.; Busschots, S.; Thery, J.; O’leary, J.J.; Hennessy, B.T.; Stordal, B. PARP Inhibitors as P-glyoprotein Substrates. J. Pharm. Sci. 2014, 103, 1913–1920. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent Review of Manufacturing Routes to Recently Approved PARP Inhibitors: Olaparib, Rucaparib, and Niraparib. Org. Process Res. Dev. 2017, 21, 1227–1244. [Google Scholar] [CrossRef]
- Kim, M.; Heinrich, F.; Haugstad, G.; Yu, G.; Yuan, G.; Satija, S.K.; Zhang, W.; Seo, H.S.; Metzger, J.M.; Azarin, S.M.J.L. Spatial Distribution of PEO–PPO–PEO Block Copolymer and PEO Homopolymer in Lipid Bilayers. Langmuir 2020, 36, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Zana, R.; Marques, C.; Johner, A. Dynamics of micelles of the triblock copolymers poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) in aqueous solution. Adv. Colloid Interface Sci. 2006, 123–126, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Binkhathlan, Z.; Ali, R.; Qamar, W.; Al-Lawati, H.; Lavasanifar, A. Pharmacokinetic and Tissue Distribution of Orally Administered Cyclosporine A-Loaded poly(ethylene oxide)-block-Poly(epsilon-caprolactone) Micelles versus Sandimmune® in Rats. Pharm. Res. 2021, 38, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, P.; Barthelemy, C.; Piva, F.; Joiris, E.; Palmieri, G.F.; Martelli, S. Improved Dissolution Behavior of Fenbufen by Spherical Crystallization. Drug Dev. Ind. Pharm. 1999, 25, 1073–1081. [Google Scholar] [CrossRef]
- Kawashimax, Y.; Aoki, S.; Takenaka, H.; Miyake, Y. Preparation of Spherically Agglomerated Crystals of Aminophylline. J. Pharm. Sci. 1984, 73, 1407–1410. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef]
- Gora, S.; Mustafa, G.; Sahni, J.K.; Ali, J.; Baboota, S. Nanosizing of valsartan by high pressure homogenization to produce dissolution enhanced nanosuspension: Pharmacokinetics and pharmacodyanamic study. Drug Deliv. 2014, 23, 940–950. [Google Scholar] [CrossRef]
- Ezquer-Garin, C.; Ferriols-Lisart, R.; Martinez-López, L.M.; Sangrador-Pelluz, C.; Nicolás-Picó, J.; Alós-Almiñana, M. Stability of tedizolid phosphate-sodium rifampicin and tedizolid phosphate-meropenem admixtures in intravenous infusion bags stored at different temperatures. Die Pharm. 2020, 75, 172–176. [Google Scholar]
- Zhang, J.; Lv, H.; Jiang, K.; Gao, Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int. J. Pharm. 2011, 420, 180–188. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, S.; Yuan, M.; Li, Q.; Xiao, G.; Wu, W.; Ouyang, Y.; Huang, L.; Yao, C. PARP inhibitor re-sensitizes Adriamycin resistant leukemia cells through DNA damage and apoptosis. Mol. Med. Rep. 2018, 19, 75–84. [Google Scholar] [CrossRef]
- Los, M.J.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.-Q.; Schulze-Osthoff, K. Activation and Caspase-mediated Inhibition of PARP: A Molecular Switch between Fibroblast Necrosis and Apoptosis in Death Receptor Signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef]
- EMA. CHMP Assessment Report; Committee for Medicinal Products for Human Use (CHMP); EMA/CHMP/789139/2014; European Medicines Agency: London, UK, 2014; pp. 1–187. [Google Scholar]








| Formulations | Amount of | Physical Characterization (Mean ± SD, n = 3) | Drug Content (%) | |||
|---|---|---|---|---|---|---|
| OLA (mg) | Soluplus (%, w/v) | Particle Size (nm) | Polydispersity INDEX | Zeta Potential (mV) | ||
| NCs-1 | 25 mg | 12.5 mg (0.25%) | 176.03 ± 8.81 | 0.202 ± 0.021 | +8.59 ± 0.84 | 94.54 ± 3.95 |
| NCs-2 | 25 mg | 25 mg (0.5%) | 92.43 ± 7.02 | 0.056 ± 0.091 | +8.46 ± 1.27 | 98.67 ± 1.68 |
| OLA-AqS | 25 mg | Tween-80 (25 mg) in 10 mL of PBS | 402.63 ± 13.52 | 0.421 ± 0.064 | −4.06 ± 0.16 | 91.65 ± 3.21 |
| Parameters | Before Freeze-Drying | After Freeze-Drying with Mannitol |
|---|---|---|
| Size (nm) | 92.43 ± 7.02 | 103.13 ± 9.41 |
| Polydispersity index | 0.056 ± 0.091 | 0.104 ± 0.061 |
| Zeta Potential (mV) | +8.46 ± 1.27 | +8.67 ± 1.25 |
| Drug content (%) | 98.67 ± 1.68 | 98.52 ± 1.86 |
| Release Models | At pH 1.2 | At pH 6.8 | ||||
|---|---|---|---|---|---|---|
| R2 Values | Slope | RE | R2 Values | Slope | RE | |
| Zero order (Fraction drug released vs. Time) | 0.7497 | 0.0215 | NA | 0.8251 | 0.0349 | NA |
| First order (Log% Drug remaining vs. Time) | 0.8035 | 0.0142 | 0.0162 | 0.9592 | 0.0372 | 0.0062 |
| Korsmeyer–Peppas (Log Fraction drug released vs. log Time) | 0.9541 | 0.6413 | 0.2818 | 0.9764 | 0.6491 | 0.2784 |
| Hixon–Crowell (Mo1/3–Mt1/3 vs. Time) | 0.7861 | 0.0094 | NA | 0.9208 | 0.0206 | NA |
| Stability of OLA-NCs-2 | At Different Time Points (Mean ± SD, n = 3) | ||||
|---|---|---|---|---|---|
| Parameters | Initially | At 7th Day | At 1-Month | At 3-Month | At 6-Month |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| At 4 ± 2 °C | |||||
| Size (nm) | 103.13 ± 9.41 | 103.23 ± 9.43 | 104.33 ± 9.86 | 106.60 ± 10.85 | 108.93 ± 11.03 |
| Polydispersity index | 0.104 ± 0.061 | 0.106 ± 0.062 | 0.109 ± 0.061 | 0.131 ± 0.079 | 0.146 ± 0.084 |
| Zeta potentials (mV) | +8.67 ± 1.25 | +8.57 ± 1,25 | +8.46 ± 1.26 | +8.30 ± 1.15 | 8.31 ± 1.16 |
| Drug content (%) | 98.53 ± 1.86 | 98.44 ± 0.81 | 98.34 ± 1.74 | 98.24 ± 0.72 | 97.69 ± 1.56 |
| At 25 ± 1 °C | |||||
| Size (nm) | 103.13 ± 9.41 | 103.5 ± 9.56 | 106.43 ± 10.31 | 109.06 ± 9.96 | 111.53 ± 10.57 |
| Polydispersity index | 0.104 ± 0.061 | 0.108 ± 0.061 | 0.131 ± 0.074 | 0.137 ± 0.075 | 0.163 ± 0.081 |
| Zeta potentials (mV) | +8.67 ± 1.25 | +8.63 ± 1.11 | +8.53 ± 1.12 | +8.33 ± 1.09 | +8.17 ± 1.04 |
| Drug content (%) | 98.53 ± 1.86 | 98.04 ± 1.53 | 97.82 ± 1.37 | 97.41 ± 1.29 | 97.01 ± 1.04 |
| At 40 ± 1 °C | |||||
| Size (nm) | 103.13 ± 9.41 | 105.37 ± 9.71 | 107.26 ± 10.35 | 109.43 ± 10.64 | 112.87 ± 11.28 |
| Polydispersity index | 0.104 ± 0.061 | 0.115 ± 0.064 | 0.132 ± 0.076 | 0.139 ± 0.076 | 0.167 ± 0.082 |
| Zeta potentials (mV) | +8.67 ± 1.25 | +8.53 ± 1.11 | +8.43 ± 1.12 | +8.37 ± 1.27 | +8.16 ± 1.21 |
| Drug content (%) | 98.53 ± 1.86 | 98.01 ± 1.51 | 97.72 ± 1.49 | 97.68 ± 1.55 | 97.67 ± 1.58 |
| Pharmacokinetic Parameters | For OLA-Pure (Mean ± SD, n = 3) | For OLA-NCs (Mean ± SD, n = 3) | Enhancement Ratio |
|---|---|---|---|
| t1/2 (h) | 1.36 ± 0.04 | 1.87 ± 0.11 | 1.36 |
| Tmax (h) | 2.00 ± 0.00 | 2.00 ± 0.00 | Same |
| Cmax (ng·mL−1) | 278.08 ± 46.52 | 571.51 ± 79.01 * | 2.06 |
| AUC0–12 h (ng·mL−1·h) | 882.04 ± 136.74 | 2022.19 ± 200.67 * | 2.29 |
| AUC0-∞ (ng·mL−1·h) | 913.37 ± 137.56 | 2058.04 ± 197.62 * | 2.25 |
| AUMC0-∞ (ng·mL−1·h2) | 3081.17 ± 471.32 | 8093.86 ± 700.68 * | 2.62 |
| MRT0-∞ (h) | 3.37 ± 0.03 | 3.94 ± 0.04 | 1.16 |
| Cl/F (L·h−1) | 6.94 ± 0.96 | 3.05 ± 0.28 | 2.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alali, A.S.; Kalam, M.A.; Ahmed, M.M.; Aboudzadeh, M.A.; Alhudaithi, S.S.; Anwer, M.K.; Fatima, F.; Iqbal, M. Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor. Polymers 2022, 14, 4827. https://doi.org/10.3390/polym14224827
Alali AS, Kalam MA, Ahmed MM, Aboudzadeh MA, Alhudaithi SS, Anwer MK, Fatima F, Iqbal M. Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor. Polymers. 2022; 14(22):4827. https://doi.org/10.3390/polym14224827
Chicago/Turabian StyleAlali, Amer S., Mohd Abul Kalam, Mohammed Muqtader Ahmed, M. Ali Aboudzadeh, Sulaiman S. Alhudaithi, Md. Khalid Anwer, Farhat Fatima, and Muzaffar Iqbal. 2022. "Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor" Polymers 14, no. 22: 4827. https://doi.org/10.3390/polym14224827
APA StyleAlali, A. S., Kalam, M. A., Ahmed, M. M., Aboudzadeh, M. A., Alhudaithi, S. S., Anwer, M. K., Fatima, F., & Iqbal, M. (2022). Nanocrystallization Improves the Solubilization and Cytotoxic Effect of a Poly (ADP-Ribose)-Polymerase-I Inhibitor. Polymers, 14(22), 4827. https://doi.org/10.3390/polym14224827











