Chitosan Containing Nano Zn-Organic Framework: Synthesis, Characterization and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zn@BTC/CS Nanocomposite
2.3. Characterization
2.4. Specific Surface Area
2.5. Biological Activity Evaluation
2.5.1. Cell Culture and MTT Assay
2.5.2. Scratch Wound Healing (Migration) Assay
2.5.3. Invasion (Transwell Assay)
2.5.4. DNA Fragmentation Assay
2.5.5. RT-qPCR
3. Results and Discussion
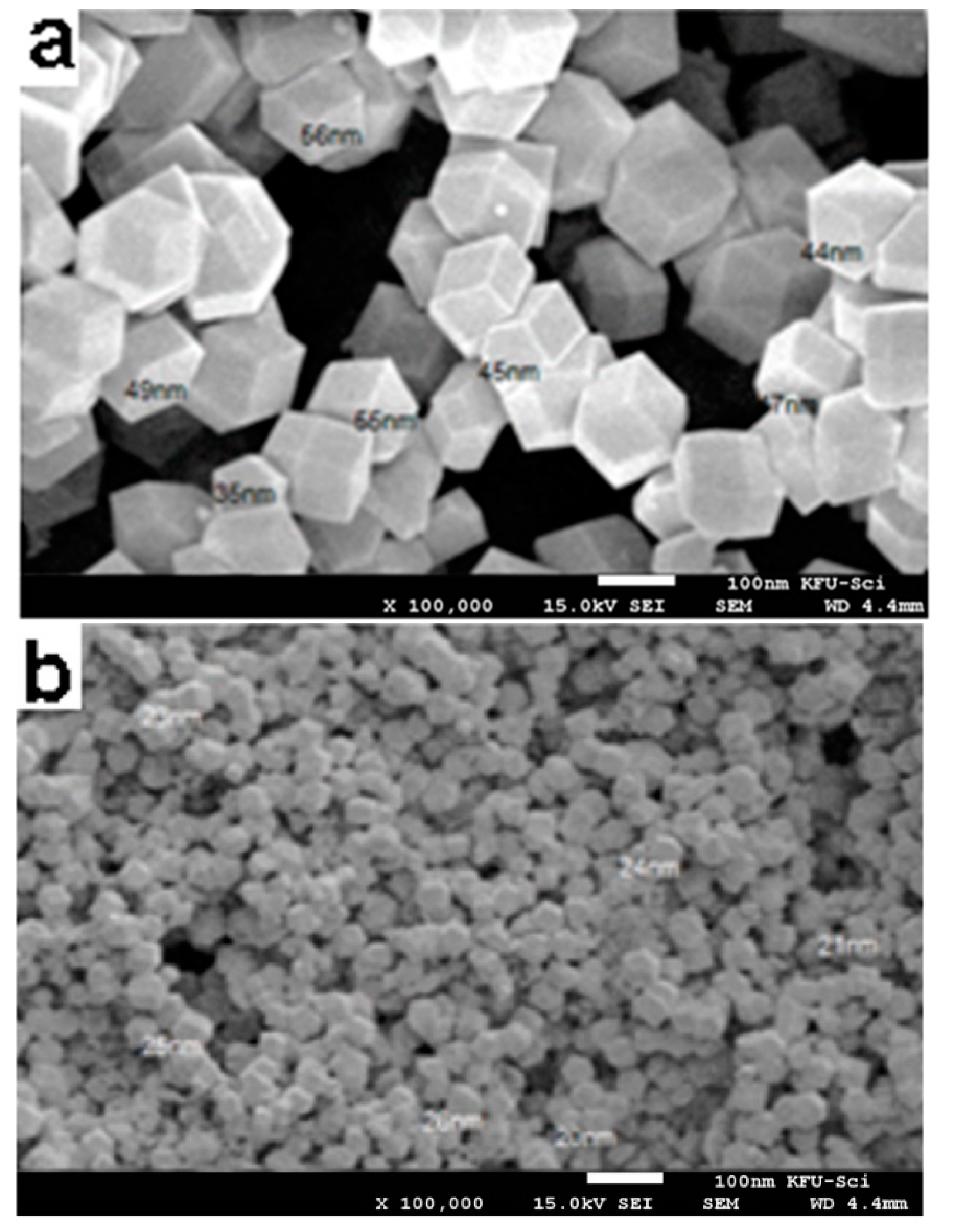
3.1. Scanning Electron Microscopy
3.2. X-ray Diffraction
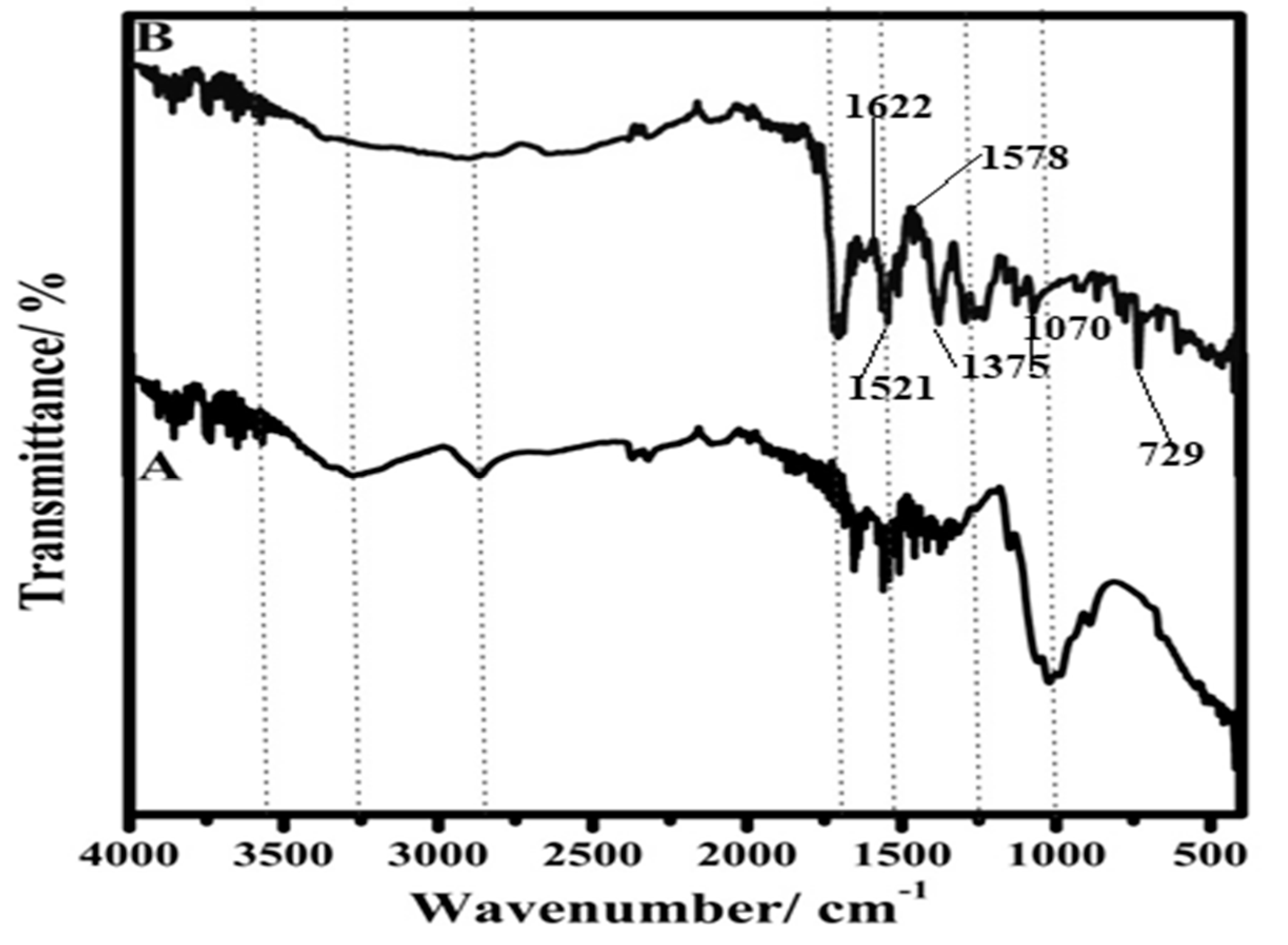
3.3. FTIR
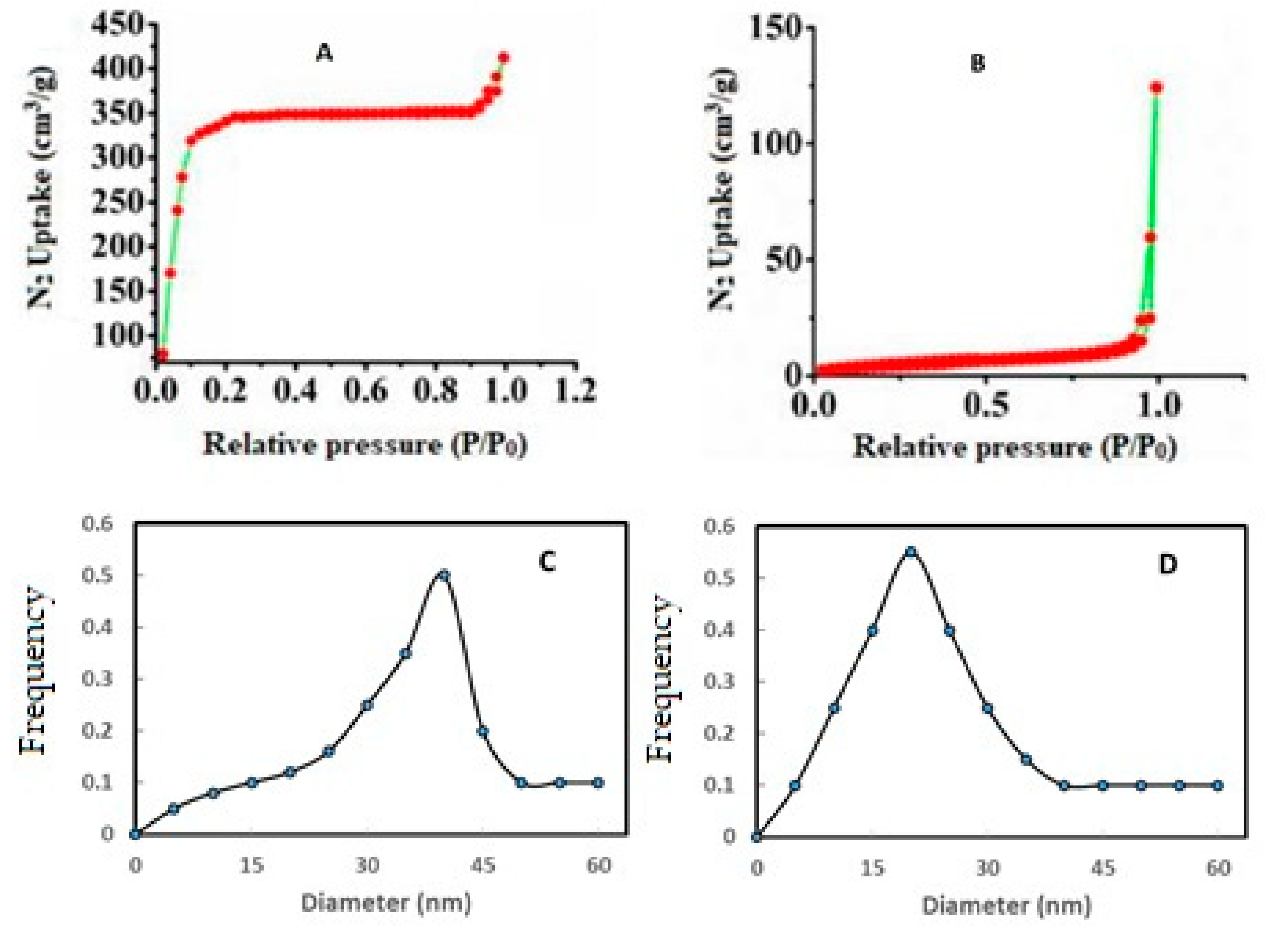
3.4. Specific Surface Area
3.5. Biological Activity Evaluation
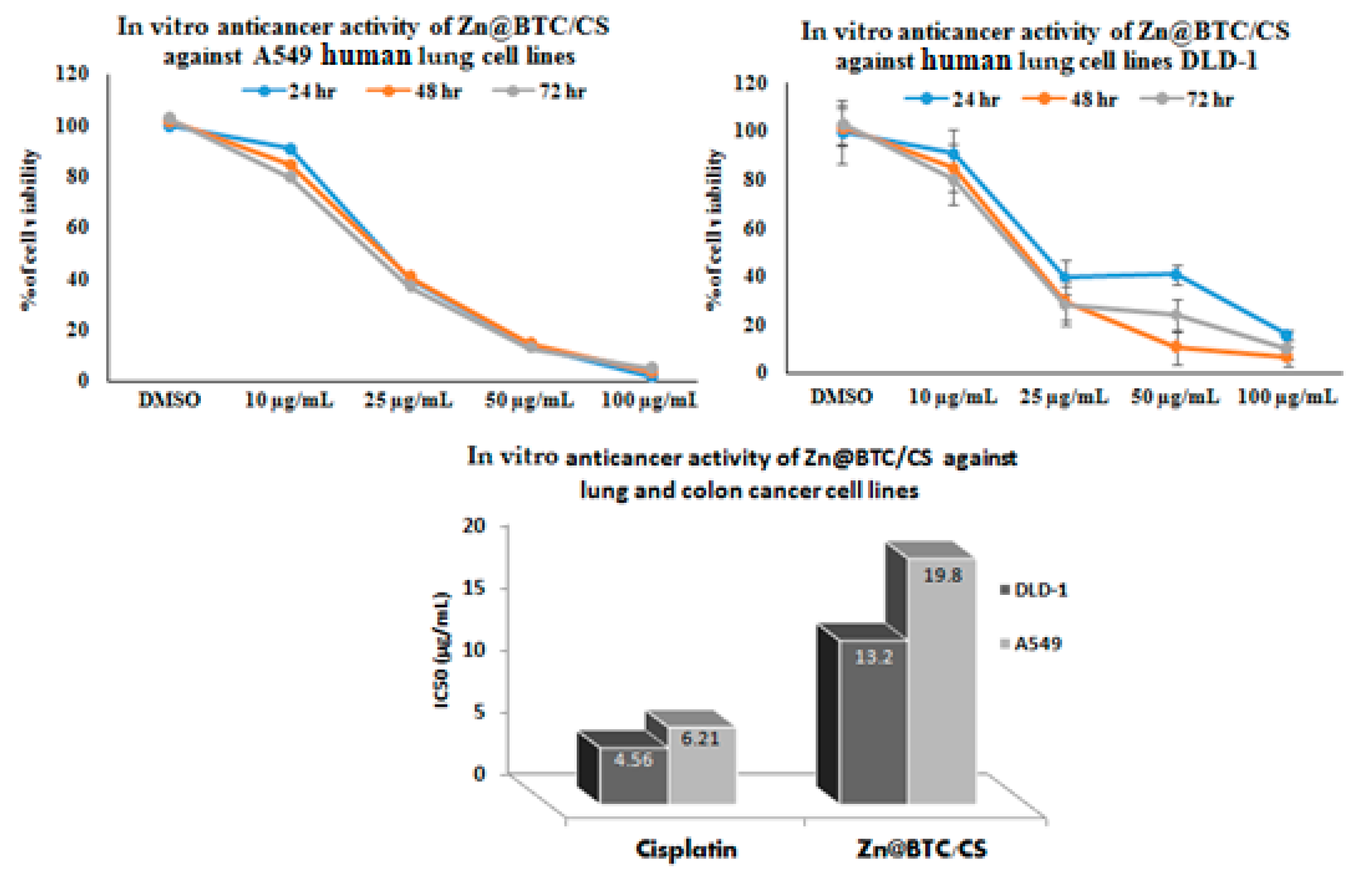
3.5.1. Anticancer Evaluation
3.5.2. DNA Fragmentation Assay
3.5.3. qRT-PCR Assessment of the Expression of p53 and Bcl-2
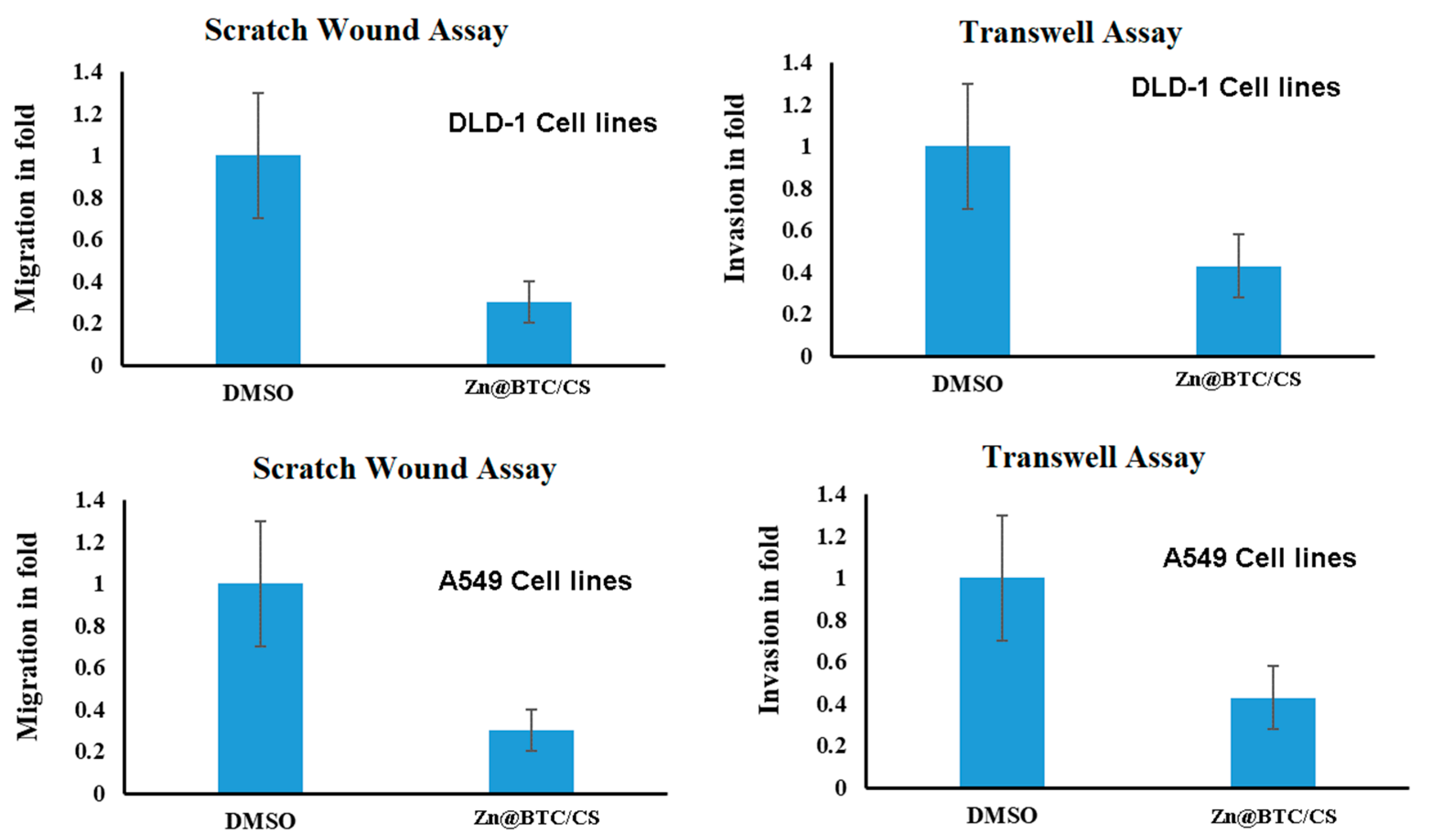
3.5.4. Migration
3.5.5. Invasion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Yan, X.; Feng, W.; Shi, W.; Wang, Q.; Zhang, Q.; Chai, L.; Liu, P.; Chen, Y.; Li, C.; et al. Risk factors of death from vascular events among cancer survivors: A SEER database analysis. Med. Clin. 2021, 156, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Veeraraghavan, V.P.; Surapaneni, K.M.; Hussain, S.; Mathanmohun, M.; Alharbi, S.A.; Aladresi, A.A.M.; Chinnathambi, A. Eugenol-piperine loaded polyhydroxy butyrate/polyethylene glycol nanocomposite-induced apoptosis and cell death in nasopharyngeal cancer (C666-1) cells through the inhibition of the PI3K/AKT/mTOR signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22700. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.; Negm, A.; Ibrahim, E.E.; Elrazak, A.A. Chemotherapeutic agents for the treatment of hepatocellular carcinoma: Efficacy and mode of action. Oncol. Rev. 2014, 8, 246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, D. Mesoporous carbon originated from non-permanent porous MOFs for gas storage and CO2/CH4 separation. Sci. Rep. 2014, 4, 5711. [Google Scholar] [CrossRef] [PubMed]
- Iqra, G.; Muhammad, I.; Samina, P.; Tasmina, K.; Salim, S.; Massimo, F.B.; Christopher, J.E.; Vamsi, K.Y.; Muhammad, R.S. Synthesis of chitosan coated metal organic frameworks (MOFs) for increasing vancomycin bactericidal potentials against resistant S. aureus strain. Mater. Sci. Eng. C 2019, 105, 110111–110121. [Google Scholar]
- Rostamnia, S.; Alamgholiloo, H.; Jafari, M. Ethylene diamine post-synthesis modification on open metal site Cr-MOF to access efficient bifunctional catalyst for the Hantzsch condensation reaction. Appl. Organomet. Chem. 2018, 32, 4370–4381. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Sabo, M.; Henschel, A.; Fröde, H.; Klemm, E.; Kaskel, S. Solution infiltration of palladium into MOF-5: Synthesis, physisorption and catalytic properties. J. Mater. Chem. 2007, 17, 3827–3832. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M. Hydrogen Physisorption in Metal-Organic Porous Crystals. Adv. Mater. 2005, 17, 538–541. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal—organic frameworks—prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Tsao, C.-S.; Ming-Sheng, Y.; Tsui-Yun, C.; Hsiu-Chu, W.; Cheng-Yu, W.; Kuei-Sen, C.; Hsin-Lung, C. Characterization of pore structure in metal-organic framework by small-angle X-ray scattering. J. Am. Chem. Soc. 2007, 129, 15997–16004. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, J.; Jhung, S.-H.; Kim, H.-K.; Chang, J.-S.; Chae, H.K. Microwave Synthesis of a Porous Metal-Organic Framework, Zinc Terephthalate MOF-5. Bull. Korean Chem. Soc. 2006, 27, 1523–1524. [Google Scholar]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Roth Stefaniak, K.; Epley, C.C.; Novak, J.J.; McAndrew, M.L.; Cornell, H.D.; Zhu, J.; McDaniel, D.K.; Davis, J.L.; Allen, I.C.; Morris, A.J.; et al. Photo-triggered release of 5-fluorouracil from a MOF drug delivery vehicle. Chem. Commun. 2018, 54, 7617–7620. [Google Scholar] [CrossRef]
- He, C.; Liu, D.; Lin, W. Nanomedicine Applications of Hybrid Nanomaterials Built from Metal–Ligand Coordination Bonds: Nanoscale Metal–Organic Frameworks and Nanoscale Coordination Polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-X.; Yang, Y.-W. Metal–Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sabouni, R.; Husseini, G. Anti-cancer Drug Delivery Using Metal Organic Frameworks (MOFs). Curr. Med. Chem. 2017, 24, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A. The applications of metal-organic-frameworks in controlled release of drugs. Rev. J. Chem. 2016, 7, 1–22. [Google Scholar] [CrossRef]
- Li, L.; Han, S.; Yang, C.; Liu, L.; Zhao, S.; Wang, X.; Liu, B.; Pan, H.; Liu, Y. Glycyrrhetinic acid modified MOFs for the treatment of liver cancer. Nanotechnology 2020, 31, 325602. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, T.; Giménez-Marqués, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; González-Fernández, A.; et al. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 43099. [Google Scholar] [CrossRef]
- Shunzhi, W.; McGuirk, M.C.; d’Aquino, A.; Mason, A.J.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv.Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef]
- Meng, L.; Yu, B.; Qin, Y. Templated interfacial synthesis of metal-organic framework (MOF) nano-and micro-structures with precisely controlled shapes and sizes. Commun. Chem. 2021, 4, 82. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Hojnik Podrepsek, G.; Knez, Z.; Leitgeb, M. Development of Chitosan Functionalized Magnetic Nanoparticles with Bioactive Compounds. Nanomaterials 2020, 10, 1913. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Can, M.V.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and Chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kiarostami, K.; Khatir, M.N.; Rezania, S.; Muhamad, I.I. Green synthesis of Mg0.99 Zn0.01O nanoparticles for the fabrication of κ-Carrageenan/NaCMC hydrogel in order to deliver catechin. Polymers 2020, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Mushtaq, A.; Li, L.; Grondahl, L. Chitosan Nanomedicine in Cancer Therapy: Targeted Delivery and Cellular Uptake. Macromol. Biosci. 2021, 21, e2100005. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hanieh, H.; Mohafez, O.; Hairul-Islam, V.I.; Alzahrani, A.; Bani Ismail, M.; Thirugnanasambantham, K. Novel Aryl Hydrocarbon Receptor Agonist Suppresses Migration and Invasion of Breast Cancer Cells. PLoS ONE 2017, 11, e0167650. [Google Scholar] [CrossRef]
- Kim, T.; Jung, U.; Cho, D.-Y.; Chung, A.-S. Se-Methylselenocysteine induces apoptosis through caspase activation in HL-60 cells. Carcinogenesis 2001, 22, 559–565. [Google Scholar] [CrossRef]
- Zuo, Y.; Shields, S.K.; Chakraborty, C. Enhanced intrinsic migration of aggressive breast cancer cells by inhibition of Rac1 GTPase. Biochem. Biophys. Res. Commun. 2006, 351, 361–367. [Google Scholar] [CrossRef]
- Li, H.; Zhai, Z.; Liu, G.; Tang, T.; Lin, Z.; Zheng, M.; Qin, A.; Dai, K. Sanguinarine inhibits osteoclast formation and bone resorption via suppressing RANKL-induced activation of NF-κB and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2013, 430, 951–956. [Google Scholar] [CrossRef]
- Porichi, O.; Nikolaidou, M.E.; Apostolaki, A.; Tserkezoglou, A.; Arnogiannaki, N.; Kassanos, D.; Margaritis, L.; Panotopoulou, E. BCL-2, BAX and P53 expression profiles in endometrial carcinoma as studied by real-time PCR and immunohistochemistry. Anticancer Res. 2009, 29, 3977–3982. [Google Scholar] [PubMed]
- Javanbakht, S. Incorporating Cu-based metal-organic framework/drug nanohybrids into gelatin microsphere for ibuprofen oral delivery. Mater. Sci. Eng. C 2019, 96, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Baksa, V.; Bege, M.; Talas, L.; Borbas, A.; Bereczki, I.; Banfalvi, G.; Szeman-Nagy, G. MTT Test and Time-lapse Microscopy to Evaluate the Antitumor Potential of Nucleoside Analogues. Anticancer Res. 2021, 41, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Dragostin, O.M.; Tatia, R.; Samal, S.K.; Oancea, A.; Zamfir, A.S.; Dragostin, I.; Lisa, E.L.; Apetrei, C.; Zamfir, C.L. Designing of Chitosan Derivatives Nanoparticles with Antiangiogenic Effect for Cancer Therapy. Nanomaterials 2020, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Tandon, S. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by Ruta graveolens in human colon cancer cells. Homeopathy 2015, 104, 36–47. [Google Scholar] [CrossRef]
- Li, Q.X.; Yu, D.H.; Liu, G.; Ke, N.; McKelvy, J.; Wong-Staal, F. Selective anticancer strategies via intervention of the death pathways relevant to cell transformation. Cell Death Differ. 2008, 15, 1197–1210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bauer, J.H.; Helfand, S.L. New tricks of an old molecule: Lifespan regulation by p53. Aging Cell 2006, 5, 437–440. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef]
- Reed, J.C. Bcl-2-family proteins and hematologic malignancies: History and future prospects. Blood 2008, 111, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Vicente-Manzanares, M.; Potvin-Trottier, L.; Wiseman, P.W.; Horwitz, A.R. The integrin-ligand interaction regulates adhesion and migration through a molecular clutch. PLoS ONE 2012, 7, e40202. [Google Scholar] [CrossRef] [PubMed]
- Stachurska, A.; Elbanowski, J.; Kowalczynska, H.M. Role of alpha5beta1 and alphavbeta3 integrins in relation to adhesion and spreading dynamics of prostate cancer cells interacting with fibronectin under in vitro conditions. Cell Biol. Int. 2012, 36, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.V.; Vlaykova, T.I. Significance of tenascin-C, fibronectin, laminin, collagen IV, alpha5beta1 and alpha9beta1 integrins and fibrotic capsule formation around liver metastases originating from cancers of the digestive tract. Neoplasma 2006, 53, 372–383. [Google Scholar]
- Shi, M.; Cao, M.; Song, J.; Liu, Q.; Li, H.; Meng, F.; Pan, Z.; Bai, J.; Zheng, J. PinX1 inhibits the invasion and metastasis of human breast cancer via suppressing NF-κB/MMP-9 signaling pathway. Mol. Cancer 2015, 14, 66. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, M.; Ibrahim, H.-I.M.; Negm, A. Chitosan Containing Nano Zn-Organic Framework: Synthesis, Characterization and Biological Activity. Polymers 2022, 14, 1276. https://doi.org/10.3390/polym14071276
Gouda M, Ibrahim H-IM, Negm A. Chitosan Containing Nano Zn-Organic Framework: Synthesis, Characterization and Biological Activity. Polymers. 2022; 14(7):1276. https://doi.org/10.3390/polym14071276
Chicago/Turabian StyleGouda, Mohamed, Hairul-Islam Mohamed Ibrahim, and Amr Negm. 2022. "Chitosan Containing Nano Zn-Organic Framework: Synthesis, Characterization and Biological Activity" Polymers 14, no. 7: 1276. https://doi.org/10.3390/polym14071276
APA StyleGouda, M., Ibrahim, H.-I. M., & Negm, A. (2022). Chitosan Containing Nano Zn-Organic Framework: Synthesis, Characterization and Biological Activity. Polymers, 14(7), 1276. https://doi.org/10.3390/polym14071276







