New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer
Abstract
:1. Introduction

2. Experimental
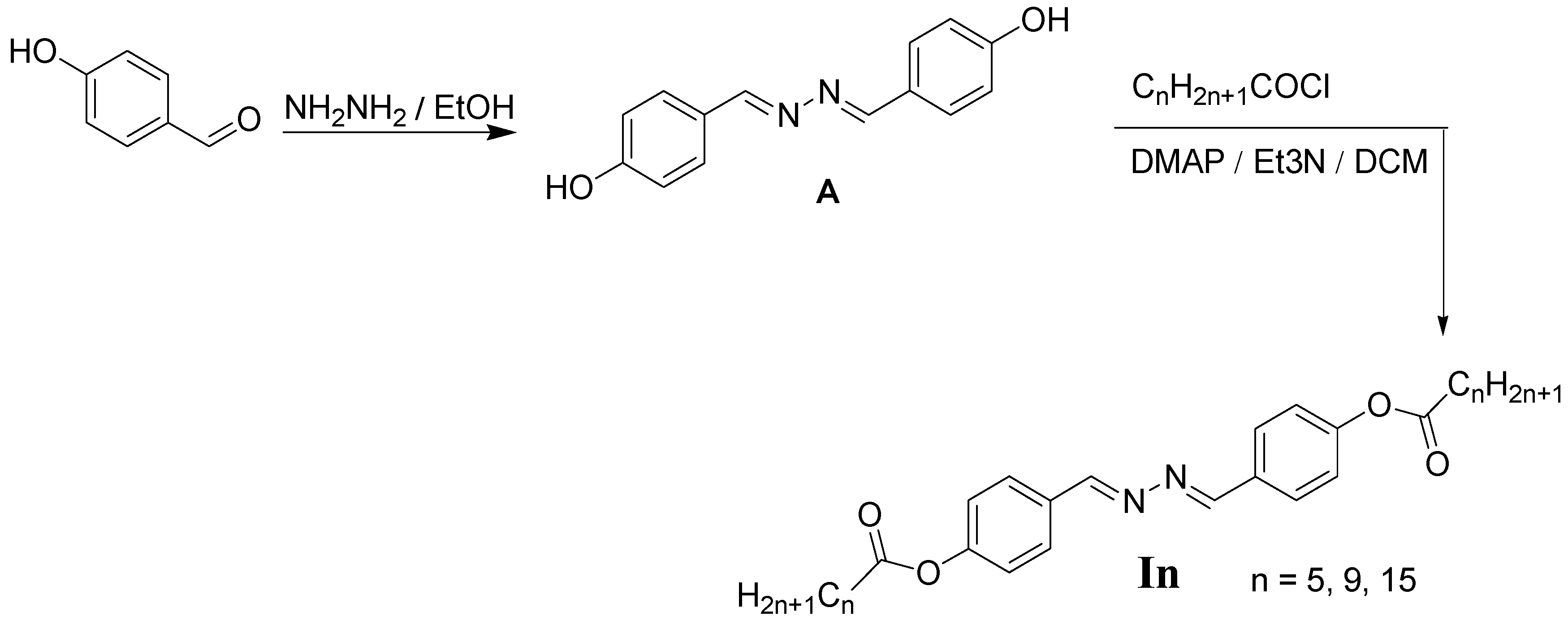
Synthesis
3. Results and Discussion
3.1. Chemistry
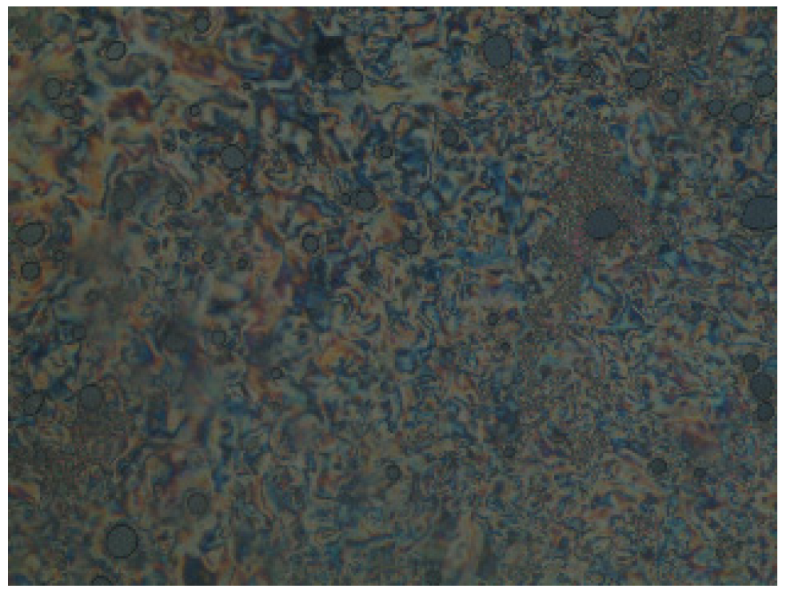
3.2. Liquid Crystalline Investigations
3.3. Computational Calculations
3.3.1. Thermal and Geometrical Parameters
3.3.2. Frontier Molecular Orbitals (FMOs)
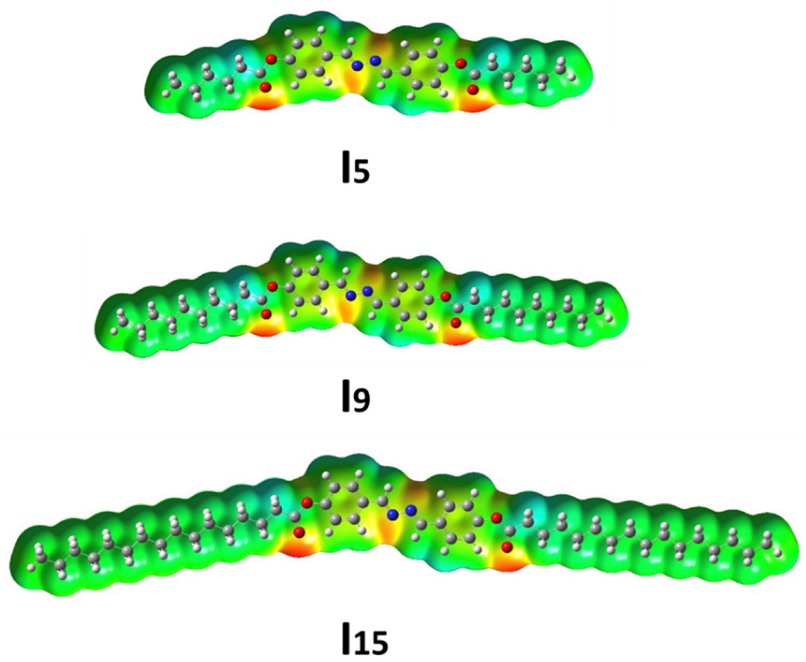
3.3.3. Molecular Electrostatic Potential (MEP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arjmand, F.; Sayeed, F.; Muddassir, M. Synthesis of new chiral heterocyclic Schiff base modulated Cu (II)/Zn (II) complexes: Their comparative binding studies with CT-DNA, mononucleotides and cleavage activity. J. Photochem. Photobiol. B Biol. 2011, 103, 166–179. [Google Scholar] [CrossRef]
- Chinnasamy, R.P.; Sundararajan, R.; Govindaraj, S. Synthesis, characterization, and analgesic activity of novel schiff base of isatin derivatives. J. Adv. Pharm. Technol. Res. 2010, 1, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Chandramouli, C.; Shivanand, M.; Nayanbhai, T.; Bheemachari, B.; Udupi, R. Synthesis and biological screening of certain new triazole Schiff bases and their derivatives bearing substituted benzothiazole moiety. J. Chem. Pharm. Res. 2012, 4, 1151–1159. [Google Scholar]
- JOMBO, G.T.A. Synthesis, Characterisation and Anti Microbial Activity of Various Schiff Base Complexes of Zinc (II) and Copper (II) Ions. Asian J. Pharm. Health Sci. 2011, 1, 8–11. [Google Scholar]
- Talukder, J.R.; Lee, Y.-H.; Wu, S.-T. Photo-responsive dye-doped liquid crystals for smart windows. Opt. Express 2019, 27, 4480–4487. [Google Scholar] [CrossRef] [Green Version]
- Sondhi, S.M.; Singh, N.; Kumar, A.; Lozach, O.; Meijer, L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Biorg. Med. Chem. 2006, 14, 3758–3765. [Google Scholar] [CrossRef]
- Mounika, K.; Pragathi, A.; Gyanakumari, C. Synthesis¸ Characterization and Biological Activity of a Schiff Base Derived from 3-Ethoxy Salicylaldehyde and 2-Amino Benzoic acid and its Transition Metal Complexes. J. Sci. Res. 2010, 2, 513. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, S.; Lei, S.; Ma, H.; Yu, R.; Liu, D. Investigation on some Schiff bases as HCl corrosioninhibitors for copper. Corros. Sci. 1999, 41, 1273–1287. [Google Scholar] [CrossRef]
- Yurt, A.; Balaban, A.; Kandemir, S.; Bereket, G.; Erk, B. Investigation on some Schiff bases as HCl corrosion inhibitors for carbon steel. Mater. Chem. Phys. 2004, 85, 420–426. [Google Scholar] [CrossRef]
- Dong, R.; Zhou, Y.; Huang, X.; Zhu, X.; Lu, Y.; Shen, J. Functional Supramolecular Polymers for Biomedical Applications. Adv. Mater. 2014, 27, 498–526. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Chen, R.; Mo, L.; Li, J.; Hu, M.; Wu, S.-T. Submillisecond-Response Polymer Network Liquid Crystal Phase Modulators. Polymers 2020, 12, 2862. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kang, Y.; Wang, Z.; Zhang, X. 25th Anniversary Article: Reversible and Adaptive Functional Supramolecular Materials: “Noncovalent Interaction”. Matters. Adv. Mater. 2013, 25, 5530–5548. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, S.T. Recent advances on polymer-stabilized blue phase liquid crystal materials and devices. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Friot, B.; Boyd, D.; Willis, K.; Donnio, B.; Ungar, G.; Bruce, D.W. Hydrogen-bonded polycatenar mesogens. Liq. Cryst. 2000, 27, 605–611. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Kato, T.; Kamikawa, Y. Hydrogen-bonded systems: Discrete defined aggregates by intermolecular H-bonding, amides, carboxylic acids, and heterocycles. Handb. Liq. Cryst. 2014, 1–28. [Google Scholar]
- Alaasar, M.; Poppe, S.; Dong, Q.; Liu, F.; Tschierske, C. Mirror symmetry breaking in cubic phases and isotropic liquids driven by hydrogen bonding. Chem. Commun. 2016, 52, 13869–13872. [Google Scholar] [CrossRef] [Green Version]
- Jansze, S.M.; Martínez-Felipe, A.; Storey, J.M.D.; Marcelis, A.T.M.; Imrie, C.T. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. 2014, 127, 653–656. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.P.; Martinez-Felipe, A.; Paterson, D.A.; Forsyth, E.; Lawrence, G.B.; Henderson, P.A.; Storey, J.M.D.; Gorecka, E.; et al. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; El-Atawy, M.A. Synthesis, optical and DFT characterizations of laterally fluorinated phenyl cinnamate liquid crystal non-symmetric system. Symmetry 2021, 13, 1145. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Naoum, M.M.; Al-Zahrani, S.A.; Ahmed, H.A. New nitro-laterally substituted azomethine derivatives; Synthesis, mesomorphic and computational characterizations. Molecules 2021, 26, 1927. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Bedowr, N.S.; Naoum, M.M.; Mostafa, A.M.; Al-Kadhi, N.S. New Liquid Crystals Based on Terminal Fatty Chains and Polymorphic Phase Formation from Their Mixtures. Crystals 2022, 12, 350. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Alhaddad, O.A.; Ahmed, H.A. Experimental and geometrical structure characterizations of new synthesized laterally fluorinated nematogenic system. Liq. Cryst. 2021, 48, 1–11. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Ahmed, H.A.; El-Atawy, M.A.; Abu Al-Ola, K.A.; Omar, A.Z. Synthetic, mesomorphic, and DFT investigations of new nematogenic polar naphthyl benzoate ester derivatives. Materials 2021, 14, 2587. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-Atawy, M.A. Synthesis, mesomorphic and geometrical approaches of new non-symmetrical system based on central naphthalene moiety. Liq. Cryst. 2021, 48, 1940–1952. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Senior, S.; El-Atawy, M.A.; El-Sadany, S.K.; Hamed, E.A. DFT calculations of 2,4,6-trinitrophenylbenzoate derivatives: Structure, ground state properties and spectral properties. J. Mol. Struct. 2011, 1006, 303–311. [Google Scholar] [CrossRef]
- Omar, A.Z.; Mahmoud, M.N.; El-Sadany, S.K.; Hamed, E.A.; El-Atawy, M.A. A combined experimental and DFT investigation of mono azo thiobarbituric acid based chalcone disperse dyes. Dye. Pigment. 2020, 185, 108887. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Omar, A.Z.; Hagar, M.; Shashira, E.M. Transalkylidation reaction: Green, catalyst-free synthesis of thiosemicarbazones and solving the NMR conflict between their acyclic structure and intramolecular cycloaddition products. Green Chem. Lett. Rev. 2019, 12, 364–376. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Hegazi, A.H.; Al Khalaf, M.; Amer, A. The structure elucidation of the isomeric mixture of 3-[L-threo-2,3,4-tri-hydroxy-1-(phenyl-hydrazono) butyl] quinoxalin-2 (1H)-one in dimethyl sulfoxide solution revisited: Experimental and theoretical study. Struct. Chem. 2020, 31, 1065–1072. [Google Scholar] [CrossRef]
- Omar, A.Z.; Mosa, T.M.; Elsadany, S.K.; Hamed, E.A.; Elatawy, M. Novel Piperazine Based Compounds as Potential Inhibitors for SARS-CoV-2 Protease Enzyme: Synthesis and Molecular Docking Study. J. Mol. Struct. 2021, 1245, 131020. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.K.; Dubey, M. Li+-Induced fluorescent metallogel: A case of ESIPT-CHEF and ICT phenomenon. Phys. Chem. Chem. Phys. 2018, 20, 23762–23772. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Khushaim, M.S.; Gomh, S.M.; Ahmed, H.A.; Naoum, M.M. Mesophase behavior of four ring ester/azomethine/ester liquid crystals in pure and mixed states. Liq. Cryst. 2022, 1–8. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Ahmed, H.A.; Khan, M.T.; Al-Ola, K.A.; AL-Refai, H.; El-Atawy, M.A. New Self-Organizing Optical Materials and Induced Polymorphic Phases of Their Mixtures Targeted for Energy Investigations. Polymers 2022, 14, 456. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision a. 02; Gaussian Inc.: Wallingford, CT, USA, 2009; 200p. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, version 5; Semichem Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Gray, G.W. Molecular Structure and the Properties of Liquid Crystals; Academic Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Ahmed, H.A.; Aboelnaga, A. Synthesis and mesomorphic study of new phenylthiophene liquid crystals. Liq. Cryst. 2021, 1–8. [Google Scholar] [CrossRef]
- Naoum, M.; Mohammady, S.Z.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-)methyl phenyl-4’-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Elshakre, M.E.; Alalawy, H.H.; Awad, M.I.; El-Anadouli, B.E. On the role of the electronic states of corrosion inhibitors: Quantum chemical-electrochemical correlation study on urea derivatives. Corros. Sci. 2017, 124, 121–130. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Al-Mutabagani, L.A.; Gomha, S.M.; Ahmed, H.A.; Popoola, S.A.; Shaban, M. Novel sulphonic acid liquid crystal derivatives: Experimental, computational and optoelectrical characterizations. RSC Adv. 2021, 11, 27937–27949. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Popoola, S.A.; Aboelnaga, A. Synthesis, Phase Behavior and Computational Simulations of a Pyridyl-Based Liquid Crystal System. Molecules 2021, 26, 6416. [Google Scholar] [CrossRef]
- Alamro, F.; Ahmed, H.; Popoola, S.; Altaleb, H.; Abu Al-Ola, K.; Gomha, S. Effect of the Relative Positions of Di-Laterally Substituted Schiff Base Derivatives: Phase Transition and Computational Investigations. Crystals 2021, 11, 870. [Google Scholar] [CrossRef]
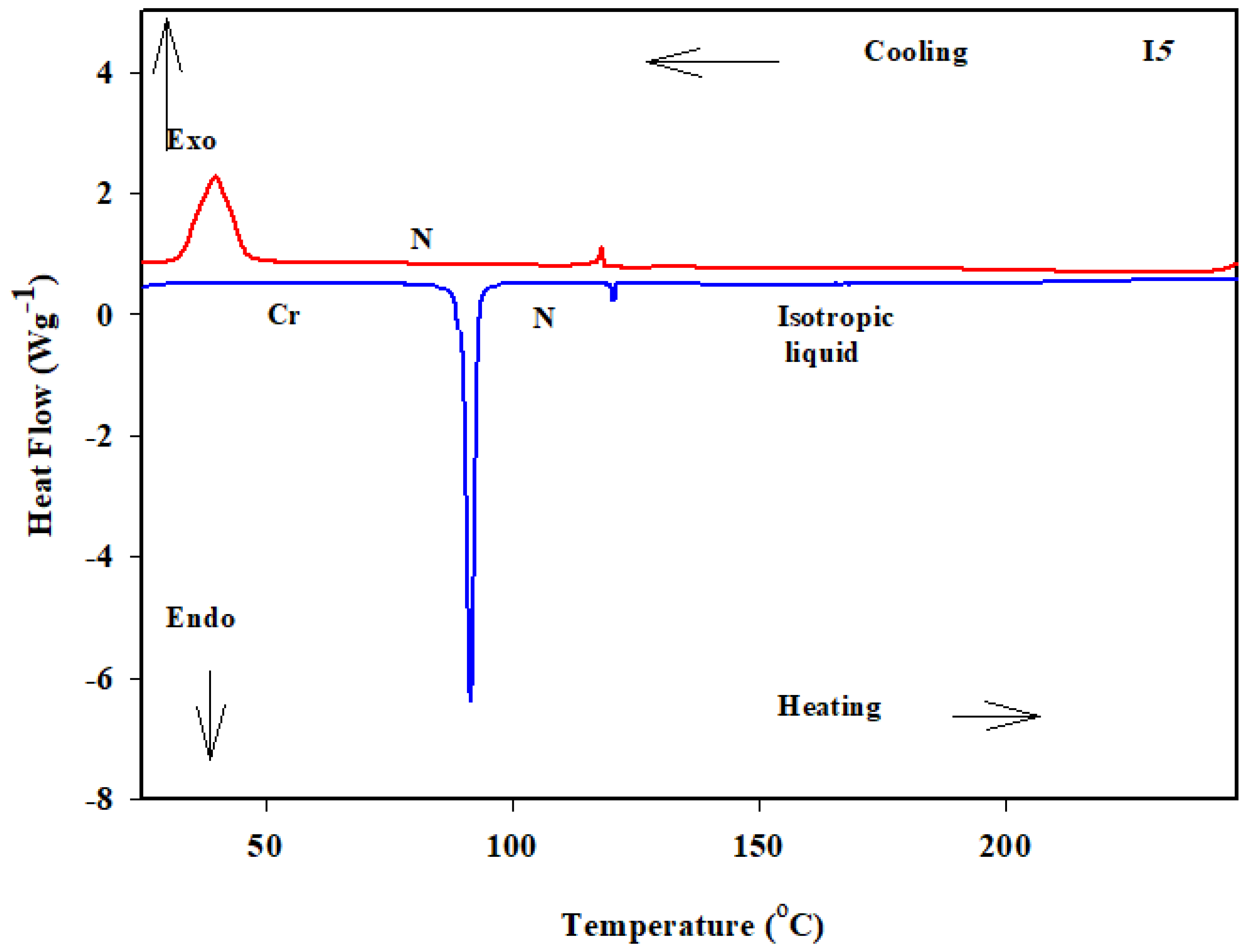
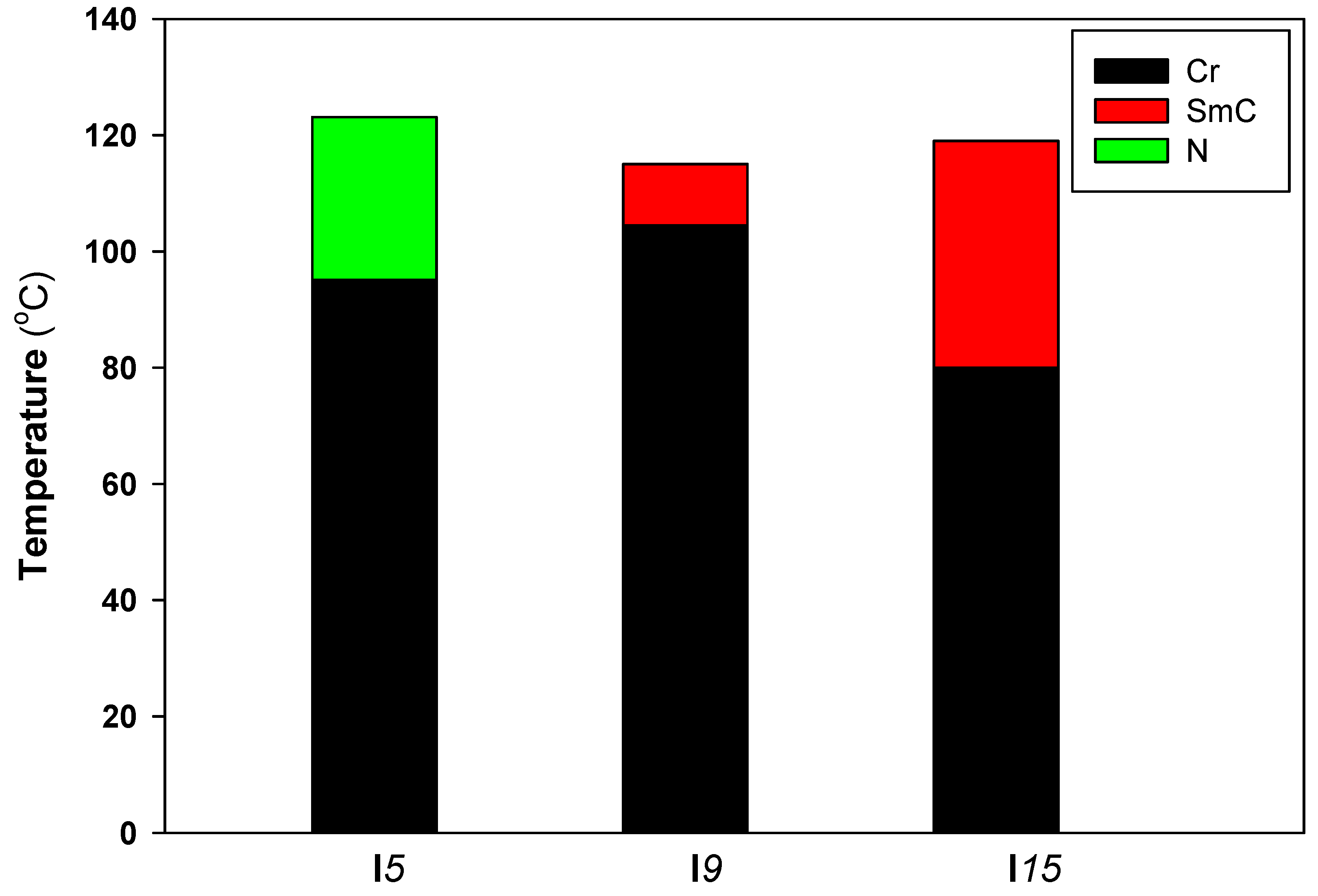
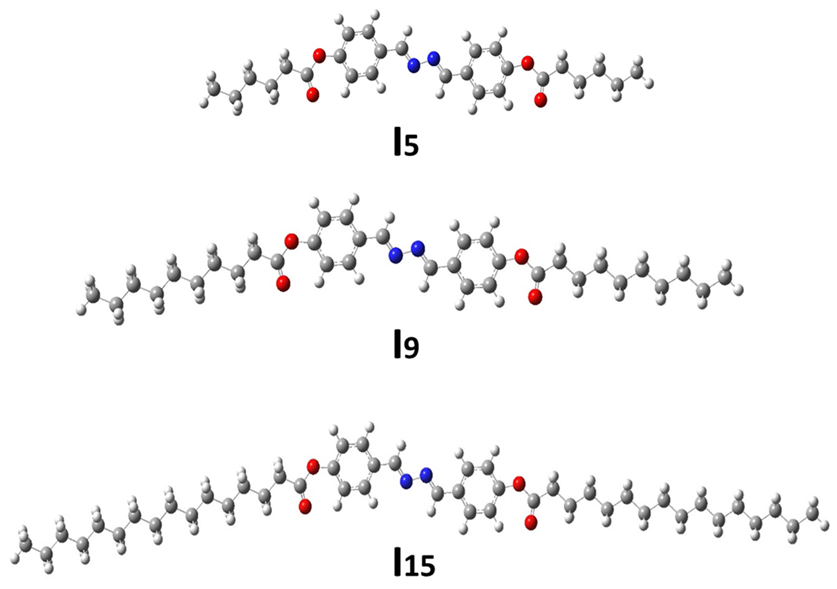

| Comp. | TCr–SmC | ∆HCr–SmC | TCr–N | ∆HCr–N | TSmC–I | ∆HSmC–I | TN–I | ∆HN–I | ΔS/R |
|---|---|---|---|---|---|---|---|---|---|
| I5 | - | - | 95.1 | 47.3 | - | - | 122.6 | 1.9 | 0.58 |
| I9 | 104.5 | 42.1 | - | - | 115.3 | 2.3 | - | - | 0.71 |
| I15 | 80.3 | 43.7 | - | - | 118.5 | 2.1 | - | - | 0.64 |
| Compound | ZPE (Kcal/Mol) | Thermal Energy (Kcal/Mol) | Enthalpy (Kcal/Mol) | Gibbs-Free Energy (Kcal/Mol) | Entropy (Cal mol.k) |
|---|---|---|---|---|---|
| I5 | 336.356 | 357.302 | 357.894 | 289.931 | 227.950 |
| I9 | 479.617 | 507.397 | 507.990 | 422.723 | 285.985 |
| I15 | 694.540 | 732.568 | 733.161 | 621.898 | 373.176 |
| Compound | Total Energy (Ha) | EHOMO (ev) | EluMO (ev) | ∆E (ev) | Dipole Moment (D) | IE (ev) | EA (ev) | Polarizability Bohr3 |
|---|---|---|---|---|---|---|---|---|
| I5 | −1419.805 | −6.158 | −2.239 | 3.919 | 2.9783 | 6.158 | 2.239 | 389.71 |
| I9 | −1734.063 | −6.155 | −2.237 | 3.918 | 2.9384 | 6.155 | 2.237 | 486.30 |
| I15 | −2205.449 | −6.154 | −2.236 | 3.918 | 2.9489 | 6.154 | 2.236 | 628.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, F.S.; Ahmed, H.A.; Bedowr, N.S.; Khushaim, M.S.; El-atawy, M.A. New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer. Polymers 2022, 14, 1256. https://doi.org/10.3390/polym14061256
Alamro FS, Ahmed HA, Bedowr NS, Khushaim MS, El-atawy MA. New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer. Polymers. 2022; 14(6):1256. https://doi.org/10.3390/polym14061256
Chicago/Turabian StyleAlamro, Fowzia S., Hoda A. Ahmed, Noha S. Bedowr, Muna S. Khushaim, and Mohamed A. El-atawy. 2022. "New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer" Polymers 14, no. 6: 1256. https://doi.org/10.3390/polym14061256
APA StyleAlamro, F. S., Ahmed, H. A., Bedowr, N. S., Khushaim, M. S., & El-atawy, M. A. (2022). New Advanced Liquid Crystalline Materials Bearing Bis-Azomethine as Central Spacer. Polymers, 14(6), 1256. https://doi.org/10.3390/polym14061256







