Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits
Abstract
:1. Introduction
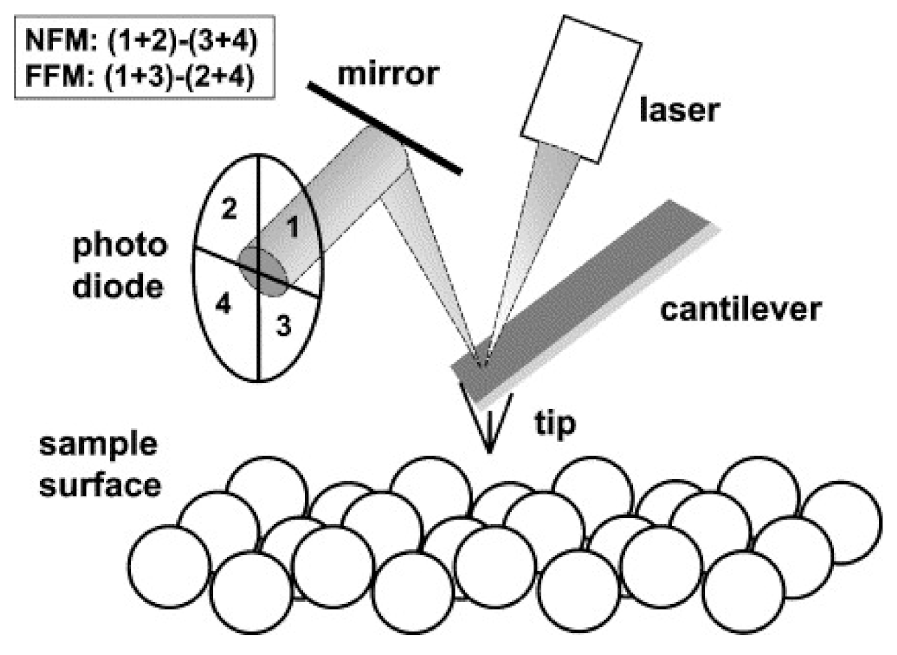
2. AFM Techniques
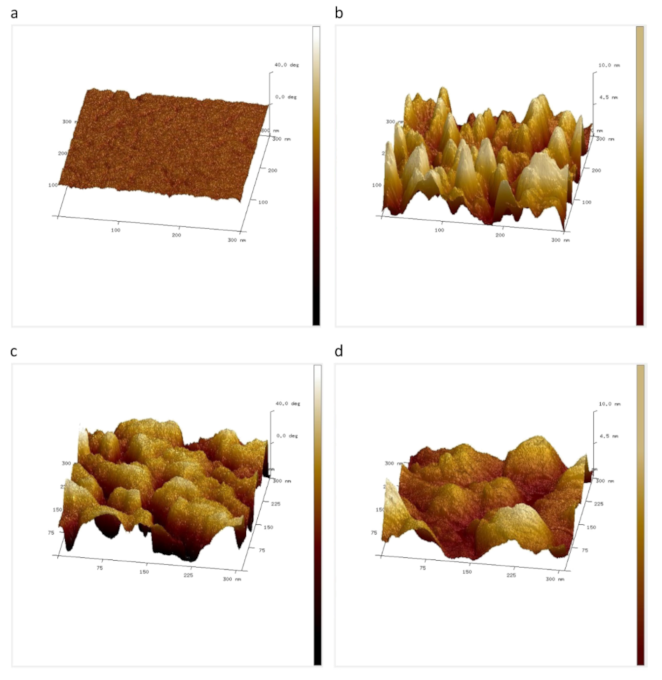
2.1. Topography and Roughness
2.2. Phase Imaging
2.3. Attractive and Repulsive Interaction Regimes
2.4. Nanoindentation
2.5. Peak Force Quantitative Nanomechanical Mapping (PeakForce QNM)
2.6. Hardness Measurements
2.7. Adhesion Measurements
2.8. Kelvin Probe Force Microscopy
2.9. Conductive AFM
3. Biopolymers and Hydrogels
3.1. Carrageenan
3.2. Chitosan
3.3. Alginate
3.4. Silica Hydrogels
4. Applications
4.1. Sample Preparation
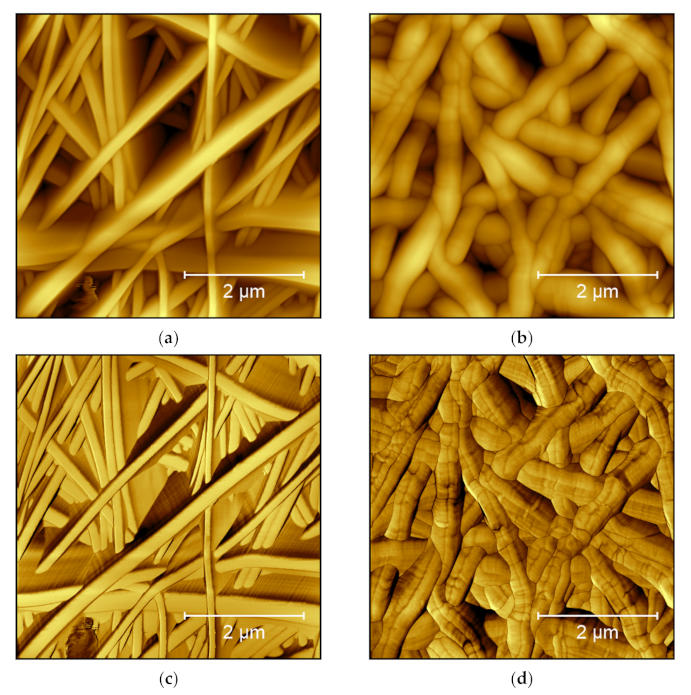
4.2. Carrageenan
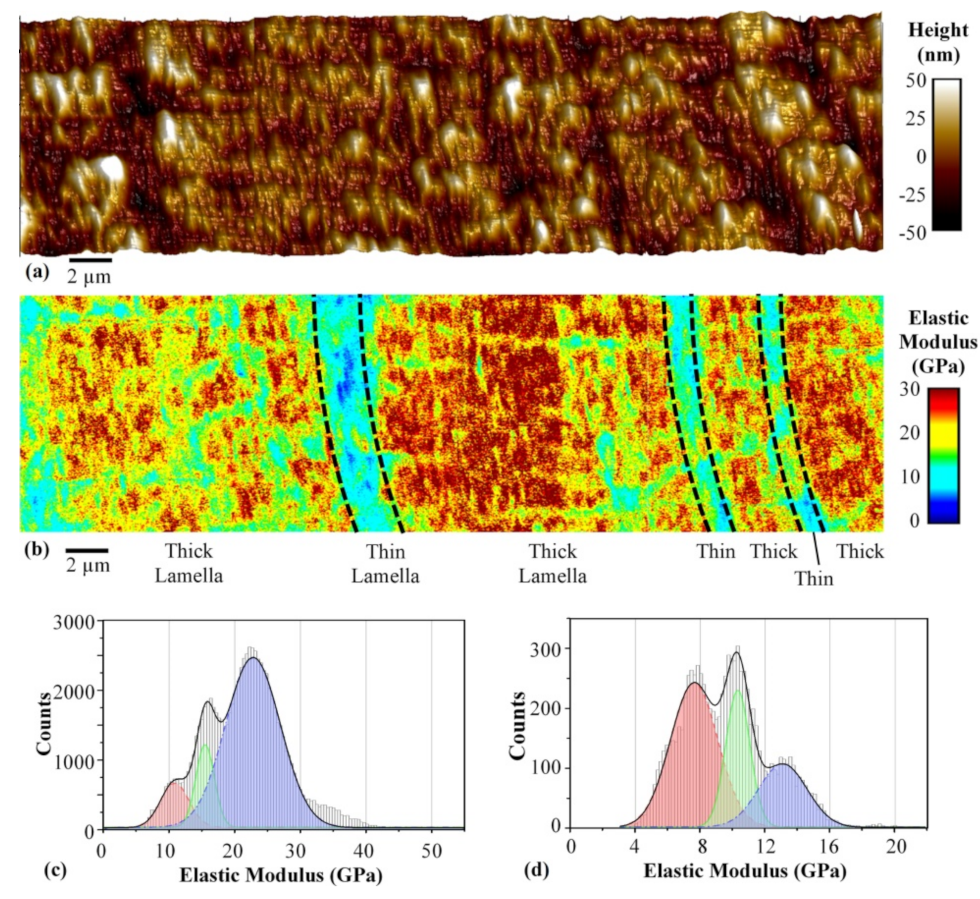
4.3. Chitosan
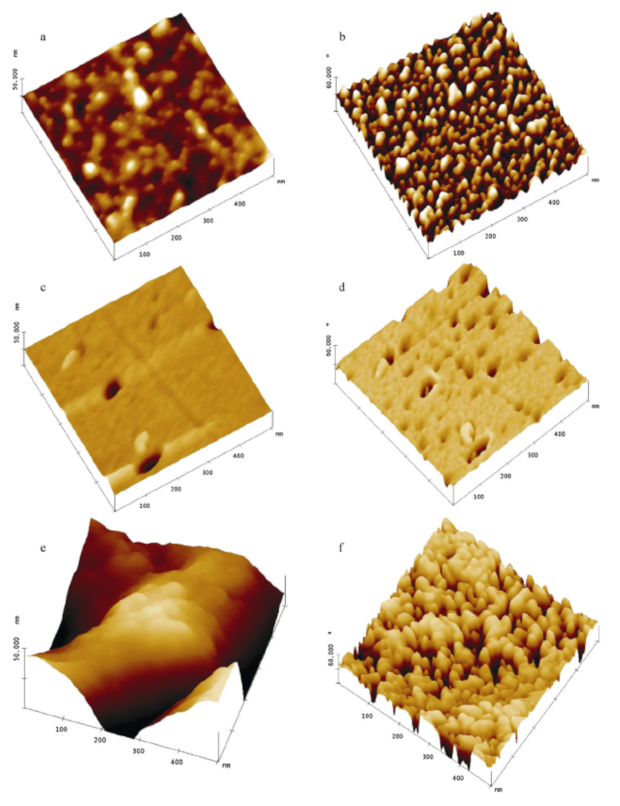
4.4. Alginate
4.5. Silica Hydrogels
4.6. Biopolymer Hydrogels
4.7. Other Biopolymers and Hydrogels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amos, B. Lessons from the history of light microscopy. Nat. Cell Biol. 2000, 2, E151–E152. [Google Scholar] [CrossRef]
- Haguenau, F.; Hawkes, P.W.; Hutchison, J.L.; Satiat-Jeunemaitre, B.; Simon, G.T.; Willams, D.B. Key events in the history of electron microscopy. Microsc. Microanal. 2003, 9, 96–138. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [Green Version]
- Parot, P.; Dufrene, Y.F.; Hinterdorfer, P.; Le Grimellec, C.; Navajas, D.; Pellequer, J.-L.; Scheuring, S. Past, present and future of atomic force microscopy in life sciences and medicine. J. Mol. Recognit. 2007, 20, 418–431. [Google Scholar] [CrossRef]
- Hoogenboom, B.W. Stretching the resolution limit of atomic force microscopy. Nat. Struct. Mol. Biol. 2021, 28, 629–630. [Google Scholar] [CrossRef]
- Roediger, P.; Wanzenboeck, H.D.; Hochleitner, G.; Bertagnolli, E. Evaluation of chamber contamination in a scanning electron microscope. J. Vac. Sci. Technol. B 2009, 27, 2711. [Google Scholar] [CrossRef]
- Jandt, K.D. Atomic force microscopy of biomaterials surfaces and interfaces. Surf. Sci. 2001, 491, 303–332. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wang, Y.F. Application of AFM in microbiology: A review. Scanning 2010, 32, 61–73. [Google Scholar] [CrossRef]
- Yang, C.-W.; Hwang, I.-S.; Chen, Y.F.; Chang, C.S.; Tsai, D.P. Imaging of soft matter with tapping-mode atomic force microscopy and non-contact-mode atomic force microscopy. Nanotechnology 2007, 18, 084009. [Google Scholar] [CrossRef]
- Morris, V.J.; Mackie, A.R.; Wilde, P.J.; Kirby, A.R.; Mills, E.C.N.; Gunning, P. Atomic force microscopy as a tool for interpreting the rheology of food biopolymers at the molecular level. LWT—Food Sci. Technol. 2001, 34, 3–10. [Google Scholar] [CrossRef]
- Morris, V.J.; Gunning, A.P.; Kirby, A.R.; Mackie, A.R.; Wilde, P.J. Viewing biopolymer networks, their formation and breakdown by AFM. Hydrocolloids 2000, 1, 99–109. [Google Scholar]
- Hansma, H.G.; Pietrasanta, L.I.; Auerbach, I.D.; Sorenson, C.; Golan, R.; Holden, P.A. Probing biopolymers with the atomic force microscope: A review. J. Biomater. Sci. Polym. Ed. 2000, 11, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.S.; Allen, S.; Chim, Y.T.A.; Robersts, C.J.; Tendler, S.J.B.; Davies, M.C. Molecular-scale studies on biopolymers using atomic force microscopy. In Pohymer Therapeutics II; Springer: Berlin/Heidelberg, Germany, 2005; pp. 123–172. [Google Scholar]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.L.; Goldmann, W.H. Feeling the forces: Atomic force microscopy in cell biology. Life Sci. 2003, 72, 2553–2560. [Google Scholar] [CrossRef]
- Alsteens, D.; Dague, E.; Rouxhet, P.G.; Baulard, A.R.; Dufrene, Y.F. Direct measurement of hydrophobic forces on cell surfaces using AFM. Langmuir 2007, 23, 11977–11979. [Google Scholar] [CrossRef]
- Raghavan, D.; Gu, X.; Nguyen, T.; Van Landingham, M.; Karim, A. Mapping polymer heterogeneity using atomic force microscopy phase imaging and nanoscale indentation. Macromolecules 2000, 33, 2573–2583. [Google Scholar] [CrossRef]
- Sabantina, L.; Klöcker, M.; Wortmann, M.; Rodríguez-Mirasol, J.; Cordero, T.; Moritzer, E.; Finsterbusch, K.; Ehrmann, A. Stabilization of polyacrylonitrile nanofiber mats obtained by needleless electrospinning using dimethyl sulfoxide as solvent. J. Ind. Text. 2020, 50, 224–239. [Google Scholar] [CrossRef]
- Sabantina, L.; Böttjer, R.; Wehlage, D.; Grothe, T.; Klöcker, M.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Ehrmann, A. Morphological study of stabilization and carbonization of polyacrylonitrile/TiO2 nanofiber mats. J. Eng. Fibers Fabr. 2019, 14, 1558925019862242. [Google Scholar] [CrossRef]
- Haugstad, G.; Jones, R.R. Mechanisms of dynamic force microscopy on polyvinyl alcohol: Region-specific non-contact and intermittent contact regimes. Ultramicroscopy 1999, 76, 77–86. [Google Scholar] [CrossRef]
- García, R.; San Paulo, A. Attractive and repulsive tip-sample interaction regimes in tapping-mode atomic force microscopy. Phys. Rev. B 1999, 60, 4961–4967. [Google Scholar] [CrossRef] [Green Version]
- García, R.; San Paulo, A. Amplitude curves and operating regimes in dynamic atomic force microscopy. Ultramicroscopy 2000, 82, 79–83. [Google Scholar] [CrossRef]
- Round, A.N.; Miles, M.J. Exploring the consequences of attractive and repulsive interaction regimes in tapping mode atomic force microscopy of DNA. Nanotechnology 2004, 15, S176–S183. [Google Scholar] [CrossRef]
- San Paulo, A.; García, R. High-resolution imaging of antibodies by tapping-mode atomic force microscopy: Attractive and repulsive tip-sample interaction regimes. Biophys. J. 2000, 78, 1599–1605. [Google Scholar] [CrossRef] [Green Version]
- Zitzler, L.; Herminghaus, S.; Mugele, F. Capillary forces in tapping mode atomic force microscopy. Phys. Rev. B 2002, 66, 155436. [Google Scholar] [CrossRef] [Green Version]
- He, M.Y.; Szuchmacher Blum, A.; Aston, D.E.; Buenviaje, C.; Overney, R.M. Critical phenomena of water bridges in nanoasperity contacts. J. Chem. Phys. 2001, 114, 1355. [Google Scholar] [CrossRef] [Green Version]
- Stifter, T.; Marti, O.; Bhushan, B. Theoretical investigation of the distance dependence of capillary and van der Waals forces in scanning force microscopy. Phys. Rev. B 2000, 62, 13667–13673. [Google Scholar] [CrossRef]
- Maragliano, C.; Glia, A.; Stefancich, M.; Chiesa, M. Effective AFM cantilever tip size: Methods for in-situ determination. Meas. Sci. Technol. 2015, 26, 015002. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. Hertz model or Oliver & Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Mater. Res. Express 2020, 7, 033001. [Google Scholar]
- Kontomaris, S.V.; Stylianou, A.; Nikita, K.S.; Malamou, A. A discussion regarding the application of the Hertz contact theory on biological samples in AFM nanoindentation experiments. Micro Nanosyst. 2021, 13, 42–48. [Google Scholar] [CrossRef]
- Qian, L.; Zhao, H.W. Nanoindentation of soft biological materials. Micromachines 2018, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Vlassov, S.; Oras, S.; Antsov, M.; Sosnin, I.; Polyakov, B.; Shutka, A.; Krauchanka, M.Y.; Dorogin, L.M. Adhesion and mechanical properties of PDMS-based materials probed with AFM: A review. Rev. Adv. Mater. Sci. 2018, 56, 62–78. [Google Scholar] [CrossRef]
- Sokolov, I.; Dokukin, M.E.; Guz, N.V. Method for quantitative measurements of the elastic modulus of biological cells in AFM indentation experiments. Methods 2013, 60, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.K.; Roos, W.H. AFM nanoindentation experiments on protein shells: A protocol. In Atomic Force Microscopy; Santos, N., Carvalho, F., Eds.; Humana Press: New York, NY, USA, 2018; pp. 243–257. [Google Scholar]
- Maghsoudy-Louyeh, S.; Kropf, M.; Tittmann, B.R. Review of progress in atomic force microscopy. Open Neuroimaging J. 2018, 12, 86–104. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Quantitative mapping of the elastic modulus of soft materials with HarmoniX and PeakForce QNM AFM modes. Langmuir 2012, 28, 16060–16071. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Kastner, M.J.; Tighe, T.B.; Du, J. Elastic modulus mapping for bovine cortical bone from submillimeter- to submicron-scales using PeakForce Tapping atomic force microscopy. Extreme Mech. Lett. 2020, 41, 101031. [Google Scholar] [CrossRef]
- Schön, P.; Bagdi, K.; Molnár, K.; Markus, P.; Pukánszky, B.; Vancso, G.J. Quantitative mapping of elastic moduli at the nanoscale in phase separated polyurethanes by AFM. Eur. Polym. J. 2011, 47, 692–698. [Google Scholar] [CrossRef]
- Calabri, L.; Pugno, N.; Menozzi, C.; Valeri, S. AFM nanoindentation: Tip shape and tip radius of curvature effect on the hardness measurement. J. Phys. Condens. Matter 2008, 20, 474208. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Koinkar, V.N. Nanoindentation hardness measurements using atomic force microscopy. Appl. Phys. Lett. 1994, 64, 1653–1655. [Google Scholar] [CrossRef]
- Balooch, M.; Wu-Magidi, I.-C.; Balazs, A.; Lundkvist, A.S.; Marshall, S.J.; Marshall, G.W.; Siekhaus, W.J.; Kinney, J.H. Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J. Biomed. Mater. Res. 1998, 40, 539–544. [Google Scholar] [CrossRef]
- Erath, J.; Schmidt, S.; Fery, A. Characterization of adhesion phenomena and contact of surfaces by soft colloidal probe AFM. Soft Matter 2010, 6, 1432–1437. [Google Scholar] [CrossRef]
- Dong, Y.H.; An, R.; Zhao, S.L.; Cao, W.; Huang, L.L.; Zhuang, W.; Lu, L.H.; Lu, X.H. Molecular Interactions of Protein with TiO2 by the AFM-Measured Adhesion Force. Langmuir 2017, 33, 11626–11634. [Google Scholar] [CrossRef] [PubMed]
- Wojcikiewicz, E.P.; Zhang, X.H.; Moy, V.T. Force and Compliance Measurements on Living Cells Using Atomic Force Microscopy (AFM). Biol. Proced. Online 2004, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, S.C.; Crow, A.K.; Lam, W.A.; Bertozzi, C.R.; Fletcher, D.A.; Francis, M.B. DNA-Coated AFM Cantilevers for the Investigation of Cell Adhesion and the Patterning of Live Cells. Angew. Chem. 2008, 120, 8601–8605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Huang, J.X.; Wang, Y.W.; Liu, R.; Huai, X.L.; Jiang, J.J.; Anfuso, C. Atomic force microscopy for two-dimensional materials: A tutorial review. Opt. Commun. 2018, 406, 3–17. [Google Scholar] [CrossRef]
- Gouveia, R.F.; Galembeck, F. Electrostatic charging of hydrophilic particles due to water adsorption. J. Am. Chem. Soc. 2009, 131, 11381–11386. [Google Scholar] [CrossRef]
- Zhang, W.; Hughes, J.; Chen, Y.S. Impacts of Hematite Nanoparticle Exposure on Biomechanical, Adhesive, and Surface Electrical Properties of Escherichia coli Cells. Appl. Environ. Microbiol. 2012, 78, 3905–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S.Y. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Nony, L.; Foster, A.S.; Bocquet, F.; Loppacher, C. Understanding the Atomic-Scale Contrast in Kelvin Probe Force Microscopy. Phys. Rev. Lett. 2009, 103, 036802. [Google Scholar] [CrossRef] [Green Version]
- Collins, L.; Kilpatrick, J.I.; Kalinin, S.V.; Rodriguez, B.J. Towards nanoscale electrical measurements in liquid by advanced KPFM techniques: A review. Rep. Prog. Phys. 2018, 81, 086101. [Google Scholar] [CrossRef]
- Frammelsberger, W.; Benstetter, G.; Kiely, J.; Stamp, R. C-AFM-based thickness determination of thin and ultra-thin SiO2 films by use of different conductive-coated probe tips. Appl. Surf. Sci. 2007, 253, 3615–3626. [Google Scholar] [CrossRef]
- Alvarez, J.; Ngo, I.; Gueunier-Farret, M.-E.; Kleider, J.-P.; Yu, L.W.; Cabarrocas, P.R.; Perraud, S.; Rouvière, E.; Celle, C.; Mouchet, C.; et al. Conductive-probe atomic force microscopy characterization of silicon nanowire. Nano Express 2011, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Kelley, T.W.; Granstrom, E.L.; Frisbie, C.D. Conducting Probe Atomic Force Microscopy: A Characterization Tool for Molecular Electronics. Adv. Mater. 1999, 11, 261–264. [Google Scholar] [CrossRef]
- Haugier, F.; Alamarguy, D.; Viel, P.; Noel, S.; Filoramo, A.; Huc, V.; Houzé, F.; Palacin, S. Conductive-probe AFM characterization of graphene sheets bonded to gold surfaces. Appl. Surf. Sci. 2012, 258, 2920–2926. [Google Scholar] [CrossRef]
- Layson, A.; Gadad, S.; Teeters, D. Resistance measurements at the nanoscale: Scanning probe ac impedance spectroscopy. Electrochim. Acta 2003, 48, 2207–2213. [Google Scholar] [CrossRef]
- Ishida, T.; Mizutani, W.; Liang, T.-T.; Azehara, H.; Miyake, K.; Sasaki, S.; Tokumoto, H. Conductive Probe AFM Measurements of Conjugated Molecular Wires. Ann. N. Y. Acad. Sci. 2003, 1006, 164–186. [Google Scholar] [CrossRef]
- Elnashar, M. Biotechnology of Biopolymers; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists, 3rd ed.; Woodhead Publishing: Duxford, UK, 2019. [Google Scholar]
- Therkelsen, G.H. Carrageenan. In Industrial Gums, 3rd ed.; Whistler, R.L., Bemiller, J.N., Eds.; Academic Press: Cambridge, MA, USA, 1993; pp. 145–180. [Google Scholar]
- Ahmed, A.B.A.; Adel, M.; Karimi, P.; Peidayesh, M. Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Adv. Food Nutr. Res. 2014, 73, 197–220. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry: FAO Fisheries Technical Paper 441; Food and Agriculture Organization of the Uited Nations: Rome, Italy, 2003; Available online: www.fao.org (accessed on 29 July 2017).
- Sokolova, E.; Chusovitin, E.; Barabanova, A.O.; Balagan, S.; Galkin, N.; Yermak, I. Atomic force microscopy imaging of carrageenans from red algae of Gigartinaceae and Tichocarpaceae families. Carbohydr. Polym. 2013, 93, 458–465. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Natural chelating polymers; alginic acid, chitin and chitosan. In Natural Chelating Polymers; Alginic Acid, Chitin and Chitosan; Pergamon Press: Oxford, UK, 1973. [Google Scholar]
- Das, S.; Ganesh, E.A. Extraction of chitin from trash crabs (Podophthalmus vigil) by an eccentric method. Curr. Res. Biol. Sci. 2010, 2, 72–75. [Google Scholar]
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Kaya, M.; Akyuz, L.; Sargin, I.; Mujtaba, M.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Koc, B.; Baran, T.; Ceter, T. Incorporation of sporopollenin enhances acid–base durability, hydrophobicity, and mechanical, antifungal and antioxidant properties of chitosan films. J. Ind. Eng. Chem. 2017, 47, 236–245. [Google Scholar] [CrossRef]
- Lorenz, S.; Humbert, P.; Patel, A.V. Chitin increases drying survival of encapsulated Metarhizium pemphigi blastospores for Ixodes ricinus control. Ticks Tick-Borne Dis. 2020, 11, 101537. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Maleki, M.; Karami, M. The effect of chitosan molecular weight on the properties of alginate/chitosan microparticles containing prednisolone. Trop. J. Pharm. Res. 2009, 8, 53–61. [Google Scholar] [CrossRef]
- Vandenberg, G.W.; Drolet, C.; Scott, S.L.; de la Noue, J. Factors affecting protein release from alginate–chitosan coacervate microparticles during production and gastric/intestinal simulation. J. Control. Release 2001, 77, 297–307. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tsuchiya, T.; Iijima, M.; Takeuchi, H.; Kamiya, H. Characterization of Surface Interaction between Chitosan-modified Liposomes and Mucin Layer by Using CNT Probe AFM Method. Yakugaku Zasshi 2018, 138, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Grimmelsmann, N.; Grothe, T.; Homburg, S.V.; Ehrmann, A. Electrospinning and stabilization of chitosan nanofiber mats. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102006. [Google Scholar] [CrossRef]
- Grimmelsmann, N.; Homburg, S.V.; Ehrmann, A. Needleless electrospinning of pure and blended chitosan. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012098. [Google Scholar] [CrossRef] [Green Version]
- Grimmelsmann, N.; Homburg, S.V.; Ehrmann, A. Electrospinning chitosan blends for nonwovens with morphologies between nanofiber mat and membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012007. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, Y.; Qin, Y.M.; Shen, P.L.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Chang, E.-S.; Lim, T.-K.; Voo, W.-P.; Pogaku, R.; Tey, B.T.; Zhang, Z.B. Effect of formulation of alginate beads on their mechanical behavior and stiffness. Particuology 2011, 9, 228–234. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Lim, L.-T.; Marcone, M.F. Effect of Various Gelling Cations on the Physical Properties of “Wet” Alginate Films. J. Food Sci. 2014, 79, E562–E567. [Google Scholar] [CrossRef] [PubMed]
- Hermann, K.M.; Grünberger, A.; Patel, A.V. Formalin-casein enhances water absorbency of calcium alginate beads and activity of encapsulated Metarhizium brunneum and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2021, 37, 156. [Google Scholar] [PubMed]
- Riseh, R.S.; Skorik, Y.A.; Thakur, V.K.; Pour, M.M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef] [PubMed]
- Souza-Alonso, P.; Rocha, M.; Rocha, I.; Ma, Y.; Freitas, H.; Oliveira, R.S. Encapsulation of Pseudomonas libanensis in alginate beads to sustain bacterial viability and inoculation of Vigna unguiculata under drought stress. 3 Biotech 2021, 11, 293. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, K.; Rasheed, T.; Bilal, M. Alginate-based nanobiosorbents for bioremediation of environmental pollutants. In Nano-Biosorbents for Decontamination of Water, Air, and Soil Pollution; Denizli, A., Ali, N., Bilal, M., Khan, A., Anh Nguyen, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 479–502. [Google Scholar]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar]
- Di Girolamo, S.; Puorger, C.; Lipps, G. Stable and selective permeable hydrogel microcapsules for high-throughput cell cultivation and enzymatic analysis. Microb. Cell Fact. 2020, 19, 170. [Google Scholar] [CrossRef]
- Pauly, J.; Gröger, H.; Patel, A.V. Catalysts Encapsulated in Biopolymer Hydrogels for Chemoenzymatic One-Pot Processes in Aqueous Media. ChemCatChem 2019, 11, 1503–1509. [Google Scholar] [CrossRef]
- Pettignano, A.; Aguilera, D.A.; Tanchoux, N.; Bernardi, L.; Quignard, F. Alginate: A Versatile Biopolymer for Functional Advanced Materials for Catalysis. Stud. Surf. Sci. Catal. 2019, 178, 357–375. [Google Scholar]
- Häring, M.; Tautz, M.; Alegre-Requena, J.V.; Saldías, C.; Díaz Díaz, D. Non-enzyme entrapping biohydrogels in catalysis. Tetrahedron Lett. 2018, 59, 3293–3306. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.Q.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.L.; Han, Q.; Lin, H.B.; Li, Q.; Hua, J.; Liu, F. Anticoagulant dialyzer with enhanced Ca2+ chelation and hydrophilicity for heparin free hemodialysis. J. Membr. Sci. 2020, 604, 118082. [Google Scholar] [CrossRef]
- Elkady, M.; Salama, E.; Amer, W.A.; Ebeid, E.-Z.M.; Ayad, M.M.; Shokry, H. Novel eco-friendly electrospun nanomagnetic zinc oxide hybridized PVA/alginate/chitosan nanofibers for enhanced phenol decontamination. Environ. Sci. Pollut. Res. 2020, 27, 43077–43092. [Google Scholar] [CrossRef] [PubMed]
- Abdu, B.; Munirasu, S.; Kallem, P.; Hasan, S.W.; Banat, F. Investigating the effect of various foulants on the performance of intrinsically superhydrophobic polyvinylidene fluoride membranes for direct contact membrane distillation. Sep. Purif. Technol. 2020, 252, 117416. [Google Scholar] [CrossRef]
- Wasim, M.; Shafiq, M.; Khan, R.U.; Sabir, A. Crosslinked integrally skinned asymmetric composite membranes for dye rejection. Appl. Surf. Sci. 2019, 478, 514–521. [Google Scholar] [CrossRef]
- Daud, H.; Ghani, A.; Iqbal, D.N.; Ahmad, N.; Nazir, S.; Muhammad, M.J.; Hussain, E.A.; Nazir, A.; Iqbal, M. Preparation and characterization of guar gum based biopolymeric hydrogels for controlled release of antihypertensive drug. Arab. J. Chem. 2021, 14, 103111. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Yu, Z.P.; Lin, K.S.; Yang, S.F.; Dai, L.; Liu, J.F.; Mao, L.; Yuan, F.; Gao, Y.X. Enhanced stability, structural characterization and simulated gastrointestinal digestion of coenzyme Q10 loaded ternary nanoparticles. Food Hydrocoll. 2019, 94, 333–344. [Google Scholar] [CrossRef]
- Li, J.R.; Zou, J.; Xiao, H.N.; He, B.H.; Hou, X.B.; Qian, L.Y. Preparation of Novel Nano-Sized Hydrogel Microcapsules via Layer-By-Layer Assembly as Delivery Vehicles for Drugs onto Hygiene Paper. Polymers 2018, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.W.; Chen, X.Q.; Wen, Y.S.; Liu, C.Y.; Cao, S.R.; Yan, H.Q.; Lin, Q. Synthesis and self-assembly behavior of decyl alginate ester derivative via bimolecular nucleophilic substitution reaction. Colloids Polym. Sci. 2021, 299, 1743–1755. [Google Scholar] [CrossRef]
- Chen, G.Y.; He, L.B.; Zhang, P.; Zhang, J.; Mei, X.F.; Wang, D.H.; Zhang, Y.Y.; Ren, X.L.; Chen, Z.H. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Sow, L.C.; Peh, Y.R.; Pekerti, B.N.; Fu, C.L.; Bansal, N.; Yang, H.S. Nanostructural analysis and textural modification of tilapia fish gelatin affected by gellan and calcium chloride addition. LWT—Food Sci. Technol. 2017, 85, 137–145. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, J.L.; Wang, X.D.; Huo, X.N.; Xu, Y.W.; Li, Q.Q.; Wang, L. Improving the hydrophilic and antifouling properties of polyvinylidene fluoride membrane by incorporation of novel nanohybrid GO@SiO2 particles. Chem. Eng. J. 2017, 314, 266–276. [Google Scholar] [CrossRef]
- BinAhmed, S.; Hozalski, R.M.; Romero-Vargas Castrillón, S. Feed Temperature Effects on Organic Fouling of Reverse Osmosis Membranes: Competition of Interfacial and Transport Properties. ACS EST Eng. 2021, 1, 591–602. [Google Scholar] [CrossRef]
- Hauck, N.; Seixas, N.; Centeno, S.P.; Schlüßler, R.; Cojoc, G.; Müller, P.; Guck, J.; Wöll, D.; Wessjohann, L.A.; Thiele, J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Homburg, S.V.; Venkanna, D.; Kraushaar, K.; Kruse, O.; Kroke, E.; Patel, A.V. Entrapment and growth of Chlamydomonas reinhardtii in biocompatible silica hydrogels. Colloids Surf. B Biointerfaces 2019, 173, 233–241. [Google Scholar] [CrossRef]
- Homburg, S.V.; Kruse, O.; Patel, A.V. Growth and photosynthetic activity of Chlamydomonas reinhardtii entrapped in lens-shaped silica hydrogels. J. Biotechnol. 2019, 302, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.M.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol-Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Sing, B.; Na, J.B.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Le Ouay, B.; Coradin, T.; Laberty-Robert, C. Silica–carbon hydrogels as cytocompatible bioelectrodes. J. Mater. Chem. B 2013, 1, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.F.; Yang, X.-Y.; Rooke, J.C.; Su, B.-L. Biofuel cells Based on the Immobilization of Photosynthetically Active Bioentities. ChemCatChem 2011, 3, 476–488. [Google Scholar] [CrossRef]
- Holzmeister, I.; Schamel, M.; Groll, J.; Gbureck, U.; Vorndran, E. Artificial inorganic biohybrids: The functional combination of microorganisms and cells with inorganic materials. Acta Biomater. 2018, 74, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Ally, J.; Bley, K.; Kappl, M.; Landfester, K.; Weiss, C.K. Direct visualization of the interfacial position of colloidal particles and their assemblies. Nanoscale 2014, 6, 6879–6885. [Google Scholar] [CrossRef] [Green Version]
- Cozens, E.J.; Kong, D.X.; Roohpour, N.; Gautrot, J.E. The physico-chemistry of adhesions of protein resistant and weak polyelectrolyte brushes to cells and tissues. Soft Matter 2020, 16, 505–522. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.W.; Li, L.; Xu, Z.Z.; Sun, Y.P.; Wu, Y.; Dai, C.L. Biomimetic functional hydrogel particles with enhanced adhesion characteristics for applications in fracture conformance control. J. Ind. Eng. Chem. 2022, 106, 482–491. [Google Scholar] [CrossRef]
- Liu, C.; Thormann, E.; Tyrode, E.; Claesson, P.M. Charge regulation and energy dissipation while compressing and sliding a cross-linked chitosan hydrogel layer. J. Colloid Interface Sci. 2015, 443, 162–169. [Google Scholar] [CrossRef]
- Wang, L.P.; Jang, G.; Ban, D.K.; Sant, V.; Seth, J.; Kazmi, S.; Patel, N.; Yang, Q.Q.; Lee, J.; Janetanakit, W.; et al. Multifunctional stimuli responsive polymer-gated iron and gold-embedded silica nano golf balls: Nanoshuttles for targeted on-demand theranostics. Bone Res. 2017, 5, 17051. [Google Scholar] [CrossRef]
- Best, J.P.; Cui, J.W.; Müllner, M.; Caruso, F. Tuning the Mechanical Properties of Nanoporous Hydrogel Particles via Polymer Cross-Linking. Langmuir 2013, 29, 9824–9831. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Simple preparation of maltose-functionalized dendrimer/graphene quantum dots as a pH-sensitive biocompatible carrier for targeted delivery of doxorubicin. Int. J. Biol. Macromol. 2020, 156, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Bosnar, S.; Bronic, J.; Sikiric, M.D.; Misic, T.; Svetlicic, V.; Mao, J.-J.; Antonic Jelic, T.; Hadzija, M.; Subotic, B. Role of Subcolloidal (Nanosized) Precursor Species in the Early Stage of the Crystallization of Zeolites in Heterogeneous Systems. Langmuir 2014, 30, 8570–8579. [Google Scholar] [CrossRef] [PubMed]
- Mo, A.H.; Landon, P.B.; Gomez, K.S.; Kang, H.M.; Lee, J.; Zhang, C.; Janetanakit, W.; Sant, V.; Lu, T.; Colburn, D.A.; et al. Magnetically-responsive silica–gold nanobowls for targeted delivery and SERS-based sensing. Nanoscale 2016, 8, 11840–11850. [Google Scholar] [CrossRef] [PubMed]
- Cejková, J.; Hanus, J.; Stepánek, F. Investigation of internal microstructure and thermo-responsive properties of composite PNIPAM/silica microcapsules. J. Colloids Interface Sci. 2010, 346, 352–360. [Google Scholar] [CrossRef]
- Dey, T. Colloidal crystalline array of hydrogel-coated silica nanoparticles: Effect of temperature and core size on photonic properties. J. Sol-Gel Sci. Technol. 2011, 57, 132–141. [Google Scholar] [CrossRef]
- Dey, T. Polymeric Nanocomposites as Photonic Sensors. In Photonics North 2010; SPIE: Bellingham, WA, USA, 2010; Volume 7750, p. 77500P. [Google Scholar]
- Sahiner, N. Colloidal nanocomposite hydrogel particles. Colloid Polym. Sci. 2007, 285, 413–421. [Google Scholar] [CrossRef]
- Roman, C.; Valencia, C.; Franco, J.M. AFM and SEM Assessment of Lubricating Grease Microstructures: Influence of Sample Preparation Protocol, Frictional Working Conditions and Composition. Tribol. Lett. 2016, 63, 20. [Google Scholar] [CrossRef] [Green Version]
- McIntire, T.M.; Brant, D.A. Imaging of individual biopolymers and supramolecular assemblies using noncontact atomic force microscopy. Biopolymers 1997, 42, 133–146. [Google Scholar] [CrossRef]
- McIntire, T.M.; Penner, R.M.; Brant, D.A. Observations of a circular, triple-helical polysaccharide using noncontact atomic force microscopy. Macromolecules 1995, 28, 6375–6377. [Google Scholar] [CrossRef]
- Baalousha, M.; Lead, J.R. Characterization of natural and manufactured nanoparticles by atomic force microscopy: Effect of analysis mode, environment and sample preparation. Physicochem. Eng. Asp. 2013, 419, 238–247. [Google Scholar] [CrossRef]
- Klinov, D.V.; Neretina, T.V.; Prokhorov, V.V.; Dobrynina, T.V.; Aldarov, K.G.; Demin, V.V. High-resolution atomic force microscopy of DNA. Biochemistry 2009, 74, 1150–1154. [Google Scholar] [CrossRef]
- Flamia, R.; Zhdan, P.A.; Martino, M.; Castle, J.E.; Tamburro, A.M. AFM study of the elastin-like biopolymer poly(ValGlyGlyValGly). Biomacromolecules 2004, 5, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Balnois, E.; Wilkinson, K.J. Sample preparation techniques for the observation of environmental biopolymers by atomic force microscopy. Colloid Surf. A Physicochem. Eng. Asp. 2002, 207, 229–242. [Google Scholar] [CrossRef]
- Matsko, N.; Mueller, M. AFM of biological material embedded in epoxy resin. J. Struct. Biol. 2004, 146, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.; Adamcik, J.; Mezzenga, R. Formation of Higher Structural Levels in λ-Carrageenan Induced by the Antimalarial Drug Chloroquine. ACS Macro Lett. 2020, 9, 1310–1317. [Google Scholar] [CrossRef]
- Volod’ko, A.V.; Davydova, V.N.; Nedashkovskaya, O.I.; Terentieva, N.A.; Chusovitin, E.A.; Galkin, N.G.; Yermak, I.M. Morphology, electrokinetic characteristics and the effect on biofilm formation of carrageenan:chitosan polyelectrolyte complexes. Int. J. Biol. Macromol. 2018, 117, 1118–1124. [Google Scholar] [CrossRef]
- Yang, D.Y.; Yang, H.S. The temperature dependent extraction of polysaccharides from eucheuma and the rheological synergistic effect in their mixtures with kappa carrageenan. LWT 2020, 129, 109515. [Google Scholar] [CrossRef]
- Webber, J.L.; Namivandi-Zangeneh, R.; Drozde, S.; Wilk, K.A.; Boyer, C.; Wong, E.H.H.; Bradshaw-Hajek, B.H.; Krasowska, M.; Beattie, D.A. Incorporation and antimicrobial activity of nisin Z within carrageenan/chitosan multilayers. Sci. Rep. 2021, 11, 1690. [Google Scholar] [CrossRef]
- Zhuikova, Y.V.; Zhuikov, V.A.; Zubareva, A.A.; Akhmedova, S.A.; Sviridova, I.K.; Sergeeva, N.S.; Varlamov, V.P. Physicochemical and biological characteristics of chitosan/κ-carrageenan thin layer-by-layer films for surface modification of nitinol. Micron 2020, 138, 102922. [Google Scholar] [CrossRef]
- Swain, S.; Bal, T. Microwave irradiated Carrageenan-Guar gum micro-porous IPN: A novel material for isotropic tissue scaffolding. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 796–804. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W. Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications. ACS Appl. Polym. Mater. 2021, 3, 6437–6445. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Kumar, B.A.; Rajasekar, A.; Annaraj, J.; Pruncu, C.I. The benefits of k-Carrageenan-gelatin hybrid composite coating on the medical grade stainless steel (SS304) used as anticorrosive barrier. Mater. Chem. Phys. 2021, 258, 123909. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Costa, T.B.; Cerqueira, M.A.; de Castro, C.M.M.B.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Construction of a Biocompatible and Antioxidant Multilayer Coating by Layer-by-Layer Assembly of κ-Carrageenan and Quercetin Nanoparticles. Food Bioprocess Technol. 2018, 11, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Alam, J.; Alhoshan, M.; Shukla, A.K.; Aldalbahi, A.; Ali, F.A.A. k-Carrageenan—A versatile biopolymer for the preparation of a hydrophilic PVDF composite membrane. Eur. Polym. J. 2019, 120, 109219. [Google Scholar] [CrossRef]
- Schoeler, B.; Delorme, N.; Doench, I.; Sukhorukov, G.B.; Fery, A.; Glinel, K. Polyelectrolyte Films Based on Polysaccharides of Different Conformations: Effects on Multilayer Structure and Mechanical Properties. Biomacromolecules 2006, 7, 2065–2071. [Google Scholar] [CrossRef]
- Maldonado, L.; Chough, S.; Bonilla, J.; Kim, K.H.; Kokini, J. Mechanism of fabrication and nano-mechanical properties of α-lactalbumin/chitosan and BSA/κ-carrageenan nanotubes through layer-by-layer assembly for curcumin encapsulation and determination of in vitro cytotoxicity. Food Hydrocoll. 2019, 93, 293–307. [Google Scholar] [CrossRef]
- Schefer, L.; Adamcik, J.; Diener, M.; Mezzenga, R. Supramolecular chiral self-assembly and supercoiling behavior of carrageenans at varying salt conditions. Nanoscale 2015, 7, 16182–16188. [Google Scholar] [CrossRef]
- Simkovic, I.; Gucmann, F.; Mendichi, R.; Schieroni, A.G.; Piovani, D.; Dobrocka, E.; Hricovíni, M. Extraction and characterization of polysaccharide films prepared from Furcellaria lumbricalis and Gigartina skottsbergii seaweeds. Cellulose 2021, 28, 9567–9588. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Kumar, S.; Nigam, N.; Ghosh, T.; Dutta, P.K.; Singh, S.P.; Datta, P.K.; An, L.; Shi, T.F. Preparation, characterization and optical properties of a novel azo-based chitosan biopolymer. Mater. Chem. Phys. 2010, 120, 361–370. [Google Scholar] [CrossRef]
- Maalige, R.N.; Aruchamy, K.; Veerababu, P.; Halakarni, M.; Mahto, A.; Mondal, D.; Nataraj, S.K. Restructuring thin film composite membrane interfaces using biopolymer as a sustainable alternative to prevent organic fouling. Carbohydr. Polym. 2021, 254, 117297. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N.; Khodakarami, Z. Nano-biopolymer effect on forward osmosis performance of cellulosic membrane: High water flux and low reverse salt. Carbohydr. Polym. 2019, 204, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.K.; Abid, N.K. Study the effect of TiO2 nanoparticles on physical properties of biopolymer blend. IOP Conf. Ser. Mater. Sci. Eng. 2020, 757, 012073. [Google Scholar] [CrossRef]
- Matienzo, L.J.; Winnacker, S.K. Dry Processes for Surface Modification of a Biopolymer: Chitosan. Macromol. Mater. Eng. 2002, 287, 871–880. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Planecka, A.; Szarszewska, P.; Krasinska, K.; Kaczmarek, B.; Kozlowska, J. Biopolymer Blends as Potential Biomaterials and Cosmetic Materials. Key Eng. Mater. 2014, 583, 95–100. [Google Scholar] [CrossRef]
- Pérez, S.; Rebollar, E.; Oujja, M.; Martín, M.; Castillejo, M. Laser-induced periodic surface structuring of biopolymers. Appl. Phys. A 2013, 110, 683–690. [Google Scholar] [CrossRef]
- Milosavljevic, N.B.; Ristic, M.D.; Peric-Grujic, A.A.; Filipovic, J.M.; Strbac, S.B.; Rakocevic, Z.L.; Kalagasidis Krusic, M.T. Sorption of zinc by novel pH-sensitive hydrogels based on chitosan, itaconic acid and methacrylic acid. J. Hazard. Mater. 2011, 192, 846–854. [Google Scholar] [CrossRef]
- Milosavljevic, N.B.; Ristic, M.D.; Peric-Grujic, A.A.; Filipovic, J.M.; Strbac, S.B.; Rakocevic, Z.L.; Kalagasidis Krusic, M.T. Removal of Cu2+ ions using hydrogels of chitosan, itaconic and methacrylic acid: FTIR, SEM/EDX, AFM, kinetic and equilibrium study. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 59–69. [Google Scholar] [CrossRef]
- Gaihre, B.; Jaasuriya, A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C 2018, 91, 330–339. [Google Scholar] [CrossRef]
- Luna, R.; Touhami, F.; Uddin, M.J.; Touhami, A. Effect of temperature and pH on nanostructural and nanomechanical properties of chitosan films. Surf. Interfaces 2022, 29, 101706. [Google Scholar] [CrossRef]
- Mendes, A.C.; Moreno, J.S.; Hanif, M.; Douglas, T.E.L.; Chen, M.; Chronakis, I.S. Morphological, Mechanical and Mucoadhesive Properties of Electrospun Chitosan/Phospholipid Hybrid Nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha Neto, J.B.M.; Taketa, T.B.; Bataglioli, R.A.; Pimentel, S.B.; Santos, D.M.; Fiamingo, A.; Costa, C.A.R.; Campana-Filho, S.P.; Carvalho, H.F.; Beppu, M.M. Tailored chitosan/hyaluronan coatings for tumor cell adhesion: Effects of topography, charge density and surface composition. Appl. Surf. Sci. 2019, 486, 508–518. [Google Scholar] [CrossRef]
- Guzmán Sierra, D.L.; Bdikin, I.; Tkach, A.; Vilarinho, P.M.; Nunes, C.; Ferreira, P. Flexible Piezoelectric Chitosan and Barium Titanate Biocomposite Films for Sensor Applications. Eur. J. Inorg. Chem. 2020, 2021, 792–803. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Lucchesi, E.G.; Gonzalez, I.; Macedo, W.A.A.; Nascimento, V.F.; Moraes, A.M.; Beppu, M.M.; Cotta, M.A. Hyaluronan/chitosan nanofilms assembled layer-by-layer and theirantibacterial effect: A study using Staphylococcus aureus andPseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2016, 141, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sing, B.; Kmar, A.; Rohit. Synthesis and characterization of alginate and sterculia gum based hydrogel for brain drug delivery applications. Int. J. Biol. Macromol. 2020, 148, 248–257. [Google Scholar] [CrossRef]
- Aburabie, J.H.; Puspasari, T.; Peinemann, K.-V. Alginate-based membranes: Paving the way for green organic solvent nanofiltration. J. Membr. Sci. 2020, 596, 117615. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 602. [Google Scholar]
- Zhang, X.L.; Weng, L.; Liu, Q.S.; Li, D.W.; Deng, B.Y. Facile fabrication and characterization on alginate microfibres with grooved structure via microfluidic spinning. R. Soc. Open Sci. 2019, 6, 181928. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Chu, Z.H.; Wang, H.L.; Cai, L.; Tu, Z.G.; Liu, H.Y.; Zhu, C.Y.; Shi, H.F.; Pan, D.H.; Pan, J.; et al. Electrostatically Assembled Multilayered Films of Biopolymer Enhanced Nanocapsules for on-Demand Drug Release. ACS Appl. Bio Mater. 2019, 2, 3429–3438. [Google Scholar] [CrossRef]
- Duckworth, P.F.; Maddocks, S.E.; Rahatekar, S.S.; Barbour, M.E. Alginate films augmented with chlorhexidine hexametaphosphate particles provide sustained antimicrobial properties for application in wound care. J. Mater. Sci. Mater. Med. 2020, 31, 33. [Google Scholar] [CrossRef] [Green Version]
- Axpe, E.; Duraj-Thatte, A.; Chan, Y.; Kaimai, D.-M.; Sanchez-Sanchez, A.; Caliskan, H.B.; Dorval Courchesne, N.-M.; Joshi, N.S. Fabrication of Amyloid Curli Fibers–Alginate Nanocomposite Hydrogels with Enhanced Stiffness. ACS Biomater. Sci. Eng. 2018, 4, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, M.; Minary-Jolandan, M. Alginate-Collagen Fibril Composite Hydrogel. Materials 2015, 8, 799–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherstova, T.; Stocke, B.T.; Skallerud, B.; Maurstad, G.; Prot, V.E. Nanoindentation and finite element modelling of chitosan–alginate multilayer coated hydrogels. Soft Matter 2016, 12, 7338–7349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hia, I.L.; Lam, W.H.; Chai, S.-P.; Chan, E.-S.; Pasbakhsh, P. Surface modified alginate multicore microcapsules and their application in self-healing epoxy coatings for metallic protection. Mater. Chem. Phys. 2018, 215, 69–80. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, L.J. Single-molecule determination of the phase- and facet-dependent adsorption of alginate on iron oxides. Environ. Sci. Nano 2020, 7, 954–962. [Google Scholar] [CrossRef]
- Ikuma, K.; Madden, A.S.; Decho, A.W.; Lau, B.L.T. Deposition of nanoparticles onto polysaccharide-coated surfaces: Implications for nanoparticle–biofilm interactions. Environ. Sci. Nano 2014, 1, 117–122. [Google Scholar] [CrossRef]
- Betiha, M.A.; Mohamed, G.G.; Negm, N.A.; Hussein, M.F.; Ahmed, H.E. Fabrication of ionic liquid-cellulose-silica hydrogels with appropriate thermal stability and good salt tolerance as potential drilling fluid. Arab. J. Chem. 2020, 13, 6201–6220. [Google Scholar] [CrossRef]
- Carvalho dos Santos, E.; Bandeira, R.M.; Vega, M.L.; Ribeiro dos Santos Junior, J. Poly(melamine-formaldehyde-silica) Composite Hydrogel for Methylene Blue Removal. Mater. Res. 2021, 24, e20200574. [Google Scholar] [CrossRef]
- Cao, Y.P.; Bolisetty, S.; Wolfisberg, G.; Adamcik, J.; Mezzenga, R. Amyloid fibril-directed synthesis of silica core–shell nanofilaments, gels, and aerogels. Proc. Natl. Acad. Sci. USA 2019, 116, 4012–4017. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hu, S.X.; Hsieh, Y.-I. Aqueous Synthesis of Compressible and Thermally Stable Cellulose Nanofibril–Silica Aerogel for CO2 Adsorption. ACS Appl. Nano Mater. 2018, 1, 6701–6710. [Google Scholar] [CrossRef]
- Becerra, N.Y.; Restrepo, L.M.; Galeano, Y.; Tobón, A.C.; Turizo, L.F.; Mesa, M. Improving Fibrin Hydrogels’ Mechanical Properties, through Addition of Silica or Chitosan-Silica Materials, for Potential Application as Wound Dressings. Int. J. Biomater. 2021, 2021, 9933331. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, G.R.; Tomova, R.; Djambova, S.T.; Nadoliiski, M.; Dimova-Malinovska, D. Functionalized aerogels–new nanomaterials for energy-efficient building. Preliminary AFM, Nanoidentation and EIS studies. J. Phys. Conf. Ser. 2010, 253, 012077. [Google Scholar] [CrossRef]
- Nalam, P.C.; Gosvami, N.N.; Caporizzo, M.A.; Composto, R.J.; Carpick, R.W. Nano-rheology of hydrogels using direct drive force modulation atomic force microscopy. Soft Matter 2015, 11, 8165–8178. [Google Scholar] [CrossRef] [PubMed]
- Nasalapure, A.V.; Chalannavar, R.K.; Kasai, D.R.; Reddy, K.R.; Raghu, A.V. Novel polymeric hydrogel composites: Synthesis, physicochemical, mechanical and biocompatible properties. Nano Express 2021, 2, 030003. [Google Scholar] [CrossRef]
- Tytgat, L.; Vagenende, M.; Declercq, H.; Martins, J.C.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; van Vlierberghe, S. Synergistic effect of κ-carrageenan and gelatin blends towards adipose tissue engineering. Carbohydr. Polym. 2018, 189, 1–9. [Google Scholar] [CrossRef]
- Song, X.Y.; Chiou, B.-S.; Xia, Y.X.; Chen, M.S.; Liu, F.; Zhong, F. The improvement of texture properties and storage stability for kappa carrageenan in developing vegan gummy candies. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Stawczyk, J.; Matyka, K.; Matyka, M.; Mroz, I.; Ciszewski, A. Surface Microstructure of Chitosan Membranes–AFM Investigations. Pol. J. Environ. Stud. 2006, 15, 84–87. [Google Scholar]
- Drabczyk, A.; Kudlacik-Kramarczyk, S.; Glab, M.; Kedzierska, M.; Jaromin, A.; Mierzwiski, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Bouali, A.B.; Montembalt, A.; David, L.; von Boxberg, Y.; Viallon, M.; Hamdi, B.; Nothias, F.; Fodil, R.; Féréol, S. Nanoscale mechanical properties of chitosan hydrogels as revealed by AFM. Prog. Biomater. 2020, 9, 187–201. [Google Scholar] [CrossRef]
- Miras, J.; Liu, C.; Blomberg, E.; Hormann, E.; Vilchez, S.; Esquena, J. pH-responsive chitosan nanofilms crosslinked with genipin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126229. [Google Scholar] [CrossRef]
- Yan, L.F.; Qian, F.; Zhu, Q. Interpolymer complex polyampholytic hydrogel of chitosan and carboxymethyl cellulose (CMC): Synthesis and ion effect. Polym. Int. 2001, 50, 1370–1374. [Google Scholar] [CrossRef]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. BioMacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Solbu, A.A.; Koernig, A.; Kjesbu, J.S.; Zaytseva-Zotova, D.; Sletmoen, M.; Strand, B.L. High resolution imaging of soft alginate hydrogels by atomic force microscopy. Carbohydr. Polym. 2022, 276, 118804. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Koh, A.J.; Danciu, T.M.; Cauley, L.K.; Ma, P.X. Preprogrammed Long-Term Systemic Pulsatile Delivery of Parathyroid Hormone to Strengthen Bone. Adv. Healthc. Mater. 2017, 6, 1600901. [Google Scholar] [CrossRef] [Green Version]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of Polyethylene Glycol on Properties and Drug Encapsulation–Release Performance of Biodegradable/Cytocompatible Agarose–Polyethylene Glycol–Polycaprolactone Amphiphilic Co-Network Gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Bera, A.; Nutan, B.; Jewrajka, S.K. Reactive compatibilizer mediated precise synthesis and application of stimuli responsive polysaccharides-polycaprolactone amphiphilic co-network gels. Polymer 2016, 99, 470–479. [Google Scholar] [CrossRef]
- Bera, A.; Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Degradable/cytocompatible and pH responsive amphiphilic conetwork gels based on agarose-graft copolymers and polycaprolactone. J. Mater. Chem. B 2015, 3, 8548–8557. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid Prepolymer-Based in Situ Formation of Degradable Poly(ethylene glycol)-Linked-Poly(caprolactone)-Linked-Poly(2-dimethylaminoethyl)methacrylate Amphiphilic Conetwork Gels Showing Polarity Driven Gelation and Bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619. [Google Scholar] [CrossRef]
- Abu-Lail, N.I.; Camesano, T.A. Role of Ionic Strength on the Relationship of Biopolymer Conformation, DLVO Contributions, and Steric Interactions to Bioadhesion of Pseudomonas putida KT2442. Biomacromolecules 2003, 4, 1000–1012. [Google Scholar] [CrossRef]
- Kwasniewska, A.; Swietlicki, M.; Prószynski, A.; Gladyszewski, G. The Quantitative Nanomechanical Mapping of Starch/Kaolin Film Surfaces by Peak Force AFM. Polymers 2021, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Chichti, E.; George, m.; Delenne, J.-Y.; Farjai, F.; Lullien-Pellerin, V. Nano-mechanical properties of starch and gluten biopolymers from atomic force microscopy. Eur. Polym. J. 2013, 49, 3788–3795. [Google Scholar] [CrossRef]
- Pelto, J.M.; Haimi, S.P.; Siljander, A.S.; Miettinen, S.S.; Tappura, K.M.; Higgins, M.J.; Wallace, G.G. Surface Properties and Interaction Forces of Biopolymer-Doped Conductive Polypyrrole Surfaces by Atomic Force Microscopy. Langmuir 2013, 29, 6099–6108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camesano, T.A.; Abu-Lail, N.I. Heterogeneity in Bacterial Surface Polysaccharides, Probed on a Single-Molecule Basis. Biomacromolecules 2002, 3, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Heedy, S.; Pineda, J.J.; Meli, V.S.; Wang, S.-W.; Yee, A.F. Nanopillar Templating Augments the Stiffness and Strength in Biopolymer Films. ACS Nano 2022, 16, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Liparoti, S.; Franco, P.; Pantani, R.; De Marco, I. Supercritical CO2 impregnation of caffeine in biopolymer films to produce anti-cellulite devices. J. Supercrit. Fluids 2022, 179, 105411. [Google Scholar] [CrossRef]
- Chu, J.; Metcalfe, P.; Linford, H.V.; Zhao, S.Y.; Goycoolea, F.M.; Chen, S.G.; Ye, Y.Q.; Holmes, M.; Orfila, C. Short-time acoustic and hydrodynamic cavitation improves dispersibility and functionality of pectin-rich biopolymers from citrus waste. J. Clean. Prod. 2022, 330, 129789. [Google Scholar] [CrossRef]
- Prokhorov, V.V.; Klinov, D.V.; Chinarev, a.; Tuzikov, A.B.; Gorokhova, I.V.; Bovin, N.V. High-Resolution Atomic Force Microscopy Study of Hexaglycylamide Epitaxial Structures on Graphite. Langmuir 2011, 27, 5879–5890. [Google Scholar] [CrossRef]
- Zemla, J.; Bobrowska, J.; Kubiak, A.; Zielinski, T.; Pabijan, J.; Pogoda, K.; Bobrowski, P.; Lekka, M. Indenting soft samples (hydrogels and cells) with cantilevers possessing various shapes of probing tip. Eur. Biophys. J. 2020, 49, 485–495. [Google Scholar] [CrossRef]
- Huth, S.; Sindt, S.; Selhuber-Unkel, C. Automated analysis of soft hydrogel microindentation: Impact of various indentation parameters on the measurement of Young’s modulus. PLoS ONE 2019, 14, e0220281. [Google Scholar] [CrossRef] [Green Version]
- Weiss, A.-V.; Fischer, T.; Iturri, J.; Benitez, R.; Toca-Herrera, J.L.; Schneider, M. Mechanical properties of gelatin nanoparticles in dependency of crosslinking time and storage. Colloids Surf. B Biointerfaces 2019, 175, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, A.; Parvinzadeh Gashti, M.; Mirjalili, M.; Parsania, M. Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, A.; Bliznakova, I.; Angelova, L.; Trifonov, A.; Declercq, H.; Buchvarov, I. Femtosecond Laser Fabrication of Engineered Functional Surfaces Based on Biodegradable Polymer and Biopolymer/Ceramic Composite Thin Films. Polymers 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemzadeh, H.; Allahverdi, A.; Ghorbani, M.; Soleymani, H.; Kocsis, A.; Fischer, M.B.; Ertl, P.; Naderi-Manesh, H. Gold Nanowires/Fibrin Nanostructure as Microfluidics Platforms for Enhancing Stem Cell Differentiation: Bio-AFM Study. Micromachines 2020, 11, 50. [Google Scholar] [CrossRef] [Green Version]






| AFM Mode | Information Given | Static/Dynamic Mode | Special Requirements |
|---|---|---|---|
| Contact mode | Topography, roughness | Static mode | Cantilever for static mode |
| Tapping mode | Topography, roughness | Dynamic mode | Cantilever for dynamic mode |
| Phase imaging | Qualitative differentiation between materials + clearer edges within single material | Dynamic mode | Cantilever for dynamic mode |
| Nanoindentation | Elastic modulus, hardness | Dynamic/static/quasistatic | Tip harder than sample |
| PeakForce QNM | Elastic modulus, adhesion | Dynamic mode | Often very broad tips are supportive, functionalization of the cantilever broadens the measurement spectrum |
| KPFM | Contact potential (work function) of a surface | Dynamic mode | Conductive tip, single or dual pass setup |
| Conductive AFM | Conductivity; local current-voltage curves | Static mode | Conductive tip |
| Material | Properties Measured by AFM | Reference |
|---|---|---|
| Agarose-polyethylene glycol-polycaprolactone gel | Topography and nano-phase separation | [195] |
| Polysaccharide-polycaprolactone gel | [196] | |
| Agarose/polycaprolactone | [197] | |
| Poly(ethylene glycol)/poly(caprolactone) | [198] | |
| Bacterial biopolymers | Adhesion between biopolymer and SiN tip | [199] |
| Starch | Mechanical properties by PeakForce AFM | [200] |
| Starch and gluten | Nano-mechanical properties | [201] |
| Biopolymer-doped polypyrrole | Adhesion, E-modulus, electrostatic force | [202] |
| Polysaccharides on bacterial cells | Stretching biopolymer molecules for force-extension tests | [203] |
| Chitosan hydrogels | Topography | [204] |
| Caffeine in polymer matrix | Topography and phase imaging | [205] |
| Pectin-rich biopolymer from citrus waste | Topography | [206] |
| Hexaglycylamide | [207] | |
| Hydrogels and cells | Indentation with different tip shapes | [208] |
| Soft hydrogels | Test of microindentation parameters | [209] |
| Gelatin | Nanoindentation | [210] |
| Gelatin nanofibers | Morphology | [211] |
| Chitosan/hydroxyapatite/nanoZrO2 | Roughness of laser-structured surfaces | [212] |
| Fibrin hydrogel with gold nanowires | Adhesion force, stiffness, elasticity | [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers 2022, 14, 1267. https://doi.org/10.3390/polym14061267
Joshi J, Homburg SV, Ehrmann A. Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers. 2022; 14(6):1267. https://doi.org/10.3390/polym14061267
Chicago/Turabian StyleJoshi, Jnanada, Sarah Vanessa Homburg, and Andrea Ehrmann. 2022. "Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits" Polymers 14, no. 6: 1267. https://doi.org/10.3390/polym14061267
APA StyleJoshi, J., Homburg, S. V., & Ehrmann, A. (2022). Atomic Force Microscopy (AFM) on Biopolymers and Hydrogels for Biotechnological Applications—Possibilities and Limits. Polymers, 14(6), 1267. https://doi.org/10.3390/polym14061267







