Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal—Application for Groundwater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
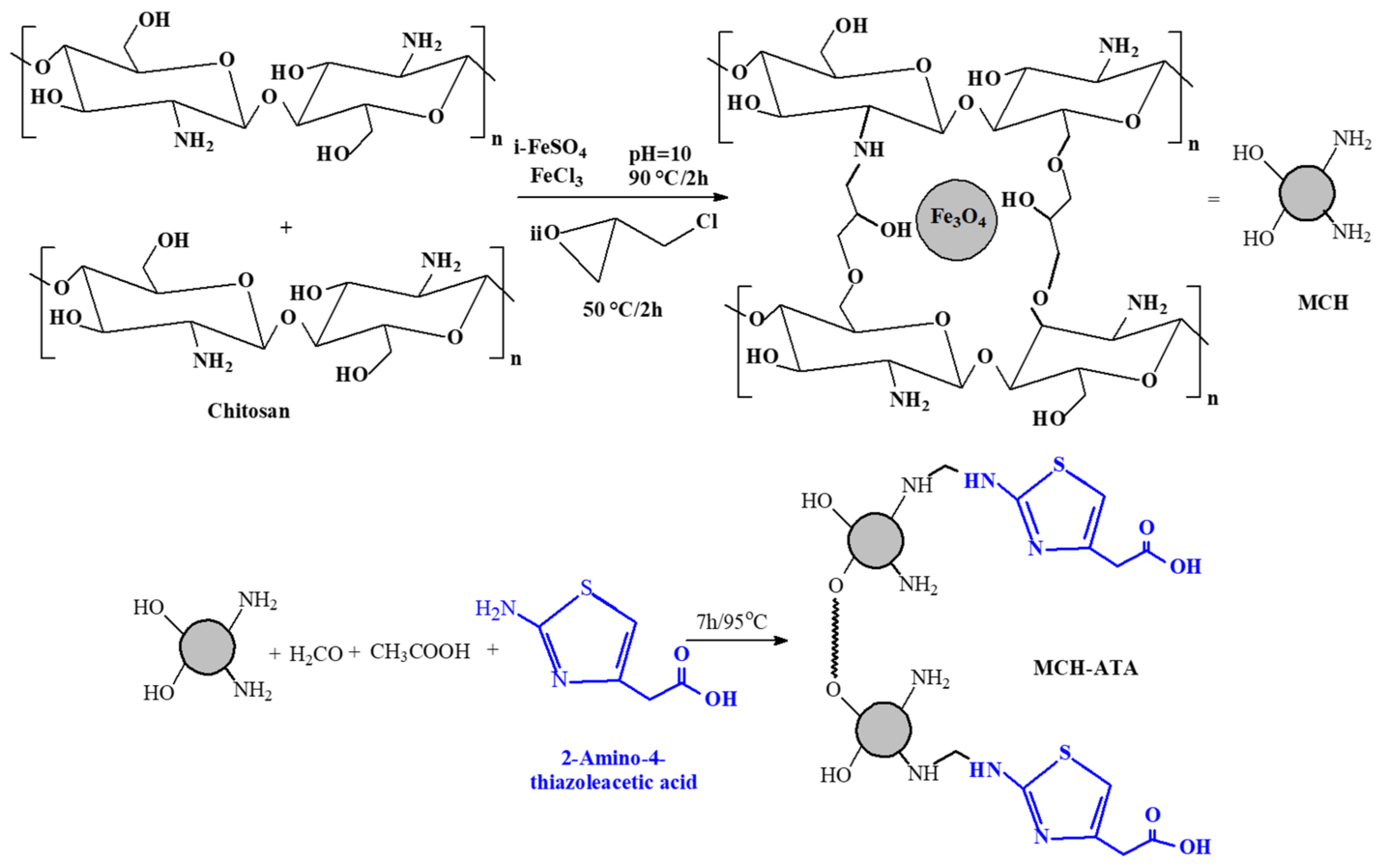
2.2. Synthesis of Sorbent
2.2.1. Synthesis of Magnetic Chitosan Nano-Particles (MG-CH)
2.2.2. Synthesis of Crosslinked Chitosan Nanoparticles (MCH)
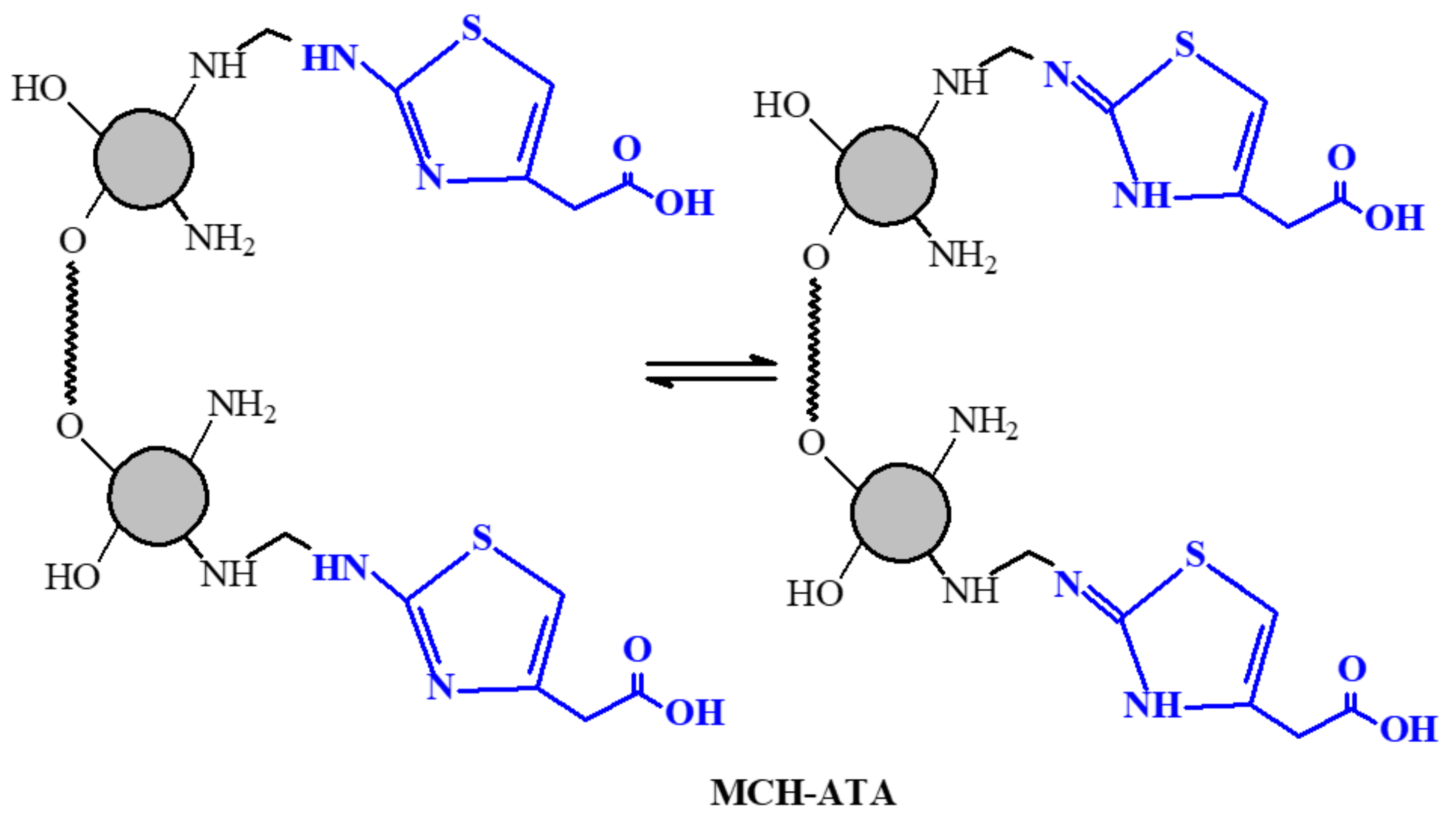
2.2.3. Synthesis of Grafted Amino Thiazol Acetic Derivative Nano Particles (MCH-ATA)
2.3. Characterization of Sorbents
2.4. Sorption Studies
2.5. Uptake Kinetics and Sorption Isotherms Models
2.6. Treatment of Real Metal-Containing Groundwater
3. Results and Discussion
3.1. Sorbent Characterization
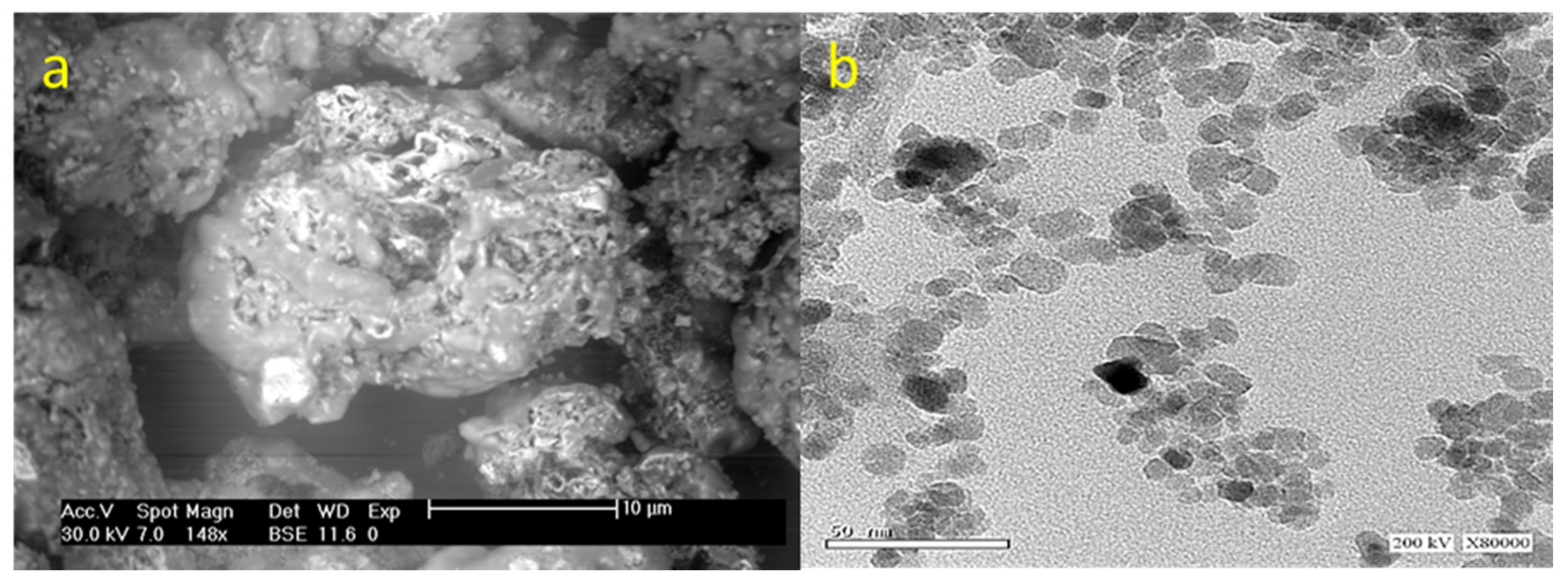
3.1.1. Morphology and Textural Properties
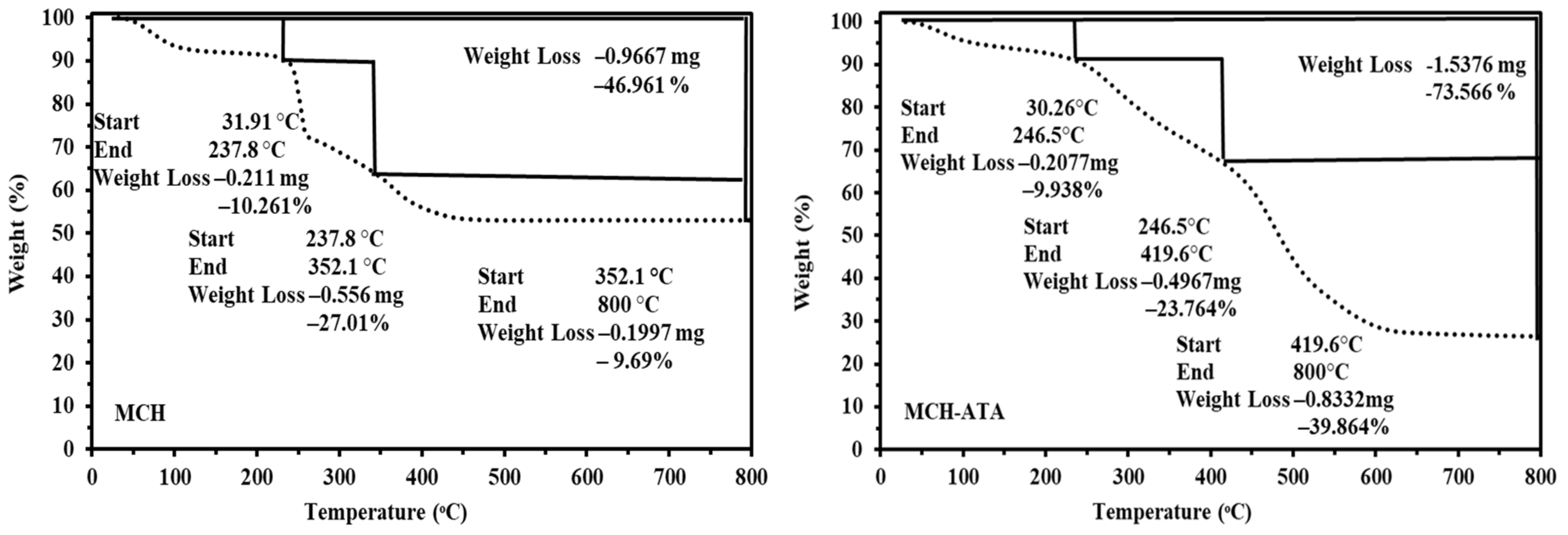
3.1.2. Thermogravimetric Analysis
- (a)
- 75.97 °C, 254.8 °C and 370.3 °C for MCH;
- (b)
- 74.4 °C, 282.6 °C, and 471.5 °C for the MCH-ATA.
3.1.3. FTIR Spectroscopy of Synthesized Sorbents
- (a)
- Decreasing the intensity peaks assigned to OH and NH with shifts from 3447 cm−1 and 3197 cm−1 to 3404 cm−1 related to sharing in the binding with metal ions;
- (b)
- Strong decreasing in the C = O stretching and amine binding vibration;
- (c)
- Decreasing of the COO− salt, and –OH bending.
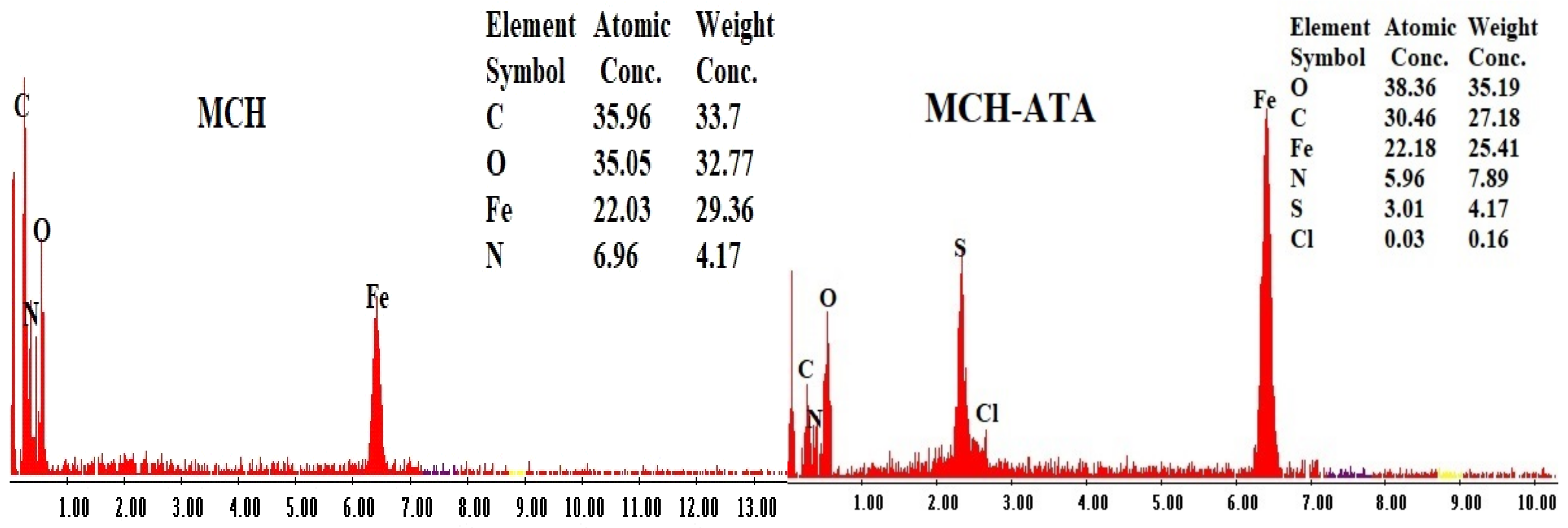
3.1.4. Elemental Analysis
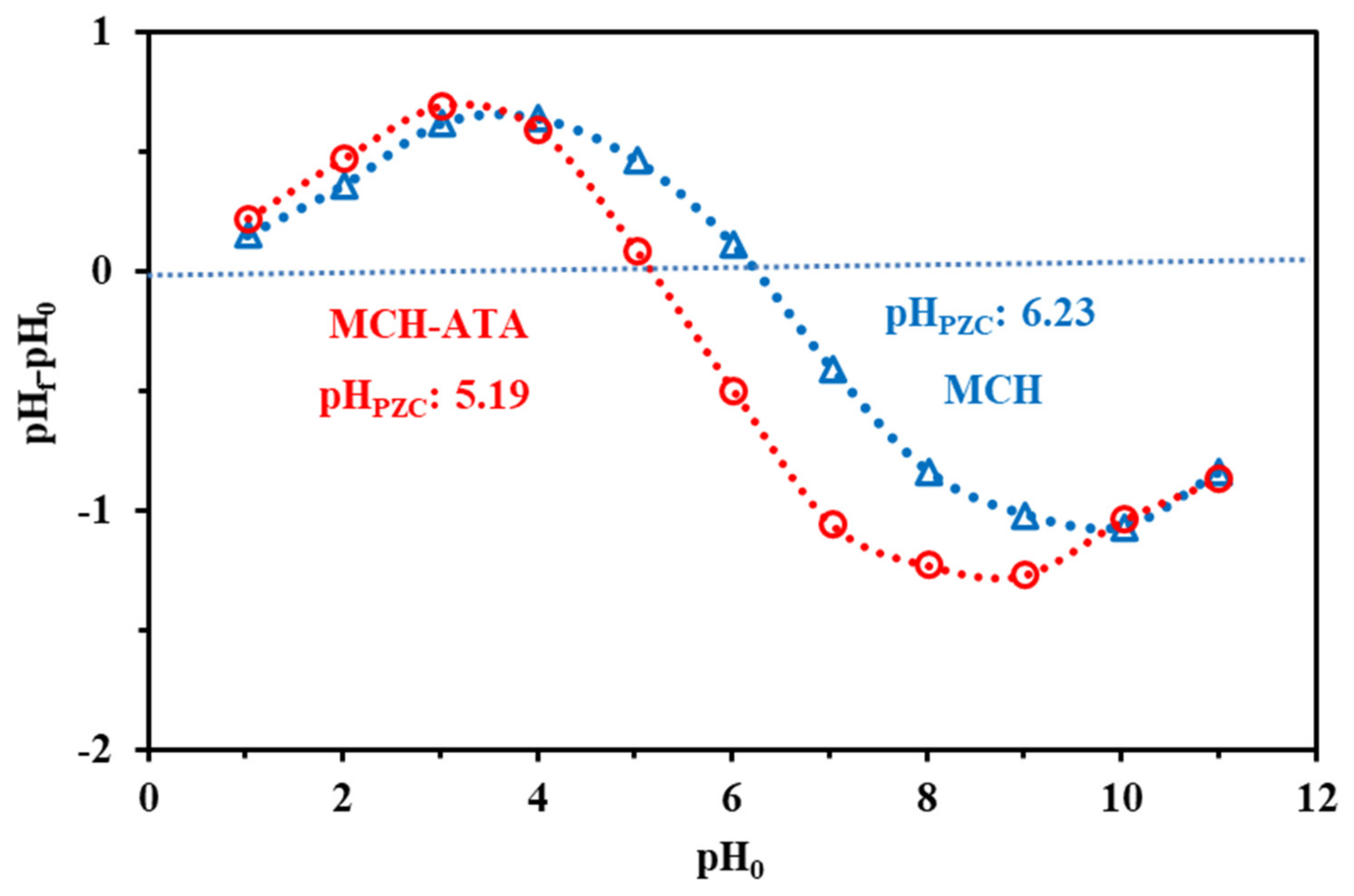
3.1.5. Surface Charge Analysis—pHPZC
3.2. Sorption Properties
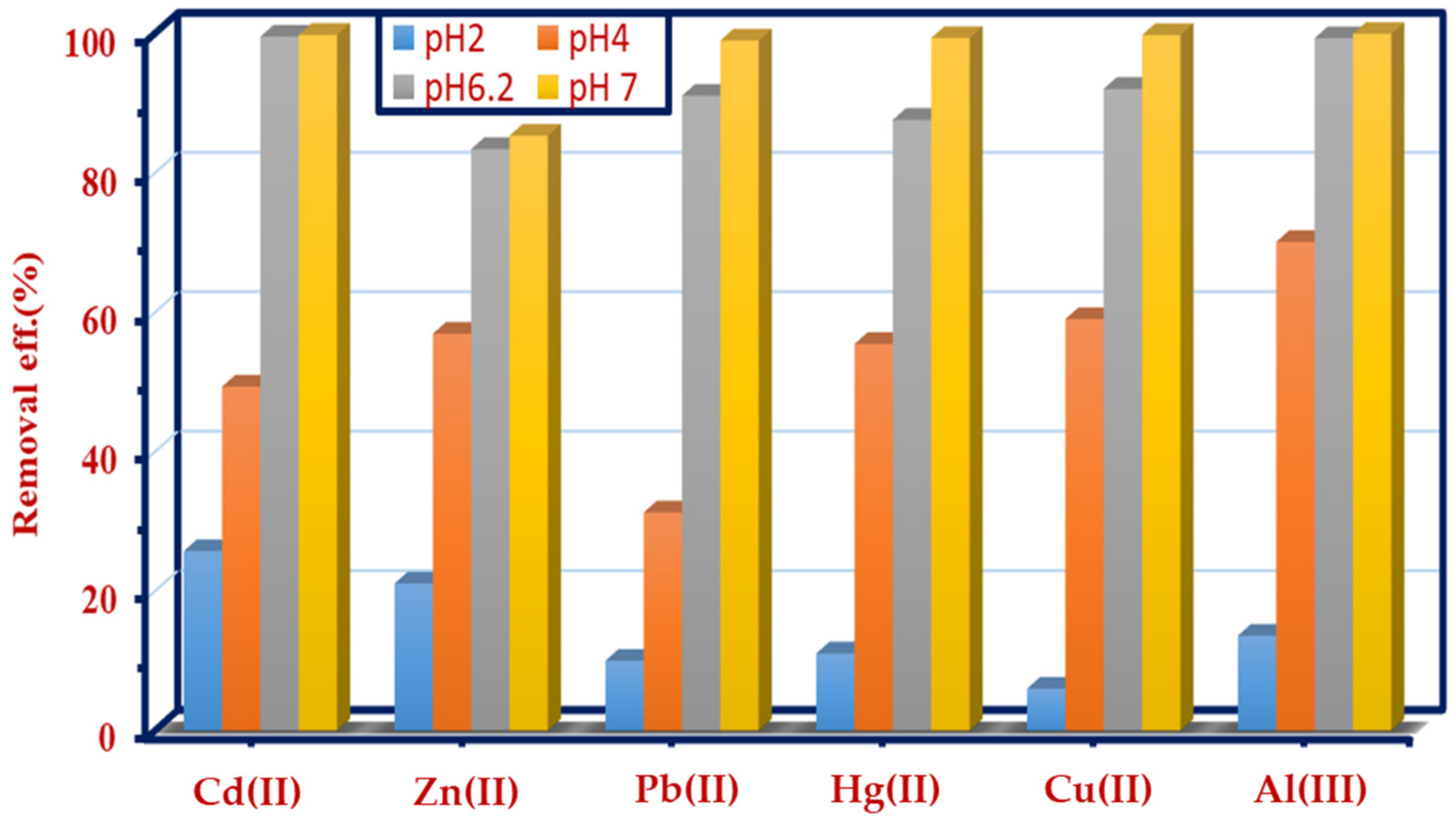
3.2.1. Effect of pH
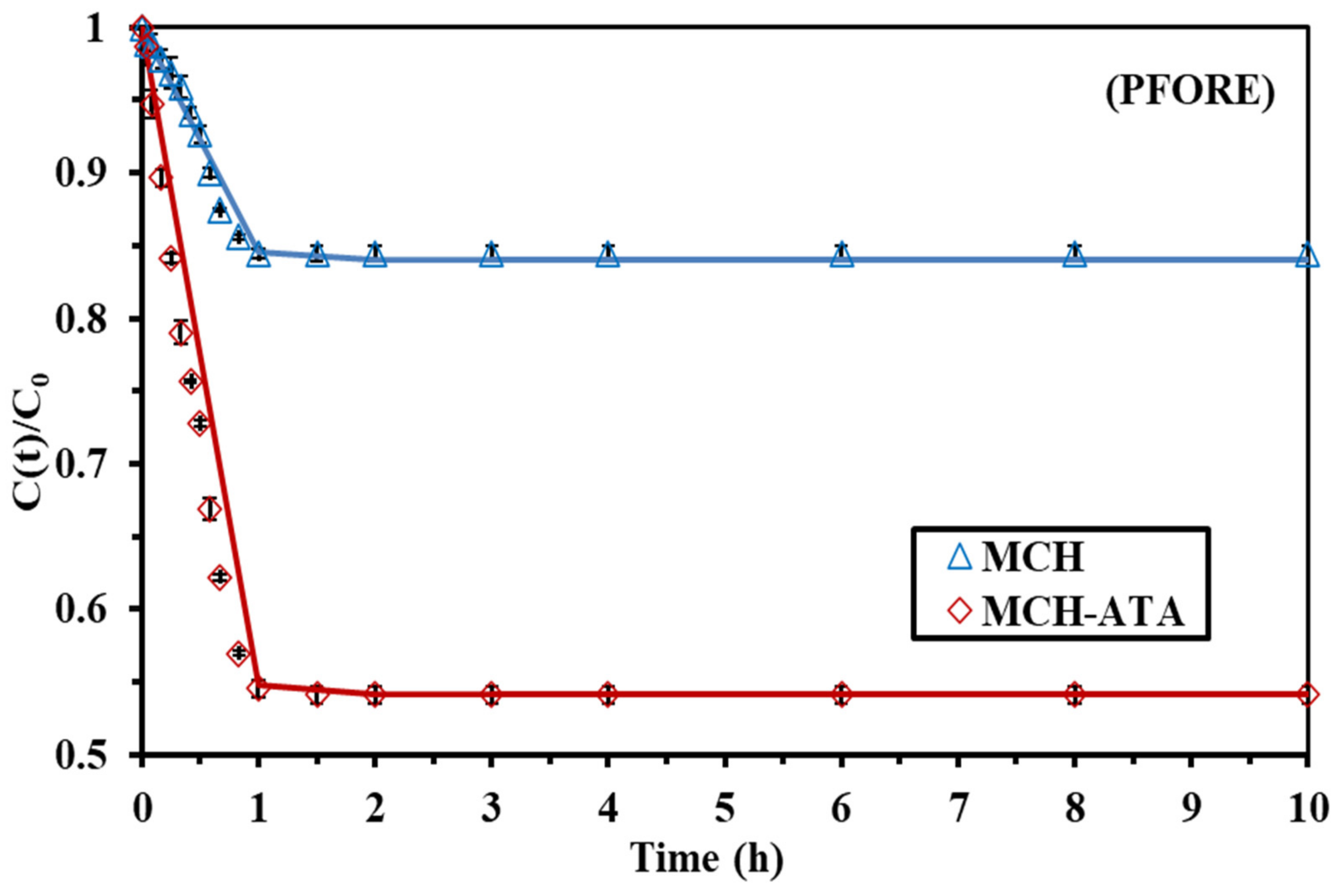
3.2.2. Uptake Kinetics
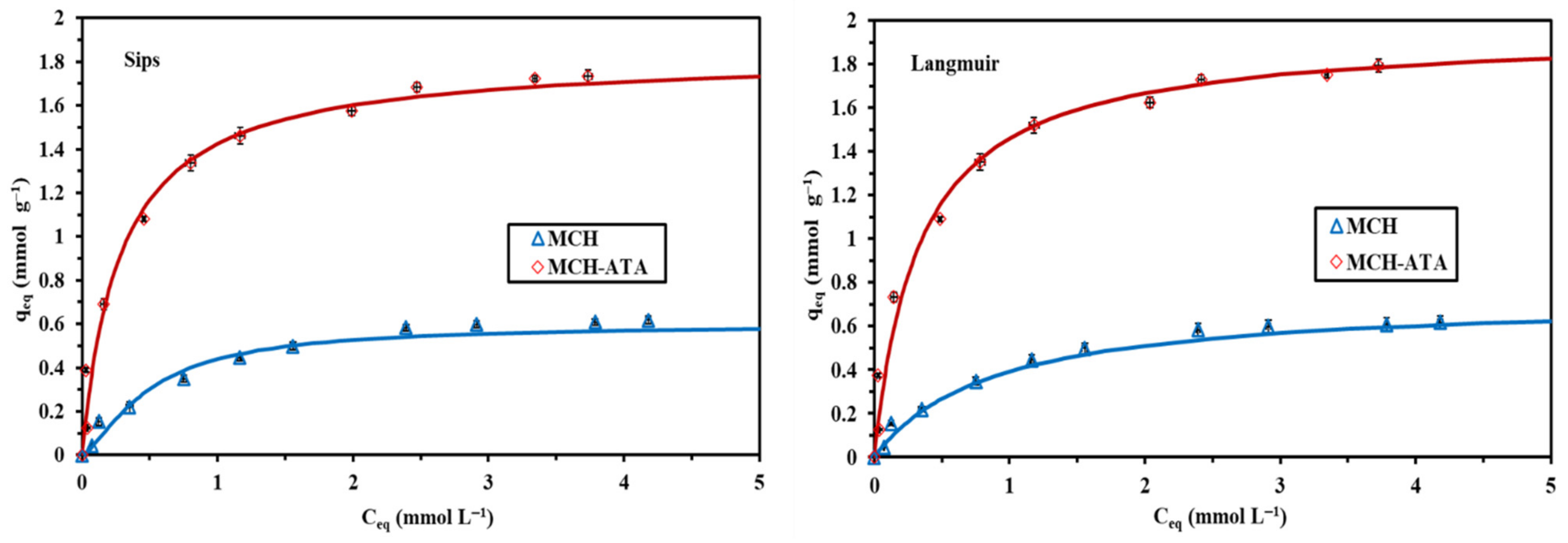
3.2.3. Sorption Isotherms
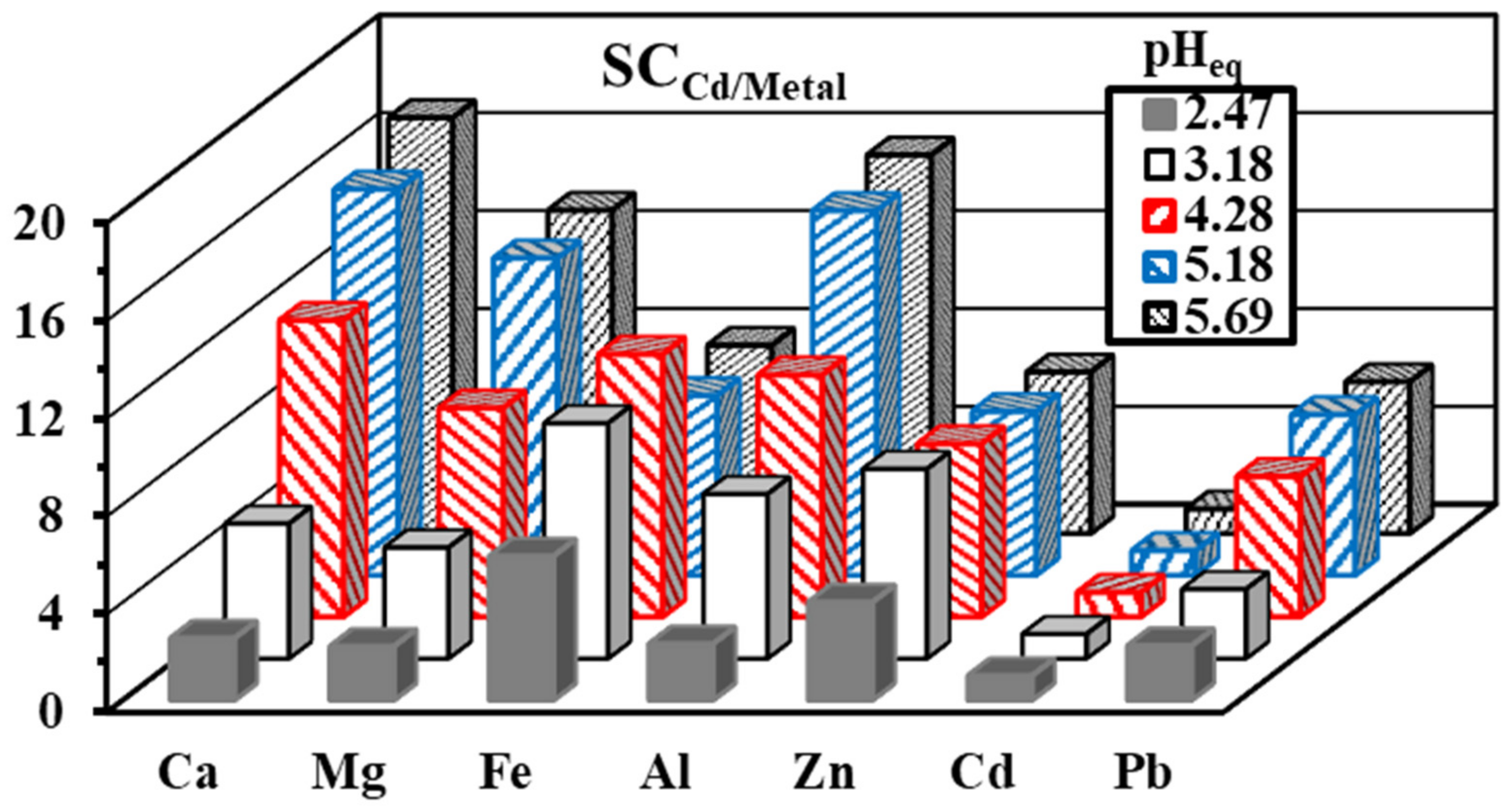
3.3. Selectivity–Sorption in Multi-Metal Solutions
3.4. Metal Desorption and Sorbent Recycling
3.5. Treatment of Contaminated Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 541. [Google Scholar]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavak, D. Removal of lead from aqueous solutions by precipitation: Statistical analysis and modeling. Desalin. Water Treat. 2013, 51, 1720–1726. [Google Scholar] [CrossRef]
- Lai, Y.C.; Lee, W.J.; Huang, K.L.; Wu, C.M. Metal recovery from spent hydrodesulfurization catalysts using a combined acid-leaching and electrolysis process. J. Hazard. Mater. 2008, 154, 588–594. [Google Scholar] [CrossRef]
- Otrembska, P.; Gega, J. Separation of nickel(II) and cadmium(II) ions with ion-exchange and membrane processes. Sep. Sci. Technol. 2016, 51, 2675–2680. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized biobased composite for metal decontamination-Insight on uranium and application to water samples collected from wells in mining areas (Sinai). Chem. Eng. J. 2021, 431, 133967. [Google Scholar] [CrossRef]
- Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd (II) and Pb (II) from Contaminated Effluents. Materials 2021, 14, 2189. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (Kw). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.; Shehal-Deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Kabay, N.; Demircioglu, M.; Yayli, S.; Gunay, E.; Yuksel, M.; Saglam, M.; Streat, M. Recovery of uranium from phosphoric acid solutions using chelating ion-exchange resins. Ind. Eng. Chem. Res. 1998, 37, 1983–1990. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, Y.; Kong, X.; Zhou, L.; Guo, H. Synthesis of phosphorus-modified poly(styrene-co-divinylbenzene) chelating resin and its adsorption properties of uranium(VI). J. Radioanal. Nucl. Chem. 2013, 298, 1137–1147. [Google Scholar] [CrossRef]
- Li, C.; Duan, H.D.; Wang, X.J.; Meng, X.; Qin, D.W. Fabrication of porous resins via solubility differences for adsorption of cadmium (II). Chem. Eng. J. 2015, 262, 250–259. [Google Scholar] [CrossRef]
- Shao, G.L.; Xiao, J.F.; Tian, Z.H.; Huang, J.J.; Yuan, S.G. Preparation and characterization of polyphenylene sulfide-based chelating resin-functionalized 2-amino-1,3,4-thiadiazole for selective removal Hg(II) from aqueous solutions. Polym. Adv. Technol. 2018, 29, 1030–1038. [Google Scholar] [CrossRef]
- Carro, L.; Barriada, J.L.; Herrero, R.; De Vicente, M.E.S. Adsorptive behaviour of mercury on algal biomass: Competition with divalent cations and organic compounds. J. Hazard. Mater. 2011, 192, 284–291. [Google Scholar] [CrossRef]
- Sinha, A.; Pant, K.K.; Khare, S.K. Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int. Biodeterior. Biodegrad. 2012, 71, 1–8. [Google Scholar] [CrossRef]
- Dutta, A.; Zhou, L.P.; Castillo-Araiza, C.O.; De Herdt, E. Cadmium(II), lead(II), and copper(II) biosorption on Baker’s yeast (Saccharomyces cerevesiae). J. Environ. Eng. 2016, 142, C6015002. [Google Scholar] [CrossRef]
- Safonov, A.; Tregubova, V.; Ilin, V.; Boldyrev, K.; Zinicovscaia, I.; Frontasyeva, M.; Khijniak, T. Comparative study of lanthanum, vanadium, and uranium bioremoval using different types of microorganisms. Water Air Soil Pollut. 2018, 229, 1–12. [Google Scholar] [CrossRef]
- Drenkova-Tuhtan, A.; Sheeleigh, E.K.; Rott, E.; Meyer, C.; Sedlak, D.L. Sorption of recalcitrant phosphonates in reverse osmosis concentrates and wastewater effluents–influence of metal ions. Water Sci. Technol. 2021, 83, 934–947. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Giraldo, J.D.; Rivas, B.L.; Elgueta, E.; Mancisidor, A. Metal ion sorption by chitosan-tripolyphosphate beads. J. Appl. Polym. Sci. 2017, 134, 45511. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Sastre, A.M.; Guibal, E. Pd and Pt recovery using chitosan gel beads: I. Influence of drying process on diffusion properties. Sep. Sci. Technol. 2002, 37, 2143–2166. [Google Scholar] [CrossRef]
- Djelad, A.; Morsli, A.; Robitzer, M.; Bengueddach, A.; Di Renzo, F.; Quignard, F. Sorption of Cu(II) ions on chitosan-zeolite X composites: Impact of gelling and drying conditions. Molecules 2016, 21, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, M.-W.; Kan, C.-C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Merrifield, J.D.; Davids, W.G.; MacRae, J.D.; Amirbahman, A. Uptake of mercury by thiol-grafted chitosan gel beads. Water Res. 2004, 38, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Roosen, J.; Binnemans, K. Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Atia, A.A. Uptake of U(VI) from aqueous media by magnetic Schiff’s base chitosan composite. J. Clean. Prod. 2014, 70, 292–302. [Google Scholar] [CrossRef]
- Liang, W.; Li, M.L.; Zhang, Z.Q.; Jiang, Y.H.; Awasthi, M.K.; Jiang, S.C.; Li, R.H. Decontamination of Hg(II) from aqueous solution using polyamine-co-thiourea inarched chitosan gel derivatives. Int. J. Biol. Macromol. 2018, 113, 106–115. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Hamza, M.F.; Abdel-Rahman, A.A.-H. Extraction studies of some hazardous metal ions using magnetic peptide resins. J. Dispers. Sci. Technol. 2015, 36, 411–422. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.-H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U (VI), Cu (II) and Zn (II)—Hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [Green Version]
- Mashaal, N.; Akagi, T.; Ishibashi, J. Hydrochemical and isotopic study of groundwater in Wadi El-Natrun, Western Desert, Egypt: Implication for salinization processes. J. Afr. Earth Sci. 2020, 172, 104011. [Google Scholar] [CrossRef]
- Hamza, M.F.; Ahmed, F.Y.; El-Aassy, I.; Fouda, A.; Guibal, E. Groundwater purification in a polymetallic mining area (SW Sinai, Egypt) using functionalized magnetic chitosan particles. Water Air Soil Pollut. 2018, 229, 360. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvao, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grondahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Abdel-Rahman, A.A.H.; Guibal, E. Magnetic glutamine—Grafted polymer for the sorption of U (VI), Nd (III) and Dy (III). J. Chem. Technol. Biotechnol. 2018, 93, 1790–1806. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 1–23. [Google Scholar]
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, characterization and anticoagulant activity of chitosan derivatives. Saudi Pharm. J. 2020, 28, 25–32. [Google Scholar] [CrossRef]
- Corazzari, I.; Nistico, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stabil. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, M.; Guo, Z.; Cui, Z. Alternatively chitosan sulfate blending membrane as methanol-blocking polymer electrolyte membrane for direct methanol fuel cell. J. Membr. Sci. 2009, 337, 318–323. [Google Scholar] [CrossRef]
- Caetano, C.S.; Caiado, M.; Farinha, J.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of free fatty acids over chitosan with sulfonic acid groups. Chem. Eng. J. 2013, 230, 567–572. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.M.; Abdel-Rahman, A.A.H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14, 7582–7626. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Krishnani, K.K.; Meng, X.G.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Peng, D.; Meng, P. Promotion effects of nitrogenous and oxygenic functional groups on cadmium (II) removal by carboxylated corn stalk. J. Clean. Prod. 2018, 201, 609–623. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blazquez, M.L.; Ballester, A.; Gonzalez, F.; Munoz, J.A. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef]
- Trakulsujaritchok, T.; Noiphom, N.; Tangtreamjitmun, N.; Saeeng, R. Adsorptive features of poly(glycidyl methacrylate-co-hydroxyethyl methacrylate): Effect of porogen formulation on heavy metal ion adsorption. J. Mater. Sci. 2011, 46, 5350–5362. [Google Scholar] [CrossRef]
- Ferrah, N.; Abderrahim, O.; Amine Didi, M.; Villemein, D. Sorption efficiency of a new sorbent towards cadmium(II): Methylphosphonic acid grafted polystyrene resins. J. Chem. 2013, 2013, 980825. [Google Scholar] [CrossRef]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 20–25. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S.; Ali, S.A. Removal of zinc and cadmium ions using a cross-linked polyaminophosphonate. J. Macromol. Sci. A 2013, 50, 375–384. [Google Scholar] [CrossRef]
- Rao, K.S.; Chaudhury, G.R.; Mishra, B.K. Kinetics and equilibrium studies for the removal of cadmium ions from aqueous solutions using Duolite ES 467 resin. Int. J. Miner. Process. 2010, 97, 68–73. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.J.; Li, J.S.; Sun, X.Y.; Han, W.Q. Adsorption behavior and mechanism of cadmium on strong-acid cation exchange resin. Trans. NonFerr. Met. Soc. China 2009, 19, 740–744. [Google Scholar] [CrossRef]
- Liu, M.Q.; Tao, Z.A.; Wang, H.C.; Zhao, F.; Sun, Q. Preparation and characterization of a series of porous anion-exchanger chelating fibers and their adsorption behavior with respect to removal of cadmium(II). RSC Adv. 2016, 6, 115222–115237. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2020, 55, 4193–4212. [Google Scholar] [CrossRef]
- Yang, Z.; Chai, Y.; Zeng, L.; Gao, Z.; Zhang, J.; Ji, H. Efficient removal of copper ion from wastewater using a stable chitosan gel material. Molecules 2019, 24, 4205. [Google Scholar] [CrossRef] [Green Version]
- Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Effect of agitation mode (mechanical, ultrasound and microwave) on uranium sorption using amine-and dithizone-functionalized magnetic chitosan hybrid materials. Chem. Eng. J. 2021, 411, 128553. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.I.; Abdel-Rahman, A.A.H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. Available online: http://www.fao.org/docrep/003/T0234E/T0234E00.htm (accessed on 20 June 2017).
- Gustafsson, J.P. Visual MINTEQ, ver. 3.1; KTH, Royal Institute of Technology: Stockholm, Sweden, 2013; Available online: https://vminteq.lwr.kth.se/ (accessed on 1 May 2017).
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Tien, C. Adsorption Calculations and Modeling; Butterworth-Heinemann: Newton, MA, USA, 1994; p. 243. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Lima, É.C.; Dehghani, M.H.; Guleria, A.; Sher, F.; Karri, R.R.; Dotto, G.L.; Tran, H.N. CHAPTER 3—Adsorption: Fundamental aspects and applications of adsorption for effluent treatment. In Green Technologies for the Defluoridation of Water; Hadi Dehghani, M., Karri, R., Lima, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–88. [Google Scholar]
- Buema, G.; Lupu, N.; Chiriac, H.; Ciobanu, G.; Bucur, R.D.; Bucur, D.; Favier, L.; Harja, M. Performance assessment of five adsorbents based on fly ash for removal of cadmium ions. J. Mol. Liq. 2021, 333, 115932. [Google Scholar] [CrossRef]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]


| Assignment | MCH | MCH-ATA | Loaded | Elution 5 Cycles | Ref. |
|---|---|---|---|---|---|
| O-H overlapped with N-H str. | 3427 | 3447, 3197 | 3404 | 3412 | [7,34,35] |
| C-H str. | 2917, 2850 | 2921, 2853 | 2847, 2917 | 2923, 2852 | [36] |
| C = O of carboxylic acid | 1728 | 1727 | [37] | ||
| C = O str. overlapped with C = C and C = N | 1638 | 1620 | 1621 | 1619 | [37,38] |
| N-H bend. | 1513 | 1511 | [37] | ||
| CH3 symm. def., C-N str. | 1383 | 1387, 1301 | 1300 | 1383 | [35,37,39] |
| C-O-C asymm. str., C-O str, and C-N str. | 1117 | 1219, | [6,34,38] | ||
| Skeletal C-O str. | 1019 | [34,35] | |||
| β-D-glucose | 886 | 1129 | 895 | ||
| C-O- epoxy | [39] | ||||
| C-O-S str. | 720 | [38] | |||
| -(CH2)n- rocking | 633 (broad) | 601 (broad) | 620 | [37] | |
| O-H out of plane bend. Fe-O and/or C-S | 572 | 583 | [40,41,42] |
| Sorbent | MCH | MCH-ATA | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | Parameter | Run No. | 1 | 2 | 3 | 1 | 2 | 3 |
| Exp. | qeq.exp. | 0.3397 | 0.3421 | 0.3467 | 1.0576 | 1.0287 | 1.0622 | |
| PFORE | qeq.1 | 0.3261 | 0.3304 | 0.3575 | 1.032 | 1.033 | 1.0979 | |
| k1 × 102 | 2.777 | 2.999 | 2.746 | 3.996 | 3.998 | 3.958 | ||
| R2 | 0.9677 | 0.9791 | 0.9971 | 0.9786 | 0.9694 | 0.9797 | ||
| AIC | −63.453 | −60.953 | −64.939 | −47.776 | −42.109 | −43.884 | ||
| PSORE | qeq.2 | 0.3082 | 0.314 | 0.3123 | 0.8918 | 0.8797 | 0.8796 | |
| k2 × 103 | 4.996 | 4.029 | 3.995 | 3.696 | 4.427 | 4.437 | ||
| R2 | 0.8177 | 0.8091 | 0.8671 | 0.7286 | 0.7994 | 0.7092 | ||
| AIC | −44.754 | −39.389 | −38.613 | −39.313 | −32.767 | −34.551 | ||
| RIDE | De × 1013 | 3.6948 | 3.745 | 3.799 | 1.161 | 1.117 | 1.1255 | |
| R2 | 0.8371 | 0.8169 | 0.8032 | 0.8073 | 0.8185 | 0.8196 | ||
| AIC | −60.276 | −57.677 | −60.994 | −44.049 | −41.867 | −40.151 | ||
| Model | Sorbent | MCH | MCH-ATA | ||||
|---|---|---|---|---|---|---|---|
| Parameter | 1 | 2 | 3 | 1 | 2 | 3 | |
| Experiment | qm,exp. | 0.607 | 0.615 | 0.621 | 1.755 | 1.792 | 1.785 |
| Langmuir | qm,L | 0.712 | 0.724 | 0.738 | 1.847 | 1.897 | 1.825 |
| bL | 1.154 | 1.394 | 1.594 | 2.794 | 2.596 | 2.453 | |
| R2 | 0.9985 | 0.9857 | 0.9687 | 0.9719 | 0.9840 | 0.9858 | |
| AIC | −83.849 | −88.094 | −80.843 | −62.457 | −66.551 | −66.4 | |
| Freundlich | kF | 0.439 | 0.364 | 0.453 | 1.194 | 1.285 | 1.353 |
| nF | 1.9747 | 2.01933 | 1.9978 | 2.503 | 2.6057 | 2.6983 | |
| R2 | 0.6767 | 0.6856 | 0.65869 | 0.7484 | 0.7746 | 0.7518 | |
| AIC | −25.316 | −24.213 | −22.225 | 8.8787 | 6.078 | 6.495 | |
| Sips | qm,S | 0.5975 | 0.6385 | 0.6982 | 1.8096 | 1.8257 | 1.8164 |
| bS | 2.7564 | 2.4837 | 2.4584 | 3.5192 | 3.4969 | 3.5014 | |
| nS | 0.7004 | 0.6957 | 0.7146 | 3.0038 | 2.9605 | 2.8758 | |
| R2 | 0.91587 | 0.90584 | 0.8918 | 0.9879 | 0.9583 | 0.9958 | |
| AIC | −76.5345 | −70.0205 | −71.78 | −62.265 | −61.279 | −63.293 | |
| Temkin | AT | 12.5718 | 14.0 | 13.01 | 23.0293 | 25.873 | 21.2771 |
| bT | 856 | 875 | 817 | 1025 | 1067 | 986 | |
| R2 | 0.6579 | 0.70371 | 0.81174 | 0.8.047 | 0.83375 | 0.81743 | |
| AIC | −12.7 | −13.97 | −12.87 | −22.37 | −23.9 | −23.837 | |
| Sorbent | PH | Equilibrium T (min) | Temp. | Initial Conc. mgL−1 | qm | Ref. |
|---|---|---|---|---|---|---|
| Rice-husk | 6 | 90 | 32± 0.5 | 200 | 0.13 | [46] |
| Carboxylate of corn stalk | 5.8 | 60 | 24.85 | 100 | 0.42 | [47] |
| EDTA-treated; Saccharomyces cerevisiae | 5 | 60 | 25 °C | 100 | 0.29 | [16] |
| beads of Ca-alginate | 6 | 480 | 23 ± 1 | 50 | 0.28 | [48] |
| Ca-alginate beads; Fucus vesiculosus | 6 | 480 | 23 ± 1 | 150 | 0.58 | [48] |
| Functionalized HEMA-PGMA resin with DETA | 5 | 50 | R.Temp | 500 | 0.32 | [49] |
| Methylphosphonic functionalized PS-resin | 5 | 180 | 19.85 | 112 | 0.34 | [50] |
| Amberlite IR (120) | 4–8 | 300 | 19.85 | 112 | 0.9 | [51] |
| Crosslinked resin polyaminophosphonate | 4 | 240 | 50 | 1000 | 0.48 | [52] |
| Duolite ES-467 resin | 4.8 | 90 | 60 | 140 | 0.15 | [53] |
| 001 × 7 cationic resin | 4–5 | 120 | 19.85 | 500 | 3.16 | [54] |
| Anion exchanger aminated fibers | 3 | 60 | 54 | 92.65 | 1.12 | [55] |
| Magnetite chitosan hydrazinyl amine (HAHZ-MG-CH) | 5 | 60 | 22 | 300 | 2.67 | [56] |
| Chitosan Alginate 1: 2 (CA#2) | 5 | 50 | 23 (±3) | 350 | 1.42 | [7] |
| Chitosan Alginate 1: 3 (CA#3) | 5 | 50 | 23 (±3) | 350 | 1.89 | [7] |
| Chitosan Alginate 1: 4 (CA#4) | 5 | 50 | 23 (±3) | 350 | 1.616 | [7] |
| Commercial activated carbon (CGAC) | 4 | 180 | 30 | 400 | 015 | [57] |
| MCH | 4 | 60 | 22 (±2) | 400 | 0.61 | This work |
| MCH-ATA | 4 | 30 | 22 (±2) | 400 | 1.78 | This work |
| Sorbent | Sorption Efficiency (%) | Desorption Efficiency (%); Using 0.2 M HCl | |||
|---|---|---|---|---|---|
| Cycle | Average | St. Dev. | Average | St. Dev. | |
| MCH | #1 | 16.15 | 1.97 | 99.439 | 1.528 |
| #2 | 15.65 | 1.83 | 100.00 | 0.069 | |
| #3 | 15.23 | 1.65 | 99.96 | 0.234 | |
| #4 | 14.91 | 1.57 | 100.00 | 0.04 | |
| #5 | 14.52 | 1.56 | 99.75 | 0.357 | |
| MCH-ATA | #1 | 46.89 | 0.53 | 99.71 | 0.319 |
| #2 | 46.41 | 0.75 | 100.00 | 0.153 | |
| #3 | 45.85 | 0.68 | 99.71 | 0.805 | |
| #4 | 45.54 | 1.03 | 99.81 | 0.552 | |
| #5 | 45.17 | 0.86 | 100.00 | 0.109 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.F.; Abdel-Rahman, A.A.-H.; Negm, A.S.; Hamad, D.M.; Khalafalla, M.S.; Fouda, A.; Wei, Y.; Amer, H.H.; Alotaibi, S.H.; Goda, A.E.-S. Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal—Application for Groundwater Treatment. Polymers 2022, 14, 1240. https://doi.org/10.3390/polym14061240
Hamza MF, Abdel-Rahman AA-H, Negm AS, Hamad DM, Khalafalla MS, Fouda A, Wei Y, Amer HH, Alotaibi SH, Goda AE-S. Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal—Application for Groundwater Treatment. Polymers. 2022; 14(6):1240. https://doi.org/10.3390/polym14061240
Chicago/Turabian StyleHamza, Mohammed F., Adel A.-H. Abdel-Rahman, Alyaa S. Negm, Doaa M. Hamad, Mahmoud S. Khalafalla, Amr Fouda, Yuezhou Wei, Hamada H. Amer, Saad H. Alotaibi, and Adel E.-S. Goda. 2022. "Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal—Application for Groundwater Treatment" Polymers 14, no. 6: 1240. https://doi.org/10.3390/polym14061240
APA StyleHamza, M. F., Abdel-Rahman, A. A.-H., Negm, A. S., Hamad, D. M., Khalafalla, M. S., Fouda, A., Wei, Y., Amer, H. H., Alotaibi, S. H., & Goda, A. E.-S. (2022). Grafting of Thiazole Derivative on Chitosan Magnetite Nanoparticles for Cadmium Removal—Application for Groundwater Treatment. Polymers, 14(6), 1240. https://doi.org/10.3390/polym14061240









