Physicochemical and Functional Properties of Modified KJ CMU-107 Rice Starches as Pharmaceutical Excipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Starch
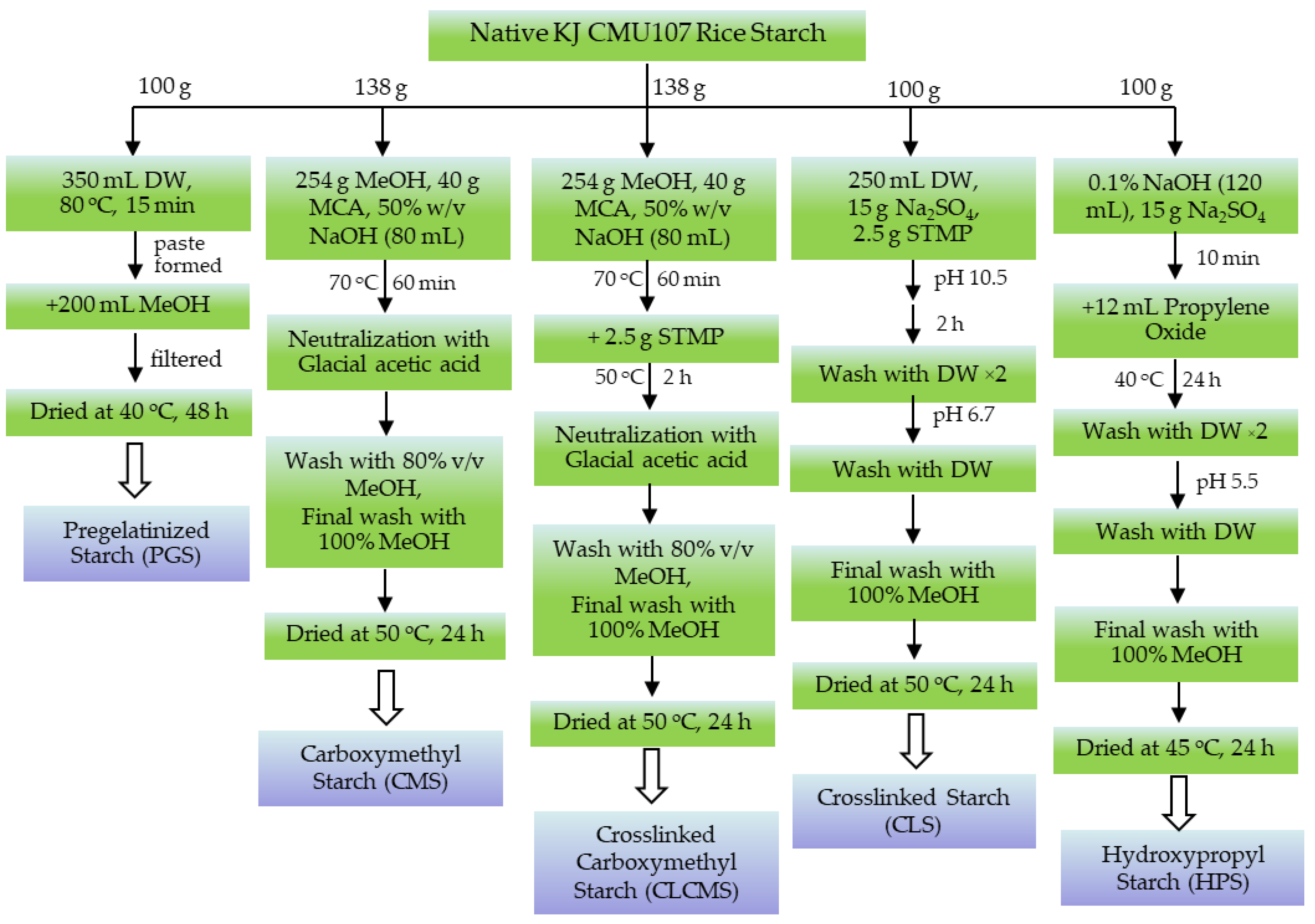
2.3. Preparation of Modified Rice Starches
2.4. Physicochemical Property Evaluation
2.4.1. Amylose Content
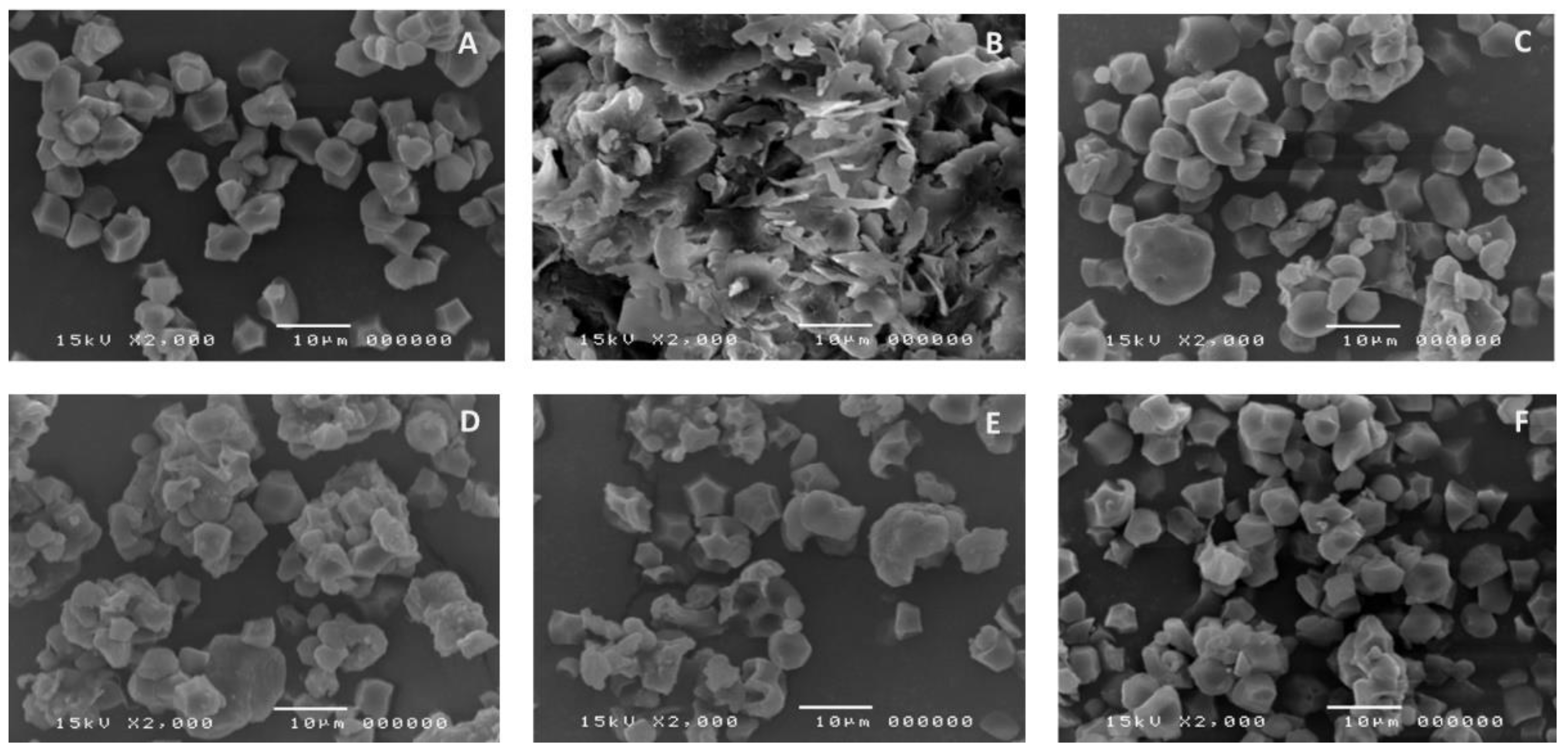
2.4.2. Scanning Electron Microscopic (SEM) with Energy-Dispersive X-ray Analysis
2.4.3. X-ray Diffraction
2.4.4. Water Solubility and Swelling Power at 70 °C
2.4.5. Free-Swelling Capacity at 37 °C
2.4.6. Moisture Content
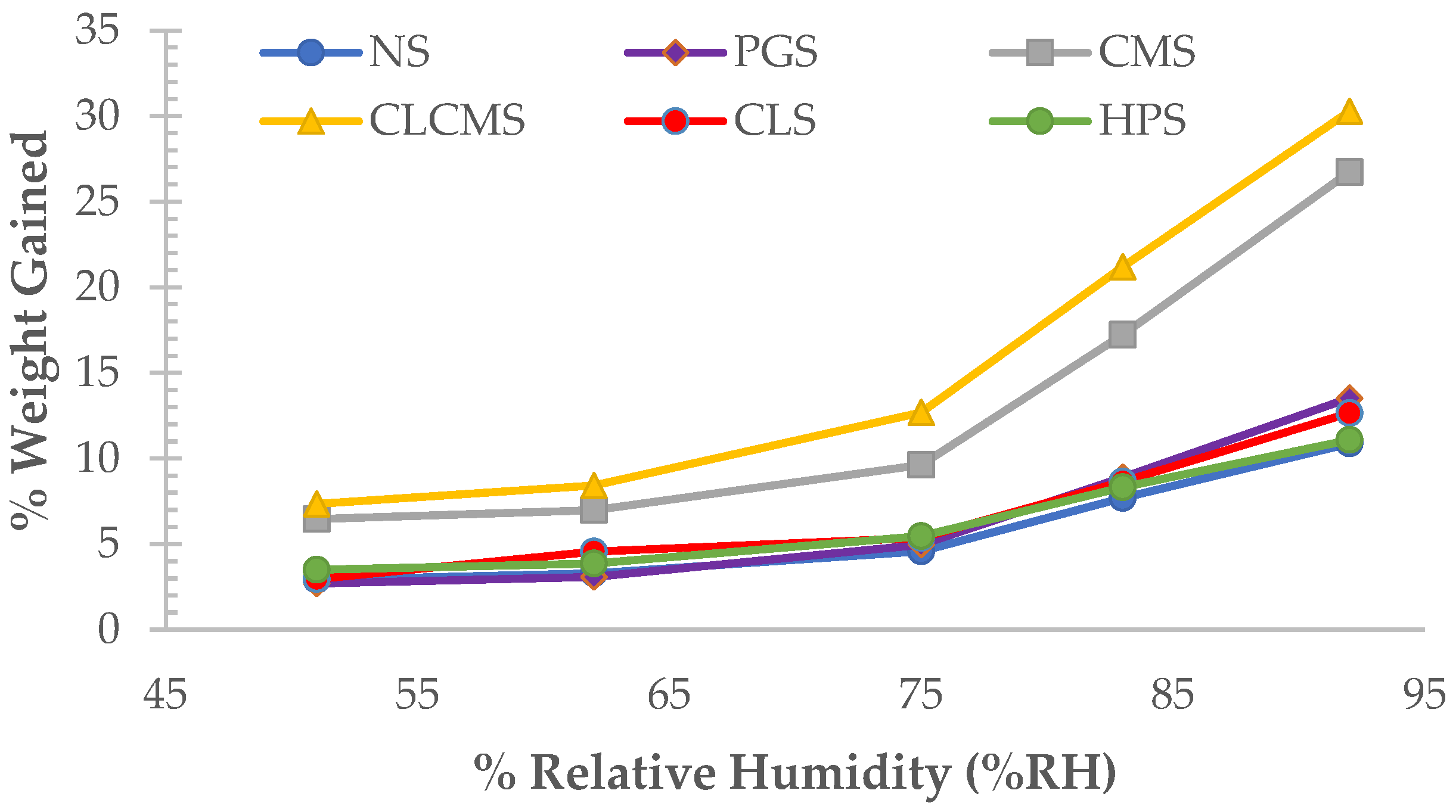
2.4.7. Moisture Sorption
2.4.8. Oil Absorption Capacity (OAC)
2.4.9. Thermal Properties
2.5. Pharmaceutical Functionality Evaluation
2.5.1. Density and Powder Flow
2.5.2. Powder Compactibility
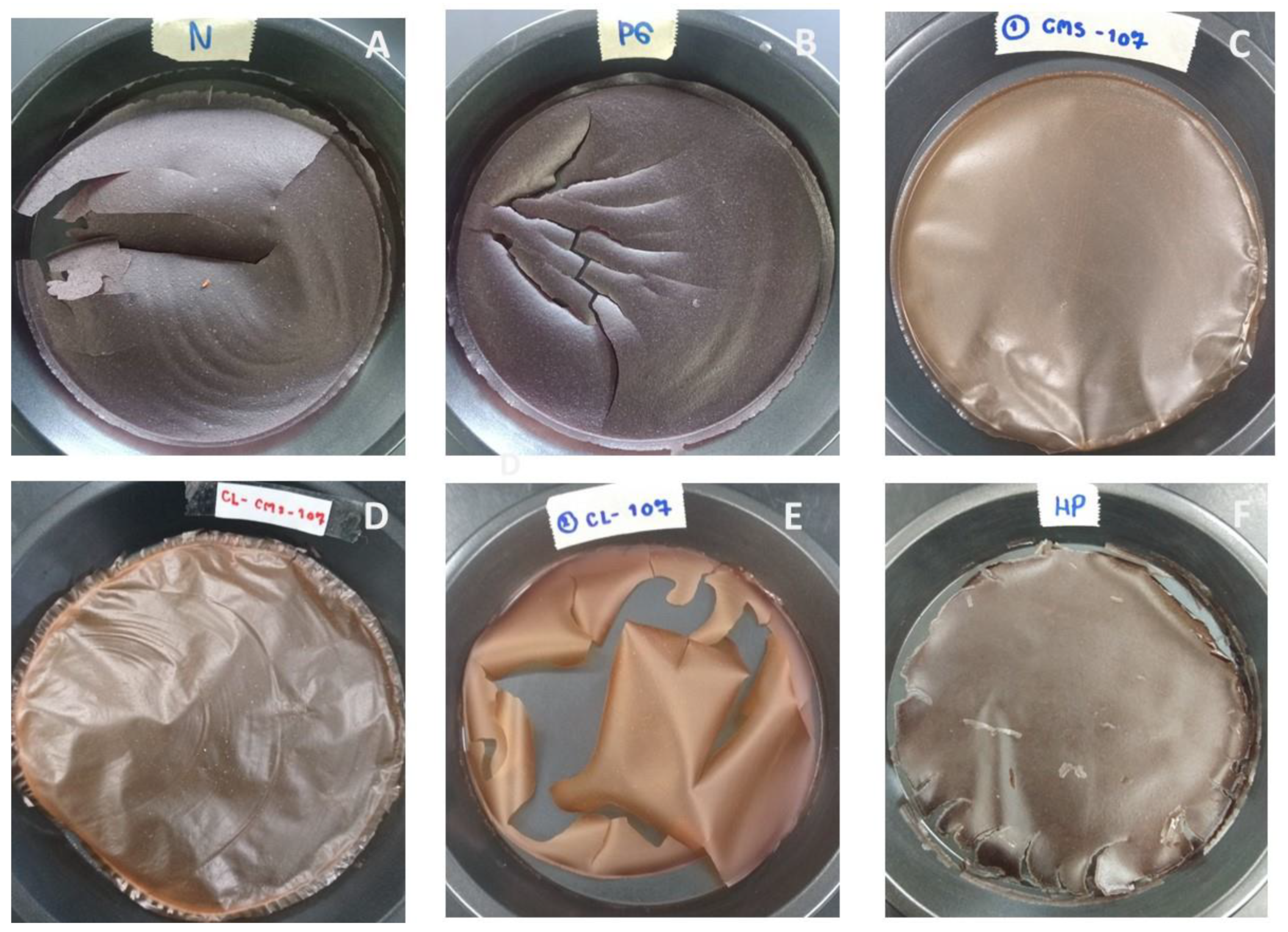
2.5.3. Film Forming Ability and Tensile Strength Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Amylose Content and General Appearances
3.2. SEM and EDX
3.3. X-ray Diffraction
3.4. Swelling Power and Solubility
3.5. Free Swelling Capacity
3.6. Oil Absorption Capacity
3.7. Moisture Content and Moisture Sorption
3.8. Thermal Properties
3.9. Pharmaceutical Functionality
3.9.1. Density and Powder Flow
3.9.2. Powder Compactibility
3.10. Film Forming Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and biomedical applications of native and modified starch: A review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O.O. Physicochemical and binder properties of starch obtained from Cyperus esculentus. AAPS PharmSciTech 2012, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kunle, O.O. Starch source and its impact on pharmaceutical applications. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Zhang, J.; Cheng, F. Effects of starches from different botanical sources and modification methods on physicochemical properties of starch-based edible films. Int. J. Biol. Macromol. 2019, 132, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.A.; Ispas-Szabo, P.; Mateescu, M.A. Structure-functions relationship of modified starches for pharmaceutical and biomedical applications. Starch-Stärke 2020, 72, 2000002. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of starch-based materials using current modification techniques and their applications: A review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Egharevba, H.O. Chemical properties of starch and its application in the food industry. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020; pp. 1–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, G. The preparation of modified nano-starch and its application in food industry. Food Res. Int. 2021, 140, 110009. [Google Scholar] [CrossRef]
- Zhi, K.; Wang, R.; Wei, J.; Shan, Z.; Shi, C.; Xia, X. Self-assembled micelles of dual-modified starch via hydroxypropylation and subsequent debranching with improved solubility and stability of curcumin. Food Hydrocoll. 2021, 118, 106809. [Google Scholar] [CrossRef]
- Azelis. Starch as a multi-functional additive in cosmetics. Sci. Beauty 2017, 6, 12–14. [Google Scholar]
- Rice Starch Market in 2022: 4.9% CAGR with Top Countries Data, Things to Focus on to Ensure Long-term Success up to 2027. Available online: https://www.marketwatch.com/press-release/rice-starch-market-in-2022–49-cagr-with-top-countries-data-things-to-focus-on-to-ensure-long-term-success-up-to-2027-latest-113-pages-report-2022–01–20 (accessed on 16 February 2022).
- Iftikhar, S.A.; Dutta, H. Use of raw and physically modified rice starches as fat replacer in whipping cream. Curr. Res. Nutr. Food Sci. 2020, 8, 122–130. [Google Scholar] [CrossRef]
- Park, H.R.; Kang, J.; Rho, S.J.; Kim, Y.R. Structural and physicochemical properties of enzymatically modified rice starch as influenced by the degree of enzyme treatment. J. Carbohydr. Chem. 2020, 39, 250–266. [Google Scholar] [CrossRef]
- Buddhasri, G.; Prom-u-Thai, C.T.; Pusadee, T.; Jamjod, S. Evaluation of advanced purple rice lines with photoperiod insensitive from Kum Doi Saket and Pathum Thani 1 for yield and grain anthocyanin content. Khon Kean Agric. J. 2021, 49, 1450–1464. [Google Scholar]
- Yamuangmorn, S.; Prom-u-Thai, C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants 2021, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Kittipongpatana, O.S.; Chaitep, W.; Kittipongpatana, N.; Laenger, R.; Sriroth, K. Physicochemical and pharmaceutical properties of carboxymethyl rice starches modified from native starches with different amylose content. Cereal Chem. 2007, 84, 331–336. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Kittipongpatana, N. Preparation and physicochemical properties of modified jackfruit starches. LWT Food Sci. Tech. 2011, 44, 1766–1773. [Google Scholar] [CrossRef]
- Johnson, D.P. Spectrophotometric determination of the hydroxypropyl groups in starch ethers. Anal. Chem. 1969, 41, 859–860. [Google Scholar] [CrossRef]
- Gibson, T.S.; Solah, V.A.; McCleary, B.V. A procedure to measure amylose in cereal starches and flours with concanavalin A. J. Cereal Sci. 1997, 25, 111–119. [Google Scholar] [CrossRef]
- Heß, C.; Hartmann, B.; Lechner, M.D.; Nierling, W.; Seidel, C.; Kulicke, W.M. Influence of soluble polymer residues in crosslinked carboxymethyl starch on some physical properties of its hydrogels. Starch-Stärke 2007, 59, 425–429. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohyd. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Standardized Tapped Density Testing for Pharmaceutical Powders. Available online: https://www.news-medical.net/whitepaper/20211208/Standardized-Tapped-Density-Testing-for-Pharmaceutical-Powders.aspx (accessed on 16 February 2022).
- Ma, H.; Liu, M.; Liang, Y.; Zheng, X.; Sun, L.; Dang, W.; Li, J.; Li, L.; Liu, C. Research progress on properties of pre-gelatinized starch and its application in wheat flour products. Grain Oil Sci. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Kittipongpatana, N. Physicochemical, in vitro digestibility and functional properties of carboxymethyl rice starch cross-linked with epichlorohydrin. Food Chem. 2013, 141, 1438–1444. [Google Scholar] [CrossRef]
- Heo, H.; Lee, Y.-K.; Chang, Y.-H. Rheological, pasting, and structural properties of potato starch by cross-linking. Int. J. Food Prop. 2017, 20, 2138–2150. [Google Scholar] [CrossRef]
- Charoenthai, N.; Sanga-ngam, T.; Kasemwong, K.; Sungthongjee, S.; Puttipipatkhachorn, S. Characterization of hydroxypropyl tapioca starch and its pregelatinized starch as tablet disintegrants. Starch-Starke 2022, in press. [Google Scholar] [CrossRef]
- Lefnaoui, S.; Moulai-Mostefa, N. Synthesis and evaluation of the structural and physicochemical properties of carboxymethyl pregelatinized starch as a pharmaceutical excipient. Saudi Pharm. J. 2015, 23, 698–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.-T.; Kasemsuwan, T.; Jane, J.-L. Characterization of phosphorous in starch by 31P-nuclear magnetic resonance spectroscopy. Cereal Chem. 1994, 71, 488–493. [Google Scholar]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; de Mello El Halal, S.L.; Lim, L.-T.; Dias, A.R.G.; da Rosa Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Alcazar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. Camp. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and chemical modifications in starch structure and reactivity. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/68720 (accessed on 16 February 2022).
- Koo, S.H.; Lee, K.Y.; Lee, H.G. Effect of cross-linking on the physicochemical and physiological properties of corn starch. Food Hydrocoll. 2010, 24, 619–625. [Google Scholar] [CrossRef]
- Adeyanju, O.; Olademehin, O.P.; Hussaini, Y.; Nwanta, U.C.; Adejoh, A.I.; Plavec, J. Synthesis and characterization of carboxymethyl Plectranthus esculentus starch. A potential disintegrant. J. Pharm. Appl. Chem. 2016, 2, 189–195. [Google Scholar] [CrossRef]
- Pal, J.; Singhal, R.S.; Kulkarni, P.R. Physicochemical properties of hydroxypropyl derivative from corn and amaranth starch. Carbohydr. Polym. 2002, 48, 49–53. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Aparicio, M.A.; Bello, L.A.; Monroy, J.A. Changes of banana starch by chemical and physical modification. Carbohydr. Polym. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Zhao, N.; Augsburger, L.L. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets. AAPS PharmSciTech 2005, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olu-Owolabi, B.I.; Afolabi, T.A.; Adebowale, K.O. Pasting, thermal, hydration, and functional properties of annealed and heat-moisture treated starch of sword bean (Canavalia gladiata). Int. J. Food Prop. 2011, 14, 157–174. [Google Scholar] [CrossRef]
- Awokoya, K.N.; Oninla, V.O.; Oni, O.D. Physicochemical properties of Carica papaya starch enhanced by etherification modification with sodium monochloroacetate. J. Agroaliment. Process Technol. 2019, 25, 157–170. [Google Scholar]
- Arueya, G.L.; Ojesanmi, A.A. Evaluation of effects of increasing molar substitution of hydroxypropylene on physicochemical, functional and morphological properties of starch from water yam (Dioscorea alata). J. Food Res. 2019, 8, 4. [Google Scholar] [CrossRef]
- Kittipongpatana, O.S.; Kittipongpatana, N. Cross-linked carboxymethyl mung bean starch as pharmaceutical gelling agent and emulsion stabilizer. Int. J. Pharm. Pharm. Sci. 2015, 7, 403–407. [Google Scholar]
- Huang, J.; Shang, Z.; Man, J.; Liu, Q.; Zhu, C.; Wei, C. Comparison of molecular structures and functional properties of high-amylose starches from rice transgenic line and commercial maize. Food Hydrocoll. 2015, 46, 172–179. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Y.; Qiao, Q.; Tao, X.; Liu, P.; Xie, F. Comparison of the structure and properties of hydroxypropylated acid-hydrolysed maize starches with different amylose/amylopectin contents. Food Hydrocoll. 2021, 110, 10613. [Google Scholar] [CrossRef]
- Xiao, H.-X.; Lin, Q.-L.; Liu, G.-Q.; Yu, F.-X. A Comparative Study of the Characteristics of cross-linked, oxidized and dual-modified rice starches. Molecules 2012, 17, 10946–10957. [Google Scholar] [CrossRef] [Green Version]
- Svačinová, P.; Mužíková, J.; Ondrejček, P. Comparison of compressibility, compactability, and lubricant sensitivity of two partially pregelatinized starches. Starch-Stärke 2021, 73, 2000166. [Google Scholar] [CrossRef]
- Leuenberger, H. The compressibility and compactibility of powder systems. Int. J. Pharm. 1982, 12, 41–55. [Google Scholar] [CrossRef]
- Nattapulwat, N.; Purkkao, N.; Suwithayapanth, O. Evaluation of native and carboxymethyl yam (Dioscorea esculenta) starches as tablet disintegrants. Silpakorn Univ. Sci. Tech. J. 2008, 2, 18–25. [Google Scholar]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Characteristics and properties of hydroxypropylated rice starch based biodegradable films. Food Hydrocoll. 2015, 50, 54–64. [Google Scholar] [CrossRef]




| Sample | Element Weight (%) | ||||
|---|---|---|---|---|---|
| C | O | Na | P | Cl | |
| NS | 54.19 ± 3.30 | 45.17 ± 3.53 | 0.02 ± 0.02 | 0.58 ± 0.71 | 0.02 ± 0.04 |
| PGS | 53.83 ± 1.04 | 45.80 ± 1.08 | 0.00 ± 0.01 | 0.31 ± 0.06 | 0.04 ± 0.03 |
| CMS | 51.54 ± 1.92 | 46.55 ± 1.68 | 1.81 ± 0.27 | 0.05 ± 0.08 | 0.01 ± 0.02 |
| CLCMS | 46.44 ± 3.29 | 49.29 ± 1.92 | 3.09 ± 1.19 | 1.12 ± 0.50 | 0.05 ± 0.06 |
| CLS | 46.10 ± 6.68 | 48.89 ± 2.92 | 3.17 ± 2.73 | 2.63 ± 0.95 | 0.02 ± 0.02 |
| HPS | 57.84 ± 1.45 | 42.03 ± 1.50 | 0.09 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.02 |
| Sample | MC (%) | SP @80 °C (g/g) | Solu (%) | FSC @37 °C (g/g) | OAC (g/g) |
|---|---|---|---|---|---|
| NS | 9.48 ± 0.56 cd | 10.78 ± 0.15 f | 2.15 ± 0.74 e | 4.53 ± 0.03 e | 1.68 ± 0.11 e |
| PGS | 10.71 ± 1.03 ab | 13.79 ± 1.32 e | 17.06 ± 0.88 c | 5.95 ± 0.49 d | 1.97 ± 0.10 d |
| CMS | 10.95 ± 0.77 a | 19.44 ± 1.81 c | 37.41 ± 0.92 a | 14.51 ± 1.48 b | 2.18 ± 0.13 c |
| CLCMS | 11.70 ± 1.12 a | 25.85 ± 0.78 a | 26.00 ± 0.51 b | 17.79 ± 0.18 a | 2.76 ± 0.14 a |
| CLS | 9.93 ± 1.25 bc | 16.84 ± 1.65 d | 2.90 ± 0.50 e | 7.12 ± 0.68 c | 2.46 ± 0.20 ab |
| HPS | 9.01 ± 0.94 d | 22.24 ± 2.23 b | 13.78 ± 1.27 d | 4.34 ± 0.19 e | 2.30 ± 0.10 bc |
| CAS | 10.82 ± 0.84 | 8.81 ± 0.69 | 5.70 ± 0.88 | 4.17 ± 0.36 | 1.84 ± 0.28 |
| Sample | Temperature (°C) | ΔT | ΔH (J/g) | ||
|---|---|---|---|---|---|
| Tonset | Tpeak | Tend | |||
| NS | 64.3 ± 1.1 b | 72.1 ± 1.1 b | 78.6 ± 0.9 b | 14.6 ± 1.7 a | 8.5 ± 0.5 b |
| PGS | 64.5 ± 0.7 b | 70.9 ± 0.3 c | 76.5 ± 1.1 c | 12.3 ± 0.8 b | 6.0 ± 0.6 d |
| CMS | N/A | ||||
| CLCMS | N/A | ||||
| CLS | 67.1 ± 0.7 a | 74.9 ± 0.6 a | 81.2 ± 0.9 a | 14.0 ± 1.6 a | 10.3 ± 0.7 a |
| HPS | 63.8 ± 0.5 c | 69.1 ± 1.0 d | 74.1 ± 1.0 d | 10.3 ± 0.9 c | 7.2 ± 1.7 c |
| CAS | 72.1 ± 0.6 | 76.2 ± 0.4 | 79.9 ± 0.6 | 7.8 ± 0.6 | 10.8 ± 0.8 |
| Sample | Density (g/cm3) | %CI | HR | AR | |
|---|---|---|---|---|---|
| Bulk | Tapped | ||||
| NS | 0.56 ± 0.01 a | 0.73 ± 0.00 a | 23.49 ± 0.72 c | 1.31 ± 0.01 b | 24.60 ± 3.52 b |
| PGS | 0.43 ± 0.01 d | 0.59 ± 0.01 c | 28.50 ± 1.06 a | 1.40 ± 0.02 a | 27.39 ± 2.08 a |
| CMS | 0.51 ± 0.01 b | 0.65 ± 0.01 b | 21.09 ± 1.03 c | 1.27 ± 0.02 c | 18.33 ± 5.12 d |
| CLCMS | 0.46 ± 0.01 c | 0.58 ± 0.01 cd | 20.81 ± 1.27 d | 1.26 ± 0.02 c | 20.73 ± 2.15 c |
| CLS | 0.43 ± 0.00 d | 0.57 ± 0.00 d | 24.30 ± 0.30 b | 1.32 ± 0.01 b | 19.27 ± 2.66 cd |
| HPS | 0.44 ± 0.04 cd | 0.57 ± 0.02 cd | 23.50 ± 4.68 bc | 1.31 ± 0.08 bc | 22.08 ± 4.51 bc |
| CAS | 0.50 ± 0.04 | 0.67 ± 0.02 | 25.39 ± 3.27 | 1.34 ± 0.04 | 24.35 ± 2.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittipongpatana, O.S.; Kittipongpatana, N. Physicochemical and Functional Properties of Modified KJ CMU-107 Rice Starches as Pharmaceutical Excipients. Polymers 2022, 14, 1298. https://doi.org/10.3390/polym14071298
Kittipongpatana OS, Kittipongpatana N. Physicochemical and Functional Properties of Modified KJ CMU-107 Rice Starches as Pharmaceutical Excipients. Polymers. 2022; 14(7):1298. https://doi.org/10.3390/polym14071298
Chicago/Turabian StyleKittipongpatana, Ornanong S., and Nisit Kittipongpatana. 2022. "Physicochemical and Functional Properties of Modified KJ CMU-107 Rice Starches as Pharmaceutical Excipients" Polymers 14, no. 7: 1298. https://doi.org/10.3390/polym14071298
APA StyleKittipongpatana, O. S., & Kittipongpatana, N. (2022). Physicochemical and Functional Properties of Modified KJ CMU-107 Rice Starches as Pharmaceutical Excipients. Polymers, 14(7), 1298. https://doi.org/10.3390/polym14071298





