Aromatic polyimide films are widely used in the microelectronic manufacturing and packaging industries, particularly as the base films of flexible printed circuits (FPC), flexible packaging substrates, such as chip on film (COF), chip scale packaging (CSP), ball grid array (BGA), and so on. Line thinning of electric circuits is rapidly progressing, as a result of miniaturization and thin filming in electrical and electronic parts. Dimensional changes in polyimide films, during long-term service, can result in accidents, such as disconnection and short-circuiting, in thinner lined circuit structures. As a result, polyimide films for microelectronic applications must have high dimensional stability, a high elastic modulus, and low moisture absorption.
2.1. Improving Film Processability of Low-CTE Polyimide Films
Advanced polyimide films for electronic applications are typically made from aromatic dianhydrides (PMDA, or/and BPDA) and aromatic diamines (ODA, or/and PDA), among other things. Their combined properties can be tuned by adjusting the aromatic dianhydride and diamine ratios in the formation of PAAs [
31]. Among the polyimide films, the PI-(BPDA/PDA) film has the highest modulus, highest
Tg, and lowest CTE (Coefficient of Thermal Expansion) value, owing to the rigid chemical structure of BPDA and PDA. ODA, on the other hand, has a relatively flexible ether linkage due to the possibility of the molecular chain bending and rotating. The elastic modulus increases from 3.8 to 7.8 GPa, when the mole ratio of [PDA]/[PDA+ODA] in the copolyimide PI-(BPDA/PDA-ODA) films increase, and
Tg increases from 290 to 420 °C, while CTE decreases from 36 to 15 × 10
−6 °C
−1. It was discovered that even if only a small amount of ODA is incorporated into the polyimide backbone, the structure is disrupted and an obvious drop in
Tg occurs, indicating that a simple component additivity could cause obvious changes in
Tg and that the effect of the component would be sensible for rigidity.
Furthermore, because PMDA is more rigid than BPDA, the polyimide PI-(PMDA/PDA) film was expected to have a higher modulus and Tg than the polyimide PI-(BPDA/PDA). Unfortunately, the mechanical properties of the polyimide PI-(PMDA/PDA) film cannot be measured because it is too brittle. The thermal and mechanical properties of the four-component copolyimide films, derived from BPDA-PMDA and PDA-ODA, were complexly related to the polymer backbone structures. The co-polyimide film, derived from BPDA-PMDA (50/50) and PDA-ODA (70/30), demonstrated the best combination of properties, with CTE of 19 × 10−6 °C−1, elongation at breakage of 60%, modulus of 5.0 GPa, and Tg of 360 °C, respectively. To some extent, the balanced properties of polyimide films can be controlled by adjusting the polyimide backbone structures.
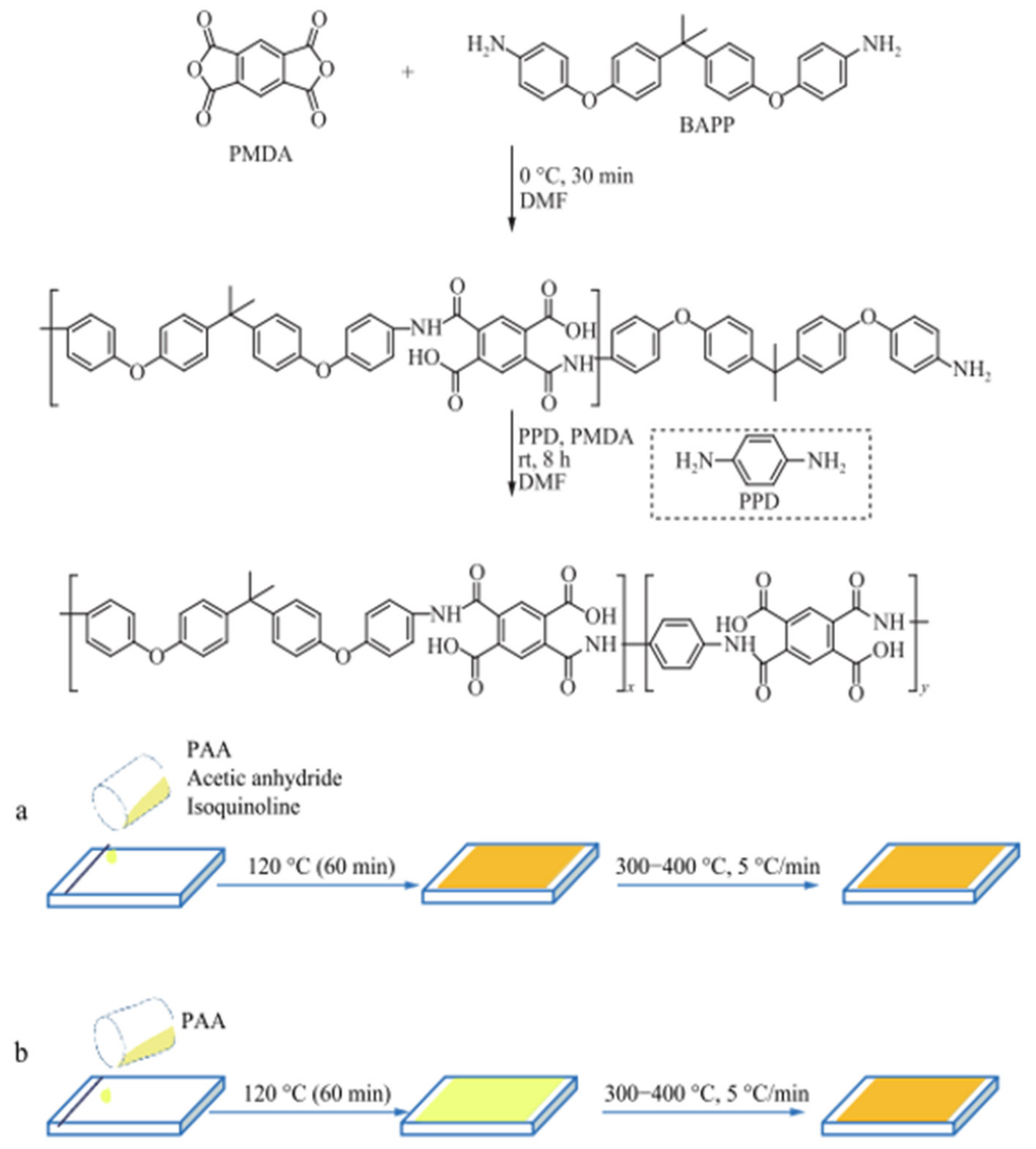
Z. H. Wang investigated the in-plane orientation and combined properties of the chemically imidized polyimide films [
32]. A two-stage film formation process is used to create a series of fully imidized TIPI and CIPI films: the solvent evaporation stage (120 °C) and the high-temperature imidization stage (300–400 °C) (
Scheme 1). Variable-temperature FTIR spectroscopy, from 40 °C to 400 °C, is used to monitor the chemical and thermal imidization processes. At the solvent evaporation stage, the imidization degree, in-plane orientation, and residual solvents of films are measured. The thermal and mechanical properties of chemically imidized polyimide (CIPI) films and thermally imidized polyimide (TIPI) films were thoroughly investigated. The experimental results show that CIPI films have significantly higher tensile strength and modulus, while having a significantly lower CTE than TIPI films. These benefits are due to the high in-plane orientation and close packing of CIPI backbones. In contrast to thermal imidization, which begins at around 140 °C, chemical imidization, activated by acetic anhydride and isoquinoline, begins cyclization at room temperature. The resulting imide rings restrict polymer chain mobility and lead to in-plane orientation with solvent evaporation. Furthermore, after chemical imidization at 120 °C, fewer small molecules remain in the films than after thermal imidization.
Figure 1 depicts a comparison of ID (Imidization Degree) values as a function of temperature. Even at room temperature, the chemically imidized film has an ID of about 35%, which does not change until the temperature rises to 100 °C, indicating that the cyclization reaction can be activated by acetic anhydride and isoquinoline. From 100 °C to 200 °C, ID increases from 35% to about 90%, with only a 10% increase at higher temperatures (>200 °C). Thermally imidized films, on the other hand, show no imidization below 140 °C. According to previous research [
33,
34], their IDs increase noticeably, as the temperature rises from 140 °C to 250 °C. Depolymerization, orientation, plasticization, and aggregation of completely imidized PI films will all be affected by the increased ID at low temperatures for chemical imidization.
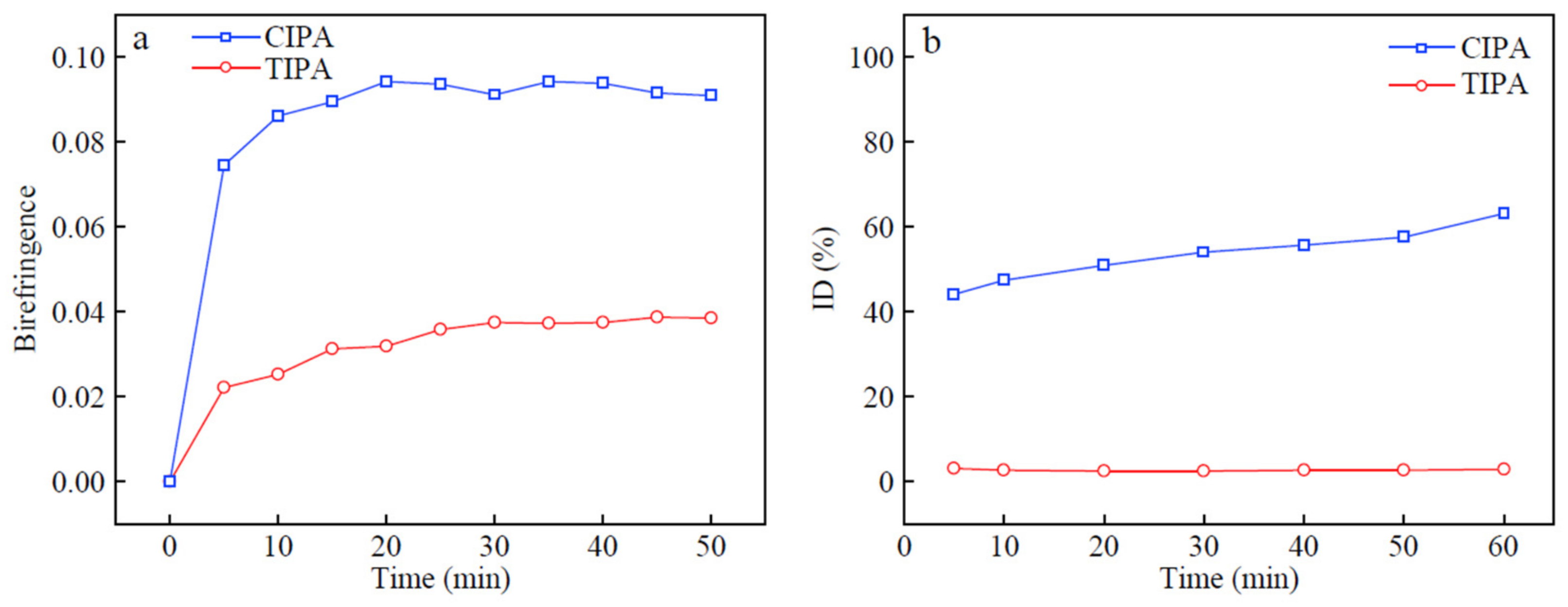
Figure 2a depicts the real-time birefringence of partially imidized films, produced by two different imidization methods during the solvent evaporation stage. Because the initial PAA films are solvent-rich, the relaxation time is reduced and the in-plane and out-of-plane refractive index values remain very close, indicating that the birefringence is close to zero. Solvents volatilize rapidly, as the standing time at 120 °C increases, and birefringence increases simultaneously, in both chemical and thermal imidizations. The IDs of TIPA-50 and CIPA-50, as a function of time at 120 °C, are depicted in
Figure 2b. When the standing time at 120 °C reaches 60 min, the ID of CIPA-50 film exceeds 60%. The resulting imide rings exhibit greater intrinsic birefringence than PAA chains.
Small molecule evaporation has been shown to plastify polymer chains and disrupt the ordered arrangement of PI films at high temperatures [
35,
36].
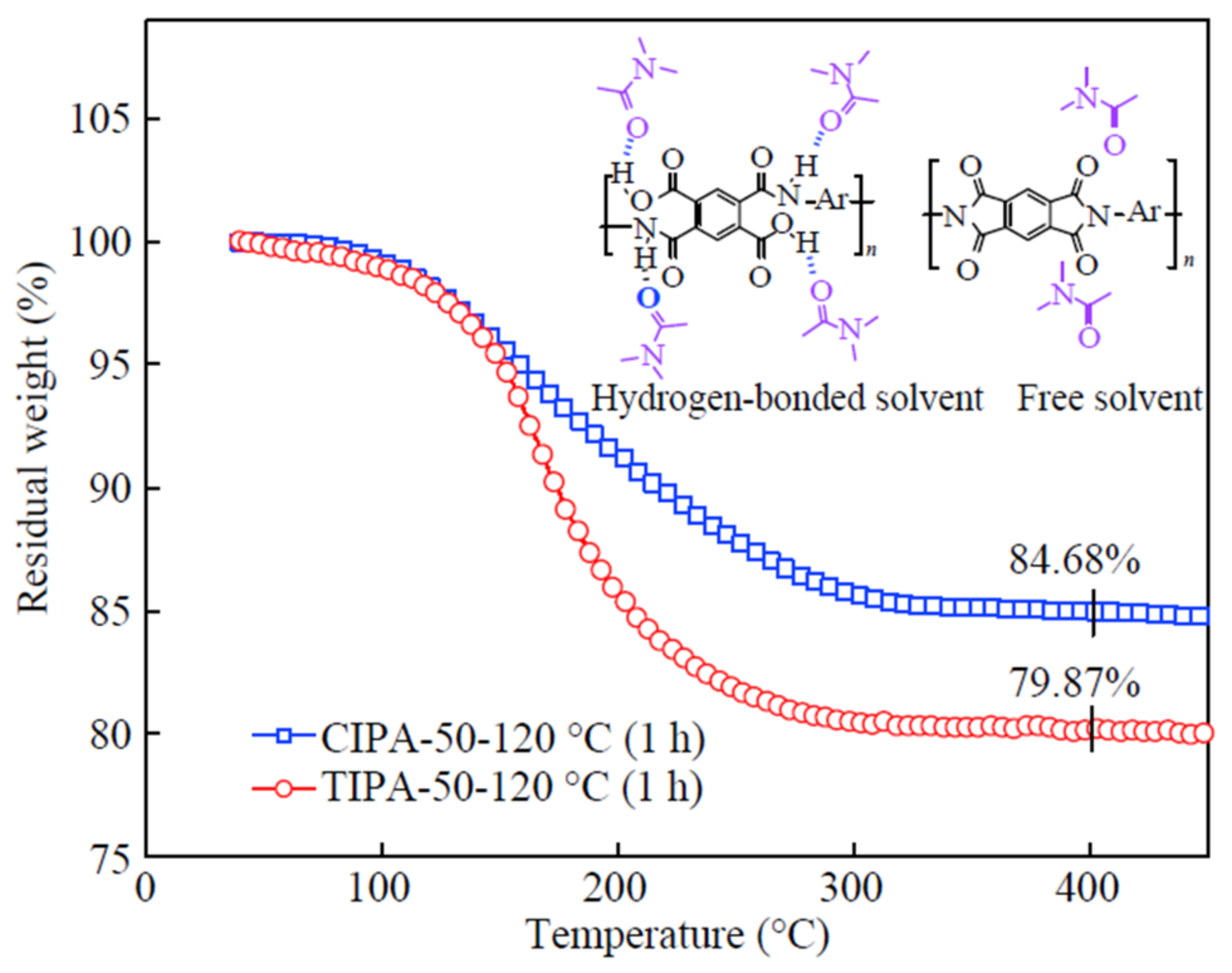
Figure 3 depicts the weight loss of PA-50 films, treated for 1 h at 120 °C, via thermal and chemical imidizations, respectively. After the solvent evaporation stage, the CIPA-50 film contains fewer residual small molecules than the TIPA-50 film. At the solvent evaporation stage, the ID of the CIPA-50 film exceeds 60%, as shown in
Figure 2b. Unlike PAA chains, imide rings cannot form hydrogen bonds with solvents, as illustrated in
Figure 3 (inset picture).
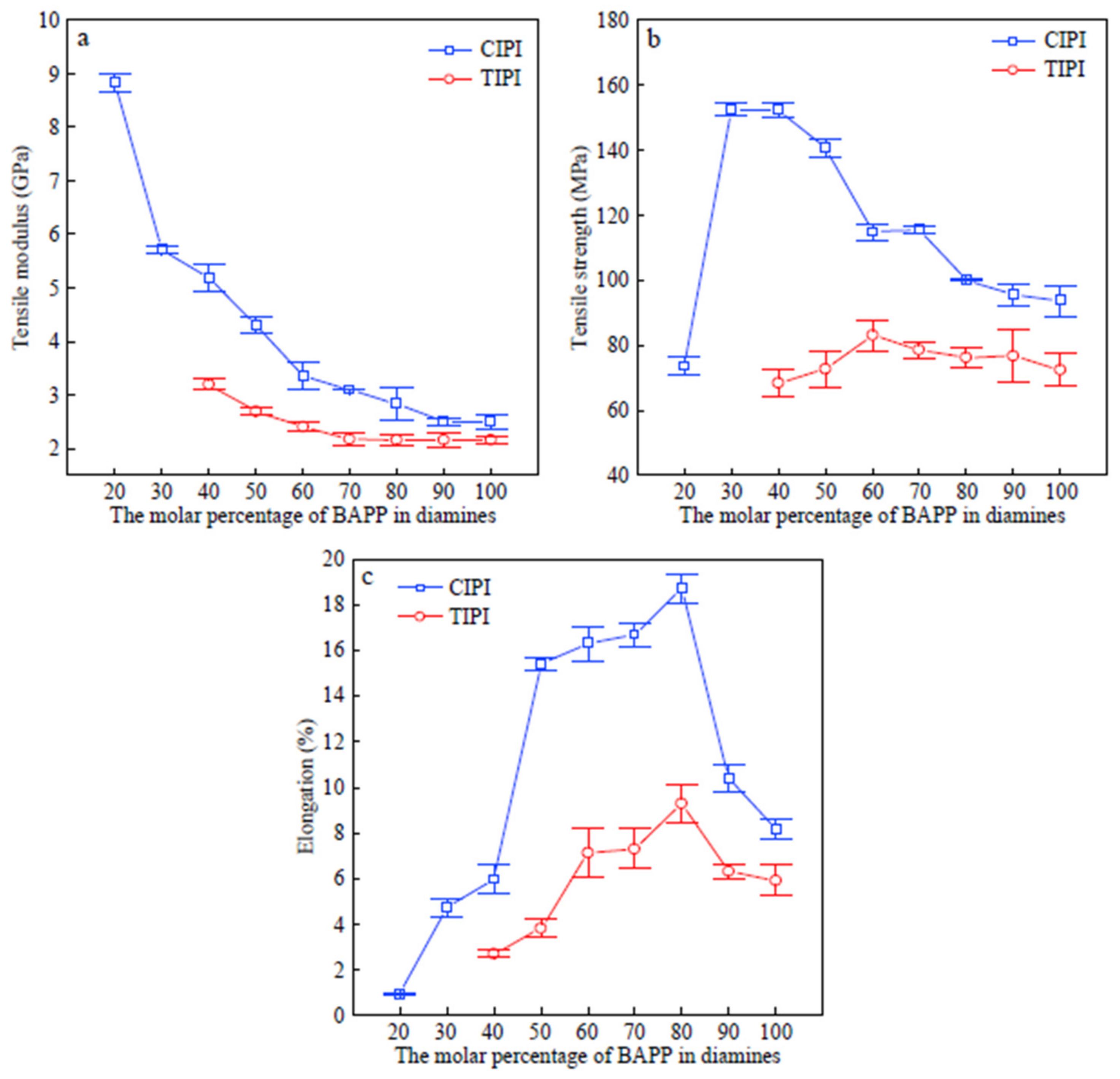
Figure 4 shows how chemical and thermal imidizations affect the mechanical characteristics of completely imidized PI films. With increased elastic modulus and tensile strength, the CIPI films have improved mechanical properties. CIPI-50, for example, has a tensile modulus of 4.5 GPa and a tensile strength of 160.4 MPa, which is 55.2% and 50.0% higher than TIPI-50. The aggregation phase structures of the PI films are responsible for the obvious improvement in mechanical properties for CIPI films. Chemical imidization causes a higher ID and in-plane orientation degree during the solvent evaporation stage, as previously stated. As a result, the CIPI films have a higher degree of in-plane orientation and a more organized molecular aggregation phase, resulting in higher tensile strength and modulus. The enhanced stability of imide rings, generated during the solvent evaporation stage of chemical imidization, may account for the increased toughness. In the subsequent imidization process, the inflexible PMDA-PPD segments and low molecular fragments cause film toughness to deteriorate and stress cracking to occur.
The wide-angle X-ray diffraction (WAXD) patterns of the PI films were measured. All PI films made by chemical or thermal imidizations have a broad peak, about 19°, showing that they are all amorphous. The matching
d-spacing was calculated using Bragg’s equation, based on the scattering angles (2
θ) at the center of the broad peaks (
Figure 5). In linear polymers, the
d-spacing represents the average distance between polymer chain segments and also indicates the free volume. It is commonly used to determine the packing density of amorphous polymer films [
37,
38]. For thermal imidized PI films, the
d-spacing varies from 4.25 Å to 4.72 Å and improves with increasing BAPP fraction in the polymer backbone. Chemically imidized PI films exhibit a similar trend. The inclusion of flexible BAPP segments, with a large free volume fraction in the polyimide backbone, will disrupt the regular ordered structures and limit the formation of the in-plane orientation of polymer chains, as seen by the
d-spacing values.
The difference between the in-plane and out-of-plane refractive indices, known as birefringence, has been utilized to define in-plane molecule orientation.
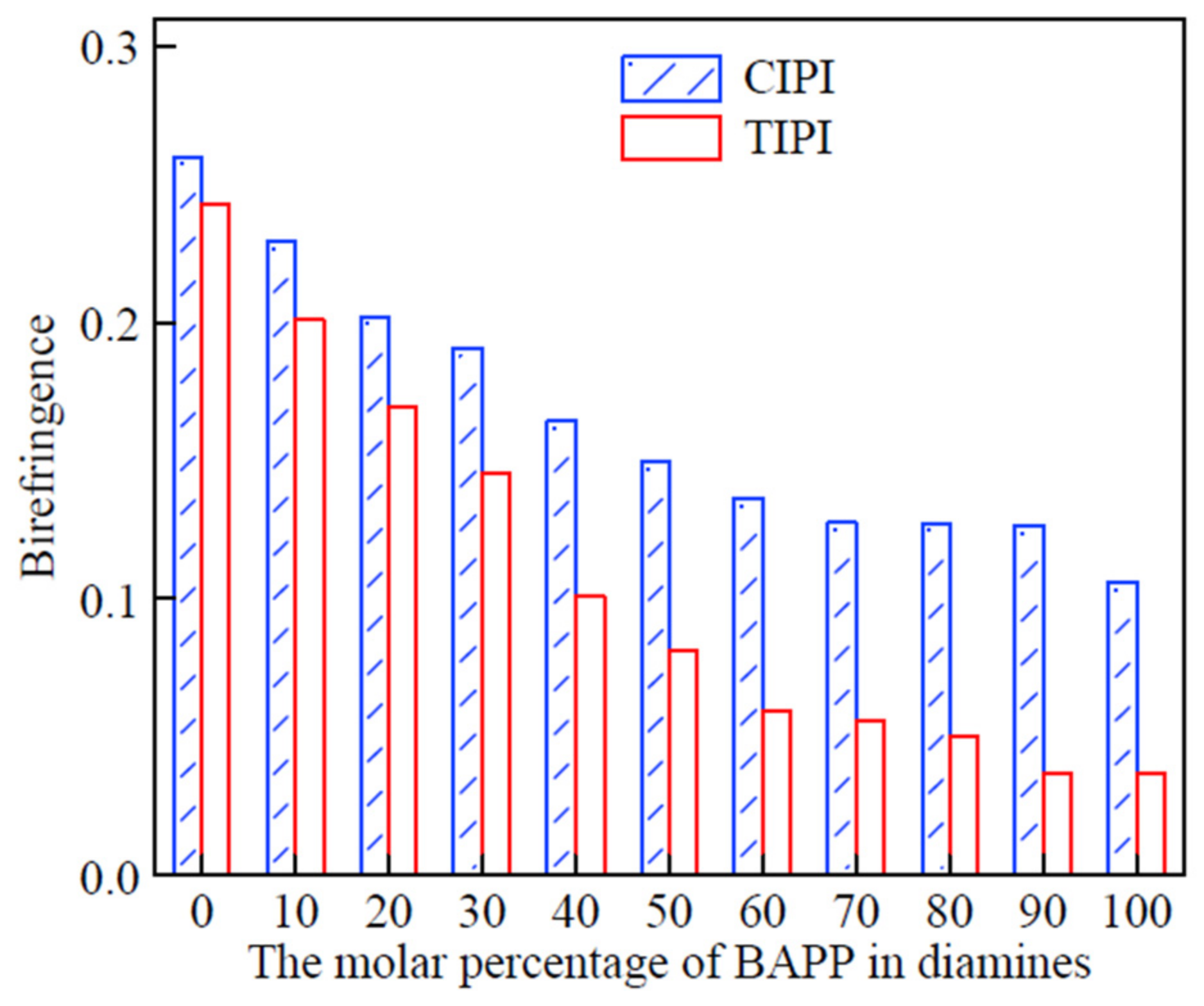
Figure 6 shows how the chemical and thermal imidizations affect the birefringence of completely imidized PI films. Chemical imidization can clearly yield PI films, with significantly better in-plane orientation than thermal imidization, especially for PI films with flexible segment concentrations more than 40 mol%.
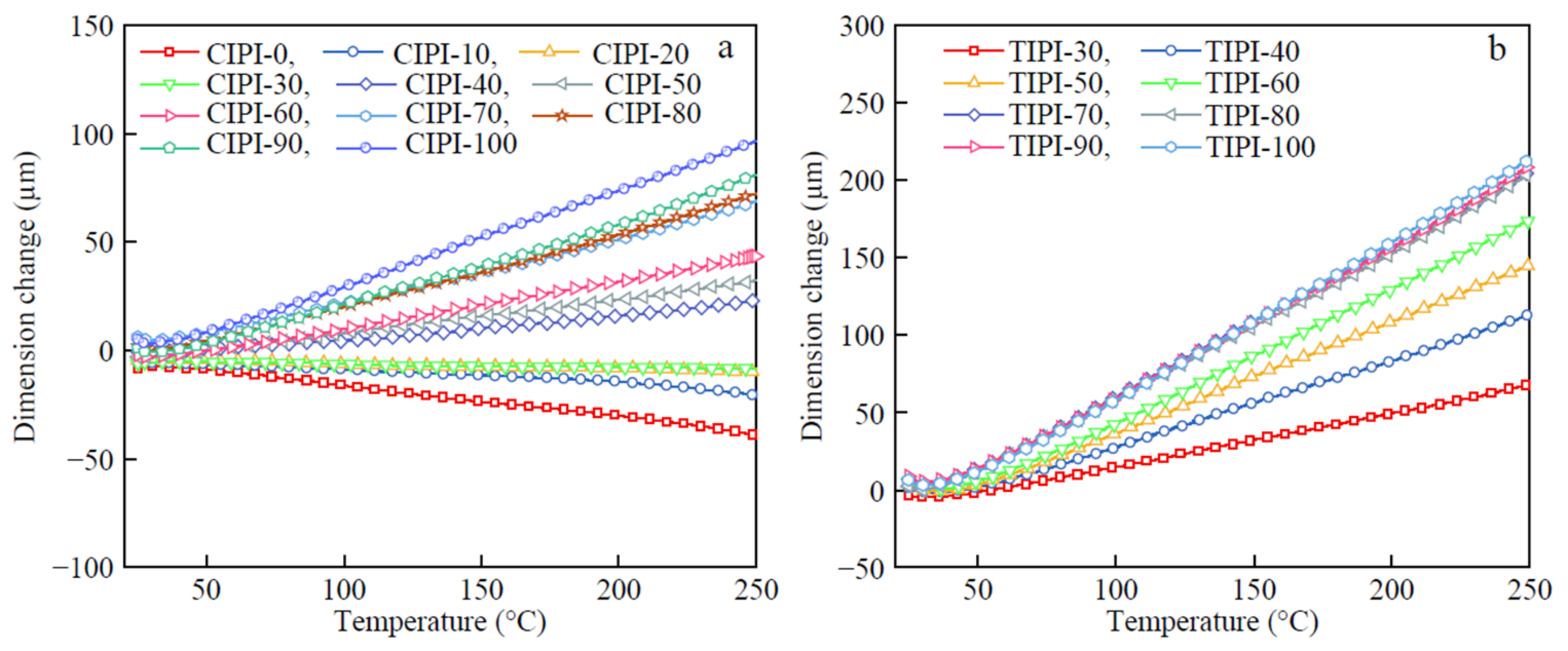
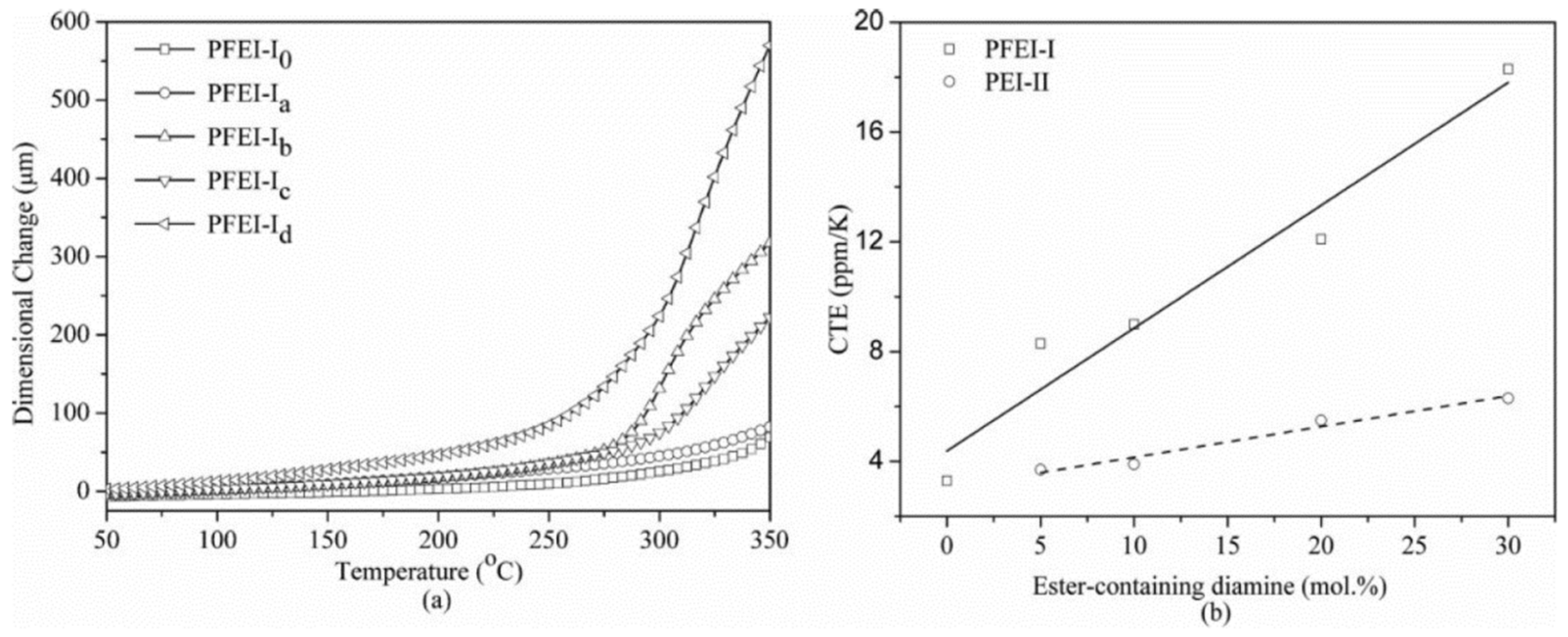
Good dimensional stability is essential for the use of PI films in the fabrication of flexible electronic substrates. TMA investigated the dimension variations of PI films as a function of temperature (
Figure 7). Regardless of chemical or thermal imidizations, CTE values rise as the flexible segment content in PI backbones increases. With increasing the contents of the flexible region from 30% to 100%, the TIPI films display CTE values of (21.7–63.7) × 10
−6 °C
−1. The PI films, on the other hand, cannot be manufactured if the flexible segment percentage is less than 30% because the films are too brittle. As a result, utilizing the thermal imidization process, obtaining PI films with CTE less than 20 × 10
−6 °C
−1, while maintaining considerable toughness, is difficult. At the same degree of flexible segment content in the PI backbones, the CIPI films have lower CTE values than the thermally imidized counterparts. For example, the CTE of the CIPI-50 film is 9.8 × 10
−6 °C
−1, which is significantly lower than that of the TIPI (CTE = 44.9 × 10
−6 °C
−1). The increased in-plane orientation favored by the more rigid imide rings, generated during the solvent evaporation stage, is clearly responsible for the improved dimensional stability of the CIPI films.
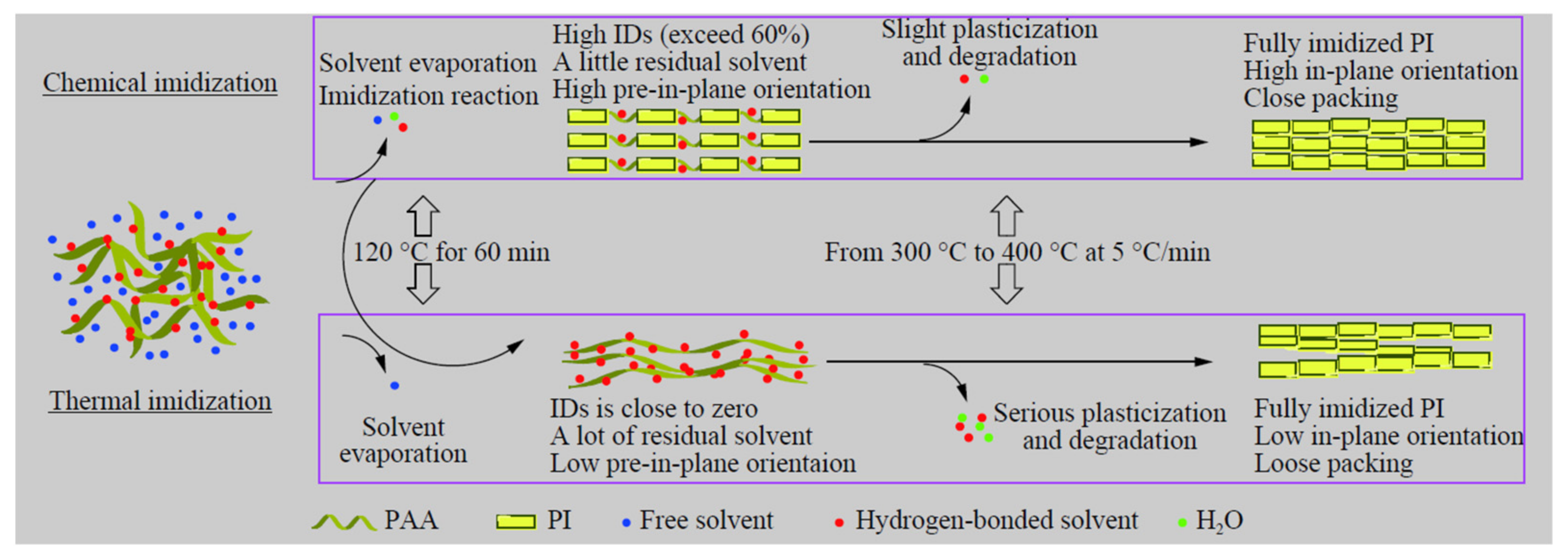
The physical and chemical alterations of the polymer chains, during the complete chemical and thermal processes, were proposed, in order to visibly and comprehensively characterize the effects of two imidization procedures on the structure and properties of fully imidized PI films (
Figure 8). Chemical imidization, which is activated by acetic anhydride and isoquinoline, initiates cyclization even at room temperature, unlike thermal imidization, which starts at around 140 °C. The orientation, plasticization, and depolymerization of completely imidized PI films will all be affected by the high ID of the partially imidized film, achieved during the solvent evaporation stage. All of these aspects will contribute to fully imidized CIPI films having a high in-plane orientation and close packing.
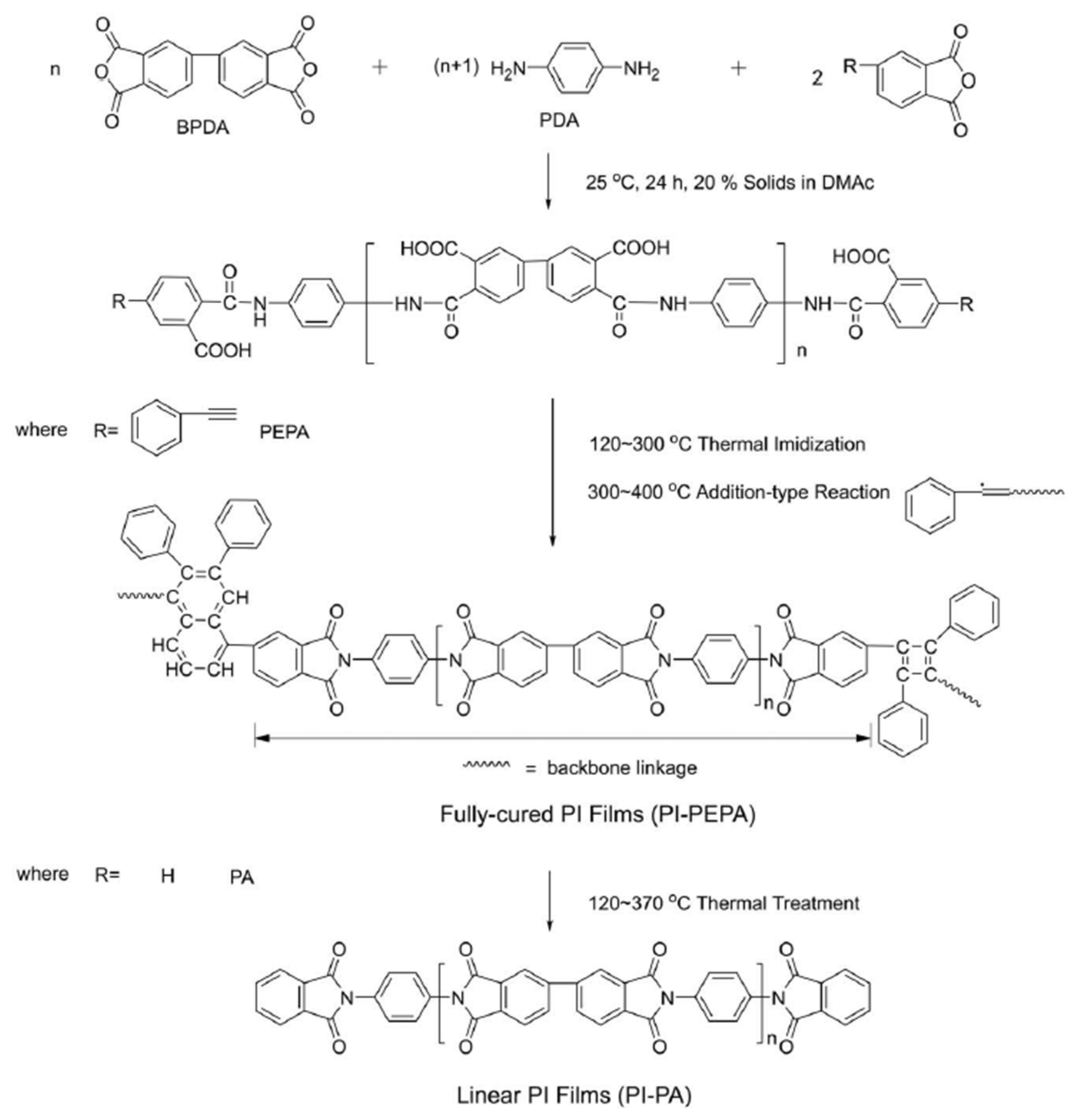
Yuan et al. [
39] prepared a series of molecular-weight-controlled polyimide precursors (PI-PEPA), with designed calculated molecular weight (Calc’d
Mw), from the reaction of BPDA and PDA, using PEPA as a chain-extendable and end-capping reagent, at concentrations of 20% in DMAc, to determine the influence of PAA molecular weights on their solution-cast processing performance (
Scheme 2). Termination main-chains, with the calculated molar fractions of PEPA, were used to regulate the molecular weights, yielding PAAs with Calc’d
Mw of 5 × 10
3 g·moL
−1 to 50 × 10
3 g·moL
−1. For comparison, an analogous series of unreactive phthalic-end-capped PAA resins (PAA-PA) was synthesized with equal regulated
Mw of 5 × 10
3 g·moL
−1−50 × 10
3 g·moL
−1.
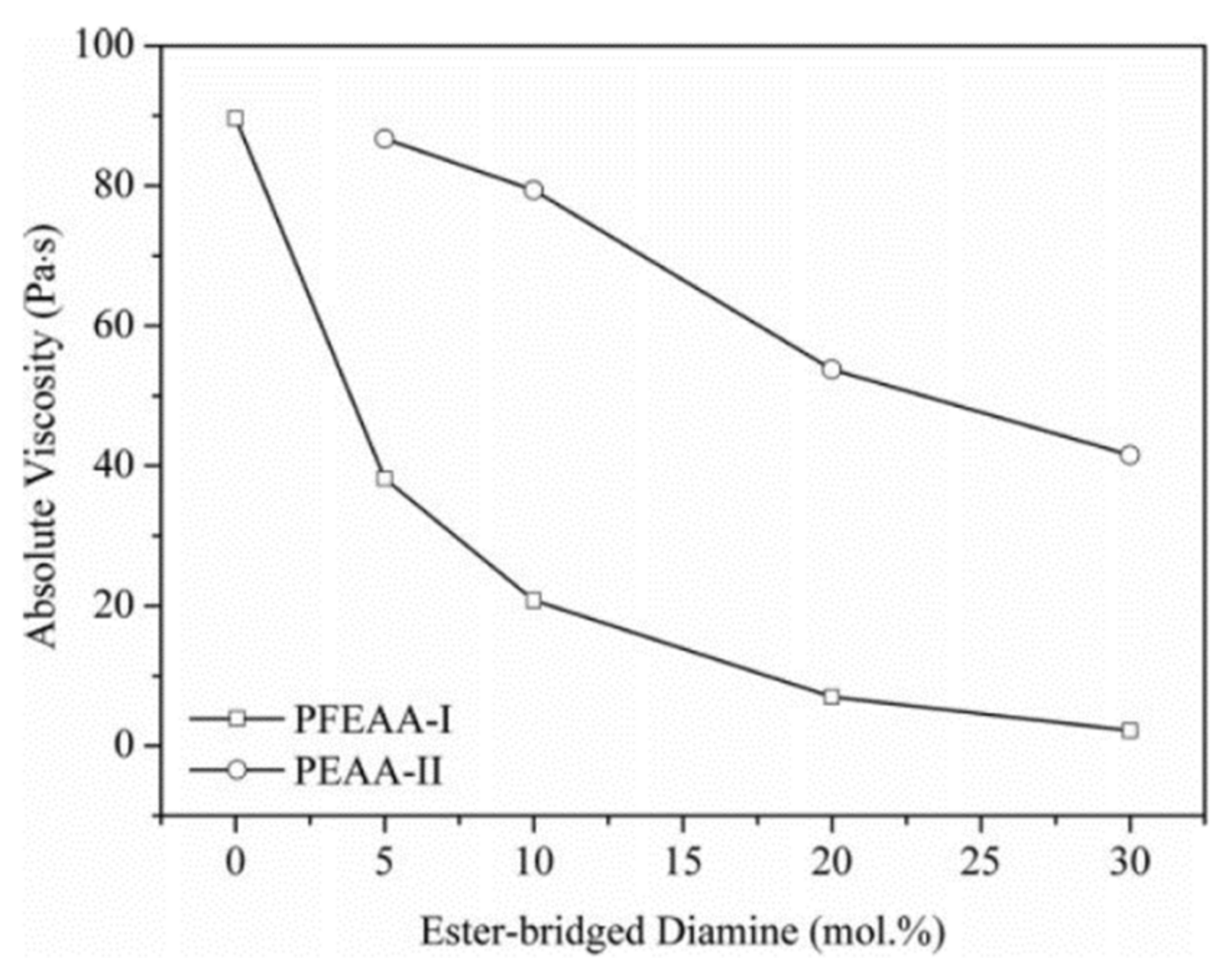
For the large-scale manufacturing of high-quality polyimide films, a reasonable apparent viscosity of PAA resin with high concentration is an important solution-cast processing characteristic.
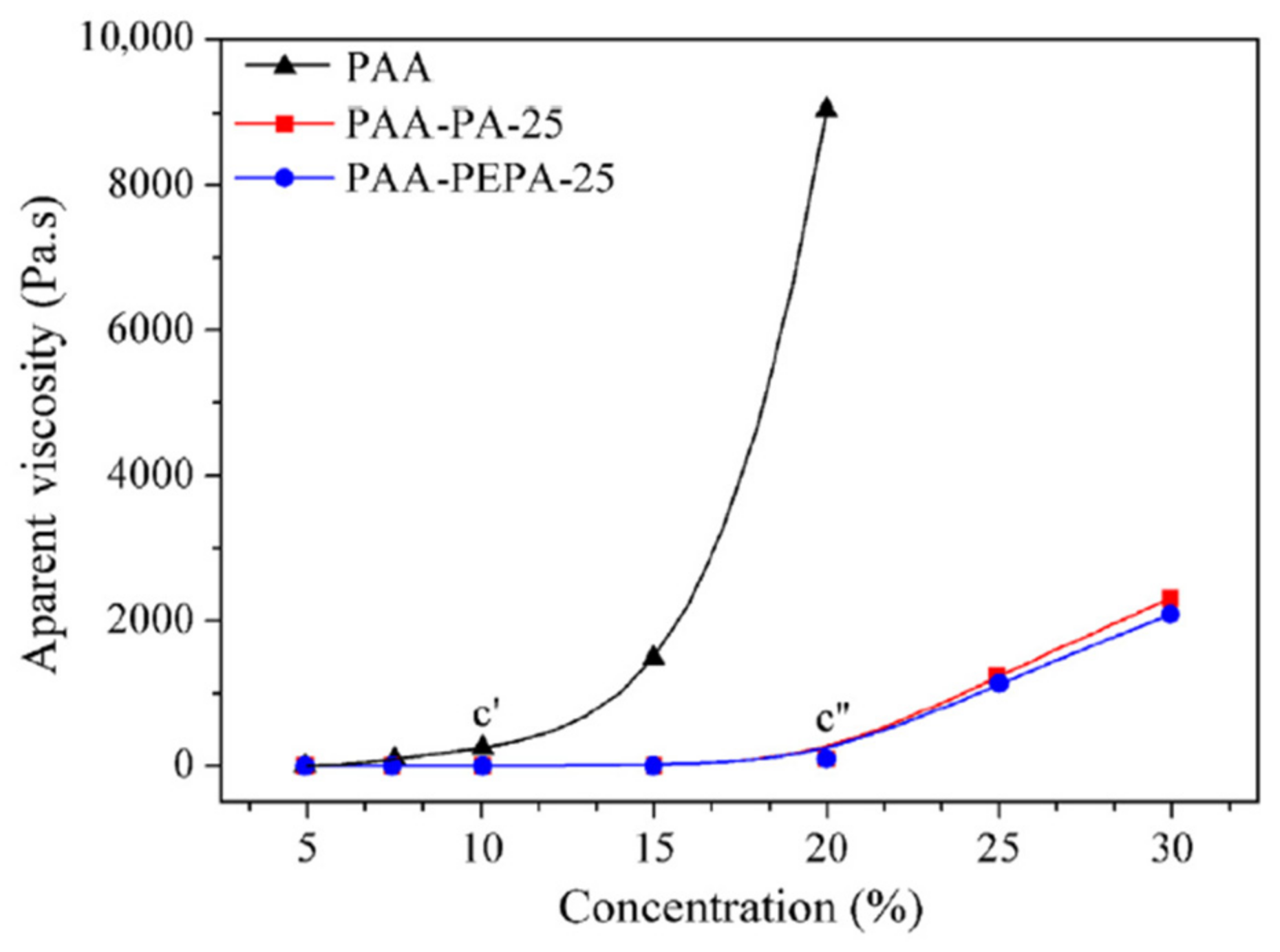
Figure 9 shows the molecular-weight-controlled PAAs with a controlled
Mw of 25 × 10
3 g·moL
−1 and the molecular-weight-uncontrolled PAAs’ solution viscosity, as a function of concentration. With increasing concentration, a systematic rising trend in apparent viscosities is observed, and two regions with very different slopes suggest critical concentrations (c* and c*′) of about 10% for PAAs (BPDA-PDA) without molecular weight limitation and 20% for molecular-weight-controlled PAAs (PAA-PEPA-25 and PAA-PA-25), respectively. When the concentration is less than 20%, the apparent viscosities of PAA-PEPA-25 and PAA-PA-25 are no more than 82 Pa·s, after which, an abrupt growth is noticed by a lesser exponential rate, proportionate to the PI precursor concentration.
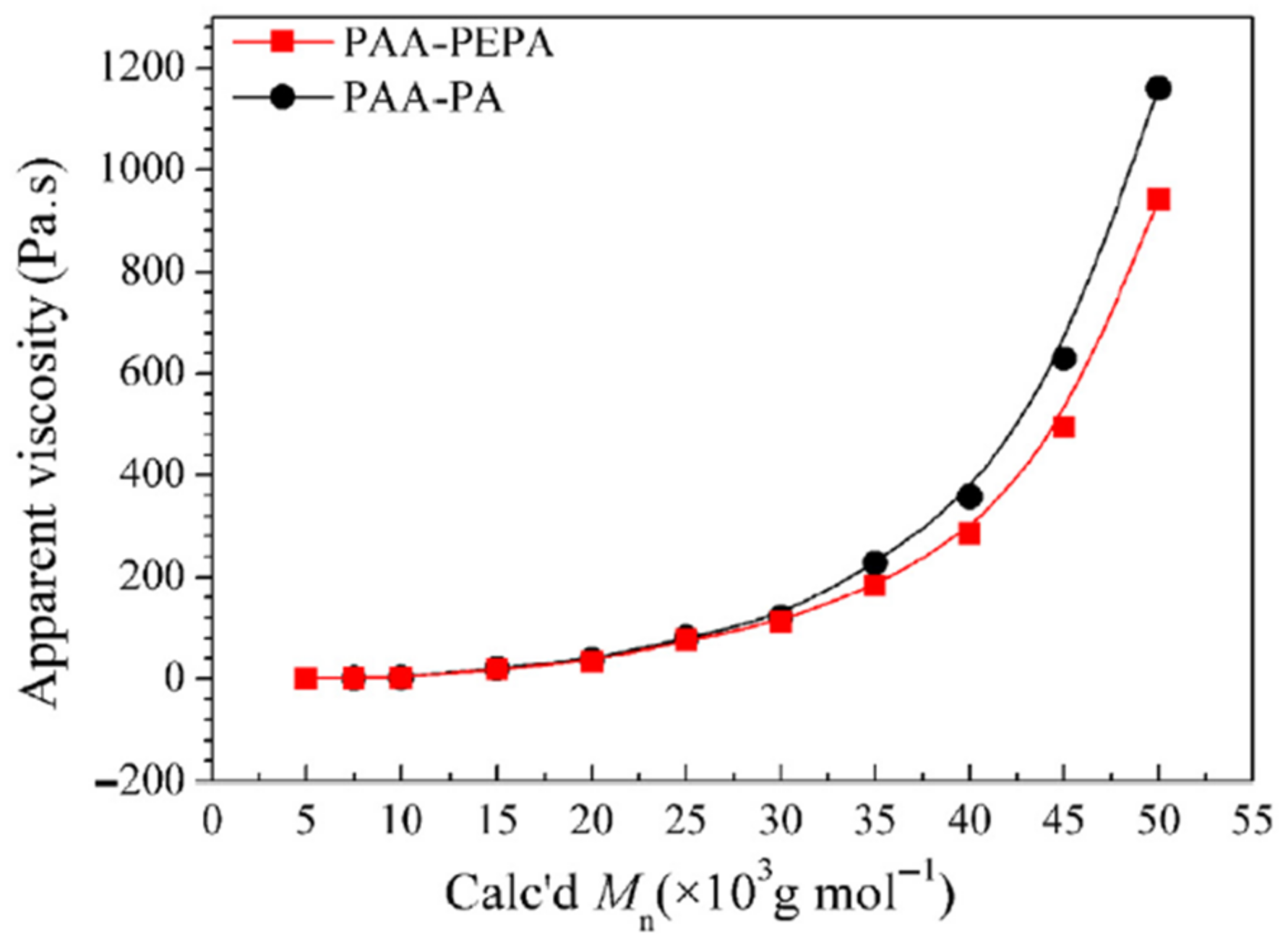
Figure 10 shows the apparent viscosity of molecular-weight-regulated PAA resins, with a concentration of 20% in DMAc at 25 °C, as a function of molecular weight. When PAA Calc’d
Mw ≤ 30 × 10
3 g·moL
−1, the apparent viscosities of both PAA-PEPA and PAA-PA gradually grow to 120 Pa·s, following which, the seemingly exponential growths to 1160 Pa·s are depicted, advancing in decreasing order: PAA-PA > PAA-PEPA.
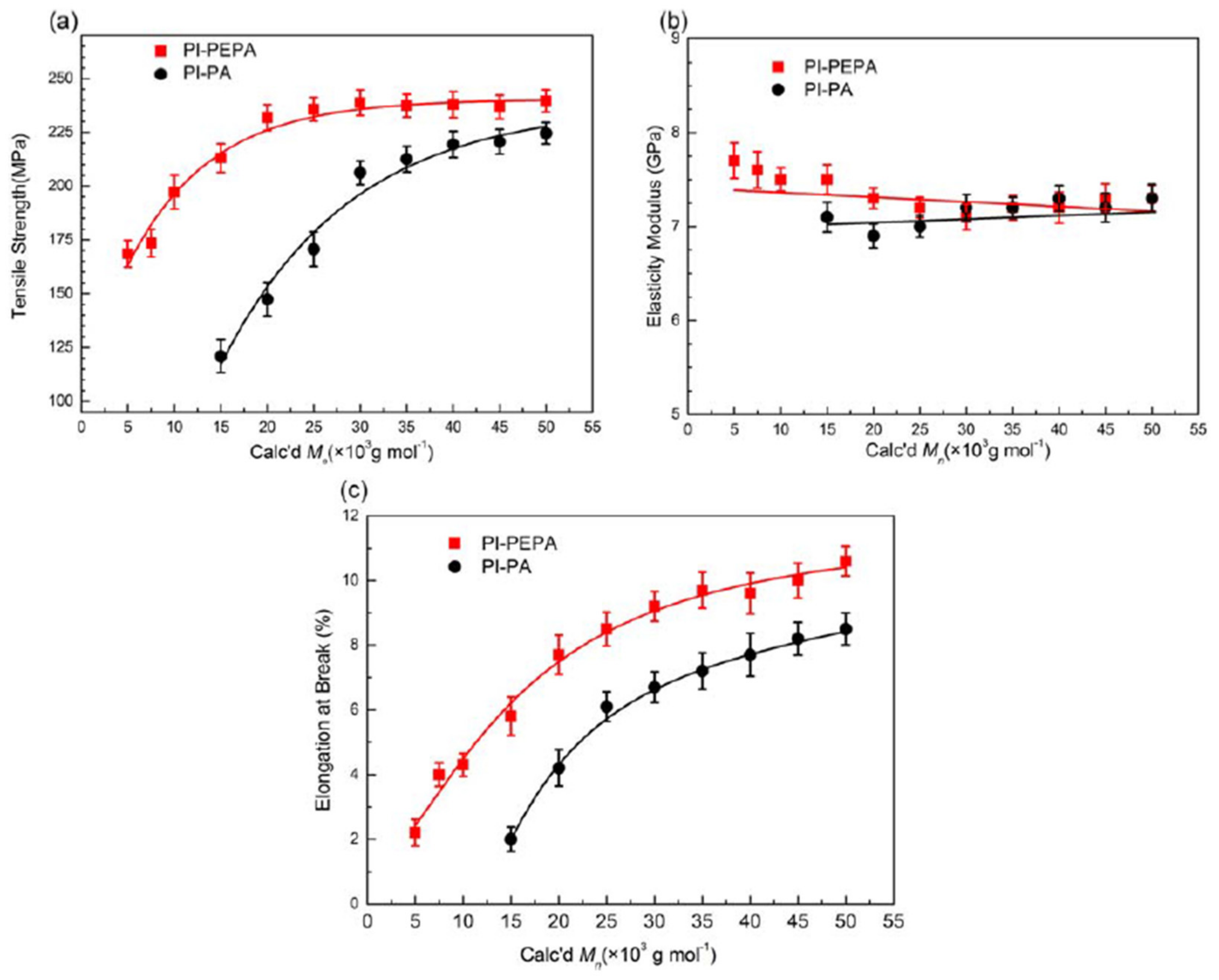
The mechanical characteristics of PI-PEPA and PI-PA films are compared in
Figure 11, as a function of PAA Calc’d
Mw. The tensile strength of PI-PEPA films increases dramatically when Calc’d
Mw rises over 20 × 10
3 g·moL
−1 (
Figure 11a), then appears to plateau at 237.6 MPa as Calc’d
Mw rises above 20 × 10
3 g·moL
−1, implying a critical Calc’d
Mw of PAA-PEPA around 20 × 10
3 g·moL
−1. PI-PA films, on the other hand, have much lower strength at the same Calc’d
Mw values. As the theoretical PAA-PEPA
Mw grows to 20 × 10
3 g·moL
−1, the elastic modulus of PI-PEPA reduces somewhat, from 7.7 to 7.3 GPa, likely due to lower chain crosslinking density (
Figure 11b); the elongations at break indicate a constant increase, with PAA Calc’d
Mw rising. Furthermore, PI-PEPA samples exhibited longer elongations than PI-PA films.
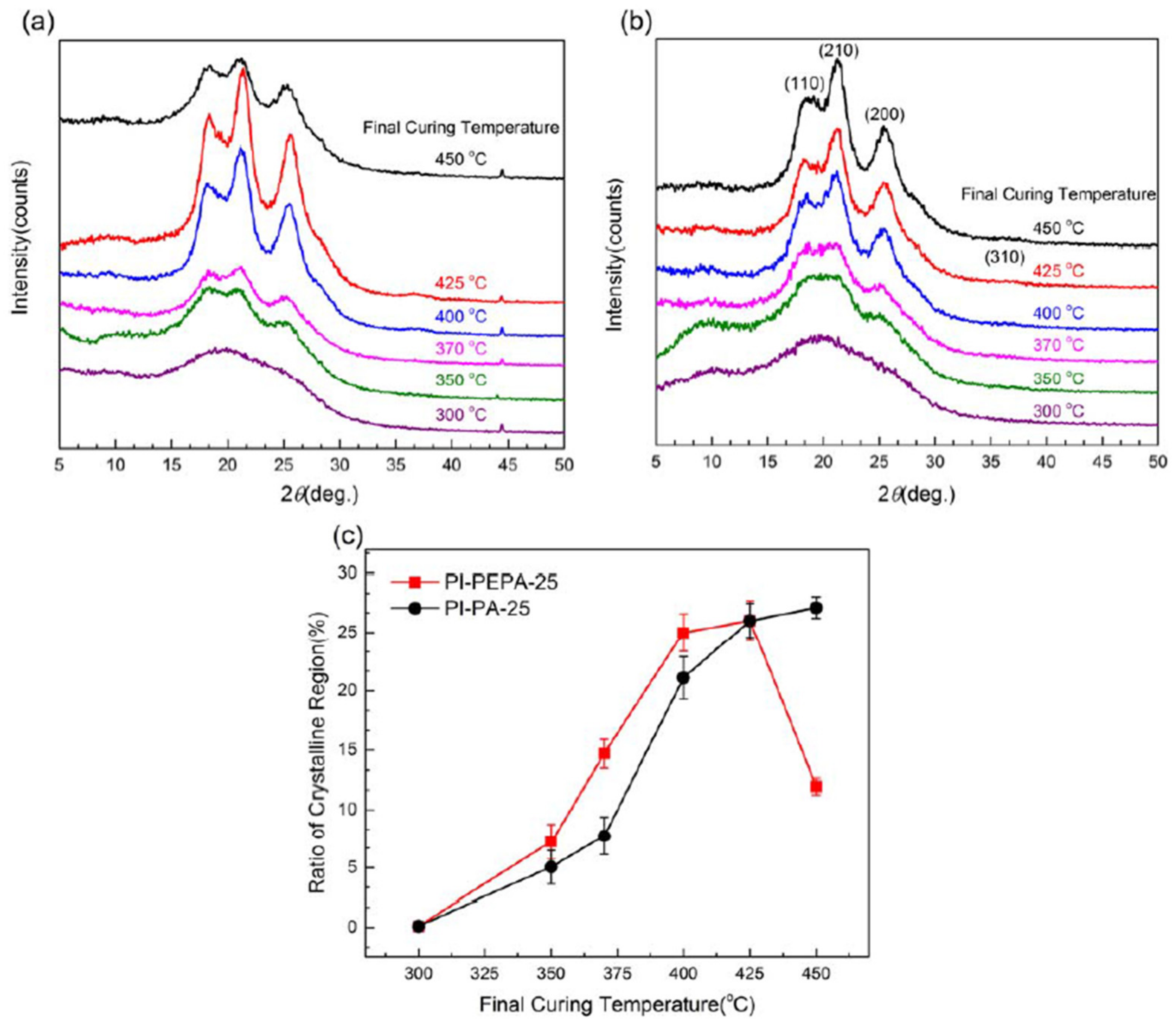
The impact of thermal-curing methods on the crystallization behavior and morphological aspects of PI-PEPA-25 and PI-PA-25 films via WAXD is depicted in
Figure 12. Both batches of films treated at lower temperatures (300 °C) show broad amorphous halos. With increasing the final curing temperature from 370 °C to 400 °C, the relative intensities of three distinguishable diffraction peaks, superimposed in the range of 18–26°, increase dramatically, and each peak’s half-width decreases, revealing a rapid enhancement in the regularity of intramolecular arrangements in polyimide films. Interestingly, when the crystalline peaks in the PI-PEPA-25 film were thermally processed at 450 °C, they became obtuse and less-structured, indicating a reduction in chain ordering, but the counterpart in the PI-PA-25 film became sharper.
Figure 12c compares the effect of different curing temperatures on the ratios of crystalline areas, determined from the diffractogram of the resulting PI films. The degrees of crystallinity in the PI-PEPA-25 film rise linearly, from 7.22% to 27.26%, as the final heating temperature is increased from 350 °C to 425 °C, showing that the curing reaction occurring in the phenylethynyl moiety improves the ability to crystallize.
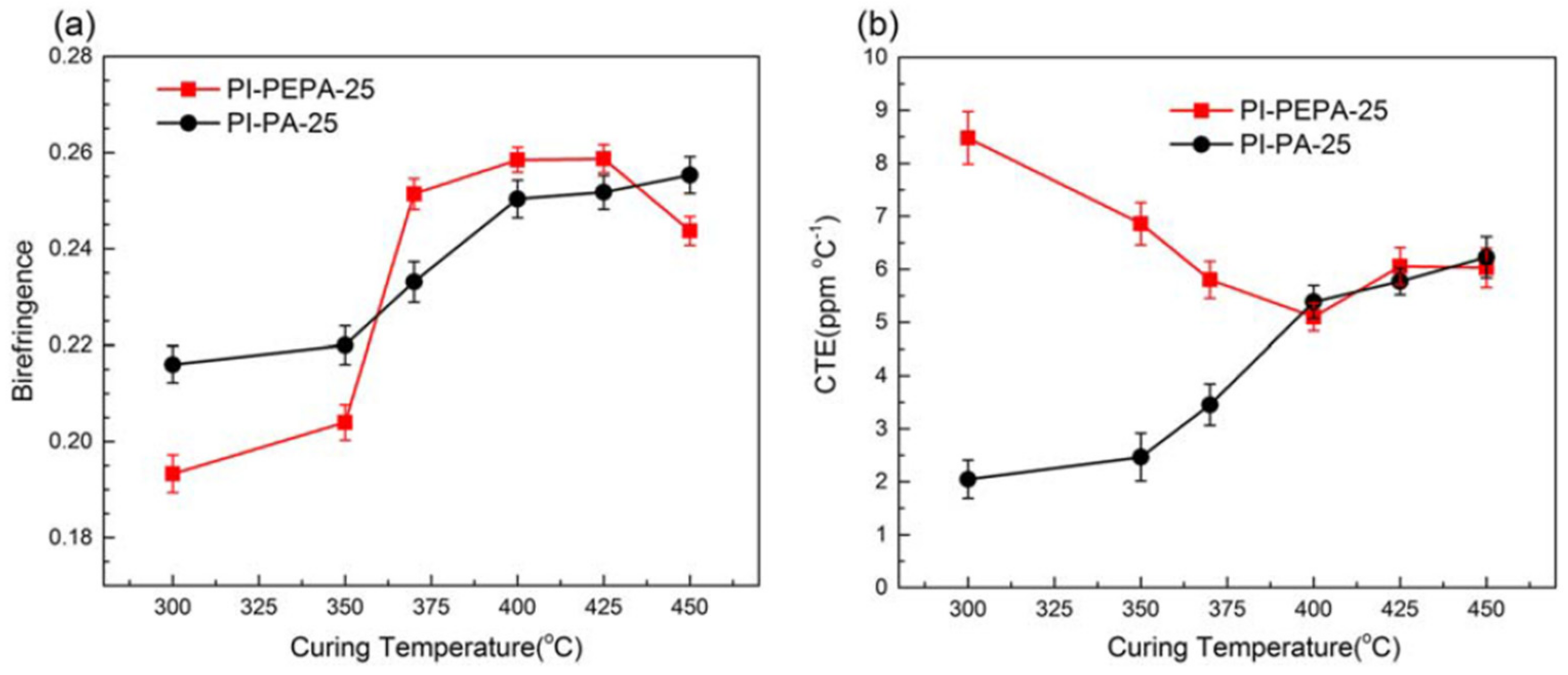
The effects of curing treatments on molecular orientation and thermal dimension stability of polyimide films (PAA-PEPA-25 and PAA-PA-25) are shown in
Figure 13. Both films with BPDA-PDA-based backbone architectures demonstrated substantial persistence lengths in the chain direction and low chain flexibility, as well as high birefringence (Δ
n) and low CTE, which are typically associated with a high level of in-plane chain orientation.
Figure 13a shows that the uncured polyimide film with reactive phenylethynyl end-caping groups has lower birefringence and a much higher CTE than the linear control film with unreactive phthalic end-capping groups, indicating that the phenylethynyl moiety has a significant influence on chain configuration via rotational hindrance. When PI-PEPA-25 was thermally treated at <370 °C, the birefringence associated with optical anisotropy rose by 33.7%, compared to 15.9% for the control PI-PA-25 film.
Figure 13b shows that when the ultimate curing temperature is raised from 350 °C to 400 °C, the CTE values of the thermally cured PAA-PEPA-25 films drop drastically from 8.5 to 5.1 × 10
−6 °C
−1, but those of the PI-PA-25 film increases.
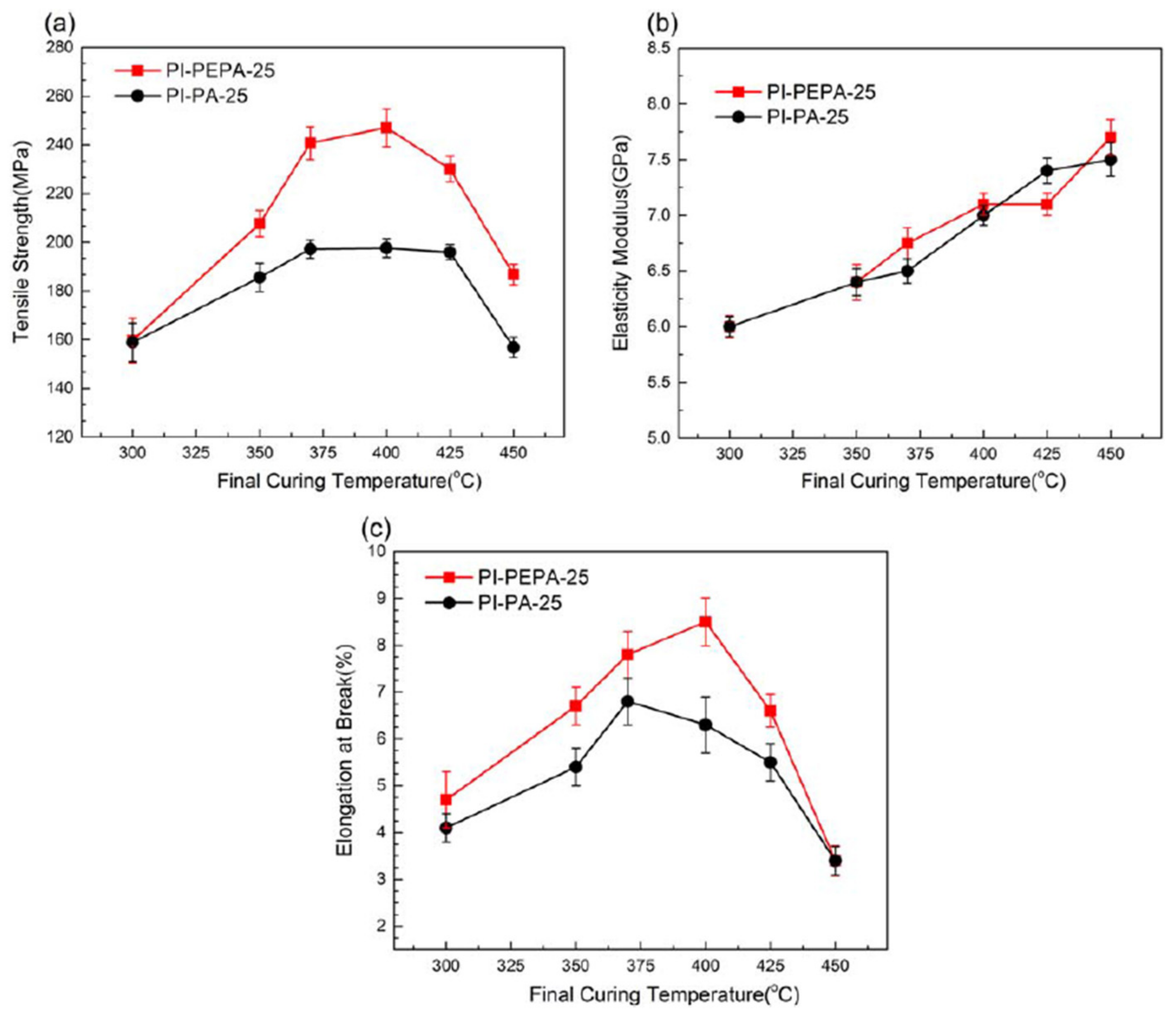
The effects of the curing treatment on the mechanical properties of PI film were studied in
Figure 14, to better understand the thermal-curing effect of phenylethynyl moieties in the comparatively high-molecular-weight PI film. For phenylethynyl-end-capped PAA resin (e.g., PAA-PEPA-25) changing to completely cured polyimide film, there is an ideal curing procedure (CP3), with an ultimate temperature as high as 400 °C (PI-PEPA-25). Advancement of the final curing temperature to 400 °C increases tensile strength and elongation at break by 55% and 83%, respectively, attaining the greatest results (247.1 MPa and 8.6%). After this, they plummet to 186.7 MPa and 3.4%, indicating that the failure mode has shifted to brittle fracture (
Figure 14a,b). The linear PI-PA-25 shows a similar tendency, with the highest strength and elongation of the material heating up to 370 °C being 25% and 26% lower than PI-PEPA-25.
As a whole, the molecular-weight-controlled PAA resins, end-capped by reactive phenylethynyl groups with calculated Mw > 20 × 103 g·moL1, demonstrated moderate viscosities and high solid concentrations, indicating enhanced wetting/spreading ability to form continuous PAA films. The PAAs are successfully converted to fully cured polyimide films after thermal curing at 400 °C/1 h, which have better mechanical properties and thermal resistances than the high-molecular-weight, unreactive phthalic, end-capped counterpart, making them suitable for high-temperature microelectronic packaging applications.
2.3. Colorless Transparent Polyimide Films for Flexible Display Applications
Aromatic polyimide films are widely utilized in the microelectronics and aerospace industries, but their yellow to brown colors limit their application in optical and display devices. The intermolecular CTC, generated as a result of molecular aggregation between polymer chains in the solid states, is principally responsible for the color of conventional aromatic polyimide films. Colorless polyimide films with good heat resistance are in high demand due to the rapid advancement of optoelectronic engineering. The processing temperature on flexible polymer film substrates may be higher than 300 °C in the manufacturing of flexible active-matrix organic light emitting display devices (AMOLEDs) [
45,
46]. At such a high processing temperature, most typical polymer optical films, such as polyethylene terephthalate (PET) or polyethylene naphthalate (PEN), lose their optical and mechanical characteristics. As a consequence, colorless and optically transparent polymer optical films with high-temperature resistance have stimulated the interest of academics and engineers alike.
One of the most complicated aspects of developing colorless transparent polyimide (CTPI) films is balancing thermal properties, optical transparency, and mechanical strength. The introduction of alicyclic substituents, including cyclobutane, cyclohexane, cardo groups (diphenylcyclohexane, adamantane, etc.); highly electronegative groups, such as trifluoromethyl (CF3); asymmetrical or twisted rigid substituents, such as asymmetrically substituted biphenyl or diphenylether moieties, are among the promising designs that could enhance both the high-temperature stability and optical transparency. These functional groups or structural segments have been commonly used to make new CTPI films, enabling in them superior thermal stability (Tg ≥ 300 °C), as well as outstanding optical transmittance (>85% in the visible light spectrum). At the same time, these substituents or structural segments may prevent the development of CTC in CTPI films caused by electron-donating and electron-withdrawing moieties.
Fluorinated polyimides synthesized from 6FDA (2,2-bis(3,4-dicarboxyphenyl) hexafluoropropanedianhydride) and aromatic diamines are transparent [
47,
48,
49,
50,
51]. Polyimides prepared from an alicyclic diamine, DCHM (4,4′-diaminodicyclohexylmethane), and aromatic dianhydrides are also transparent and thermally stable. As a result, semi-alicyclic polyimides have been thoroughly investigated, in order to obtain colorless transparent polyimide films. The fluorescence spectra and ultraviolet-visible absorption spectra of PI (6FDA/DCHM) and PI (6FDA/PDA) films were examined [
52]. In the region of the visible spectrum, both films displayed high transparency, with no absorption bands above 370 nm. The bulky group -C(CF
3)
2- precludes molecular packing in polyimide films, resulting in weak intermolecular interactions between the diamine and diimide moiety as electronic donors and acceptors. Because of the steric obstacle, introducing 6FDA into polyimide chains could reduce intermolecular charge transfer [
53]. The poor electron-donating capability of DCHM, on the other hand, would restrict not only intermolecular but also intramolecular charge transfer. An extremely transparent polyimide film was obtained by introducing 6FDA and DCHM into the polyimide chain. Both films exhibit
Tgs of around 250 °C, demonstrating that the alicyclic groups did not impair the thermal stability of polyimides.
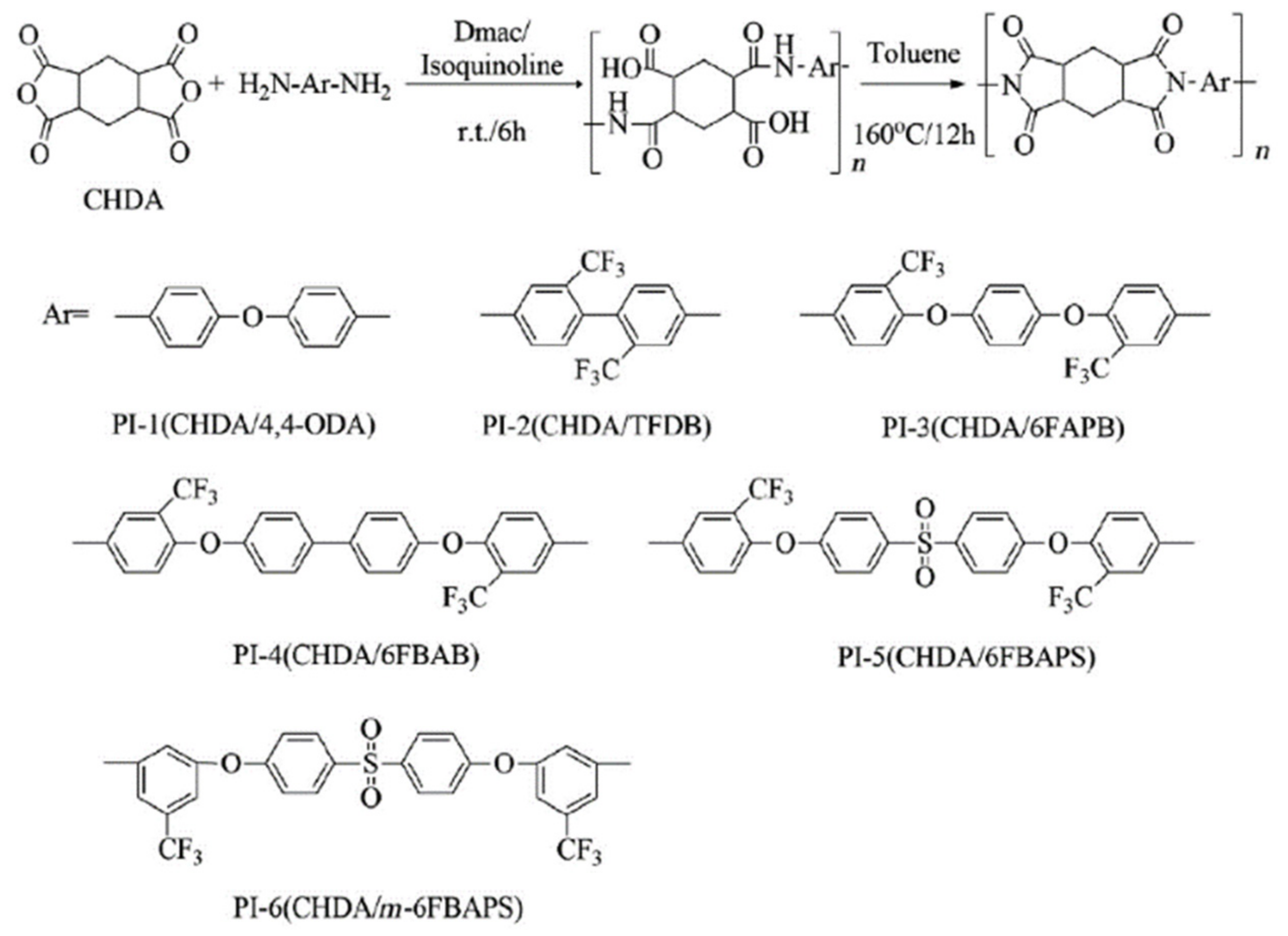
A semi-aromatic polyimide film with a high transparency and colorlessness was prepared using alicyclic dianhydride and aromatic diamine at ICCAS (
Scheme 4) [
54]. 2,2′-bis[4-(3-amino-5-trifluoromethylphenoxy) phenyl]sulfone (
m-6FBAPS), a unique
meta-substituted aromatic diamine with trifluoromethyl and sulfonyl groups in the backbone, was synthesized and utilized to react with 1,2,4,5-cyclohexanetetracarboxylic dianhydride (CHDA), to form a semi-aromatic polyimide film. A number of additional semi-aromatic polyimides were produced by employing CHDA and other different aromatic diamines for comparison.
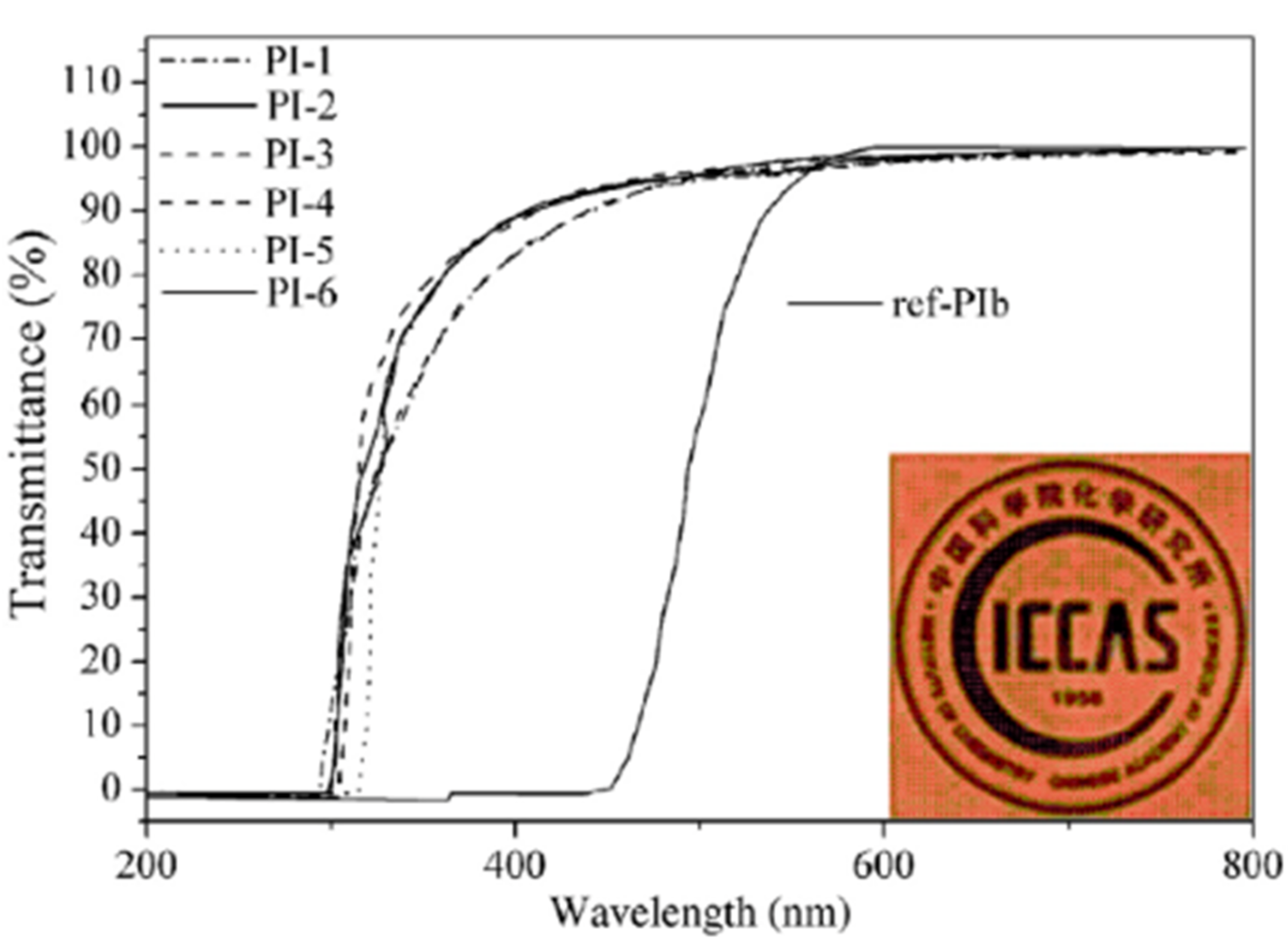
The optical transparency of semi-aromatic polyimide films is shown in
Figure 22, as measured by their physical appearance and UV-vis spectra. Even though the film thickness was as high as 70 μm, these films were transparent and basically colorless, in contrast to the deep-yellow or brown color of conventional aromatic polyimide films. The alicyclic moieties in the polymer structure are responsible for the increased optical transparency.
Table 4 summarizes the optical transparency obtained from UV-vis spectra, including cutoff wavelength (absorption edge) and transmittance at various wavelengths. All of the semi-aromatic polyimide films demonstrated exceptional optical transparency, with UV cutoff wavelengths less than 314 nm and transmittance at 450 nm, greater than 91%. In contrast, the typical aromatic polyimide film derived from PMDA and 4,4′-ODA (ref-PIb) exhibited a deep-yellow color and a 2% transmittance at 450 nm.
Polyimides, including bulky electron-withdrawing trifluoromethyl and sulfonyl groups in diamine moieties, such as PI-2, PI-5, and PI-6, formed highly transparent and colorless films, with T400 of 90% and T450 of 94%, respectively. The deformed molecular conformation, combined with the impaired electron-accepting and electron-donating capabilities of dianhydride and diamines, which considerably restricted the generation of inter-/intramolecular charge transfer interactions, are credited with their good optical features.
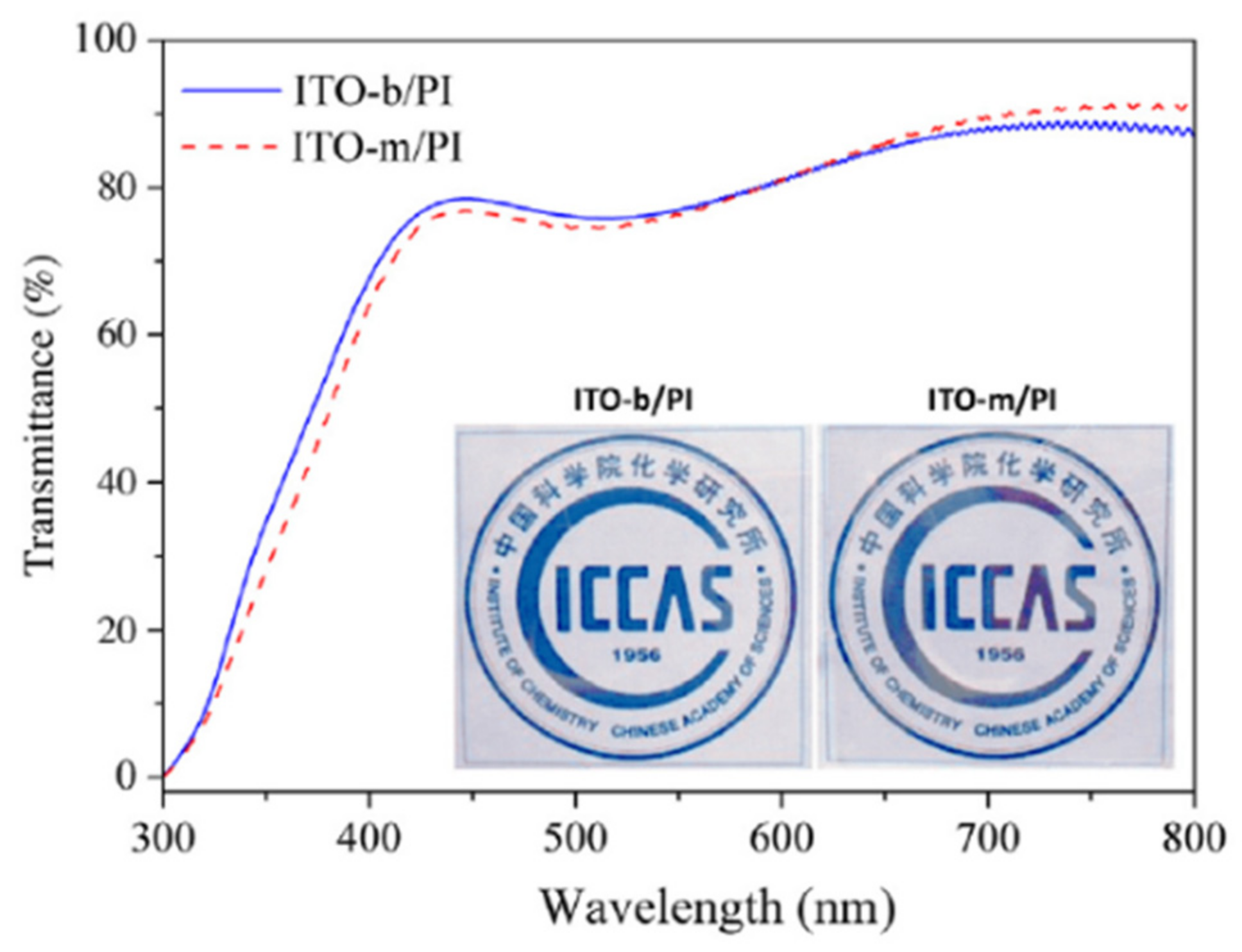
Furthermore, a high-temperature radio-frequency magnetron sputtering method for producing transparent and conductive indium tin oxide (ITO)/polyimide films has been designed at ICCAS (
Figure 23). ITO/PI films were fabricated via radio-frequency magnetron sputtering at an increased substrate temperature, using a highly transparent and thermally stable polyimide (
Figure 24). To manufacture the extremely transparent and conductive ITO/PI films, a two-step deposition approach was established, in which a thick bulk ITO layer was overlapped by deposition on a thin seed ITO layer, with a dense surface. Physical appearances, transmittance intensities were used to evaluate the optical properties of the ITO-m/PI and ITO-b/PI films. Both films were exceptionally transparent and colorless, with average transmittances of over 81% in the 400–800 nm range and
b* values of less than 3 (
Table 5). ITO-b/PI showed a resistivity of 9.03 × 10
−4 Ω·cm, which was two orders of magnitude lower than ITO-m/PI. After annealing at 240 °C, the ITO/PI film possessed an 83% transmittance and a sheet resistance of 19.7 Ω·square
−1.
A method for manufacturing colorless and transparent PI films, using a solution casting procedure, was reported by Mitsubishi Gas Chemical Company. The bi-axially stretched colorless PI films have high optical transparency, heat resistance, and low dimension changes [
55]. The films were made using soluble CTPI resin, which was synthesized from CBDA and aromatic diamines, via a one-step high-temperature polycondensation process. The resulting CTPI film had a thickness of 200 µm, a total light transmittance of 89.8%, a yellow index of 1.9, and a haze of 0.74%. The film had a 0.5% solvent residual ratio by weight. Colorless CTPI films could have a wide range of applications in optoelectronics, including transparent conductive film, transparent substrates for flexible displays, flexible solar cells, and flexible printed circuit boards (FPCB), because of their unique optical and thermal features. Similar approaches have been reported in the literature [
56].
In addition, DuPont Company [
57] and Kolon Industries [
58] revealed the ways to form colorless transparent polyimide films from fluoro-containing dianhydride copolymers. Flexible and robust CTPI films, with achromatic color and high transparency, were obtained. The Langley Research Center of NASA (National Aeronautics and Space Administration, USA) has researched molecularly orientated transparent polyimide films for space applications [
59]. A colorless and transparent polyimide film, LaRC-CP1, is prepared using soluble polyimide resin with 6FDA and fluoro-containing diamine. After stretching treatment, the tensile strength of 2.0× stretched film increased from 93.0 MPa to 145.4 MPa, and the elongation increased from 16 to 65%. The dimensional stability, toughness, elongation, and strength of the films were considerably improved after stretching, which is critical for applications in space conditions.
2.4. Inorganic Hybrid Polyimide Films
Hazardous conditions, mainly atomic oxygen (AO) and vacuum ultraviolet (VUV), can degrade polymer materials used on the exterior surfaces of a spacecraft in low Earth orbit (LEO). Typically, multi-layer thermal insulation (MLI) is used to protect the outside surfaces of a spacecraft, with the outer surface of MLI consisting of exposed polyimide films, such as Kapton H, Upilex-R, and others. The MLI performances of these polymer films are always deteriorated as a result of AO and UV-induced erosion. Optically transparent inorganic thin films have traditionally been used as overlay protective coatings to protect polyimide films from AO bombardment [
60]. The existing hard coating technology, on the other hand, has three flaws. Pinholes in protective coatings produced during the coating process are one example. Even small pinholes have been proven to cause significant undercutting in polymer layers [
61]. As a result, the number of pinholes or imperfections in the inorganic coating must be kept to a minimum, in order for the polymer films to survive. For existing thin-film techniques, this is a major technological barrier. Another issue is the formation of cracks in regions where cutting, punching, or a sharp bend has occurred. The cracks are larger than pinholes, which are the most common cause of MLI damage. Last but not least, collisions with space debris or micrometeoroids in orbit may cause gaps in the inorganic coatings, allowing AO to react with the polymer beneath. Consequently, spacecraft protective coatings with self-healing capabilities are desired in LEO environment.
Kapton polyimide film is widely applied in solar arrays, spaceship thermal blankets, and space inflatable constructions. The film is extensively damaged when exposed to AO in LEO. Incorporating polyhedral oligomeric silsesquioxane (POSS) into the polyimide backbone frameworks by copolymerizing a monomer mixture of POSS-containing diamine and other aromatic diamine with aromatic dianhydride in a polar solvent to produce a high-molecular-weight PAA solution, is then cast on a substrate surface to form a PAA film [
62]. A self-standing gel film is peeled off from the substrate surface, followed by imidization to obtain a POSS-Kapton polyimide film, after some of the solvent is removed by evaporation and partly chemical or thermal imidization (
Scheme 5). The POSS is a remarkable class of nanoscale inorganic/organic hybrid cage-like architectures, with a silicon/oxygen framework (RSiO
1.5), where R is a hydrocarbon group used to tune polymer compatibility and chemical stability. On the Materials International Space Station Experiment (MISSE) platform, POSS-Kapton films were subjected to AO in space. AO flux and fluence differed from experiment to experiment, based on exposure time, experiment period within the solar cycle, and MISSE sample tray orientation. The AO’s relative velocity to the ram surface is about 7.4 km·s
−1, equating to oxygen atoms impacting the surface at a velocity of 4.5 eV on average. After 3.9 years, the MISSE-1 flight experiment was recovered. The samples were exposed to the ram and, hence, to all constituents of the LEO environment, including AO and VUV irradiation, with the O-atom fluence being approximately 8 × 10
21 atoms·cm
−2, comparable to the fluence on the companion MISSE-2, which was accurately determined at 8.43 × 10
21 atoms·cm
−2.
At ambient temperature, the SC-POSS Kapton polyimide film was subjected to AO, with a total fluence of around 2.7 × 10
20 atoms·cm
−2. The laboratory AO erosion measurements of SC-Kapton film are included in
Table 6 [
62]. The experimental results showed that increasing the content of SC-POSS reduced surface erosion dramatically. The atomic-oxygen erosion yields of SC POSS-Kapton and MC POSS-Kapton films were comparable.
Temperature effects on the atomic-oxygen erosion yield of POSS-Kapton films with Si
8O
12 loadings of 0%, 3.5%, and 7.0% were examined.
Table 7 illustrates the AO erosion depths of MC-Kapton films in the lab at various temperatures. From 25 °C to 300 °C, the erosion depth of the 0%(
w) MC POSS-Kapton film reference increased by a factor of around 3.6, indicating the largest temperature dependence. Furthermore, the erosion of 3.5 and 7.0%(
w) Si
8O
12 MC POSS-Kapton films was less temperature dependent. With the increase in temperature, from 25 °C to 300 °C, the erosion depths of these samples increased by factors of 2.2 and 2.4, respectively.
On MISSE-1, 0%(w), 1.75%(w), and 3.5%(w) MC POSS-Kapton films were flown in ISS for 3.9 years. Throughout the flight, several photographs of the samples were obtained, and it was observed that the 0%(w) MC POSS-Kapton film reference sample was entirely eroded in less than 4 months. The 0%(w) MC POSS-Kapton film should have eroded a minimum of 240 µm, based on an estimated O-atom fluence of 8 × 1021 atoms·cm−2 and an erosion yield of 3.00 × 10−24 cm3 per atom for standard Kapton H film. The 1.75%(w) and 3.5%(w) MC POSS-Kapton films were degraded by 5.8 µm and 2.1 µm, respectively, according to the experimental data.
Miyazaki et al. researched the AO tolerance of a polysiloxane-block polyimide film [
63,
64]. The silicon-containing polyimide film (BSF30), a polysiloxane-block-polyimide, was chosen as the subject of inquiry. At JAXA’s Combined Space Effects Test Facility in Tsukuba, Japan, an AO beam was bombarded on a polysiloxane-block-polyimide film. The new layer is built with a 500 nm deep damaged area, as shown by AO irradiation of the eroded surface. The ability of the polysiloxane-block-polyimide to “self-heal” was identified, implying that polysiloxane-block-polyimide film has a great deal of potential as a space-use material, especially for LEO environment.
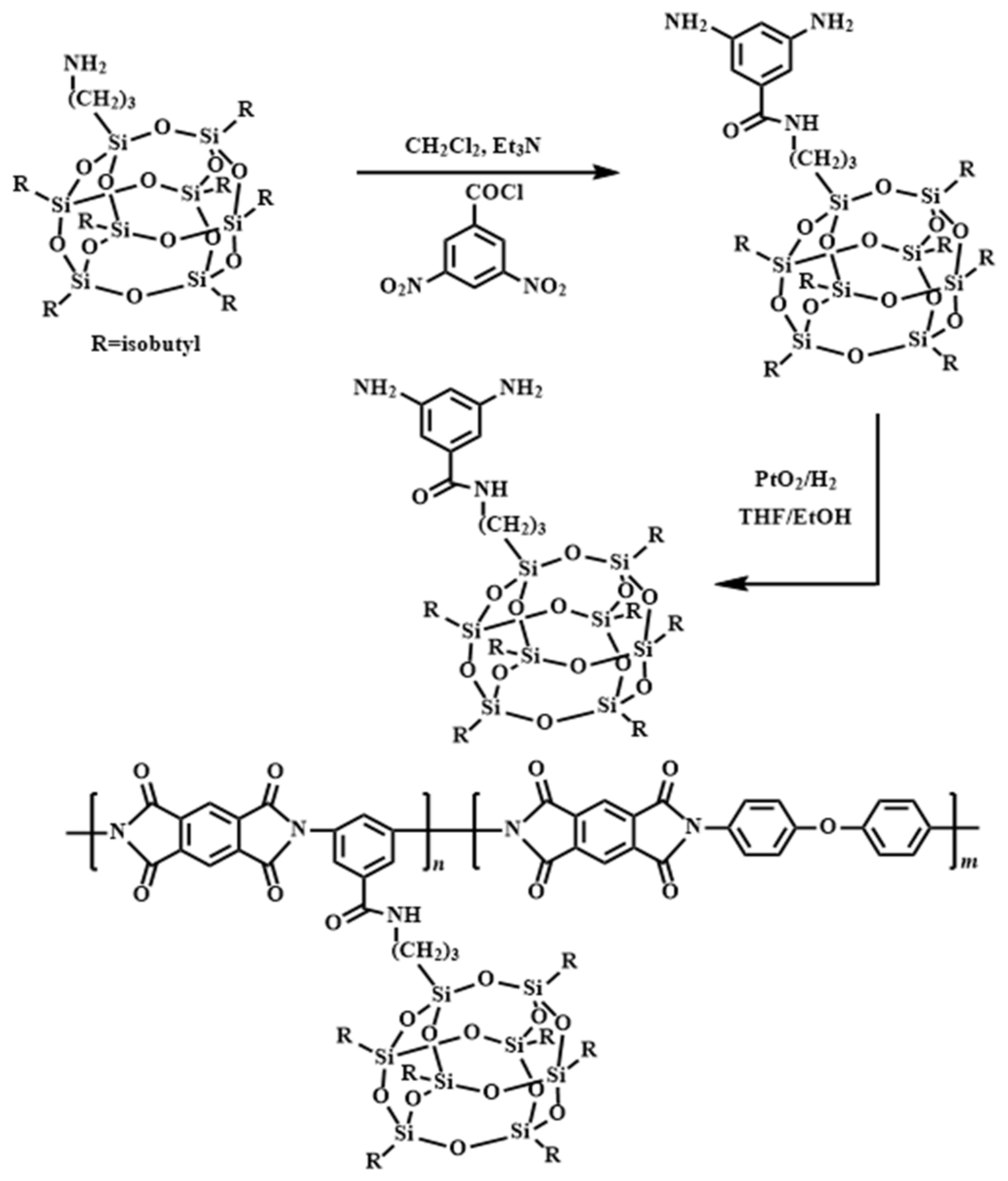
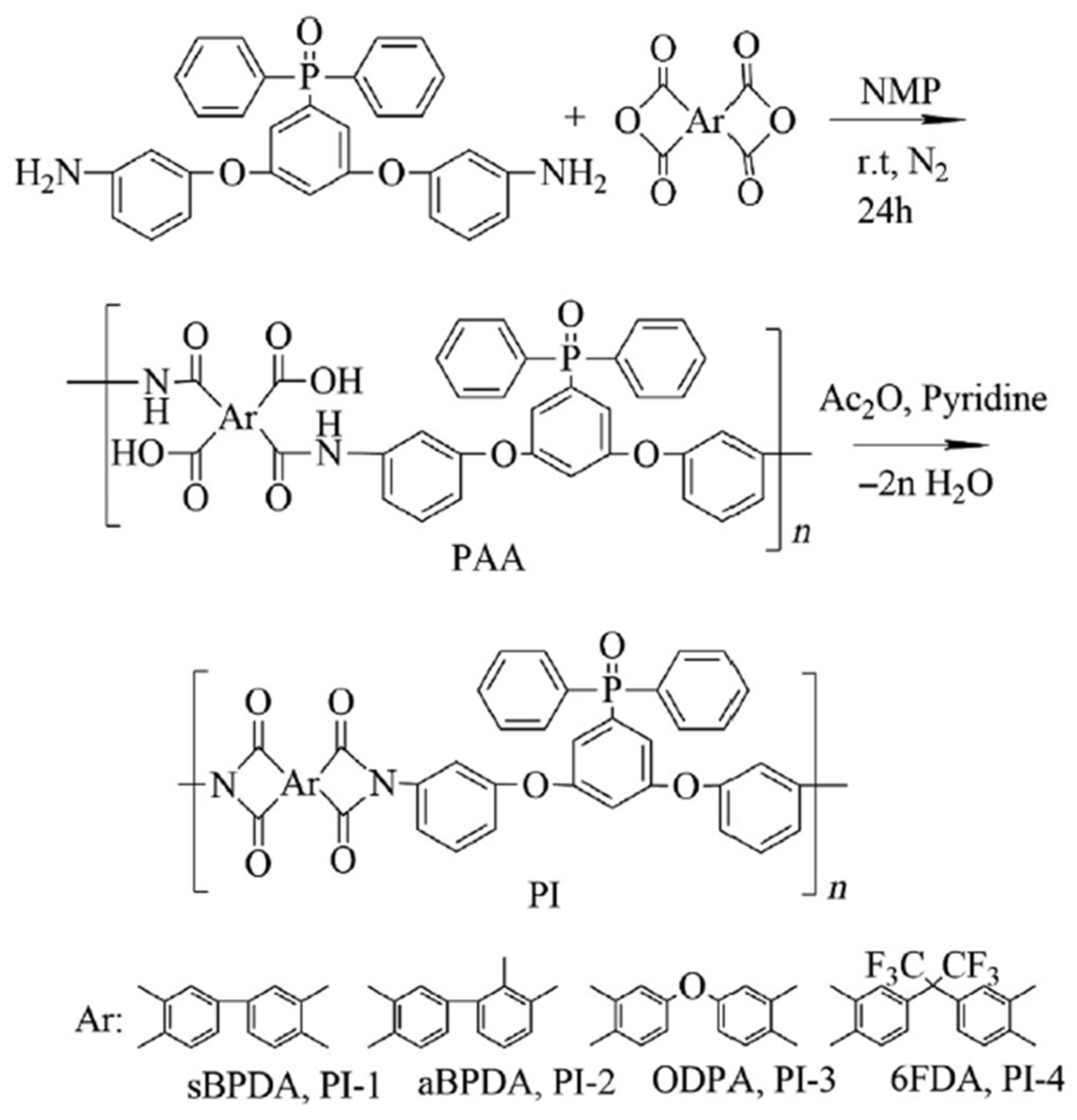
A set of AO-resistant and transparent polyimide coatings incorporating phosphine have been designed [
65]. The Williamson reaction of 3,5-difluorophenyldiphenylphosphine oxide (DFPPO) and
meta-aminophenol yielded [3,5-bis(3-aminophenoxy) phenyl]diphenylphosphine oxide (
m-BADPO), a
meta-substituted aromatic diamine. Afterwards, the aromatic diamine was polymerized using a variety of commercially available aromatic dianhydrides to produce a series of aromatic polyimides (PI-1 to PI-4) (
Scheme 6).
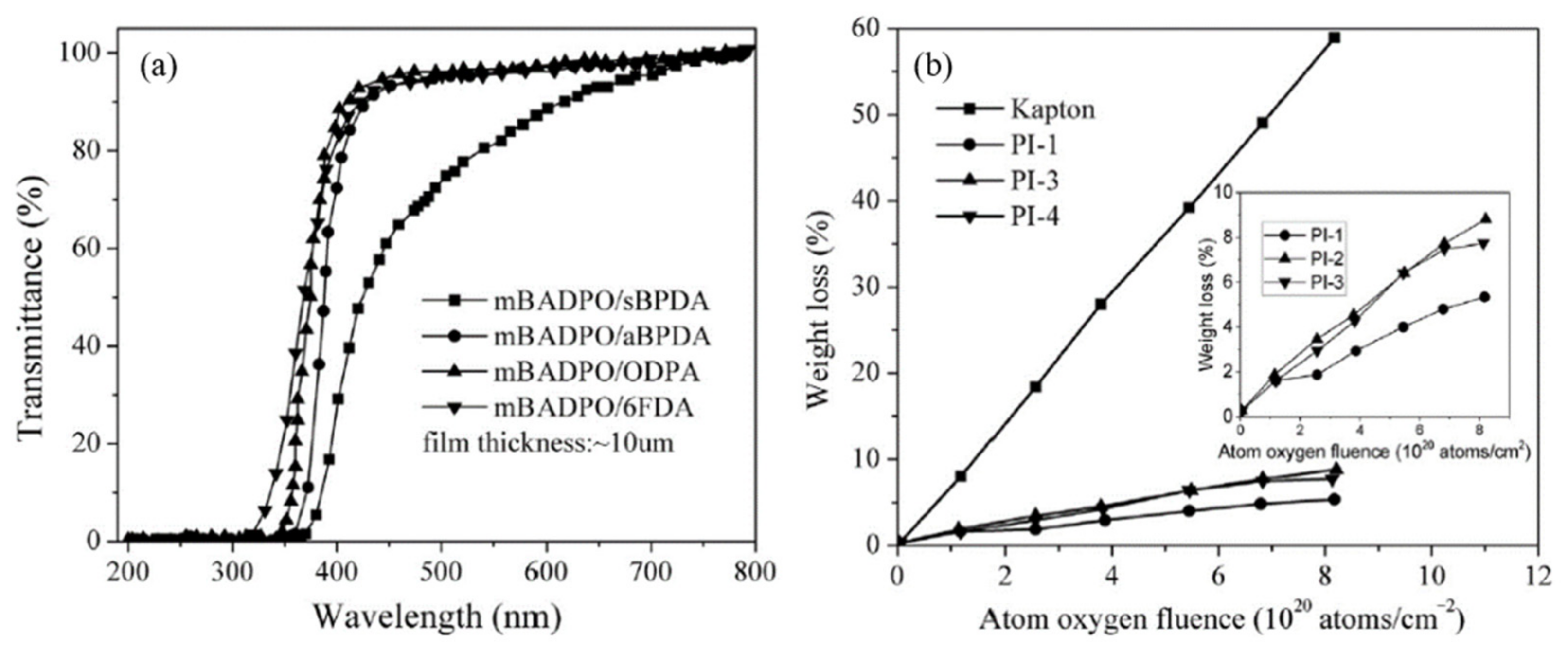
The solubility of the polyimides was found to be improved due to the synergistic effects of
meta-linked structure and bulky PPO groups, allowing for solution casting fabrication of the polyimide films. In the visible light wavelength, the films demonstrated flexible and robust features, with light color and good transparency (
Figure 25a). The films exhibited up to 87% transmittance at 400 nm. At the same thickness, PI-4 had a cutoff wavelength of 319 nm, which was 7 nm lower than polyimide from
p-BADPO and 6FDA. The PI-4 film’s transmittance at 400 nm (85%) was also higher than its
para-linked counterpart (82%). The yellow index (YI) is a commonly used criterion for assessing the coloration of a polymer film. This value describes how even a film sample’s color shifts from clear or white to yellow. A lower YI value generally suggests a polymer film with poor coloring. The loose molecular packing, driven by the bulky pendant PPO group in the diamine monomer, and the flexible ether or 6F atoms in the aromatic dianhydride in PI-3 and PI-4 films, could be attributed to their good transparency and low coloring.
At the ground-based simulation facility, the polyimide films PI-1, PI-3, and PI-4, as well as the Kapton reference (20 × 20 × 0.05 mm), were exposed to AO. As a standard, the Kapton erosion rate was set to 3.0 × 10
−24 cm
3·atom
−1. The specimens were subjected to AO, with a total exposed fluence of 8.13 × 10
20 atoms·cm
−2. The mass loss against AO fluence for the polyimide films and the Kapton reference can be seen in
Figure 25b. The polyimide samples containing the PPO group had a weight loss of 5.48–8.76%(
w), while Kapton H showed a weight loss of 56.76%(
w).
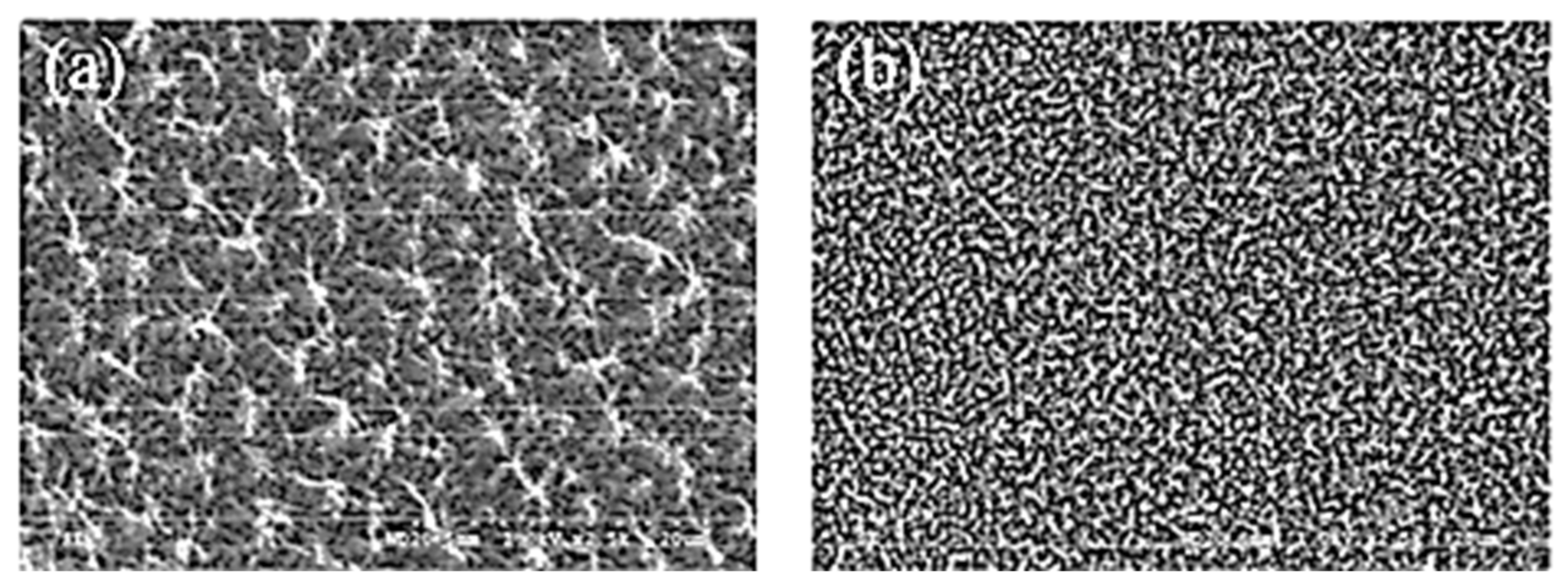
XPS was used to investigate the surface composition of the AO-exposed PI films. The results revealed that a phosphorus oxide layer formed on the surface of the PI-1 film. This inert layer may prevent the underlying polyimide film from eroding deeper. After AO exposure, SEM images of Kapton H and PI-1 samples are compared in
Figure 26. Both the surface appearances of PI-1 and Kapton films presented a typical carpet-like pattern.
F. L. Liu et al. developed carborane-containing aromatic polyimide (CPI) films, with extraordinary thermo-oxidative stability at 700 °C, formed by casting poly(amic acid) (PAA) resin solution on a glass substrate and then thermal imidizing at high temperatures [
66]. In an aprotic solvent, an aromatic dianhydride and a mixture of aromatic diamines, including carborane-containing aromatic diamine, were copolymerized to produce the PAA solution. Because of the multilayered protective layers generated on the film surface by the pyrolysis process of the carborane group into boron oxides, the CPI films demonstrated exceptional thermo-oxidative stability at 700 °C. In a high-temperature condition, the boron oxide layer increased the degradation activation energy and restricted the direct interaction of inner polymer materials with molecular oxygen, performing as a “self-healing” upmost coating layer on the polyimide materials. Even after thermo-oxidative aging at 700 °C for 5 min, the CPI-50 film remained flexible and retained 50% of its mechanical strength.
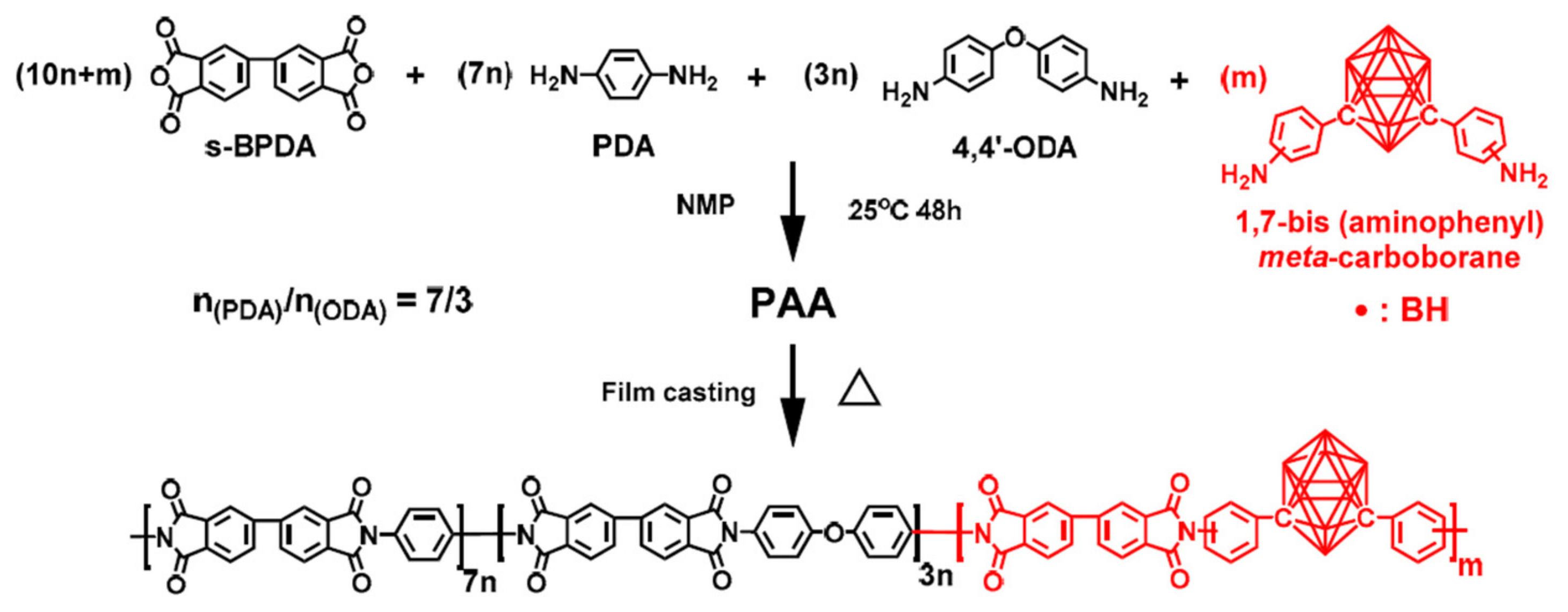
The preparation process of the CPI films is shown in
Scheme 7. Polycondensation of equimolar aromatic dianhydride (
s-BPDA), with a mixture of three distinct aromatic diamines, including PDA, 4,4′-ODA, and 1,7-bis(aminophenyl)-
meta-carborane (BACB) in NMP, afforded the polyimide precursor PAA resin solution. The researchers designed a series of CPI-
x films with different boron loadings (
x means the molar ratio of BACB in diamine monomers and is equivalent to 0, 5, 10, 20, 30, 40, and 50, respectively).
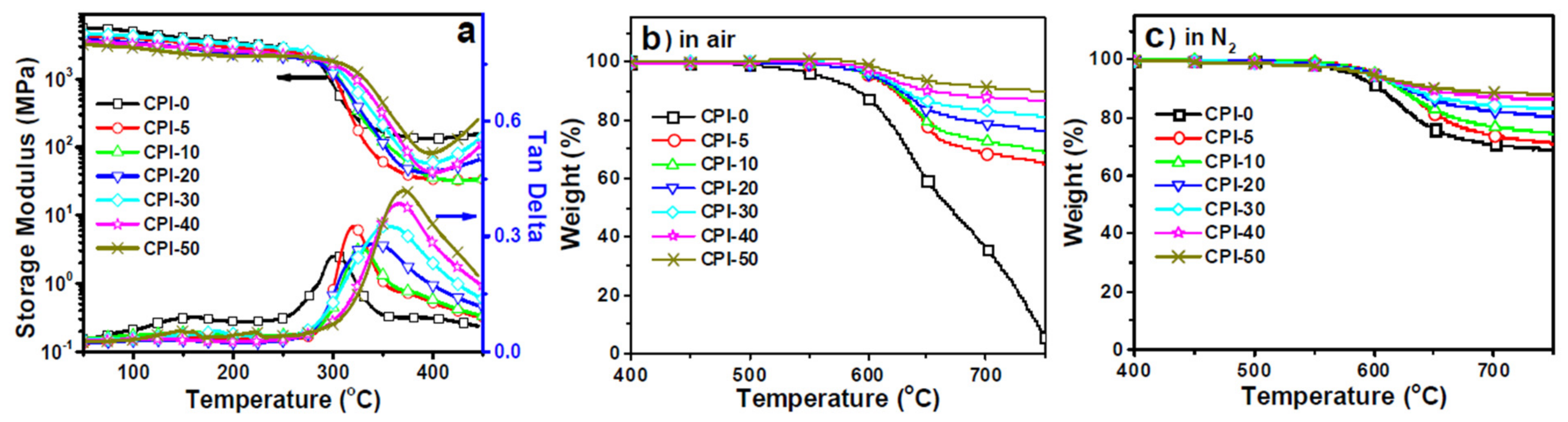
The thermal stabilities, as measured by DMA and TGA, are shown in
Figure 27. Though the bulky carborane cage structure increased the distance between polymer chains, it also restrained polymer chain mobility in the large scale due to its large steric hindrance, and the movements prompted by the larger interchain space were too tiny in size scale to change polymers from a glassy to rubbery state, leading to higher
Tg values. Incorporating carborane into the polyimide backbone structure dramatically improved polymer thermal and thermo-oxidative stabilities, as compared to the boron-free film (CPI-0) (
Figure 27b,c). Increased boron loadings enhanced both thermal and thermo-oxidative stability.
Due to the strong electron-withdrawing capability and massive steric hindrance effect of the carborane cage structure, BACB is much less reactive than the other two aromatic diamines (4,4′-ODA and PDA) during copolymerization with aromatic dianhydride, limiting the polymerization reactivity of the diamine monomer mixture. BACB interacted with dianhydride to generate a lower Mw polymer, despite its difficulty in forming a high Mw polymer. Hence, the molecular weight of PAA precursors decreased, and the polymer interchain interaction deteriorated as boron loadings increased, resulting in reduced tensile strength and modulus. In comparison to CPI-20 (TS: 161 MPa, TM: 4.9 GPa), the CPI-50 film possessed the lowest mechanical properties, with a tensile strength (TS) of 108 MPa and a tensile modulus (TM) of 3.7 GPa.
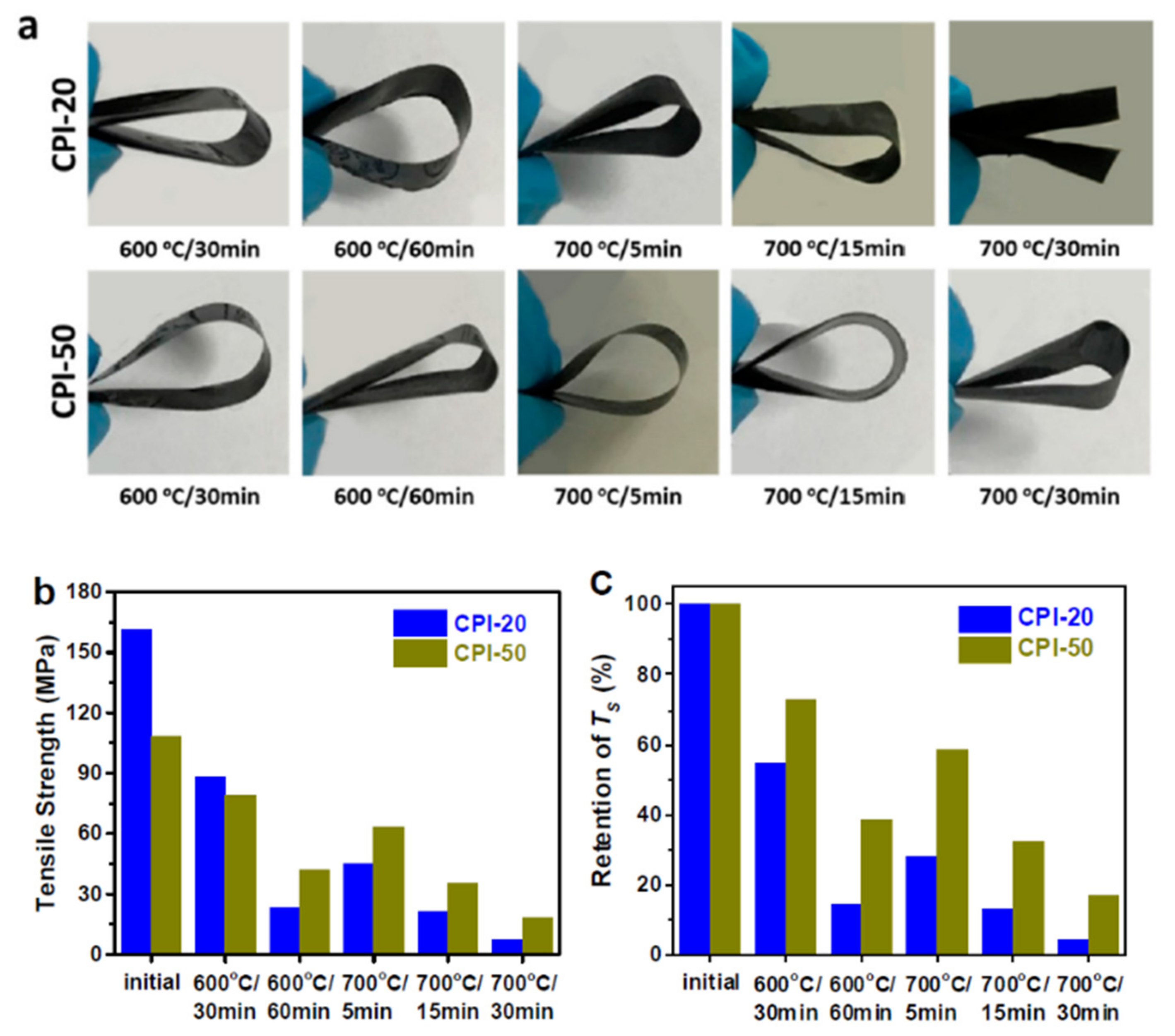
CPI films with varied boron loadings (CPI-0, CPI-20, and CPI-50) were thermally treated under a series of thermo-oxidative aging test conditions (600 °C/30 min, 600 °C/60 min, 700 °C/5 min, 700 °C/15 min, 700 °C/30 min). Bend testing was used to determine the flexibility of the CPI films, and tensile characteristics were determined on an Instron 5567 universal testing instrument, with a drawing rate of 2.0 mm·min
−1. Under the same circumstances, all of the aged boron-containing films could be bent 180°, demonstrating excellent flexibility retention (
Figure 28a). Moreover, despite the fact that the CPI-20 film was split into two pieces during bend testing, following thermo-oxidative aging at 700 °C for 30 min, the CPI-50 film still maintained good flexibility.
After thermo-oxidative aging at 600 to 700 °C, both CPI-20 and CPI-50 films exhibited appreciable tensile strength (
Figure 28b); the unaged CPI-20 and CPI-50 films possess tensile strengths of 161 MPa and 108 MPa, respectively. The tensile strength of CPI-20 and CPI-50 was degraded to 88 MPa and 79 MPa, after thermo-oxidative aging at 600 °C for 30 min. The tensile strength was degraded to 23 MPa (CPI-20) and 42 MPa (CPI-50) when the aging period was extended to 60 min at 600 °C, demonstrating that the thermo-oxidation at 600 °C increased with increasing aging duration. Furthermore, the CPI-50 film successfully retained tensile strength of 63, 35, and 18 MPa, after aging at 700 °C for 5, 15, and 30 min, and its strength retention was substantially higher than that of CPI-20 (
Figure 28c). The introduction of the carborane cage into the polymer backbone structure considerably enhanced the polyimide thermo-oxidative stability at 700 °C, when compared to boron-free films.
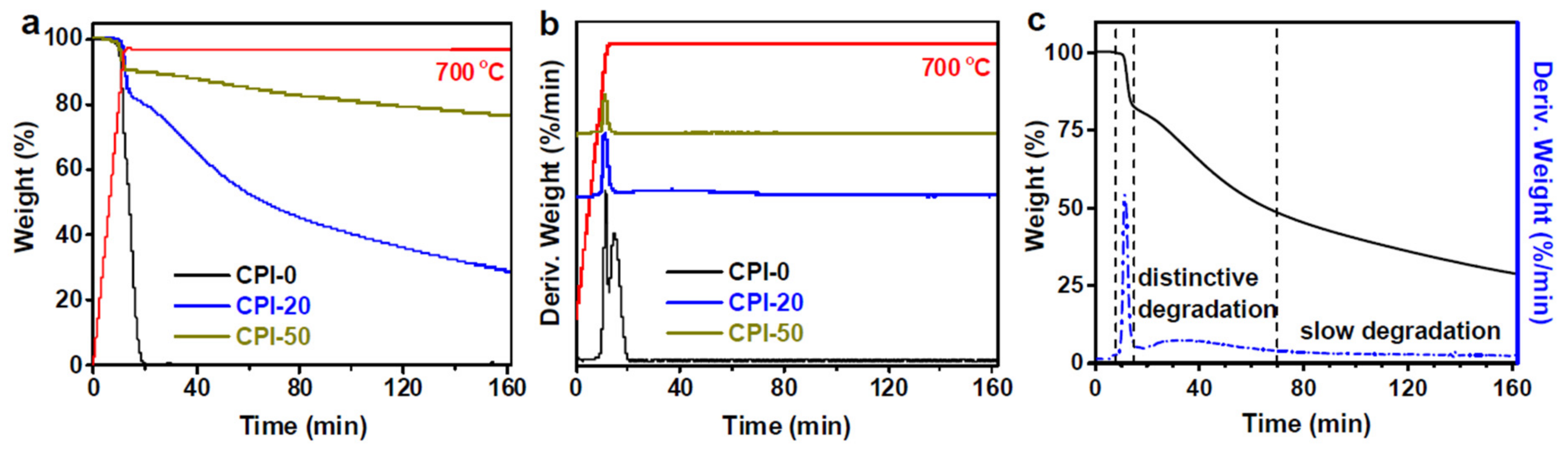
Additionally, three representatives, with differing carborane cage loadings (CPI-0, CPI-20, and CPI-50), were isothermally treated by TGA in air at 700 °C for 150 min, for mechanism investigation (
Figure 29a). Increased carborane cage loadings appear to have considerably enhanced thermo-oxidative stability. After 150 min of thermo-oxidative aging at 700 °C, the boron-free CPI film (CPI-0) was fully degraded into volatiles, while the CPI-20 and CPI-50 films still kept more than 29% and 76% weight residue, respectively. Furthermore, the boron-free CPI film (CPI-0) was easier to degrade than the carborane-containing films (CPI-20 and CPI-50), with a stronger decomposition peak. When combined with DTG curves (
Figure 29b), a second fast weight loss peak for CPI-0 in air occurred, almost immediately after the first. For CPI-50 in air, however, just one decomposition peak was seen. This might be explained by the fact that, due to the larger
Ea of the boron oxide passivation layer formed during the thermal processing, further decomposition of the CPI-50 films was postponed.
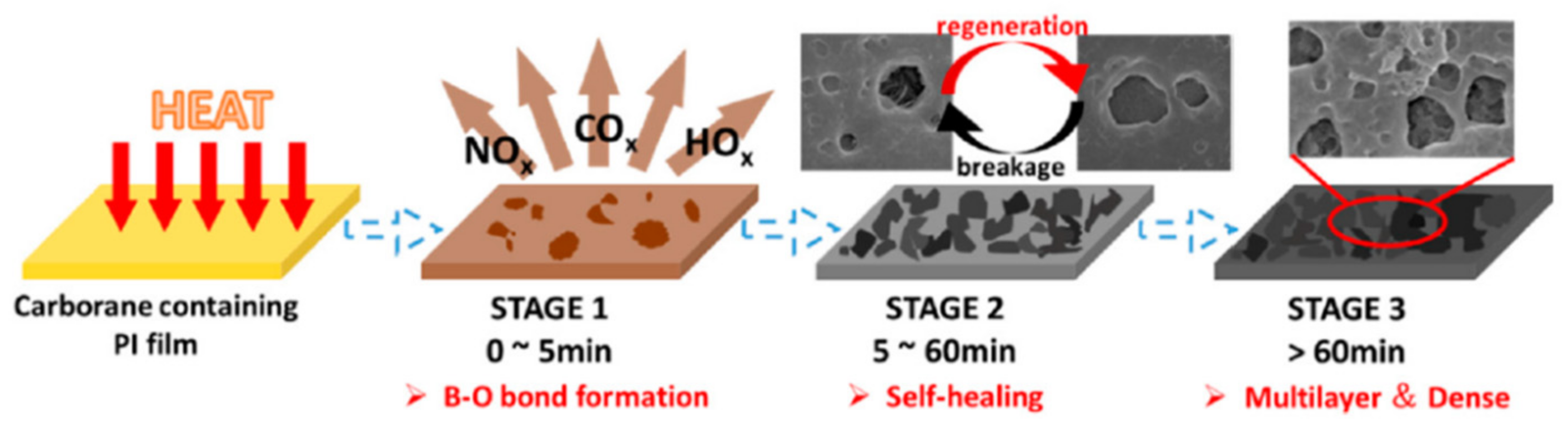
The thermal degradation can be separated into three stages, as shown in
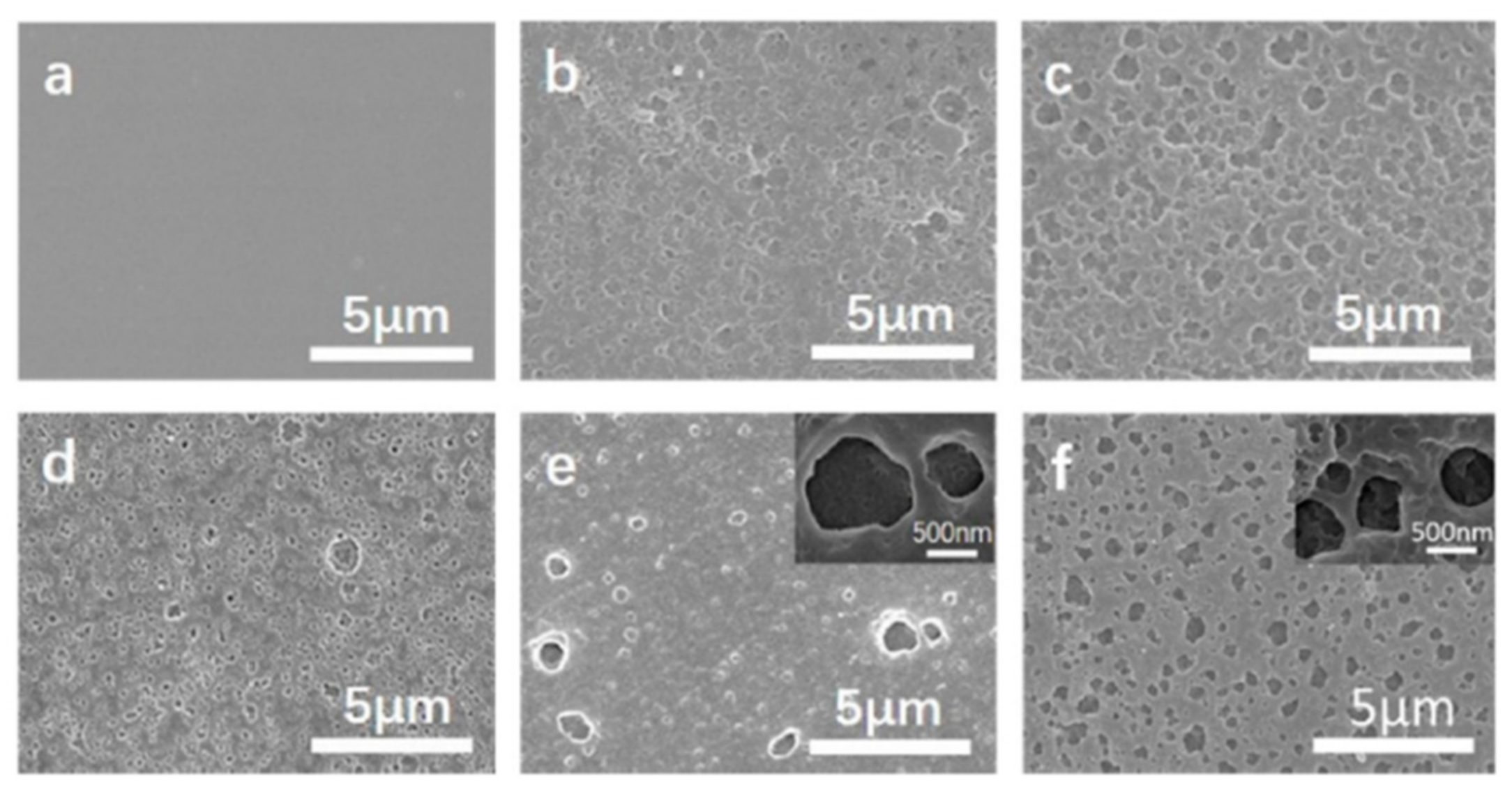
Figure 30. The first stage, according to the XPS measurement, was the degradation of the exterior polymer, with the surface becoming increasingly rough. The upmost film was reacted with oxygen in this stage, and volatiles, such as oxynitride, oxycarbide, and hydroxide, were released, along with the generation of a B-O bond. The second stage was the formation of a boron oxide protective barrier on the film surface. At first, the oxidative layer covered a portion of the surface (
Figure 31b). Accordingly, the polymer on the upmost passivation layer next to the opening hole was then degraded in situ, forming a new passivation layer, allowing the hybrid polyimide surface to “self-heal” [
67]. Fracture and regeneration of the protective layer were in a dynamic balance at this stage, but the regenerated layers progressively repaired the previous ones, eventually establishing the multilayered passivation layer (
Figure 31e). This layer encased the film surface and avoided direct interaction between the underlying polymer and oxygen, resulting in a considerable reduction in the rate of decomposition. Despite the formation of a “self-healing” multilayered dense passivation layer on the film surface in the second stage, heat could still be carried into the inside via the protective covering, causing the polymer to deteriorate slowly in the final stage.
Up to 700 °C, the carborane-containing polyimide (CPI) films demonstrated extraordinarily good thermal and thermo-oxidative stability, attributed to the multilayered protective layers generated on the film surface by the pyrolysis process of the carborane cage into boron oxides. In a high-temperature condition, the boron oxide layer increased the degradation activation energy and restricted direct interaction of interior polymer materials with oxygen molecules, functioning as a “self-healing” cover coating on the polyimide materials. Even following thermo-oxidative aging at 700 °C for 30 min, the CPI films maintained substantially higher mechanical strength and flexibility than the similar carborane-free films. This “self-healing” multilayered dense boron oxide barrier delayed the further degradation of the underlying polymer and extended the life span of materials at elevated temperatures.