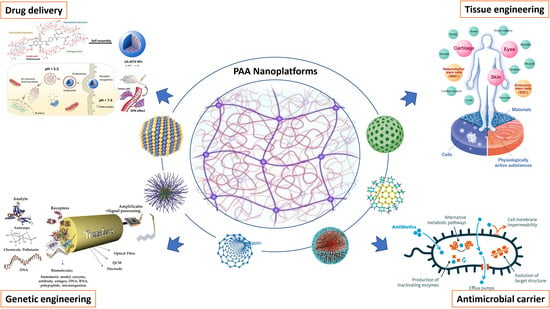
Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications
Abstract
:1. Introduction
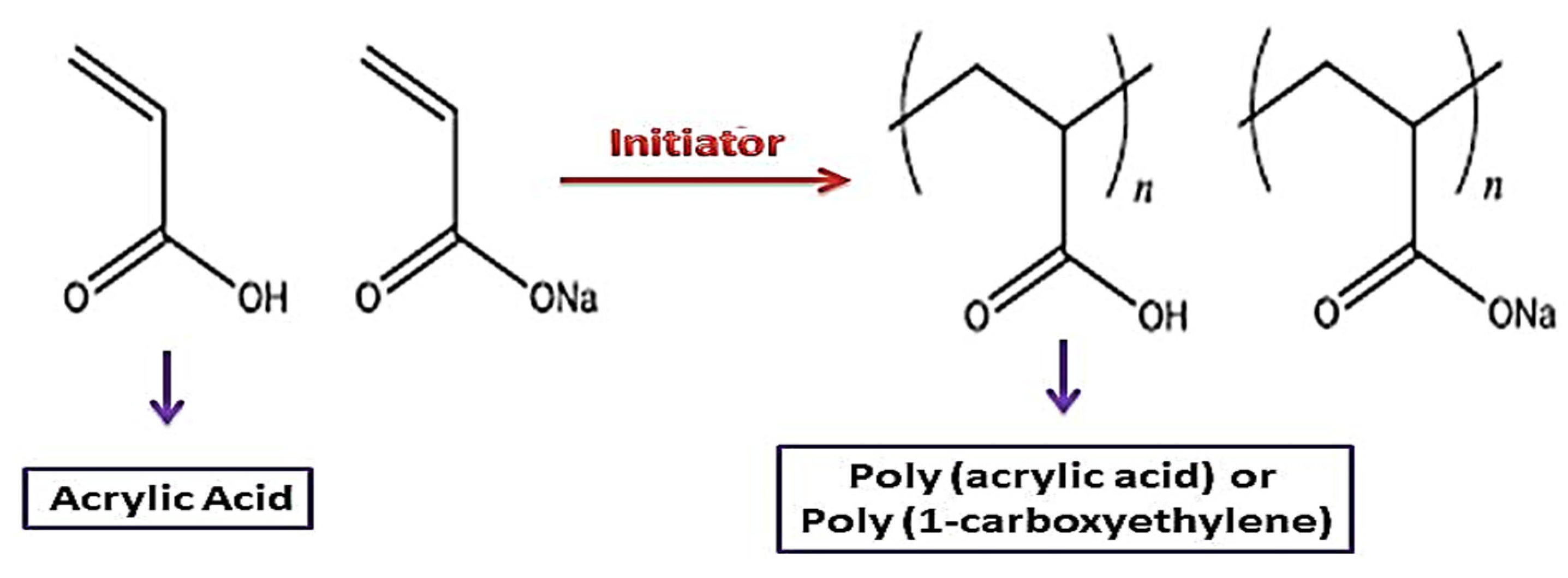
2. Synthesis and Structure Characteristics
3. Architectures of PAA
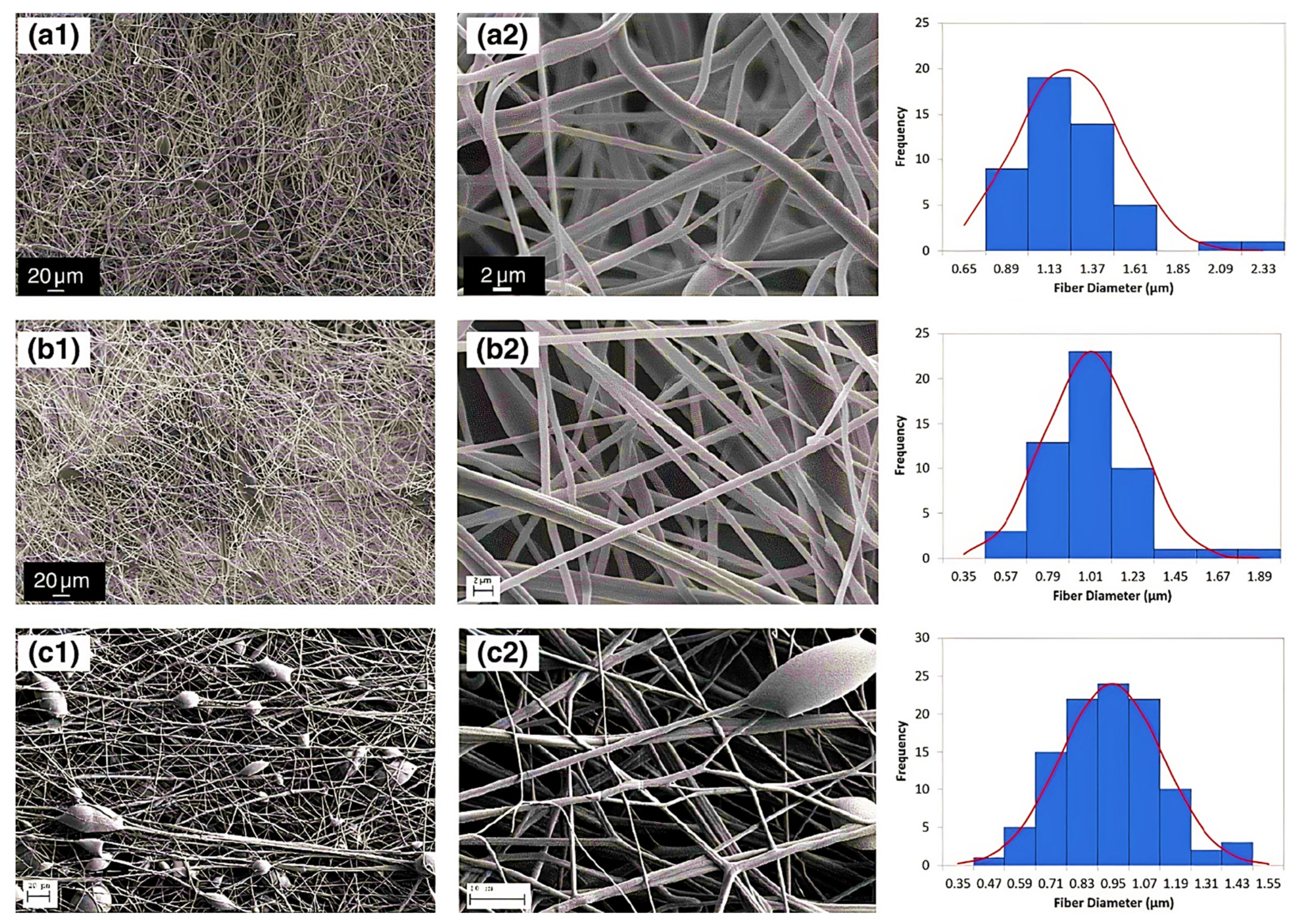
3.1. Nanofibers
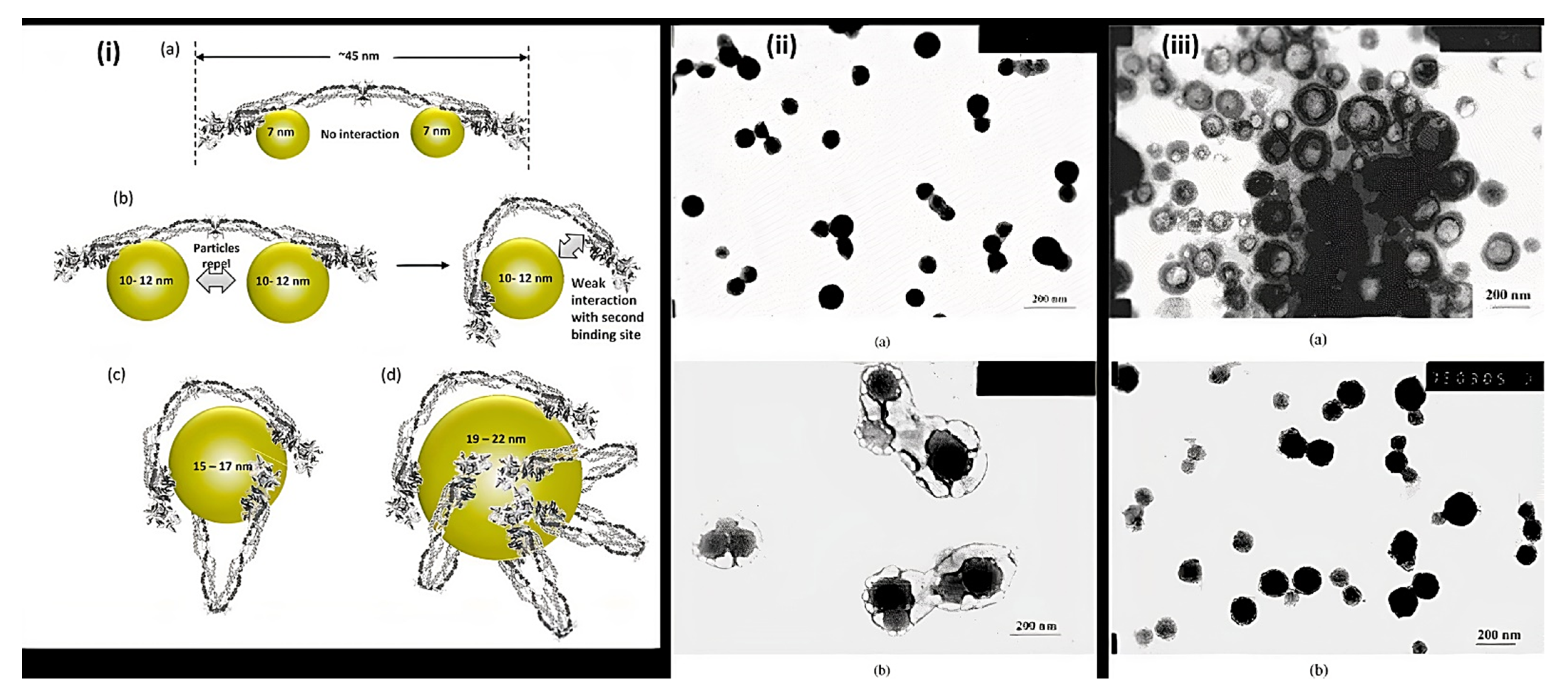
3.2. Nanoparticles
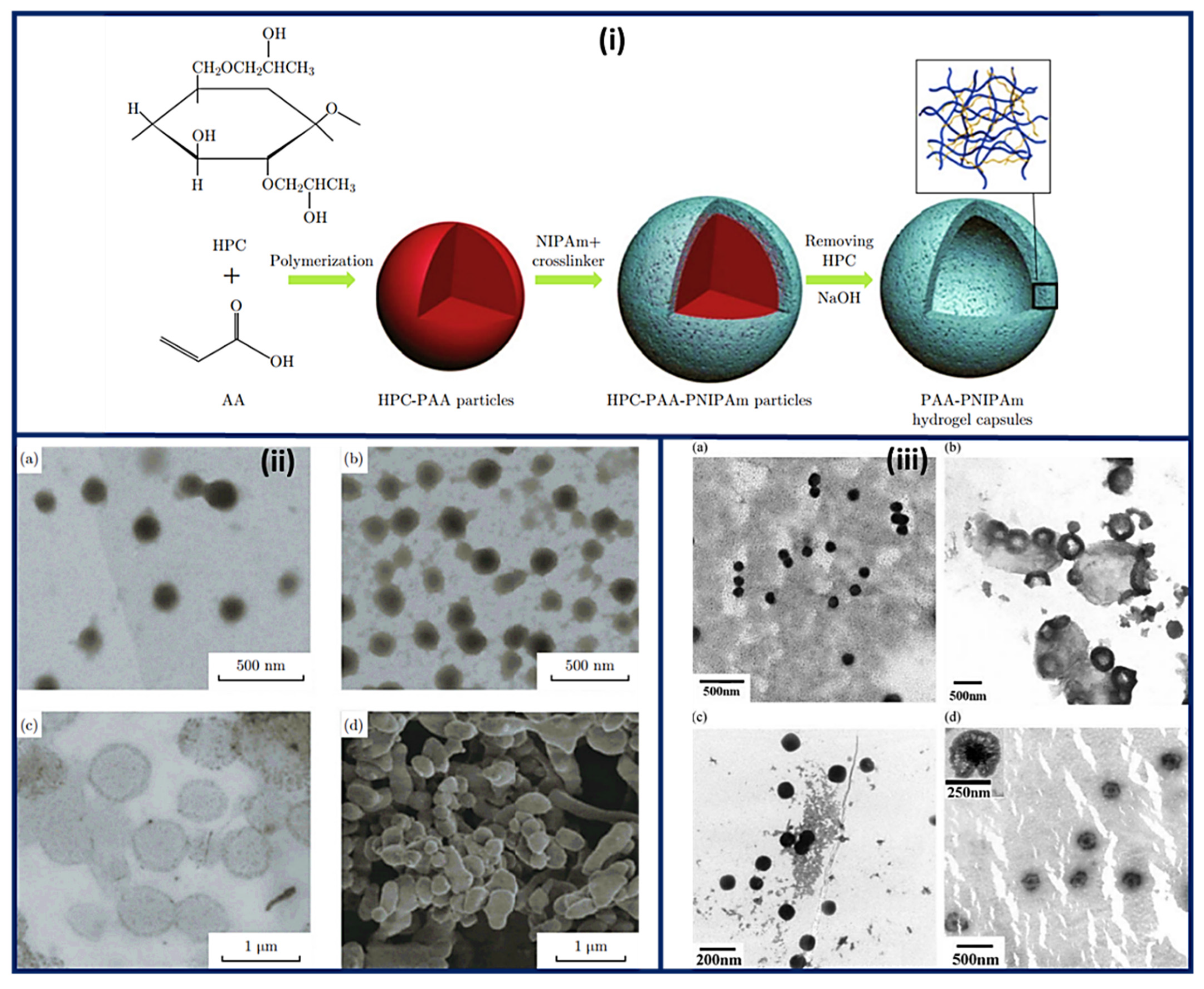
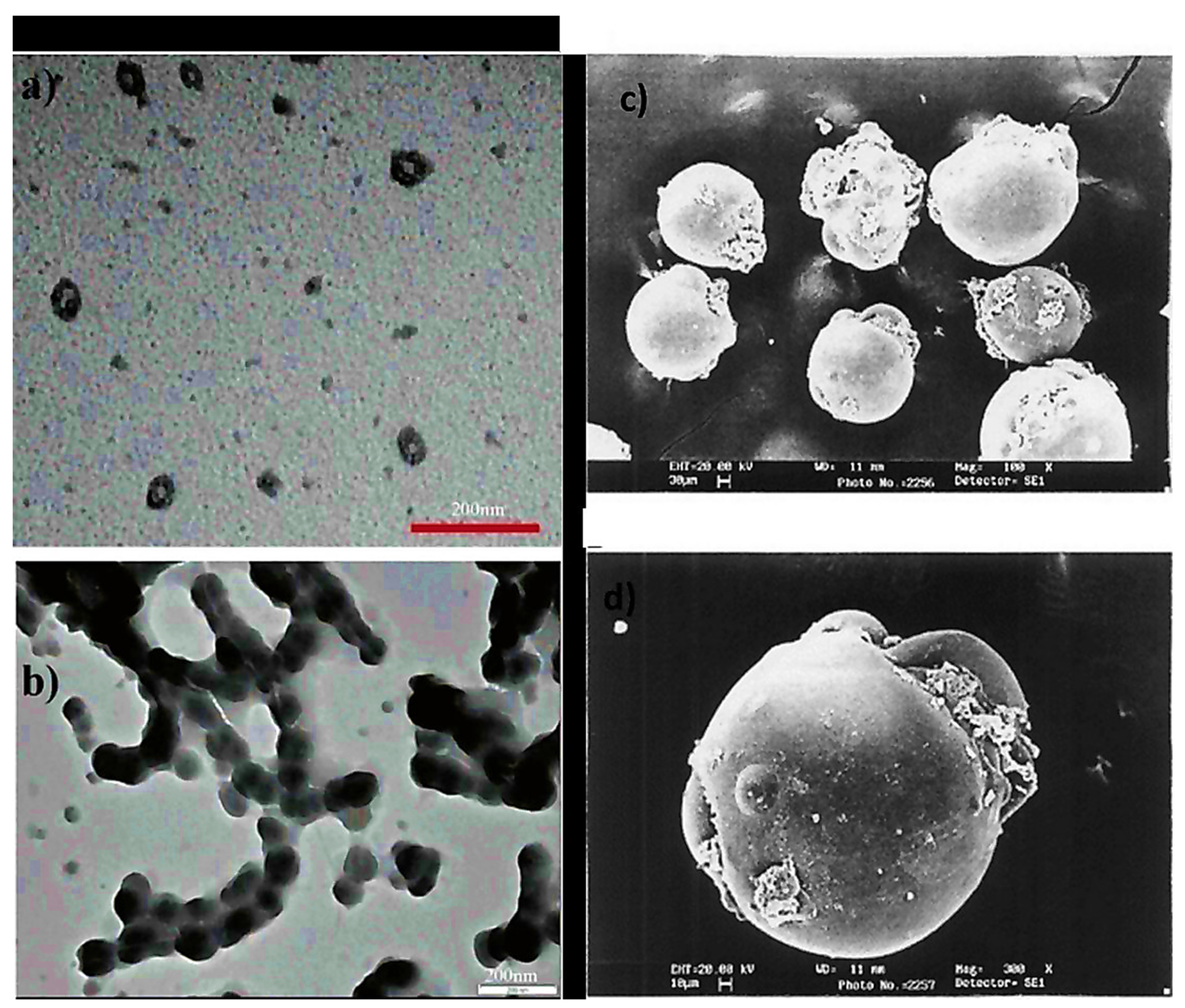
3.3. Nanocapsules
3.4. Other Structures
4. Bio-Conjugation with Other Materials
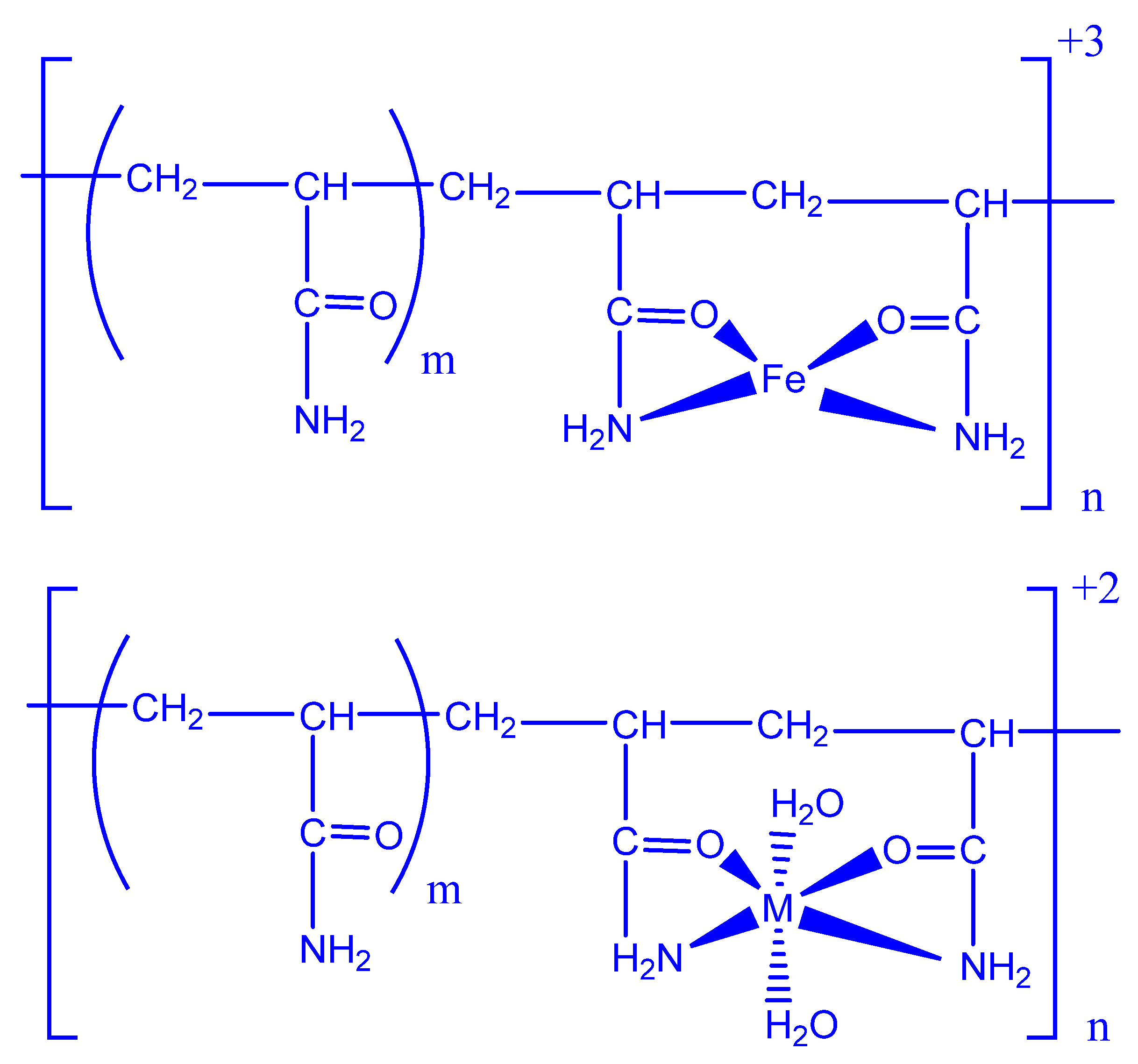
4.1. Metal Oxides
4.2. Gold Nanostructures
4.3. Silica Nanoparticles
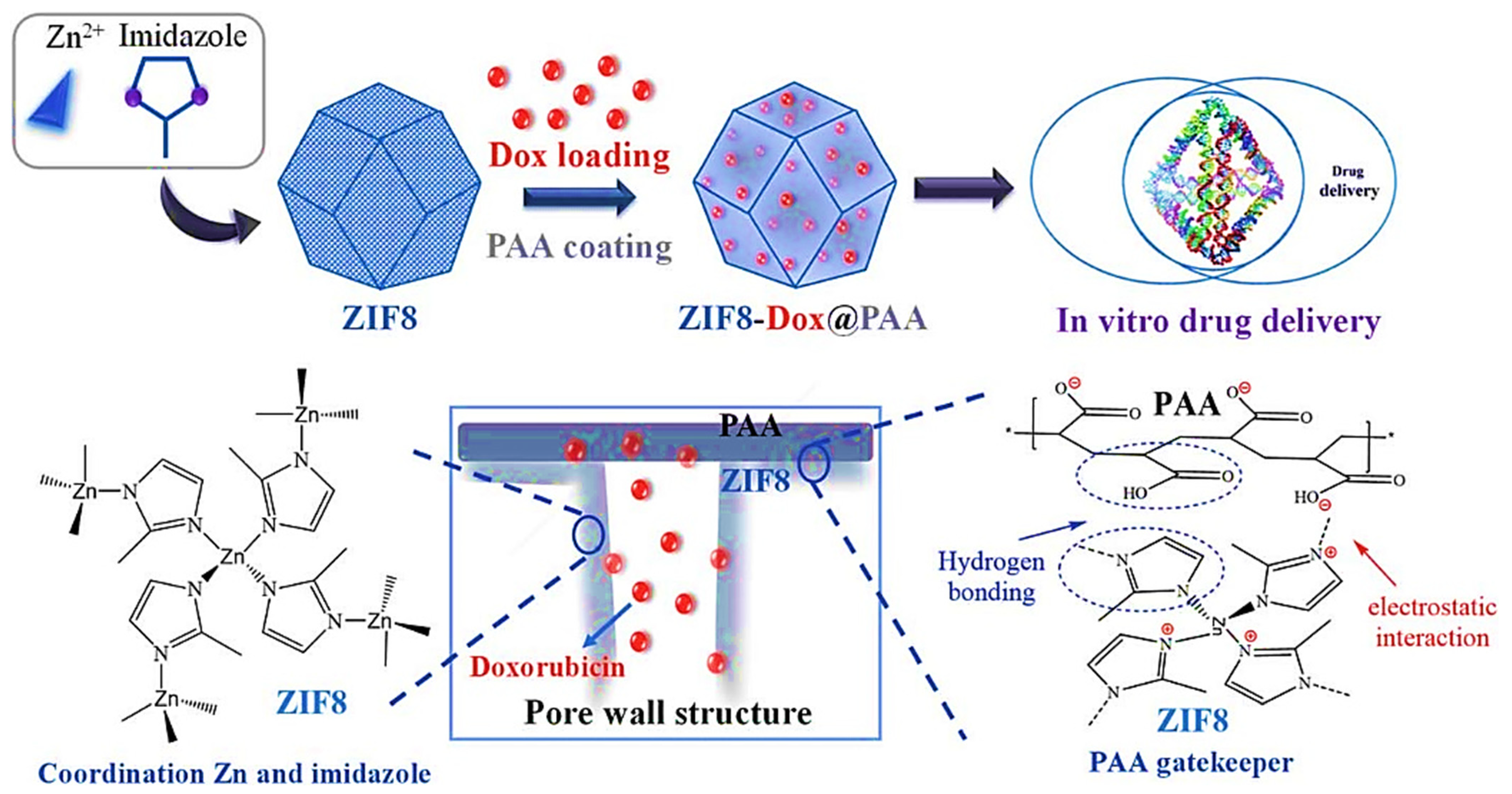
4.4. Metal-Organic Frameworks (MOFs)
4.5. Carbon Nanomaterials
4.6. Other Metals
5. Biomedical Applications
5.1. Bio-Sensing
5.2. Bio-Imaging
5.3. Cancer Therapy
5.4. Cancer Theranostic
5.5. Tissue Engineering
5.6. Antimicrobial Applications
6. Compatibility and Biodegradability
7. Conclusions, Challenges
8. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suárez-García, S.; Solórzano, R.; Alibés, R.; Busqué, F.; Novio, F.; Ruiz-Molina, D. Antitumour activity of coordination polymer nanoparticles. Coord. Chem. Rev. 2021, 441, 213977. [Google Scholar] [CrossRef]
- Daneshvari, G.; Yousefi, A.R.; Mohammadi, M.; Banibairami, S.; Shariati, P.; Rahdar, A.; Kyzas, G.Z. Controlled-release Formulations of Trifluralin Herbicide by Interfacial Polymerization as a Tool for Environmental Hazards. Biointerface Res. Appl. Chem. 2021, 11, 13866–13877. [Google Scholar]
- Rauf, A.; Tabish, T.A.; Ibrahim, I.M.; ul Hassan, M.R.; Tahseen, S.; Sandhu, M.A.; Shahnaz, G.; Rahdar, A.; Cucchiarini, M.; Pandey, S. Design of Mannose-Coated Rifampicin nanoparticles modulating the immune response and Rifampicin induced hepatotoxicity with improved oral drug delivery. Arab. J. Chem. 2021, 14, 103321. [Google Scholar] [CrossRef]
- Rahdar, S.; Rahdar, A.; Sattari, M.; Hafshejani, L.D.; Tolkou, A.K.; Kyzas, G.Z. Barium/cobalt@ polyethylene glycol nanocomposites for dye removal from aqueous solutions. Polymers 2021, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Zaquen, N.; Rubens, M.; Corrigan, N.; Xu, J.; Zetterlund, P.B.; Boyer, C.; Junkers, T. Polymer synthesis in continuous flow reactors. Prog. Polym. Sci. 2020, 107, 101256. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Polymer synthesis and processing. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Synthetic polymers. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 559–590. [Google Scholar]
- Pavlinec, J.; Novák, I.; Rychlý, J.; Kleinová, A.; Nógellová, Z.; Preťo, J.; Vanko, V.; Žigo, O.; Chodák, I. Hot melt adhesives prepared by grafting of acrylic and crotonic acids onto metallocene ethylene–octene copolymers. J. Plast. Film. Sheeting 2019, 35, 239–259. [Google Scholar] [CrossRef]
- Dashtizadeh, A.; Abdouss, M.; Khorassani, M.; Mahdavi, H. Preparation of silica-filled water-based acrylic nanocomposite paints with improved scratch resistance. J. Plast. Film. Sheeting 2012, 28, 120–135. [Google Scholar] [CrossRef]
- Savaskan Yilmaz, S.; Yildirim, N.; Misir, M.; Misirlioglu, Y.; Celik, E. Synthesis, Characterization of a New Polyacrylic Acid Superabsorbent, Some Heavy Metal Ion Sorption, the Adsorption Isotherms, and Quantum Chemical Investigation. Materials 2020, 13, 4390. [Google Scholar] [CrossRef]
- Yee, S.Y.Y. Medicinal properties of bioactive compounds and antioxidant activity in Durio zibethinus. Malays. J. Sustain. Agric. (MJSA) 2021, 5, 82–89. [Google Scholar] [CrossRef]
- Zenoozi, S.; Sadeghi, G.M.M.; Rafiee, M. Synthesis and characterization of biocompatible semi-interpenetrating polymer networks based on polyurethane and cross-linked poly (acrylic acid). Eur. Polym. J. 2020, 140, 109974. [Google Scholar] [CrossRef]
- Zuo, Y.; Yang, W.; Zhang, K.; Chen, Y.; Yin, X.; Liu, Y. Experimental and theoretical studies of carboxylic polymers with low molecular weight as inhibitors for calcium carbonate scale. Crystals 2020, 10, 406. [Google Scholar] [CrossRef]
- Mori, H.; Müller, A.H.; Klee, J.E. Intelligent colloidal hybrids via reversible pH-induced complexation of polyelectrolyte and silica nanoparticles. J. Am. Chem. Soc. 2003, 125, 3712–3713. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 2005, 105, 1401–1444. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, J.; Bhattacharjee, S.; Wijeratne, S.; Bruening, M.L.; Baker, G.L. Increased protein sorption in poly (acrylic acid)-containing films through incorporation of comb-like polymers and film adsorption at low pH and high ionic strength. Langmuir 2013, 29, 2946–2954. [Google Scholar] [CrossRef] [Green Version]
- Spagnol, C.; Rodrigues, F.H.; Pereira, A.G.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly (acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef] [Green Version]
- Takada, K.; Iida, T.; Kawanishi, Y.; Yasui, T.; Yuchi, A. An electrochemical actuator based on reversible changes in volume of poly (acrylic acid) gel induced by quinone redox. Sens. Actuators B Chem. 2011, 160, 1586–1592. [Google Scholar] [CrossRef]
- Hisamatsu, N.; Iida, T.; Yasui, T.; Takada, K.; Yuchi, A. Double-side coated electrochemical actuator based on changes in volume of poly (acrylic acid) gel. Sens. Actuators B Chem. 2014, 203, 289–295. [Google Scholar] [CrossRef]
- Changez, M.; Koul, V.; Krishna, B.; Dinda, A.K.; Choudhary, V. Studies on biodegradation and release of gentamicin sulphate from interpenetrating network hydrogels based on poly (acrylic acid) and gelatin: In vitro and in vivo. Biomaterials 2004, 25, 139–146. [Google Scholar] [CrossRef]
- Khanlari, S.; Dubé, M.A. Bioadhesives: A review. Macromol. React. Eng. 2013, 7, 573–587. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, C.; Cheng, Z.; Li, C.; Kang, X.; Yang, D.; Lin, J. pH-responsive drug delivery system based on luminescent CaF2: Ce3+/Tb3+-poly (acrylic acid) hybrid microspheres. Biomaterials 2012, 33, 2583–2592. [Google Scholar] [CrossRef]
- Babiker, D.M.; Zhu, L.; Yagoub, H.; Xu, X.; Zhang, X.; Shibraen, M.H.; Yang, S. Hydrogen-bonded methylcellulose/poly (acrylic acid) complex membrane for oil-water separation. Surf. Coat. Technol. 2019, 367, 49–57. [Google Scholar] [CrossRef]
- Abd Alsaheb, R.A.; Aladdin, A.; Othman, N.Z.; Abd Malek, R.; Leng, O.M.; Aziz, R.; El Enshasy, H.A. Recent applications of polylactic acid in pharmaceutical and medical industries. J. Chem. Pharm. Res 2015, 7, 51–63. [Google Scholar]
- Jiang, L.; Gao, L.; Wang, X.; Tang, L.; Ma, J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Swilem, A.E.; Elshazly, A.H.; Hamed, A.A.; Hegazy, E.-S.A.; Abd El-Rehim, H.A. Nanoscale poly (acrylic acid)-based hydrogels prepared via a green single-step approach for application as low-viscosity biomimetic fluid tears. Mater. Sci. Eng. C 2020, 110, 110726. [Google Scholar] [CrossRef] [PubMed]
- Partenhauser, A.; Bernkop-Schnürch, A. Mucoadhesive polymers in the treatment of dry X syndrome. Drug Discov. Today 2016, 21, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Monirifard, R.; Abolhasani, M.; Tahani, B.; Fathi, A.; Choobdaran, A. Relationship of Personality Traits and Patient Satisfaction with Fixed Implant Prosthodontic Treatments. J. Iran. Dent. Assoc. 2019, 31, 182–188. [Google Scholar] [CrossRef]
- Mosharraf, R.; Molaei, P.; Fathi, A.; Isler, S. Investigating the Effect of Nonrigid Connectors on the Success of Tooth-and-Implant-Supported Fixed Partial Prostheses in Maxillary Anterior Region: A Finite Element Analysis (FEA). Int. J. Dent. 2021, 2021, 5977994. [Google Scholar] [CrossRef]
- Maalekipour, M.; Safari, M.; Barekatain, M.; Fathi, A. Effect of Adhesive Resin as a Modeling Liquid on Elution of Resin Composite Restorations. Int. J. Dent. 2021, 2021, 3178536. [Google Scholar] [CrossRef]
- Abolhasani, M.; Givehchian, P.; Fathi, A.; Goudarzi, S. Relationship of Life Satisfaction and Satisfaction with Fixed Implant-Supported Prostheses in the Elderly. J. Iran. Dent. Assoc. 2021, 33, 17–21. [Google Scholar]
- Roostaee, M.; Sheikhshoaie, I. Low-temperature synthesis of hetero-structures of magnetically separable iron oxide@ Au-rGO nanocomposite for efficient degradation of organic dye under visible light irradiation. Environ. Res. 2022, 205, 112510. [Google Scholar] [CrossRef]
- Roostaee, M.; Sheikhshoaie, I. Fabrication of a sensitive sensor for determination of xanthine in the presence of uric acid and ascorbic acid by modifying a carbon paste sensor with Fe3O4@ Au core–shell and an ionic liquid. J. Food Meas. Charact. 2022, 16, 731–739. [Google Scholar] [CrossRef]
- Hooshmand, S.; Hayat, S.M.G.; Ghorbani, A.; Khatami, M.; Pakravanan, K.; Darroudi, M. Preparation and Applications of Superparamagnetic Iron Oxide Nanoparticles in Novel Drug Delivery Systems: An Overview Article. Curr. Med. Chem. 2022, 28, 777–799. [Google Scholar] [CrossRef]
- Roostaee, M.; Sheikhshoaie, I. A novel, sensitive and selective nanosensor based on graphene nanoribbon–cobalt ferrite nanocomposite and 1-methyl-3-butylimidazolium bromide for detection of vanillin in real food samples. J. Food Meas. Charact. 2022, 16, 523–532. [Google Scholar] [CrossRef]
- Roostaee, M.; Sheikhshoaei, I.; Karimi-Maleh, H. Fe3O4@ Au-rGO Nanocomposite/Ionic Liquid Modified Sensor for Ultrasensitive and Selective Sensing of Doxorubicin. Top. Catal. 2022, 1–10. [Google Scholar] [CrossRef]
- Ebadian, B.; Fathi, A.; Savoj, M. In Vitro Evaluation of the Effect of Different Luting Cements and Tooth Preparation Angle on the Microleakage of Zirconia Crowns. Int. J. Dent. 2021, 2021, 8461579. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Roostaee, M.; Sheikhshoaie, I. Magnetic nanoparticles; synthesis, properties and electrochemical application: A review. Curr. Biochem. Eng. 2020, 6, 91–102. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly (acrylic acid)-functionalization is superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef]
- Jing, Z.; Xu, A.; Liang, Y.-Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable poly (acrylic acid-co-acrylamide)/poly (vinyl alcohol) double network hydrogels with tunable mechanics and high self-healing performance. Polymers 2019, 11, 952. [Google Scholar] [CrossRef] [Green Version]
- Mutar, M.A.; Kmal, R.K. Preparation of copolymer of acrylamide and acrylic acid and its application for slow release sodium nitrate fertilizer. Al-Qadisiyah J. Pure Sci. 2012, 17, 71–83. [Google Scholar]
- Gao, J.; Penlidis, A. A comprehensive simulator/database package for reviewing free-radical copolymerizations. J. Macromol. Sci. Part C 1998, 38, 651–780. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties-A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Ishizone, T.; Sugiyama, K.; Hirao, A. Anionic Polymerization of Protected Functional Monomers; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Satoh, K.; Kamigaito, M.; Sawamoto, M. Transition Metal Complexes for Metal-Catalyzed Atom Transfer Controlled/Living Radical Polymerization; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Matyjaszewski, K.; Spanswick, J. Copper-Mediated Atom Transfer Radical Polymerization; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kausar, A. Poly (acrylic acid) nanocomposites: Design of advanced materials. J. Plast. Film Sheeting 2020, 36, 409–428. [Google Scholar] [CrossRef]
- Masuda, T.; Takai, M. Structure and properties of thermoresponsive gels formed by RAFT polymerization: Effect of the RAFT agent content. Polym. J. 2020, 52, 1407–1412. [Google Scholar] [CrossRef]
- Chanprapanon, W.; Chitpong, N. Functionalized nonwoven Nylon fabrics for cadmium ion exchange applications. Eng. Appl. Sci. Res. 2020, 47, 222–231. [Google Scholar]
- Chrisostomo, D.A.; Strazzi-Sahyon, H.B.; Briso, A.L.F.; Dos Santos, P.H. Efficacy of Polyacrylic Acid as a Conditioning Agent on the Bond Strength of Self-adhesive Resin Cements to Dental Enamel. Oral Health Prev. Dent. 2020, 18, 747–756. [Google Scholar]
- Jing, X.; Feng, P.; Chen, Z.; Xie, Z.; Li, H.; Peng, X.-F.; Mi, H.-Y.; Liu, Y. Highly Stretchable, Self-Healable, Freezing-Tolerant, and Transparent Polyacrylic Acid/Nanochitin Composite Hydrogel for Self-Powered Multifunctional Sensors. ACS Sustain. Chem. Eng. 2021, 9, 9209–9220. [Google Scholar] [CrossRef]
- Schilli, C.M.; Zhang, M.; Rizzardo, E.; Thang, S.H.; Chong, Y.; Edwards, K.; Karlsson, G.; Müller, A.H. A new double-responsive block copolymer synthesized via RAFT polymerization: Poly (N-isopropylacrylamide)-b lock-poly (acrylic acid). Macromolecules 2004, 37, 7861–7866. [Google Scholar] [CrossRef]
- Yigit, F.; Güven, O. A kinetic investigation of radiation induced bulk polymerization of acrylic acid. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1989, 33, 97–101. [Google Scholar] [CrossRef]
- Kahveci, M.; Yagci, Y.; Avgeropoulos, A.; Tsitsilianis, C. Well-Defined Block Copolymers; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Patten, T.E.; Matyjaszewski, K. Atom transfer radical polymerization and the synthesis of polymeric materials. Adv. Mater. 1998, 10, 901–915. [Google Scholar] [CrossRef]
- Couvreur, L.; Lefay, C.; Belleney, J.; Charleux, B.; Guerret, O.; Magnet, S. First Nitroxide-Mediated Controlled Free-Radical Polymerization of Acrylic Acid. Macromolecules 2003, 36, 8260. [Google Scholar] [CrossRef]
- Ladavière, C.; Dörr, N.; Claverie, J.P. Controlled radical polymerization of acrylic acid in protic media. Macromolecules 2001, 34, 5370–5372. [Google Scholar] [CrossRef]
- Sütekin, S.D.; Güven, O. Preparation of poly (tert-butyl acrylate)-poly (acrylic acid) amphiphilic copolymers via radiation-induced reversible addition–fragmentation chain transfer mediated polymerization of tert-butyl acrylate. Polym. Int. 2020, 69, 693–701. [Google Scholar] [CrossRef]
- Yan, L.; Ji, J.; Xie, D.; Li, W.; Zhang, G. Surfactant-free synthesis of amphiphilic copolymer of poly (styrene-co-acrylamide) in aqueous emulsion with the assistance of ultrasound. Polym. Adv. Technol. 2008, 19, 221–228. [Google Scholar] [CrossRef]
- Loiseau, J.; Doërr, N.; Suau, J.; Egraz, J.; Llauro, M.; Ladavière, C.; Claverie, J. Synthesis and characterization of poly (acrylic acid) produced by RAFT polymerization. Application as a very efficient dispersant of CaCO3, kaolin, and TiO2. Macromolecules 2003, 36, 3066–3077. [Google Scholar] [CrossRef]
- Millard, P.E.; Barner, L.; Stenzel, M.H.; Davis, T.P.; Barner-Kowollik, C.; Müller, A.H. RAFT Polymerization of N-Isopropylacrylamide and Acrylic Acid under γ-Irradiation in Aqueous Media. Macromol. Rapid Commun. 2006, 27, 821–828. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Hassan, S.; Yasin, T. Synthesis of radiation crosslinked poly (acrylic acid) in the presence of phenyltriethoxysilane. Radiat. Phys. Chem. 2014, 97, 292–297. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Buback, M.; Charleux, B.; Coote, M.L.; Drache, M.; Fukuda, T.; Goto, A.; Klumperman, B.; Lowe, A.B.; Mcleary, J.B. Mechanism and kinetics of dithiobenzoate-mediated RAFT polymerization. I. The current situation. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5809–5831. [Google Scholar] [CrossRef]
- Ji, J.; Jia, L.; Yan, L.; Bangal, P.R. Efficient synthesis of poly (acrylic acid) in aqueous solution via a RAFT process. J. Macromol. Sci. Part A Pure Appl. Chem. 2010, 47, 445–451. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Z.; Liu, Y.; Yin, Z.-Z.; Sheng, Y.; Ding, C.; Kong, Y. Facile synthesis of calcium carbonate/polyacrylic acid hydrogels for pH-responsive delivery of cytarabine. J. Saudi Chem. Soc. 2021, 25, 101344. [Google Scholar] [CrossRef]
- Xiong, J.; Dupré, N.; Mazouzi, D.; Guyomard, D.; Roué, L.; Lestriez, B. Influence of the polyacrylic acid binder neutralization degree on the initial electrochemical behavior of a silicon/graphite electrode. ACS Appl. Mater. Interfaces 2021, 13, 28304–28323. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.B.; Darabonia, N.; Madras, G. Ultrasonic degradation of poly (acrylic acid). J. Appl. Polym. Sci. 2009, 112, 991–997. [Google Scholar] [CrossRef]
- Kadlubowski, S.; Grobelny, J.; Olejniczak, W.; Cichomski, M.; Ulanski, P. Pulses of fast electrons as a tool to synthesize poly (acrylic acid) nanogels. Intramolecular cross-linking of linear polymer chains in additive-free aqueous solution. Macromolecules 2003, 36, 2484–2492. [Google Scholar] [CrossRef]
- Kumar, A.; Maity, D.; Vyas, G.; Bhatt, M.; Bhatt, S.; Paul, P. Polyacrylic acid@ zeolitic imidazolate framework-8 nanoparticles for detection and absorptive removal of cyanide from aqueous media with high efficiency. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126358. [Google Scholar] [CrossRef]
- Laraib, U.; Sargazi, S.; Rahdar, A.; Khatami, M.; Pandey, S. Nanotechnology-based approaches for effective detection of tumor markers: A comprehensive state-of-the-art review. Int. J. Biol. Macromol. 2021, 195, 356–383. [Google Scholar] [CrossRef]
- De la Garza, D.; De Santiago, F.; Materon, L.; Chipara, M.; Alcoutlabi, M. Fabrication and characterization of centrifugally spun poly (acrylic acid) nanofibers. J. Appl. Polym. Sci. 2019, 136, 47480. [Google Scholar] [CrossRef]
- Jin, X.; Hsieh, Y.-L. pH-responsive swelling behavior of poly (vinyl alcohol)/poly (acrylic acid) bi-component fibrous hydrogel membranes. Polymer 2005, 46, 5149–5160. [Google Scholar] [CrossRef]
- Santiago-Morales, J.; Amariei, G.; Letón, P.; Rosal, R. Antimicrobial activity of poly (vinyl alcohol)-poly (acrylic acid) electrospun nanofibers. Colloids Surf. B Biointerfaces 2016, 146, 144–151. [Google Scholar] [CrossRef]
- Duan, Z.; Yin, Q.; Li, C.; Dong, L.; Bai, X.; Zhang, Y.; Yang, M.; Jia, D.; Li, R.; Liu, Z. Milling force and surface morphology of 45 steel under different Al 2 O 3 nanofluid concentrations. Int. J. Adv. Manuf. Technol. 2020, 107, 1277–1296. [Google Scholar] [CrossRef]
- Greindl, M.; Bernkop-Schnürch, A. Development of a novel method for the preparation of thiolated polyacrylic acid nanoparticles. Pharm. Res. 2006, 23, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Molnar, R.M.; Bodnar, M.; Hartmann, J.F.; Borbely, J. Preparation and characterization of poly (acrylic acid)-based nanoparticles. Colloid Polym. Sci. 2009, 287, 739–744. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Schrinner, M.; Ballauff, M.; Möller, M.W.; Breu, J. In situ formation of Ag nanoparticles in spherical polyacrylic acid brushes by UV irradiation. J. Phys. Chem. C 2007, 111, 7676–7681. [Google Scholar] [CrossRef]
- Müller, C.; Leithner, K.; Hauptstein, S.; Hintzen, F.; Salvenmoser, W.; Bernkop-Schnürch, A. Preparation and characterization of mucus-penetrating papain/poly (acrylic acid) nanoparticles for oral drug delivery applications. J. Nanopart. Res. 2013, 15, 1353. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.; Toth, I.; Monteiro, M.J.; Minchin, R.F. Molecular interaction of poly (acrylic acid) gold nanoparticles with human fibrinogen. ACS Nano 2012, 6, 8962–8969. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic acid-coated iron oxide magnetic nanoparticles: The polymer molecular weight influence. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Jiang, X.; Ding, Y.; Ge, H.; Yuan, Y.; Yang, C. Synthesis and characterization of chitosan–poly (acrylic acid) nanoparticles. Biomaterials 2002, 23, 3193–3201. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Wu, S.-Y.; Wu, T.; Chang, Y.-J.; Hua, M.-Y.; Chen, J.-P. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials 2009, 30, 3343–3351. [Google Scholar] [CrossRef]
- Mahdavian, A.R.; Mirrahimi, M.A.-S. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 2010, 159, 264–271. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Vilas-Boas, V.; Dias, I.; Porto, G.; Lopez-Quintela, M.A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E. Interaction of polyacrylic acid coated and non-coated iron oxide nanoparticles with human neutrophils. Toxicol. Lett. 2014, 225, 57–65. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Pinheiro, A.C.; Silva, H.D.; Ramos, P.E.; Azevedo, M.A.; Flores-López, M.L.; Rivera, M.C.; Bourbon, A.I.; Ramos, Ó.L.; Vicente, A.A. Design of bio-nanosystems for oral delivery of functional compounds. Food Eng. Rev. 2014, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hsu, B.Y.W.; Ren, C.; Li, X.; Wang, J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Mansour, O.; Carbonnier, B. Promising sub-100 nm tailor made hollow chitosan/poly (acrylic acid) nanocapsules for antibiotic therapy. J. Colloid Interface Sci. 2018, 522, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Chen, Y.; Li, R.; Wang, J.; Liu, M.; Wang, C.; Chu, F. Polymeric hydrogel nanocapsules: A thermo and pH Dual-responsive carrier for sustained drug release. Nano-Micro Lett. 2014, 6, 200–208. [Google Scholar] [CrossRef]
- Wang, R.M.; Li, G.; Zhang, H.F.; He, Y.F.; He, N.P.; Lei, Z. Preparation of albumin—PAA nanocapsules and their controlled release behavior for drugs. Polym. Adv. Technol. 2010, 21, 685–690. [Google Scholar] [CrossRef]
- Aqil, A.; Detrembleur, C.; Gilbert, B.; Jérôme, R.; Jérôme, C. Controlled RAFT synthesis of polyacrylonitrile-b-poly (acrylic acid) diblocks as precursors of carbon nanocapsules with assistance of gold nanoparticles. Chem. Mater. 2007, 19, 2150–2154. [Google Scholar] [CrossRef]
- Scarioti, G.D.; Lubambo, A.; Feitosa, J.P.; Sierakowski, M.R.; Bresolin, T.M.; de Freitas, R.A. Nanocapsule of cationic liposomes obtained using “in situ” acrylic acid polymerization: Stability, surface charge and biocompatibility. Colloids Surf. B Biointerfaces 2011, 87, 267–272. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Yan, Y.-Z.; Nagappan, S.; He, S.; Ha, C.-S.; Jin, Y.-S. Dual (thermo-/pH-) responsive P (NIPAM-co-AA-co-HEMA) nanocapsules for controlled release of 5-fluorouracil. J. Macromol. Sci. Part A 2021, 58, 860–871. [Google Scholar] [CrossRef]
- Perkin, K.K.; Turner, J.L.; Wooley, K.L.; Mann, S. Fabrication of hybrid nanocapsules by calcium phosphate mineralization of shell cross-linked polymer micelles and nanocages. Nano Lett. 2005, 5, 1457–1461. [Google Scholar] [CrossRef]
- Kurkuri, M.D.; Aminabhavi, T.M. Poly (vinyl alcohol) and poly (acrylic acid) sequential interpenetrating network pH-sensitive microspheres for the delivery of diclofenac sodium to the intestine. J. Control. Release 2004, 96, 9–20. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef] [PubMed]
- He, W.-D.; Sun, X.-L.; Wan, W.-M.; Pan, C.-Y. Multiple morphologies of PAA-b-PSt assemblies throughout RAFT dispersion polymerization of styrene with PAA macro-CTA. Macromolecules 2011, 44, 3358–3365. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile polydopamine platforms: Synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R.; Jurga-Stopa, J.; Markiewicz, R.; Coy, E.L.; Jurga, S.; Woźniak, A. Assessment of polydopamine coated magnetic nanoparticles in doxorubicin delivery. RSC Adv. 2016, 6, 5936–5943. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Porto, G.; Lopez-Quintela, M.A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E. Polyacrylic acid-coated and non-coated iron oxide nanoparticles induce cytokine activation in human blood cells through TAK1, p38 MAPK and JNK pro-inflammatory pathways. Arch. Toxicol. 2015, 89, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-L.; Lo, Y.-L.; Chen, H.-Y.; Wang, L.-F. Hybrid polyethylenimine and polyacrylic acid-bound iron oxide as a magnetoplex for gene delivery. Langmuir 2012, 28, 3542–3552. [Google Scholar] [CrossRef]
- González-Gómez, M.A.; Belderbos, S.; Yañez-Vilar, S.; Piñeiro, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of superparamagnetic nanoparticles coated with polyacrylic acid and aluminum hydroxide as an efficient contrast agent for multimodal imaging. Nanomaterials 2019, 9, 1626. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhuang, L.; Lin, Y.; Yan, M.; Lv, J.; Li, X.; Lin, H.; Zhu, P.; Lin, Q.; Xu, Y. Novel drug delivery system based on hollow mesoporous magnetic nanoparticles for head and neck cancers--targeted therapy in vitro and in vivo. Am. J. Cancer Res. 2020, 10, 350. [Google Scholar]
- Rodrigues, D.; Freitas, M.; Marisa Costa, V.; Arturo Lopez-Quintela, M.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E.; Silva, P. Quantitative histochemistry for macrophage biodistribution on mice liver and spleen after the administration of a pharmacological-relevant dose of polyacrylic acid-coated iron oxide nanoparticles. Nanotoxicology 2017, 11, 256–266. [Google Scholar] [CrossRef]
- Arkaban, H.; Ebrahimi, A.K.; Yarahmadi, A.; Zarrintaj, P.; Barani, M. Development of a multifunctional system based on CoFe2O4@ polyacrylic acid NPs conjugated to folic acid and loaded with doxorubicin for cancer theranostics. Nanotechnology 2021, 32, 305101. [Google Scholar] [CrossRef]
- Foroushani, M.S.; Niroumand, N.; Shervedani, R.K.; Yaghoobi, F.; Kefayat, A.; Torabi, M. A theranostic system based on nanocomposites of manganese oxide nanoparticles and a pH sensitive polymer: Preparation, and physicochemical characterization. Bioelectrochemistry 2019, 130, 107347. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Shervedani, R.K.; Yaghoobi, F.; Kefayat, A. A nanocomposite theranostic system, consisting of AuNPs@ MnCO3/Mn3O4 coated with PAA and integrated with folic acid, doxorubicin, and propidium iodide: Synthesis, characterization and examination for capturing of cancer cells. Inorg. Chem. Commun. 2021, 128, 108566. [Google Scholar] [CrossRef]
- Cho, M.H.; Choi, E.-S.; Kim, S.; Goh, S.-H.; Choi, Y. Redox-responsive manganese dioxide nanoparticles for enhanced MR imaging and radiotherapy of lung cancer. Front. Chem. 2017, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Mehboob, H.; Abd Razak, S.I.; Yahya, M.Y.; Mohd Yusof, A.H.; Ramlee, M.H.; Sahaya Anand, T.J.; Hassan, R.; Aziz, A.; Amin, R. Development of polymeric nanocomposite (xyloglucan-co-methacrylic acid/hydroxyapatite/sio2) scaffold for bone tissue engineering applications—In-vitro antibacterial, cytotoxicity and cell culture evaluation. Polymers 2020, 12, 1238. [Google Scholar] [CrossRef]
- Pan, S.; Xing, H.; Fu, X.; Yu, H.; Yang, Z.; Yang, Y.; Sun, W. The effect of photothermal therapy on osteosarcoma with polyacrylic acid–coated gold nanorods. Dose-Response 2018, 16, 1559325818789841. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Zhang, L.; Zhang, M.; Li, S.; Li, L.; Wang, T.; Wang, C. Facile approach to synthesize gold nanorod@ polyacrylic acid/calcium phosphate yolk–shell nanoparticles for dual-mode imaging and pH/NIR-responsive drug delivery. Nano-Micro Lett. 2018, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, L.; Zhang, L.; Wang, T.; Wang, C.; Su, Z. Multifunctional spherical gold nanocluster aggregate@ polyacrylic acid@ mesoporous silica nanoparticles for combined cancer dual-modal imaging and chemo-therapy. J. Mater. Chem. B 2015, 3, 2421–2425. [Google Scholar] [CrossRef]
- Tian, C.; Zhu, L.; Lin, F.; Boyes, S.G. Poly (acrylic acid) bridged gadolinium metal–organic framework–gold nanoparticle composites as contrast agents for computed tomography and magnetic resonance bimodal imaging. ACS Appl. Mater. Interfaces 2015, 7, 17765–17775. [Google Scholar] [CrossRef]
- Mazloomi-Rezvani, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Fabricating core (Au)-shell (different stimuli-responsive polymers) nanoparticles via inverse emulsion polymerization: Comparing DOX release behavior in dark room and under NIR lighting. Colloids Surf. B Biointerfaces 2018, 166, 144–151. [Google Scholar] [CrossRef]
- Song, C.; Dou, Y.; Yuwen, L.; Sun, Y.; Dong, C.; Li, F.; Yang, Y.; Wang, L. A gold nanoflower-based traceable drug delivery system for intracellular SERS imaging-guided targeted chemo-phototherapy. J. Mater. Chem. B 2018, 6, 3030–3039. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Zhang, L.; Wang, T.; Yu, H.; Wang, C.; Su, Z. Multifunctional magnetic–fluorescent eccentric-(concentric-Fe3O4@ SiO2)@ polyacrylic acid core–shell nanocomposites for cell imaging and pH-responsive drug delivery. Nanoscale 2013, 5, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Fu, Y.; Gu, T.; Li, Y.; Liu, H.; Ren, Z.; Li, X.; Han, G. Fibrous CaF2: Yb, Er@ SiO2-PAA ‘tumor patch’with NIR-triggered and trackable DOX release. Mater. Des. 2017, 119, 85–92. [Google Scholar] [CrossRef]
- Alahri, M.B.; Arshadizadeh, R.; Raeisi, M.; Khatami, M.; Sajadi, M.S.; Abdelbasset, W.K.; Akhmadeev, R.; Iravani, S. Theranostic applications of metal–organic frameworks (MOFs)-based materials in brain disorders: Recent advances and challenges. Inorg. Chem. Commun. 2021, 134, 108997. [Google Scholar] [CrossRef]
- Bazzazzadeh, A.; Dizaji, B.F.; Kianinejad, N.; Nouri, A.; Irani, M. Fabrication of poly (acrylic acid) grafted-chitosan/polyurethane/magnetic MIL-53 metal organic framework composite core-shell nanofibers for co-delivery of temozolomide and paclitaxel against glioblastoma cancer cells. Int. J. Pharm. 2020, 587, 119674. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.A.; Lee, S.-W. pH-triggered degradation and release of doxorubicin from zeolitic imidazolate framework-8 (ZIF8) decorated with polyacrylic acid. RSC Adv. 2021, 11, 9222–9234. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Sharifi, M.; Torkamani, H.; Vancaeyzeele, C. Synthesis of magnetic multi walled carbon nanotubes hydrogel nanocomposite based on poly (acrylic acid) grafted onto salep and its application in the drug delivery of tetracyceline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126350. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, Y.; Liu, B. Polyacrylic acid functionalized nanographene as a nanocarrier for loading and controlled release of doxorubicin hydrochloride. J. Nanomater. 2013, 2013, 345738. [Google Scholar] [CrossRef] [Green Version]
- Sgarlata, C.; D’Urso, L.; Consiglio, G.; Grasso, G.; Satriano, C.; Forte, G. pH sensitive functionalized graphene oxide as a carrier for delivering Gemcitabine: A computational approach. Comput. Theor. Chem. 2016, 1096, 1–6. [Google Scholar] [CrossRef]
- Ghazanfari, A.; Marasini, S.; Miao, X.; Park, J.A.; Jung, K.-H.; Ahmad, M.Y.; Yue, H.; Ho, S.L.; Liu, S.; Jang, Y.J. Synthesis, characterization, and X-ray attenuation properties of polyacrylic acid-coated ultrasmall heavy metal oxide (Bi2O3, Yb2O3, NaTaO3, Dy2O3, and Gd2O3) nanoparticles as potential CT contrast agents. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 73–81. [Google Scholar] [CrossRef]
- Jia, X.; Yin, J.; He, D.; He, X.; Wang, K.; Chen, M.; Li, Y. Polyacrylic acid modified upconversion nanoparticles for simultaneous pH-triggered drug delivery and release imaging. J. Biomed. Nanotechnol. 2013, 9, 2063–2072. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; You, M.; Wang, S.; Dong, Y.; Jin, G.; Lin, M.; Wang, Q.; Li, A.; Zhang, X. Labeling and long-term tracking of bone marrow mesenchymal stem cells in vitro using NaYF4: Yb3+, Er3+ upconversion nanoparticles. Acta Biomater. 2016, 42, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Chen, H.; Miao, Z.; He, G.; Zhou, J.; Zha, Z. Polyacrylic acid functionalized Co0. 85Se nanoparticles: An ultrasmall pH-responsive nanocarrier for synergistic photothermal-chemo treatment of cancer. ACS Biomater. Sci. Eng. 2018, 4, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, L.; Zhang, L.; Wang, T.; Wang, C.; Zhu, D.; Su, Z. Designed preparation of polyacrylic acid/calcium carbonate nanoparticles with high doxorubicin payload for liver cancer chemotherapy. CrystEngComm 2015, 17, 4768–4773. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, Y.; Li, J.; Liu, X.; He, S. Poly (Acrylic Acid)-Modified MoS2 Nanoparticle-Based Transdermal Delivery of Atenolol. Int. J. Nanomed. 2020, 15, 5517. [Google Scholar] [CrossRef] [PubMed]
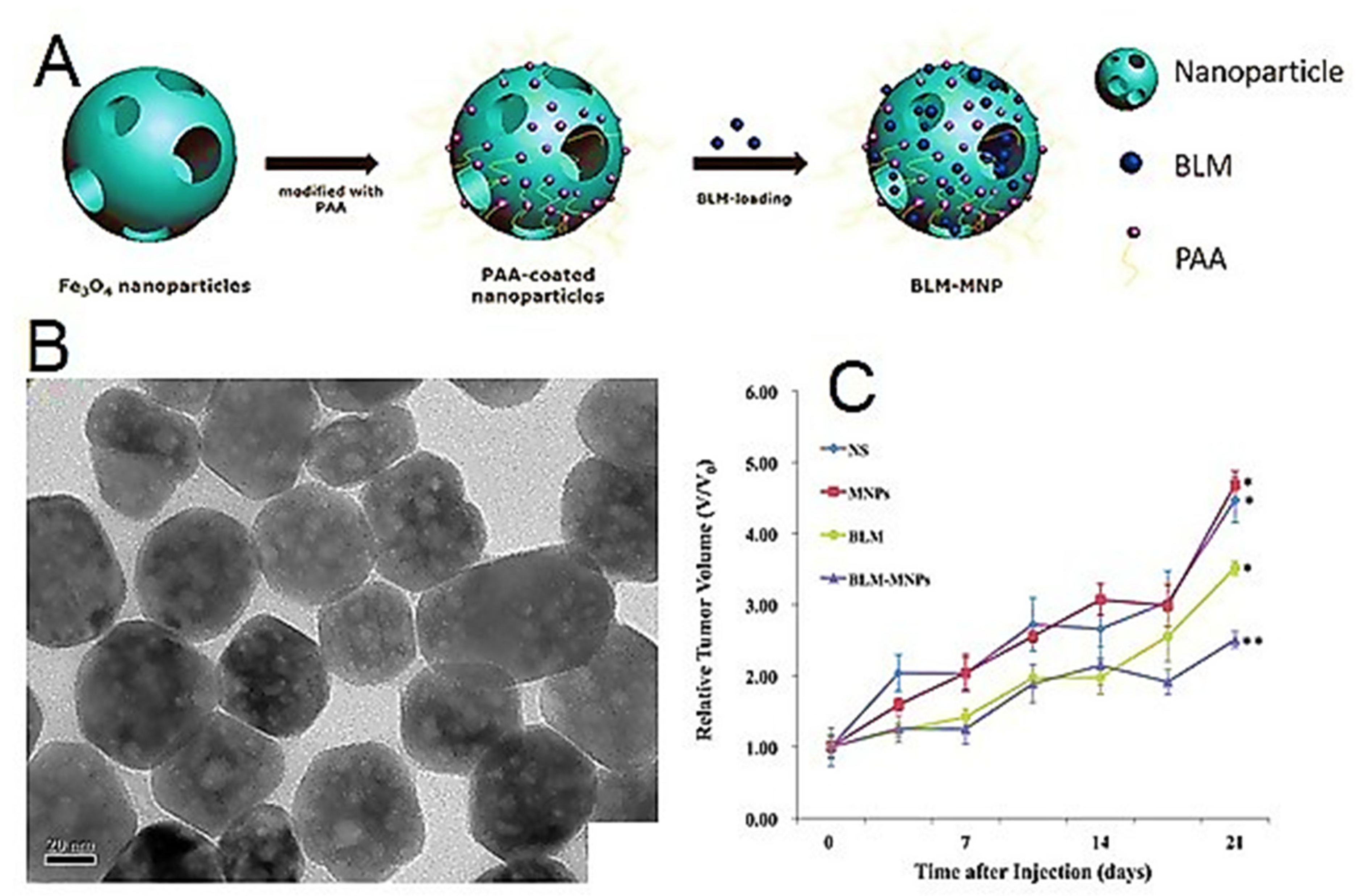
- Xu, Y.; Lin, Y.; Zhuang, L.; Lin, J.; Lv, J.; Huang, Q.; Sun, J. Bleomycin loaded magnetite nanoparticles functionalized by polyacrylic acid as a new antitumoral drug delivery system. BioMed Res. Int. 2013, 2013, 462589. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, C.; Luo, R.; Liu, H.; Zhang, Z.; Xu, T.; Zhang, Y.; Wang, D. Noninvasive monitoring of early antiangiogenic therapy response in human nasopharyngeal carcinoma xenograft model using MRI with RGD-conjugated ultrasmall superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 5671. [Google Scholar] [CrossRef] [Green Version]
- Giraldo-Villegas, M.; Urquijo, J.; Arnache-Olmos, O.L.; Rojas-López, M. Polyacrylic acid-coated iron oxide nanoparticles could be a useful tool for tracking inflammatory monocytes. Future Sci. OA 2019, 5, FSO423. [Google Scholar] [CrossRef] [Green Version]
- Padwal, P.; Bandyopadhyaya, R.; Mehra, S. Polyacrylic acid-coated iron oxide nanoparticles for targeting drug resistance in mycobacteria. Langmuir 2014, 30, 15266–15276. [Google Scholar] [CrossRef]
- Lojk, J.; Bregar, V.B.; Rajh, M.; Miš, K.; Kreft, M.E.; Pirkmajer, S.; Veranič, P.; Pavlin, M. Cell type-specific response to high intracellular loading of polyacrylic acid-coated magnetic nanoparticles. Int. J. Nanomed. 2015, 10, 1449. [Google Scholar]
- Bian, R.; Wang, T.; Zhang, L.; Li, L.; Wang, C. A combination of tri-modal cancer imaging and in vivo drug delivery by metal–organic framework based composite nanoparticles. Biomater. Sci. 2015, 3, 1270–1278. [Google Scholar] [CrossRef]
- Kang, X.J.; Dai, Y.L.; Ma, P.A.; Yang, D.M.; Li, C.X.; Hou, Z.Y.; Cheng, Z.Y.; Lin, J. Poly (acrylic acid)-modified Fe3O4 microspheres for magnetic-targeted and ph-triggered anticancer drug delivery. Chem. Eur. J. 2012, 18, 15676–15682. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, L.; Zhang, L.; Xing, S.; Wang, T.; Wang, Y.A.; Wang, C.; Su, Z. Designed fabrication of unique eccentric mesoporous silica nanocluster-based core–shell nanostructures for pH-responsive drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 7282–7290. [Google Scholar] [CrossRef]
- Esfahanian, M.; Ghasemzadeh, M.A.; Razavian, S.M.H. Synthesis, identification and application of the novel metal-organic framework Fe3O4@ PAA@ ZIF-8 for the drug delivery of ciprofloxacin and investigation of antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2024–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Nan, J.; Lu, Y.; Wang, C.; Chu, F.; Gu, Z. Hybrid Fe3O4-poly (acrylic acid) nanogels for theranostic cancer treatment. J. Biomed. Nanotechnol. 2015, 11, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Moscoso-Londoño, O.; Gonzalez, J.S.; Muraca, D.; Hoppe, C.E.; Alvarez, V.A.; López-Quintela, A.; Socolovsky, L.M.; Pirota, K. Structural and magnetic behavior of ferrogels obtained by freezing thawing of polyvinyl alcohol/poly (acrylic acid)(PAA)-coated iron oxide nanoparticles. Eur. Polym. J. 2013, 49, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- Iversen, N.K.; Frische, S.; Thomsen, K.; Laustsen, C.; Pedersen, M.; Hansen, P.B.; Bie, P.; Fresnais, J.; Berret, J.-F.; Baatrup, E. Superparamagnetic iron oxide polyacrylic acid coated γ-Fe2O3 nanoparticles do not affect kidney function but cause acute effect on the cardiovascular function in healthy mice. Toxicol. Appl. Pharmacol. 2013, 266, 276–288. [Google Scholar] [CrossRef] [Green Version]
- Surendra, M.K.; Annapoorani, S.; Ansar, E.B.; Varma, P.H.; Rao, M.R. Magnetic hyperthermia studies on water-soluble polyacrylic acid-coated cobalt ferrite nanoparticles. J. Nanopart. Res. 2014, 16, 2773. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; Alshahrani, T.; Ibrahim, E.H.; Kilany, M.; Ahmad, Z.; Manthrammel, M.A.; AlFaify, S.; Kateb, B.; Kaushik, A. One-spot fabrication and in-vivo toxicity evaluation of core-shell magnetic nanoparticles. Mater. Sci. Eng. C 2021, 122, 111898. [Google Scholar] [CrossRef]
- Irfan, M.; Dogan, N.; Bingolbali, A.; Aliew, F. Synthesis and characterization of NiFe2O4 magnetic nanoparticles with different coating materials for magnetic particle imaging (MPI). J. Magn. Magn. Mater. 2021, 537, 168150. [Google Scholar] [CrossRef]
- Foroushani, M.S.; Zahmatkeshan, A.; Arkaban, H.; Shervedani, R.K.; Kefayat, A. A drug delivery system based on nanocomposites constructed from metal-organic frameworks and Mn3O4 nanoparticles: Preparation and physicochemical characterization for BT-474 and MCF-7 cancer cells. Colloids Surf. B Biointerfaces 2021, 202, 111712. [Google Scholar] [CrossRef] [PubMed]
- Talusan, T.J.E.; Baltazar, M.C.P.; Usman, K.A.S.; Payawan, L.M., Jr. Synthesis of glucose-sensitive microcapsules via layer-by-layer assembly for controlled insulin release applications. Appl. Mech. Mater. 2017, 863, 84–88. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Y.; Xue, Z.; Zeng, S. Tumor microenvironment responsive hollow mesoporous Co9S8@ MnO2-ICG/DOX intelligent nanoplatform for synergistically enhanced tumor multimodal therapy. Biomaterials 2020, 262, 120346. [Google Scholar] [CrossRef] [PubMed]
- Jian-Hua, L.; Lei, W.; ZHANG, T.-Q.; Jian-Qiu, W.; Xue, G.; Feng-Zhi, C.; ZHENG, J.-J.; Bo, L.; Zhan, S. Facile Synthesis of Biocompatible Fe3O4-Based Nanoparticles for pH-Responsive Dual-Model Magnetic Resonance Imaging-Guided Tumour Eradication by Photothermal Therapy. Chin. J. Anal. Chem. 2019, 47, 678–685. [Google Scholar]
- Zhang, Q.; Wang, W.; Zhang, M.; Wu, F.; Zheng, T.; Sheng, B.; Liu, Y.; Shen, J.; Zhou, N.; Sun, Y. A theranostic nanocomposite with integrated black phosphorus nanosheet, Fe3O4@ MnO2-doped upconversion nanoparticles and chlorin for simultaneous multimodal imaging, highly efficient photodynamic and photothermal therapy. Chem. Eng. J. 2020, 391, 123525. [Google Scholar] [CrossRef]
- Javakhishvili, I.; Hvilsted, S. Gold Nanoparticles Protected with Thiol-Derivatized Amphiphilic Poly (ϵ-caprolactone)-b-poly (acrylic acid). Biomacromolecules 2009, 10, 74–81. [Google Scholar] [CrossRef]
- Lin, I.-C.; Liang, M.; Liu, T.-Y.; Ziora, Z.M.; Monteiro, M.J.; Toth, I. Interaction of densely polymer-coated gold nanoparticles with epithelial Caco-2 monolayers. Biomacromolecules 2011, 12, 1339–1348. [Google Scholar] [CrossRef]
- Mahmoodzadeh, F.; Abbasian, M.; Jaymand, M.; Salehi, R.; Bagherzadeh-Khajehmarjan, E. A novel gold-based stimuli-responsive theranostic nanomedicine for chemo-photothermal therapy of solid tumors. Mater. Sci. Eng. C 2018, 93, 880–889. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Mizani, F.; Hosseini, S.S. pH sensitive release of doxorubicin anticancer drug from gold nanocomposite hydrogel based on poly (acrylic acid) grafted onto salep biopolymer. J. Polym. Res. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Khan, I.M.; Niazi, S.; Yu, Y.; Pasha, I.; Yue, L.; Mohsin, A.; Shoaib, M.; Iqbal, M.W.; Khaliq, A.; Wang, Z. Fabrication of PAA coated green-emitting AuNCs for construction of label-free FRET assembly for specific recognition of T-2 toxin. Sens. Actuators B Chem. 2020, 321, 128470. [Google Scholar] [CrossRef]
- Wang, L.; Pei, J.; Cong, Z.; Zou, Y.; Sun, T.; Davitt, F.; Garcia-Gil, A.; Holmes, J.D.; O’Driscoll, C.M.; Rahme, K. Development of anisamide-targeted PEGylated gold nanorods to deliver epirubicin for chemo-photothermal therapy in tumor-bearing mice. Int. J. Nanomed. 2019, 14, 1817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jin, X.Q.; Sun, J.H.; Bai, S.Y.; Wu, X. PAA-grafted surface and fractal feature of dense nanosilica spheres for ibuprofen delivery. Mater. Chem. Phys. 2017, 195, 213–223. [Google Scholar] [CrossRef]
- Tian, F.; Kaňka, J.; Yang, F.; Min, J.; Hammond, P.T. Role of silica nanoparticles in monitoring and prolonging release of drug-eluting polyelectrolyte coatings using long-period fiber grating platform. Sens. Actuators B Chem. 2017, 252, 831–839. [Google Scholar] [CrossRef]
- Runowski, M.; Lis, S. Synthesis, surface modification/decoration of luminescent–magnetic core/shell nanomaterials, based on the lanthanide doped fluorides (Fe3O4/SiO2/NH2/PAA/LnF3). J. Lumin. 2016, 170, 484–490. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Li, G.; Wang, F.; Wang, W. Synthesis of hollow α-Fe2O3–silica composites templated by poly (acrylic acid) colloidal aggregates. J. Coat. Technol. Res. 2014, 11, 595–600. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Q.; Sun, J.; Panezai, H.; Bai, S.; Wu, X. Dual (pH-and temperature-) stimuli responsive nanocarrier with bimodal mesoporous silica nanoparticles core and copolymer shell for controlled ibuprofen-releasing: Fractal feature and diffusion mechanism. Microporous Mesoporous Mater. 2017, 254, 77–85. [Google Scholar] [CrossRef]
- Cabana, S.; Lecona-Vargas, C.S.; Meléndez-Ortiz, H.I.; Contreras-García, A.; Barbosa, S.; Taboada, P.; Magarinos, B.; Bucio, E.; Concheiro, A.; Alvarez-Lorenzo, C. Silicone rubber films functionalized with poly (acrylic acid) nanobrushes for immobilization of gold nanoparticles and photothermal therapy. J. Drug Deliv. Sci. Technol. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Han, J.-H.; Jung, S.-W. Encapsulating metal-organic frameworks (MOFs) with calcium phosphate (CaP) for pH-responsive drug delivery. Korea Ind. Chem. Soc. 2019, 2019, 194. [Google Scholar]
- Wu, L.; Hu, Y.; Tang, P.; Wang, H.; Bin, Y. High stretchable, pH-sensitive and self-adhesive rGO/CMCNa/PAA composite conductive hydrogel with good strain-sensing performance. Compos. Commun. 2021, 24, 100669. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Wang, R.; Zhou, X. A split aptamer (SPA)-based sandwich-type biosensor for facile and rapid detection of streptomycin. J. Hazard. Mater. 2021, 403, 123941. [Google Scholar] [CrossRef]
- Ortega, V.A.; Cameron, M.S.; Stafford, J.L.; Goss, G.G.; Donald, J.A.; Schultz, A.G. Polyacrylic acid coated nanoparticles elicit endothelial cell apoptosis and diminish vascular relaxation in ex vivo perfused iliac arteries of the cane toad (Rhinella marina). Environ. Sci. Nano 2020, 7, 1912–1926. [Google Scholar] [CrossRef]
- Morita, K.; Suzuki, T.; Nishimura, Y.; Matsumoto, K.; Numako, C.; Sato, K.; Nakayama, M.; Sasaki, R.; Ogino, C.; Kondo, A. In vivo tissue distribution and safety of polyacrylic acid-modified titanium peroxide nanoparticles as novel radiosensitizers. J. Biosci. Bioeng. 2018, 126, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.; El-Kemary, M.; Leporatti, S. Reduction diameter of CaCO3 crystals by using poly acrylic acid might improve cellular uptake of encapsulated curcumin in breast cancer. J. Nanomed. Res 2018, 7, 235–239. [Google Scholar]
- Baldim, V.; Yadav, N.; Bia, N.; Graillot, A.; Loubat, C.; Singh, S.; Karakoti, A.S.; Berret, J.-F. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42056–42066. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-M.; Nazir, U.; Sohail, M.; Selim, M.M.; Lee, J.-R. Enhancement in thermal energy and solute particles using hybrid nanoparticles by engaging activation energy and chemical reaction over a parabolic surface via finite element approach. Fractal Fract. 2021, 5, 119. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Zhang, Y.; Wang, Y.; Jia, D.; Yang, M.; Zhang, N.; Wu, Q.; Han, Z.; Sun, K. Heat transfer performance of MQL grinding with different nanofluids for Ni-based alloys using vegetable oil. J. Clean. Prod. 2017, 154, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhang, Y.; Li, B.; Yang, M.; Zhang, X.; Guo, S.; Liu, G. Experimental evaluation of the lubrication properties of the wheel/workpiece interface in MQL grinding with different nanofluids. Tribol. Int. 2016, 99, 198–210. [Google Scholar] [CrossRef]
- Yang, Y.; Gong, Y.; Li, C.; Wen, X.; Sun, J. Mechanical performance of 316 L stainless steel by hybrid directed energy deposition and thermal milling process. J. Mater. Process. Technol. 2021, 291, 117023. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, F.; Cao, C.; Wang, Q.; Zou, Q. Single-cell RNA analysis reveals the potential risk of organ-specific cell types vulnerable to SARS-CoV-2 infections. Comput. Biol. Med. 2022, 140, 105092. [Google Scholar] [CrossRef]
- Zha, T.-H.; Castillo, O.; Jahanshahi, H.; Yusuf, A.; Alassafi, M.O.; Alsaadi, F.E.; Chu, Y.-M. A fuzzy-based strategy to suppress the novel coronavirus (2019-NCOV) massive outbreak. Appl. Comput. Math. 2021, 20, 160–176. [Google Scholar]
- Nazeer, M.; Hussain, F.; Khan, M.I.; El-Zahar, E.R.; Chu, Y.-M.; Malik, M. Theoretical study of MHD electro-osmotically flow of third-grade fluid in micro channel. Appl. Math. Comput. 2022, 420, 126868. [Google Scholar] [CrossRef]
- Zhao, T.H.; Khan, M.I.; Chu, Y.M. Artificial neural networking (ANN) analysis for heat and entropy generation in flow of non-Newtonian fluid between two rotating disks. Math. Methods Appl. Sci. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Wang, F.; Khan, M.N.; Ahmad, I.; Ahmad, H.; Abu-Zinadah, H.; Chu, Y.-M. Numerical solution of traveling waves in chemical kinetics: Time-fractional fishers equations. Fractals 2022, 1, 2240051. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Zhao, T.-H.; Chu, Y.-M.; Zhang, X.-H. Optimal evaluation of a Toader-type mean by power mean. J. Inequalities Appl. 2015, 2015, 408. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.-M.; Shankaralingappa, B.; Gireesha, B.; Alzahrani, F.; Khan, M.I.; Khan, S.U. Combined impact of Cattaneo-Christov double diffusion and radiative heat flux on bio-convective flow of Maxwell liquid configured by a stretched nano-material surface. Appl. Math. Comput. 2022, 419, 126883. [Google Scholar] [CrossRef]
- Guo, S.; Li, C.; Zhang, Y.; Wang, Y.; Li, B.; Yang, M.; Zhang, X.; Liu, G. Experimental evaluation of the lubrication performance of mixtures of castor oil with other vegetable oils in MQL grinding of nickel-based alloy. J. Clean. Prod. 2017, 140, 1060–1076. [Google Scholar] [CrossRef]
- Lai, W.-F. Development of Hydrogels with Self-Healing Properties for Delivery of Bioactive Agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Liu, X.; Chang, M.; He, B.; Meng, L.; Wang, X.; Sun, R.; Ren, J.; Kong, F. A one-pot strategy for preparation of high-strength carboxymethyl xylan-g-poly (acrylic acid) hydrogels with shape memory property. J. Colloid Interface Sci. 2019, 538, 507–518. [Google Scholar] [CrossRef]
- Posha, B.; Sandhyarani, N.J. Highly sensitive endotoxin detection using a gold nanoparticle loaded layered molybdenum disulfide-polyacrylic acid nanocomposite. Analyst 2020, 145, 3939–3947. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; He, L.; Peng, D.; Yan, F.; Wang, M.; Zhao, J.; Zhang, H.; Fang, S. Feasible electrochemical biosensor based on plasma polymerization-assisted composite of polyacrylic acid and hollow TiO2 spheres for sensitively detecting lysozyme. Biosens. Bioelectron. 2015, 74, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Feng, F.; Ma, Z. Metal ions doped chitosan–poly (acrylic acid) nanospheres: Synthesis and their application in simultaneously electrochemical detection of four markers of pancreatic cancer. Biosens. Bioelectron. 2016, 75, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Zore, O.V.; Thilakarathne, V.K.; Briand, V.A.; Lenehan, P.J.; Lei, Y.; Kasi, R.M.; Kumar, C.V. “Stable-on-the-table” biosensors: Hemoglobin-poly (acrylic acid) nanogel bioelectrodes with high thermal stability and enhanced electroactivity. Sensors 2015, 15, 23868–23885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.C.; Mendez, F.; Serpe, M.J. Detecting solution pH changes using poly (N-isopropylacrylamide)-co-acrylic acid microgel-based etalon modified quartz crystal microbalances. Anal. Chim. Acta 2012, 739, 83–88. [Google Scholar] [CrossRef]
- Akkahat, P.; Mekboonsonglarp, W.; Kiatkamjornwong, S.; Hoven, V.P. Surface-grafted poly (acrylic acid) brushes as a precursor layer for biosensing applications: Effect of graft density and swellability on the detection efficiency. Langmuir 2012, 28, 5302–5311. [Google Scholar] [CrossRef]
- Akkahat, P.; Hoven, V.P. Introducing surface-tethered poly (acrylic acid) brushes as 3D functional thin film for biosensing applications. Colloids Surf. B Biointerfaces 2011, 86, 198–205. [Google Scholar] [CrossRef]
- Yin, Z.; Cui, R.; Liu, Y.; Jiang, L.; Zhu, J.-J. Ultrasensitive electrochemical immunoassay based on cadmium ion-functionalized PSA@ PAA nanospheres. Biosens. Bioelectron. 2010, 25, 1319–1324. [Google Scholar] [CrossRef]
- Liu, A.; Watanabe, T.; Honma, I.; Wang, J.; Zhou, H. Effect of solution pH and ionic strength on the stability of poly (acrylic acid)-encapsulated multiwalled carbon nanotubes aqueous dispersion and its application for NADH sensor. Biosens. Bioelectron. 2006, 22, 694–699. [Google Scholar] [CrossRef]
- Rather, A.; Wani, G.M.; Robbani, I. Multidetector-Row Computed Tomography and Colour Doppler Imaging in the Evaluation of Patients with Extrahepatic Portal Hypertension: A Prospective Study. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 86–93. [Google Scholar]
- Zhao, Y.; Wu, R.; Yu, H.; Li, J.; Liu, L.; Wang, S.; Chen, X.; Chan, T.-W.D. Magnetic solid-phase extraction of sulfonamide antibiotics in water and animal-derived food samples using core-shell magnetite and molybdenum disulfide nanocomposite adsorbent. J. Chromatogr. A 2020, 1610, 460543. [Google Scholar] [CrossRef]
- Nuñez, N.O.; Cussó, F.; Cantelar, E.; Martin-Gracia, B.; de la Fuente, J.M.; Corral, A.; Balcerzyk, M.; Ocaña, M. Bimodal Nd-doped luVo4 nanoprobes functionalized with polyacrilic acid for x-ray computed tomography and NIR luminescent imaging. Nanomaterials 2020, 10, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, O.M.; Okasha, A.; Hosni, H.M.; Ali, A.E.-H. Biocompatible Water Soluble Polyacrylic Acid Coated CdSe/Cu Quantum Dot Conjugates for Biomolecule Detection. J. Fluoresc. 2018, 28, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, N.O.; García, M.; García-Sevillano, J.; Rivera-Fernández, S.; de la Fuente, J.M.; Ocaña, M. One-Step Synthesis and Polyacrylic Acid Functionalization of Multifunctional Europium-Doped NaGdF4 Nanoparticles with Selected Size for Optical and MRI Imaging. Eur. J. Inorg. Chem. 2014, 2014, 6075–6084. [Google Scholar] [CrossRef]
- Escudero, A.; Carrillo-Carrión, C.; Zyuzin, M.V.; Ashraf, S.; Hartmann, R.; Núñez, N.O.; Ocaña, M.; Parak, W.J. Synthesis and functionalization of monodisperse near-ultraviolet and visible excitable multifunctional Eu 3+, Bi 3+: REVO 4 nanophosphors for bioimaging and biosensing applications. Nanoscale 2016, 8, 12221–12236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Z.; Zeng, S.; Hao, J. Non-invasive through-skull brain vascular imaging and small tumor diagnosis based on NIR-II emissive lanthanide nanoprobes beyond 1500 nm. Biomaterials 2018, 171, 153–163. [Google Scholar] [CrossRef]
- Sargazi, G.; Ebrahimi, A.K.; Afzali, D.; Badoei-dalfard, A.; Malekabadi, S.; Karami, Z. Fabrication of PVA/ZnO fibrous composite polymer as a novel sorbent for arsenic removal: Design and a systematic study. Polym. Bull. 2019, 76, 5661–5682. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z. Biological stability of water-based cutting fluids: Progress and application. Chin. J. Mech. Eng. 2022, 35, 3. [Google Scholar] [CrossRef]
- Zhang, Z.; Sui, M.; Li, C.; Zhou, Z.; Liu, B.; Chen, Y.; Said, Z.; Debnath, S.; Sharma, S. Residual stress of MoS2 nano-lubricant grinding cemented carbide. Int. J. Adv. Manuf. Technol. 2021, 1, 1–15. [Google Scholar] [CrossRef]
- Nnaemeka, O.; Phyllis, N.; Chinaza, O. The Use of Herbal Medicines in Pregnancy: A Cross-sectional Analytic Study. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 66–72. [Google Scholar]
- Shangloo, P.; Gupte, B.; Syed, M. Histopathological Effect of Arsenic in Drinking Water on Liver and Kidney of Albino Rat: A Light Microscopic Study. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 166–170. [Google Scholar]
- Mohammadi, L.; Pal, K.; Bilal, M.; Rahdar, A.; Fytianos, G.; Kyzas, G.Z. Green nanoparticles to treat patients with Malaria disease: An overview. J. Mol. Struct. 2021, 1229, 129857. [Google Scholar] [CrossRef]
- Zong, X.; Xiao, X.; Shen, B.; Jiang, Q.; Wang, H.; Lu, Z.; Wang, F.; Jin, M.; Min, J.; Wang, F. The N 6-methyladenosine RNA-binding protein YTHDF1 modulates the translation of TRAF6 to mediate the intestinal immune response. Nucleic Acids Res. 2021, 49, 5537–5552. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Sultana, G.; Khan, R.; Islam, A.; Mahmud, H.; Raihan, S. Study on Mitochondrial ATPase6 Gene Polymorphisms as a Genetic Risk Factor for Breast Cancer in Bangladeshi Women. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 18–22. [Google Scholar]
- Jamali, S.; Kasraei, E.; Rezazadeh Kalashami, A.; Barri Dizaj, M. Strategy for Treating the Gastric Cancer: A Systematic Review and Meta-analysis. Int. J. Sci. Res. Dent. Med. Sci. 2020, 2, 6–11. [Google Scholar]
- Jamali, S.; Marcella, C.; Prakash, P.; Moradkhani, A.; Kasraei, E. Prevalence of Malignancy and Chronic Obstructive Pulmonary Disease among Patients with COVID-19: A Systematic Review and Meta-analysis. Int. J. Sci. Res. Dent. Med. Sci. 2020, 2, 52–58. [Google Scholar]
- Dinita Devi, N.; Chyrmang, D.; Baidya, K.; Devi, Y.S. Askin Tumor: A Case Report of a Rare Tumor. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 153–155. [Google Scholar]
- Hamidian, K.; Sarani, M.; Sheikhi, E.; Khatami, M. Cytotoxicity evaluation of green synthesized ZnO and Ag-doped ZnO nanoparticles on brain glioblastoma cells. J. Mol. Struct. 2022, 1251, 131962. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Li, Y.; Iqbal, Z.; Yu, L.; Liu, J.; Wang, H.; He, P. The thermal/pH-sensitive drug delivery system encapsulated by PAA based on hollow hybrid nanospheres with two silicon source. J. Biomater. Sci. Polym. Ed. 2021, 32, 695–713. [Google Scholar] [CrossRef]
- Bagheri, N.; Lakouraj, M.M.; Nabavi, S.R.; Tashakkorian, H.; Mohseni, M. Synthesis of bioactive polyaniline-b-polyacrylic acid copolymer nanofibrils as an effective antibacterial and anticancer agent in cancer therapy, especially for HT29 treatment. RSC Adv. RSC Adv. 2020, 10, 25290–25304. [Google Scholar] [CrossRef]
- Li, Y.; Wan, J.; Wang, F.; Guo, J.; Wang, C. Effect of increasing liver blood flow on nanodrug clearance by the liver for enhanced antitumor therapy. Biomater. Sci. 2019, 7, 1507–1515. [Google Scholar] [CrossRef]
- Reddy, B.N.; Rauta, P.R.; Lakshmi, V.V.; Sreenivasa, S. Development, formulation, and evaluation of sodium alginate-g-poly (acryl amide-co-acrylic acid/cloiste-30b)/agnps hydrogel composites and their applications in paclitaxel drug delivery and anticancer activity. Int. J. Appl. Pharm. 2018, 10, 141–150. [Google Scholar]
- Tan, S.; Wang, G. Redox-responsive and pH-sensitive nanoparticles enhanced stability and anticancer ability of erlotinib to treat lung cancer in vivo. Drug Des. Dev. Ther. 2017, 11, 3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, X.-Y.; Luo, Y.-L.; Xu, F.; Chen, Y.-S.; Su, Y.-Y. Dual stimuli-responsive Fe3O4 graft poly (acrylic acid)-block-poly (2-methacryloyloxyethyl ferrocenecarboxylate) copolymer micromicelles: Surface RAFT synthesis, self-assembly and drug release applications. J. Nanobiotechnol. 2017, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L.; Hu, B.; Li, K.; Wang, Z.; Quan, Z.; et al. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf. B Biointerfaces 2017, 154, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Tang, F. Shape matters when engineering mesoporous silica-based nanomedicines. Biomater. Sci. 2016, 4, 575–591. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, J.; Sheng, J.; Hu, Y.; Jiang, Z. Paclitaxel-Loaded β-Cyclodextrin-Modified Poly (Acrylic Acid) Nanoparticles through Multivalent Inclusion for Anticancer Therapy. Macromol. Biosci. 2016, 16, 341–349. [Google Scholar] [CrossRef]
- Chiang, C.S.; Tseng, Y.H.; Liao, B.J.; Chen, S.Y. Magnetically targeted nanocapsules for PAA-cisplatin-conjugated cores in PVA/SPIO shells via surfactant-free emulsion for reduced nephrotoxicity and enhanced lung cancer therapy. Adv. Healthc. Mater. 2015, 4, 1066–1075. [Google Scholar] [CrossRef]
- Lee, K.D.; Jeong, Y.-I.; Da Hye Kim, G.-T.L.; Choi, K.-C. Cisplatin-incorporated nanoparticles of poly (acrylic acid-co-methyl methacrylate) copolymer. Int. J. Nanomed. 2013, 8, 2835. [Google Scholar]
- Zhao, Y.; Liu, Y.; Wang, Y.; Xu, B.; Zhang, S.; Liu, J.; Zhang, T.; Jin, L.; Song, S.; Zhang, H. Rapidly clearable MnCo2O4@ PAA as novel nanotheranostic agents for T1/T2 bimodal MRI imaging-guided photothermal therapy. Nanoscale 2021, 13, 16251–16257. [Google Scholar] [CrossRef]
- Li, D.; Bao, A.; Chen, X.; Li, S.; Wang, T.; Zhang, L.; Ji, J.; Li, Q.; Wang, C.; Gao, Y.; et al. Prussian Blue@ Polyacrylic Acid/Au Aggregate Janus Nanoparticles for CT Imaging-guided Chemotherapy and Enhanced Photothermal Therapy. Adv. Ther. 2020, 3, 2000091. [Google Scholar] [CrossRef]
- Zhao, S.; Tian, R.; Shao, B.; Feng, Y.; Yuan, S.; Dong, L.; Zhang, L.; Wang, Z.; You, H. One-pot synthesis of Ln 3+-doped porous BiF 3@ PAA nanospheres for temperature sensing and pH-responsive drug delivery guided by CT imaging. Nanoscale 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Wang, T.; Wu, X.; Ren, H.; Wang, C.; Su, Z. Facile and Scalable Synthesis of Novel Spherical Au Nanocluster Assemblies@ Polyacrylic Acid/Calcium Phosphate Nanoparticles for Dual-Modal Imaging-Guided Cancer Chemotherapy. Small 2015, 11, 3162–3173. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hu, S.-H.; Hung, S.-Y.; Chiang, C.-S.; Liu, H.-L.; Chiu, T.-L.; Lai, H.-Y.; Chen, Y.-Y.; Chen, S.-Y. SPIO nanoparticle-stabilized PAA-F127 thermosensitive nanobubbles with MR/US dual-modality imaging and HIFU-triggered drug release for magnetically guided in vivo tumor therapy. J. Control. Release 2013, 172, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Hajikhani, M.; Emam Djomeh, Z.; Askari, G. Lycopene loaded polylactic acid (PLA) and PLA/copolymer electrospun nanofibers, synthesis, characterization, and control release. J. Food Process. Preserv. 2021, 45, e15055. [Google Scholar] [CrossRef]
- Khajeh, H.G.; Sabzi, M.; Ramezani, S.; Jalili, A.A.; Ghorbani, M.J.C.; Physicochemical, S.A.; Aspects, E. Fabrication of a wound dressing mat based on Polyurethane/Polyacrylic acid containing Poloxamer for skin tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127891. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Zahedi, P.; Shahrousvand, M. Enhanced osteogenesis using poly (l-lactide-co-d, l-lactide)/poly (acrylic acid) nanofibrous scaffolds in presence of dexamethasone-loaded molecularly imprinted polymer nanoparticles. Int. J. Biol. Macromol. 2020, 165, 2363–2377. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Y.; Xu, M.; Qin, M.; Lan, W.; Huang, D.; Wei, Y.; Chen, W. High strength polyvinyl alcohol/polyacrylic acid (PVA/PAA) hydrogel fabricated by Cold-Drawn method for cartilage tissue substitutes. J. Biomater. Sci. Polym. Ed. 2020, 31, 1836–1851. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Haider, S.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Mun, G.A.; Sherbakova, M.V.; Ivaschenko, A.T.; Aitkhozhina, N.A. Polycomplexes of poly (acrylic acid) with streptomycin sulfate and their antibacterial activity. Eur. J. Pharm. Biopharm. 2004, 57, 245–249. [Google Scholar] [CrossRef]
- Larsson, M.; Bergstrand, A.; Mesiah, L.; Van Vooren, C.; Larsson, A. Nanocomposites of polyacrylic acid nanogels and biodegradable polyhydroxybutyrate for bone regeneration and drug delivery. J. Nanomater. 2014, 2014, 371307. [Google Scholar] [CrossRef]
- Ghorbaniazar, P.; Sepehrianazar, A.; Eskandani, M.; Nabi-Meibodi, M.; Kouhsoltani, M.; Hamishehkar, H. Preparation of poly acrylic acid-poly acrylamide composite nanogels by radiation technique. Adv. Pharm. Bull. 2015, 5, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, A.; Salehi, A. Antimicrobial resistance properties of Helicobacter pylori strains isolated from dental plaque and saliva samples. Acad. J. Health Sci. Med. Balear 2022, 37, 29–33. [Google Scholar]
- Khamisi, N.; Fathi, A.; Yari, A. Antimicrobial resistance of Staphylococcus aureus isolated from dental plaques. Acad. J. Health Sci. Med. Balear 2022, 37, 136–140. [Google Scholar]
- Ekwebene, O.; Nnamani, C.; Edeh, C.; Obidile, C.; Tyotswame, Y. Prevalence of Falciparum Malaria in Conjunction with Age, Gravidity, Abo Blood Group/Rhesus Factor, and Genotype Among Gravid Women in South-eastern Nigeria. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 12–17. [Google Scholar]
- Dilip, D.; Menon, A. Speciation, Detection of Virulence Factors and Antibiotic Susceptibility of Coagulase Negative Staphylococci. Int. J. Sci. Res. Dent. Med. Sci. 2021, 3, 122–132. [Google Scholar]
- Sanaei, Z.; Azizi, A.; Rahimi, A. The Comparison of the Effect of Photodynamic Therapy Using two Photosensitizer Indocyanine Green and Methylene Blue on the Colony Count of Staphylococcus Aureus (In Vitro). Int. J. Sci. Res. Dent. Med. Sci. 2019, 1, 13–17. [Google Scholar]
- Safari Kakroudi, M.; Rimaz, S.; Atrkar Roshan, Z.; Mobayen, M. The Frequency of Bacterial Colonization in Burn Wounds and Antibiogram pattern in Patients Hospitalized in the ICU of Velayat Burn and Reconstructive Surgery Center in Rasht City. Int. J. Sci. Res. Dent. Med. Sci. 2019, 1, 72–79. [Google Scholar]
- Nazaripour, E.; Mosazadeh, F.; Rahimi, S.S.; Alijani, H.Q.; Isaei, E.; Borhani, F.; Iravani, S.; Ghasemi, M.; Akbarizadeh, M.R.; Azizi, E. Ferromagnetic nickel (II) oxide (NiO) nanoparticles: Biosynthesis, characterization and their antibacterial activities. Rend. Lincei. Sci. Fis. E Nat. 2022, 1–8. [Google Scholar] [CrossRef]
- Gratzl, G.; Paulik, C.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial activity of poly (acrylic acid) block copolymers. Mater. Sci. Eng. C 2014, 38, 94–100. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Rivas, B.L.; Pereira, E.D.; Moreno-Villoslada, I. Water-soluble polymer–metal ion interactions. Prog. Polym. Sci. 2003, 28, 173–208. [Google Scholar] [CrossRef]
- Sethy, P.K.; Mohapatra, P.; Patra, S.; Bharatiya, D.; Swain, S.K. Antimicrobial and barrier properties of polyacrylic acid/GO hybrid nanocomposites for packaging application. Nano-Struct. Nano-Objects 2021, 26, 100747. [Google Scholar] [CrossRef]
- Gratzl, G.; Walkner, S.; Hild, S.; Hassel, A.W.; Weber, H.K.; Paulik, C. Mechanistic approaches on the antibacterial activity of poly (acrylic acid) copolymers. Colloids Surf. B Biointerfaces 2015, 126, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Chinthamreddy, A.; Koppula, S.; Kuruva, S.; Surya, S.M. ‘Biopolymer-PAA and surfactant-CTAB assistant solvothermal synthesis of Zn-based MOFs: Design, characterization for removal of toxic dyes, copper and their biological activities. Inorg. Chem. Commun. 2021, 133, 108928. [Google Scholar] [CrossRef]
- Shibraen, M.H.; Ibrahim, O.M.; Asad, R.A.; Yang, S.; El-Aassar, M.J.C.; Physicochemical, S.A.; Aspects, E. Interpenetration of metal cations into polyelectrolyte-multilayer-films via layer-by-layer assembly: Selective antibacterial functionality of cationic guar gum/polyacrylic acid-Ag+ nanofilm against resistant E. coli. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125921. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Ji, C.; Zhang, F.; Zhou, Q.; Sun, M.; Sun, Y.; Politis, C.; Shavandi, A. Poly (acrylic acid) capped iron oxide nanoparticles via ligand exchange with antibacterial properties for biofilm applications. Colloids Surf. B Biointerfaces 2021, 197, 111385. [Google Scholar] [CrossRef]
- Xu, X.; Wang, N.; Wu, M.; Wang, J.; Wang, D.; Chen, Z.; Xie, J.; Ding, C.; Li, J. Programmed antibacterial and mineralization therapy for dental caries based on zinc-substituted hydroxyapatite/alendronate-grafted polyacrylic acid hybrid material. Colloids Surf. B Biointerfaces 2020, 194, 111206. [Google Scholar] [CrossRef]
- Sharma, S.; Virk, K.; Sharma, K.; Bose, S.K.; Kumar, V.; Sharma, V.; Focarete, M.L.; Kalia, S. Preparation of gum acacia-poly (acrylamide-IPN-acrylic acid) based nanocomposite hydrogels via polymerization methods for antimicrobial applications. J. Mol. Struct. 2020, 1215, 128298. [Google Scholar] [CrossRef]
- Ji, X.-J.; Cheng, Q.; Wang, J.; Zhao, Y.-B.; Han, Z.-Z.; Zhang, F.; Li, S.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci. 2019, 13, 87–98. [Google Scholar] [CrossRef]
- Arik, N.; Inan, A.; Ibis, F.; Demirci, E.A.; Karaman, O.; Ercan, U.K.; Horzum, N. Modification of electrospun PVA/PAA scaffolds by cold atmospheric plasma: Alignment, antibacterial activity, and biocompatibility. Polym. Bull. 2019, 76, 797–812. [Google Scholar] [CrossRef]
- Dil, N.N.; Sadeghi, M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu (II) metal ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers—an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Ranjbari, E.; Bazgir, S.; Shirazi, M.M.A. Needleless electrospinning of poly (acrylic acid) superabsorbent: Fabrication, characterization and swelling behavior. Polym. Test. 2020, 84, 106403. [Google Scholar] [CrossRef]
- Mallawarachchi, S.; Mahadevan, A.; Gejji, V.; Fernando, S. Mechanics of controlled release of insulin entrapped in polyacrylic acid gels via variable electrical stimuli. Drug Deliv. Transl. Res. 2019, 9, 783–794. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Y.; Yu, W.; Li, R.; Wang, M.; Gao, Q.; Li, J.; Lin, H. Fabrication of hydrophilic and antibacterial poly (vinylidene fluoride) based separation membranes by a novel strategy combining radiation grafting of poly (acrylic acid)(PAA) and electroless nickel plating. J. Colloid Interface Sci. 2019, 543, 64–75. [Google Scholar] [CrossRef]
- Jose, J.; Shehzad, F.; Al-Harthi, M.A. Preparation method and physical, mechanical, thermal characterization of poly (vinyl alcohol)/poly (acrylic acid) blends. Polym. Bull. 2014, 71, 2787–2802. [Google Scholar] [CrossRef]
- Yang, F.; Fan, X.; Zhang, M.; Wang, C.; Zhao, W.; Zhao, C. A template-hatched method towards poly (acrylic acid) hydrogel spheres with ultrahigh ion exchange capacity and robust adsorption of environmental toxins. J. Ind. Eng. Chem. 2019, 69, 422–431. [Google Scholar] [CrossRef]
- Bin-Dahman, O.A.; Jose, J.; Al-Harthi, M.A. Compatibility of poly (acrylic acid)/starch blends. Starch-Stärke 2015, 67, 1061–1069. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin and poly (acrylic acid)-based biodegradable, non-cytotoxic, chemically cross-linked hydrogel for sustained release of ornidazole and ciprofloxacin. ACS Appl. Mater. Interfaces 2015, 7, 4791–4803. [Google Scholar] [CrossRef]

| Substrates | Substrate Form | Refs. |
|---|---|---|
| Fe3O4 | nanoparticles | [131,132,133,134,135,136,137,138,139] |
| Fe3O4 | nanogels | [140] |
| Fe3O4 | ferrogels | [141] |
| Fe3O4 | hydrogel | [142] |
| γ-Fe2O3 | nanoparticles | [143] |
| CoFe2O4 | nanoparticles | [144,145] |
| NiFe2O4 | nanoparticles | [146] |
| Multi-walled carbon nanotubes | nanocomposite | [122] |
| Mn3O4 | nanoparticles | [147] |
| MnCO3 | microcapsules | [148] |
| Co9S8@MnO2 | nanoparticles | [149] |
| Fe3O4@MnO2 | nanoparticles | [150] |
| Fe3O4@MnO2-doped NaYF4:Yb/Er/Nd | nanosheets | [151] |
| Au NPs | nanoparticles | [152,153,154,155] |
| Au NPs | hydrogel | [156] |
| Au NPs | nanoclusters | [157] |
| Au NPs | nanorods | [158] |
| SiO2 | nanoparticles | [159,160,161,162,163] |
| Au NPs@SiO2 | rubber film | [164] |
| MOF | nanoparticles | [165] |
| rGO | hydrogel | [166] |
| Mg-Ca3(PO4)2 | clusters | [167] |
| TiO2 | nanoparticles | [168,169] |
| CaCO3 | nanoparticles | [170] |
| CeO2 | nanoparticles | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. https://doi.org/10.3390/polym14061259
Arkaban H, Barani M, Akbarizadeh MR, Pal Singh Chauhan N, Jadoun S, Dehghani Soltani M, Zarrintaj P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers. 2022; 14(6):1259. https://doi.org/10.3390/polym14061259
Chicago/Turabian StyleArkaban, Hassan, Mahmood Barani, Majid Reza Akbarizadeh, Narendra Pal Singh Chauhan, Sapana Jadoun, Maryam Dehghani Soltani, and Payam Zarrintaj. 2022. "Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications" Polymers 14, no. 6: 1259. https://doi.org/10.3390/polym14061259
APA StyleArkaban, H., Barani, M., Akbarizadeh, M. R., Pal Singh Chauhan, N., Jadoun, S., Dehghani Soltani, M., & Zarrintaj, P. (2022). Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers, 14(6), 1259. https://doi.org/10.3390/polym14061259