Shape-Memory Composites Based on Ionic Elastomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rubber Compounds
2.3. Characterization Methods
3. Results and Discussion
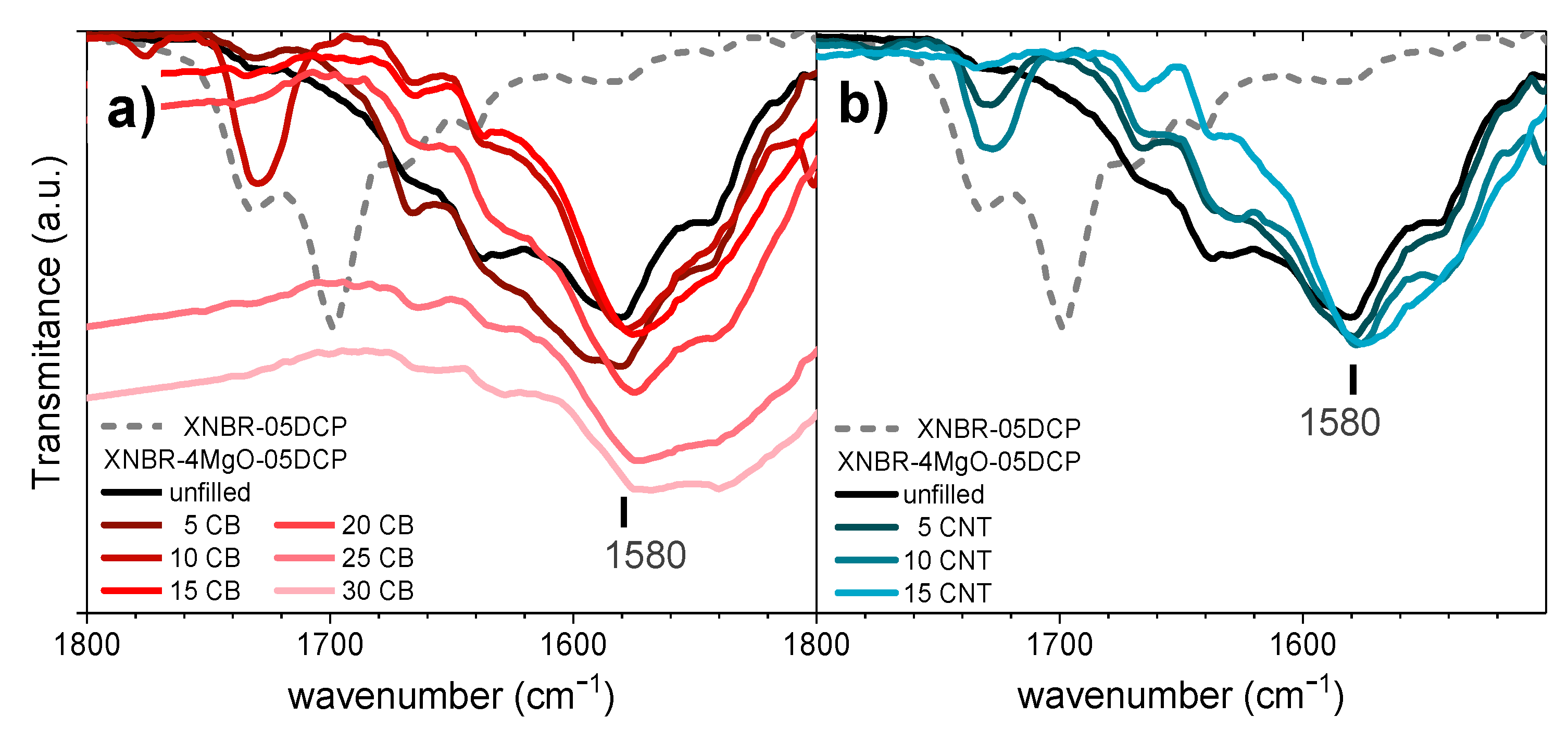
3.1. Formation of Rubber Networks
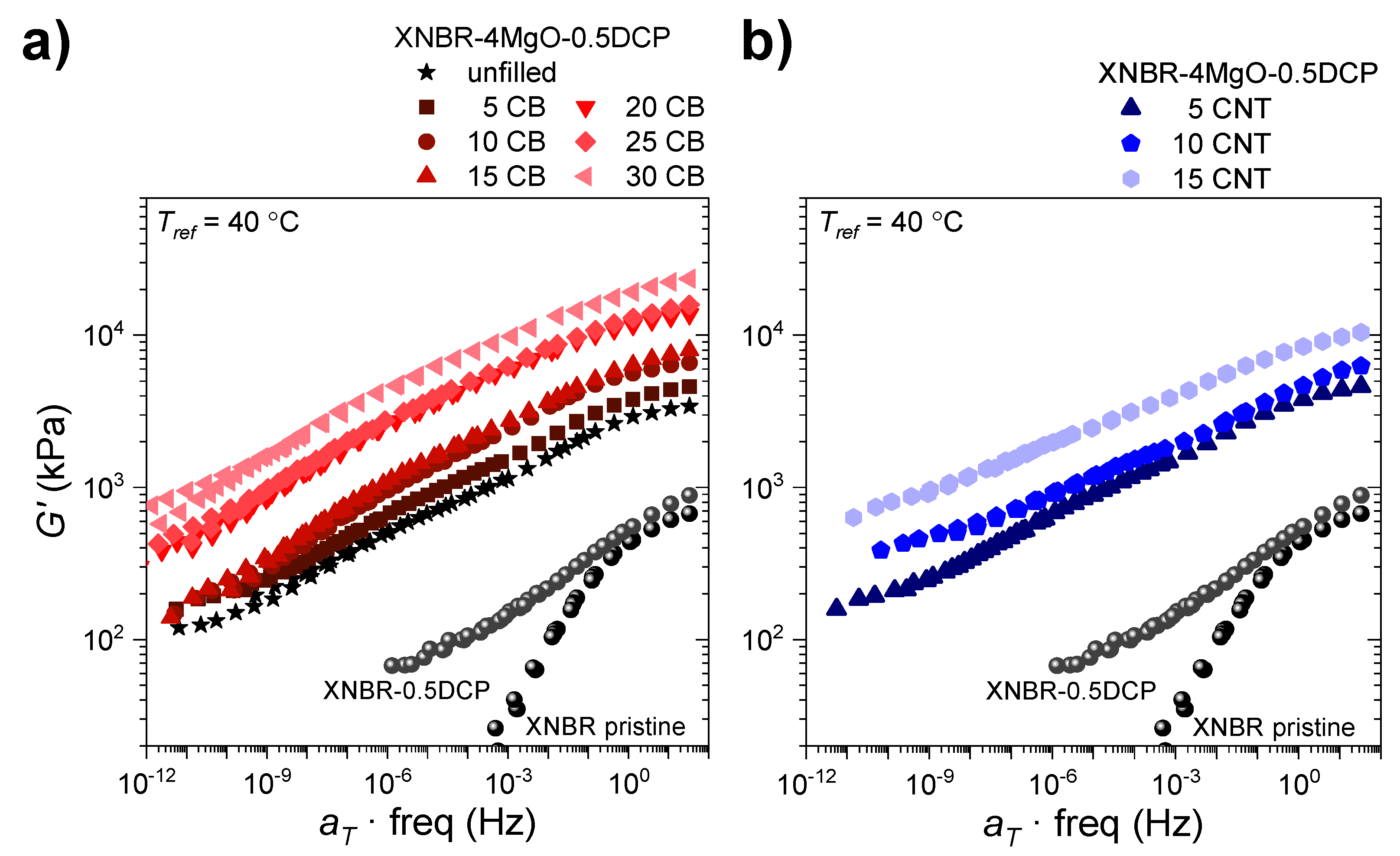
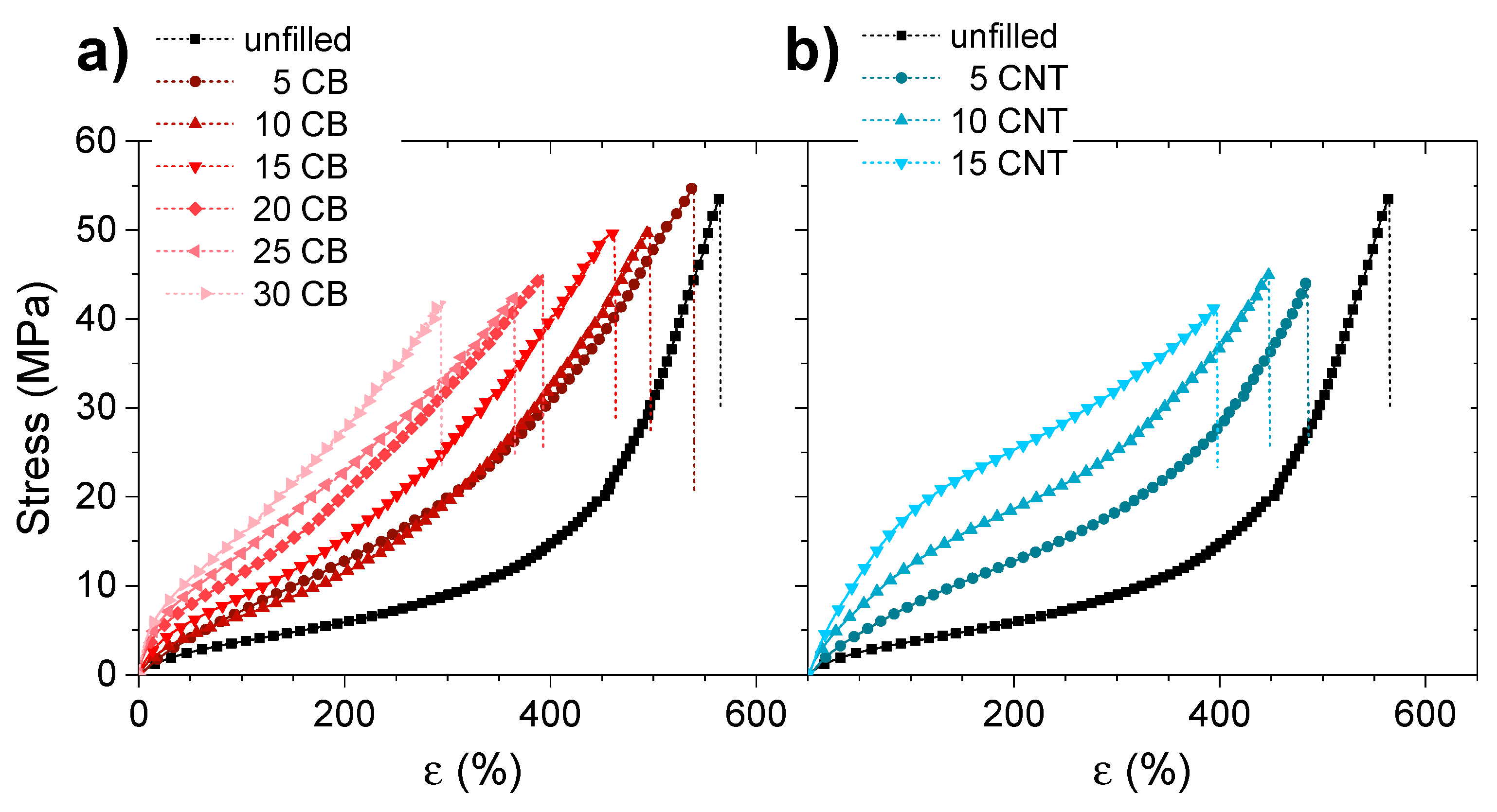
3.2. Physical Properties
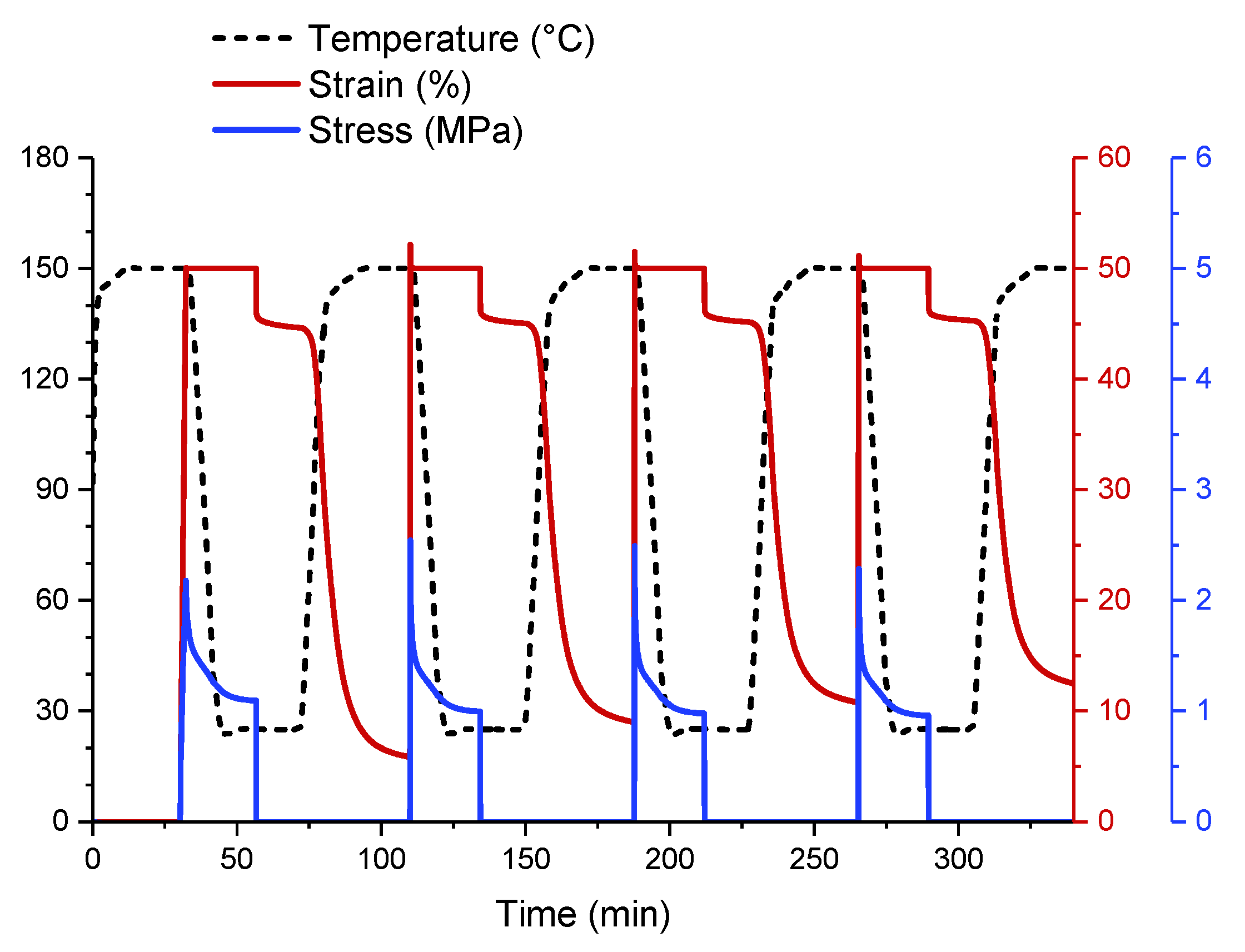
3.3. Shape-Memory Properties of Elastomeric Composites
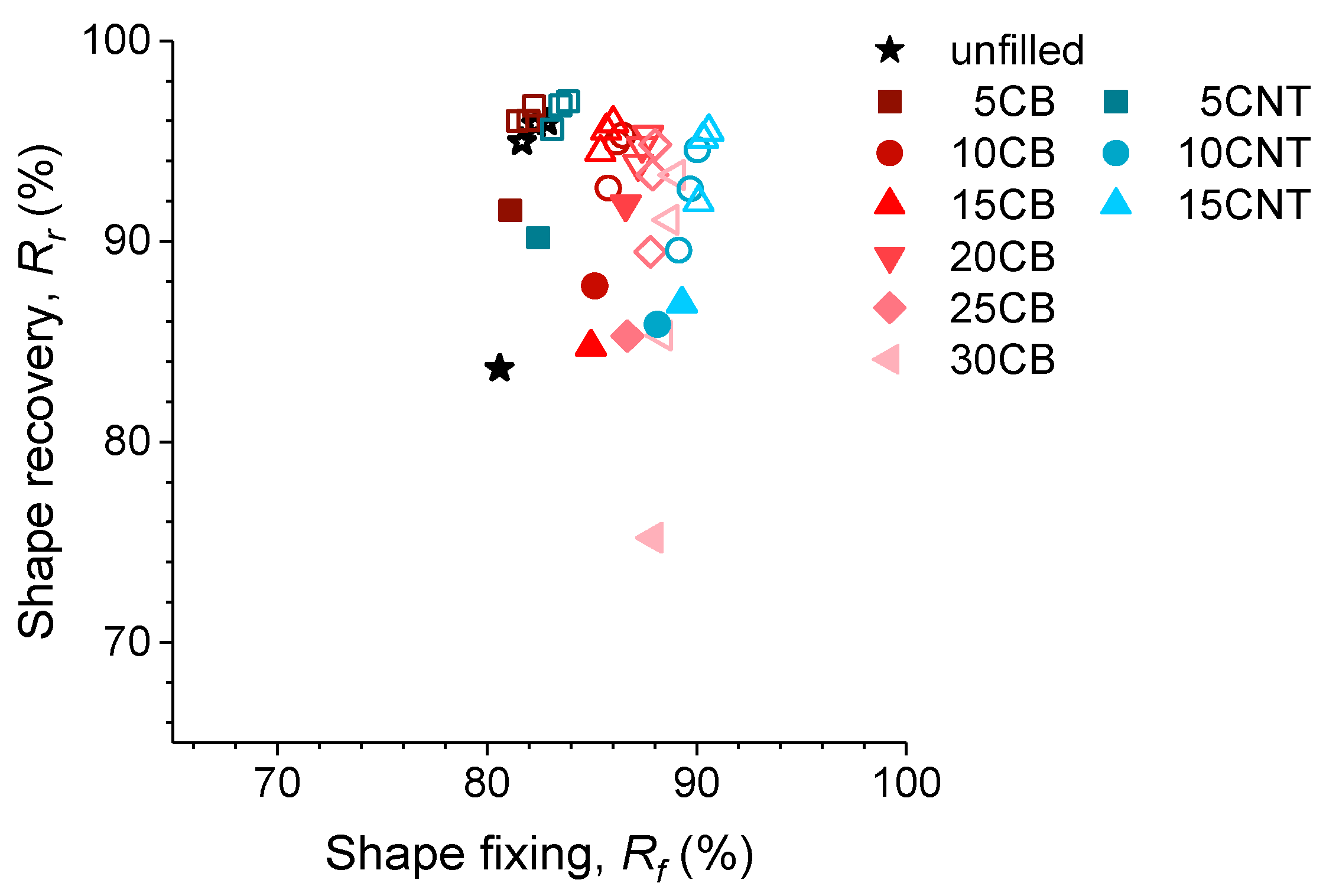
3.3.1. Effect of the Incorporation of Reinforcing Fillers on the Shape-Memory Properties of Elastomers
3.3.2. Effect of the Incorporation of Fillers on the Thermal Conductivity of Elastomeric Nanocomposites
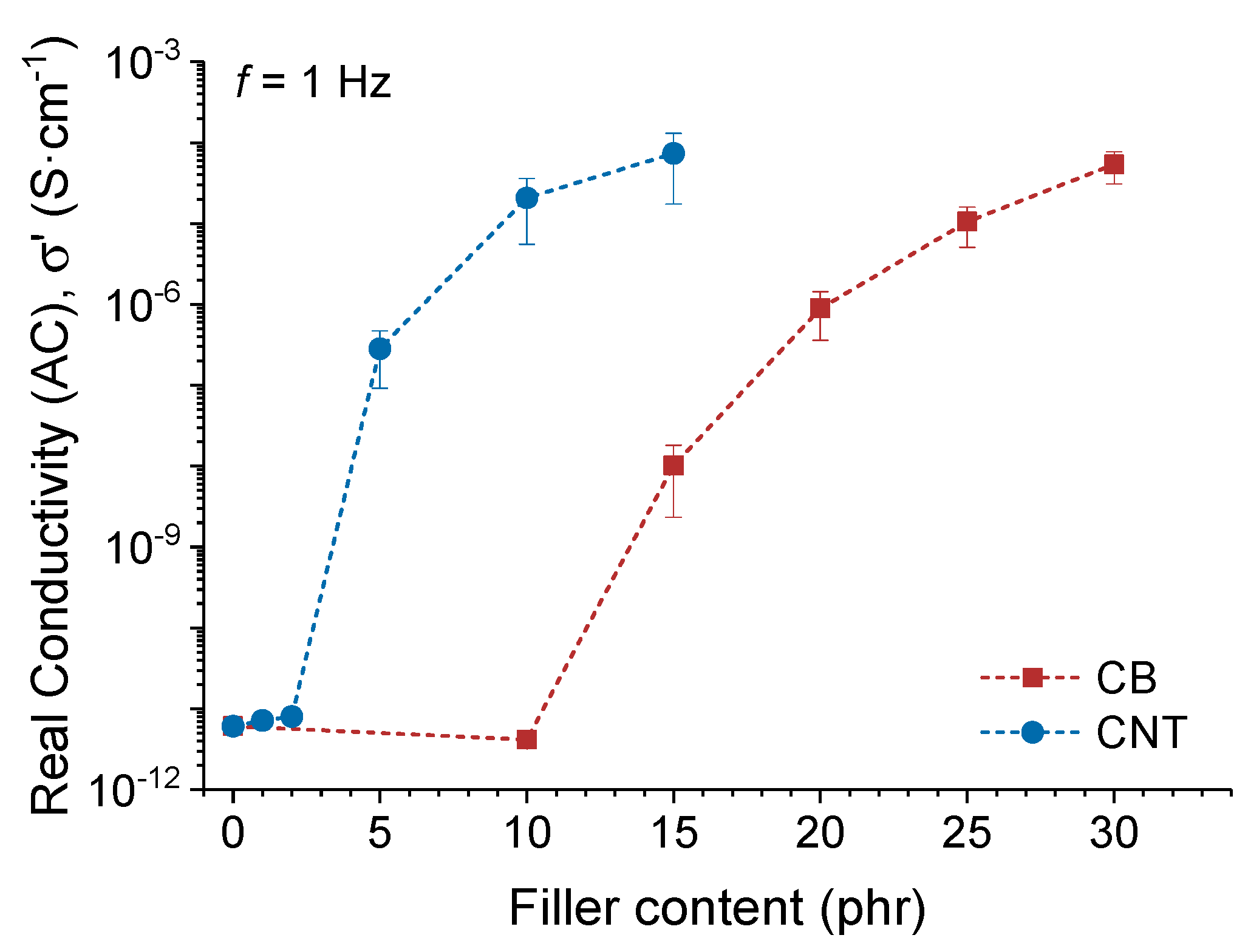
3.3.3. Electroactivation of the Shape-Memory Effect in Elastomeric Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Chung, T.; Romo-Uribe, A.; Mather, P.T. Two-Way Reversible Shape Memory in a Semicrystalline Network. Macromolecules 2008, 41, 184–192. [Google Scholar] [CrossRef]
- Li, J.; Rodgers, W.R.; Xie, T. Semi-Crystalline Two-Way Shape Memory Elastomer. Polymer 2011, 52, 5320–5325. [Google Scholar] [CrossRef]
- Heo, M.S.; Kim, T.H.; Chang, Y.W.; Jang, K.S. Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline. Polymers 2021, 13, 3984. [Google Scholar] [CrossRef] [PubMed]
- Pringpromsuk, S.; Xia, H.; Ni, Q.Q. Multifunctional Stimuli-Responsive Shape Memory Polyurethane Gels for Soft Actuators. Sens. Actuators A 2020, 313, 112207. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive Anti-Oxidant Polyurethane Elastomers with Shape Memory Property as Non-Adherent Wound Dressing to Enhance Wound Healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Gong, X.; Tan, K.; Deng, Q.; Shen, S. Athermal Shape Memory Effect in Magnetoactive Elastomers. ACS Appl. Mater. Interfaces 2020, 12, 16930–16936. [Google Scholar] [CrossRef] [PubMed]
- Testa, P.; Style, R.W.; Cui, J.; Donnelly, C.; Borisova, E.; Derlet, P.M.; Dufresne, E.R.; Heyderman, L.J. Magnetically Addressable Shape-Memory and Stiffening in a Composite Elastomer. Adv. Mater. 2019, 31, 1900561. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Xu, C.; Lin, B.; Fu, L. Enhanced, Hydrophobic, Initial-Shape Programmable Shape-Memory Composites with a Bio-Based Nano-Framework via Gradient Metal-Ligand Cross-Linking. Compos. Sci. Technol. 2022, 220, 109255. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H. Water-Induced Shape Memory Behavior of Poly (Vinyl Alcohol) and p-Coumaric Acid-Modified Water-Soluble Chitosan Blended Membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.M.; Guo, G.L.; Xie, Y.C.; Chen, J.M.; Xu, D.Y.; Wei, Y.E.; Cao, Z.R. A Novel Multi-Triggered Natural Rubber (NR)/Beeswax (BW)/Carbon Nanotube (CNT) Shape Memory Bio-Nanocomposite. J. Polym. Res. 2020, 27, 283. [Google Scholar] [CrossRef]
- Mather, P.T. Soft Answers for Hard Problems. Nat. Mater. 2006, 6, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Miriyev, A.; Stack, K.; Lipson, H. Soft Material for Soft Actuators. Nat. Commun. 2017, 8, 596. [Google Scholar] [CrossRef]
- Luo, X.; Mather, P.T. Preparation and Characterization of Shape Memory Elastomeric Composites. Macromolecules 2009, 42, 7251–7253. [Google Scholar] [CrossRef]
- Rousseau, I.A.; Mather, P.T. Shape Memory Effect Exhibited by Smectic-C Liquid Crystalline Elastomers. J. Am. Chem. Soc. 2003, 125, 15300–15301. [Google Scholar] [CrossRef]
- Cavicchi, K.A. Shape Memory Polymers from Blends of Elastomers and Small Molecule Additives. Macromol. Symp. 2015, 358, 194–201. [Google Scholar] [CrossRef]
- Xie, T.; Rousseau, I.A. Facile Tailoring of Thermal Transition Temperatures of Epoxy Shape Memory Polymers. Polymer 2009, 50, 1852–1856. [Google Scholar] [CrossRef]
- Heuwers, B.; Beckel, A.; Krieger, A.; Katzenberg, F.; Tiller, J.C. Shape-Memory Natural Rubber: An Exceptional Material for Strain and Energy Storage. Macromol. Chem. Phys. 2013, 214, 912–923. [Google Scholar] [CrossRef]
- Heuwers, B.; Quitmann, D.; Hoeher, R.; Reinders, F.M.; Tiemeyer, S.; Sternemann, C.; Tolan, M.; Katzenberg, F.; Tiller, J.C. Stress-Induced Stabilization of Crystals in Shape Memory Natural Rubber. Macromol. Rapid Commun. 2013, 34, 180–184. [Google Scholar] [CrossRef]
- Pantoja, M.; Lin, Z.; Cakmak, M.; Cavicchi, K.A. Structure-Property Relationships of Fatty Acid Swollen, Crosslinked Natural Rubber Shape Memory Polymers. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 673–688. [Google Scholar] [CrossRef]
- Mehrbakhsh, E.; Rezaei, M.; Babaie, A.; Mohammadi, A.; Mayan Sofla, R.L. Physical and Thermo-Mechanical Properties of Shape Memory Polyurethane Containing Reversible Chemical Cross-Links. J. Mech. Behav. Biomed. Mater. 2021, 116, 104336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Li, Q.; Wu, C. SBS Thermoplastic Elastomer Based on Dynamic Metal-Ligand Bond: Structure, Mechanical Properties, and Shape Memory Behavior. Macromol. Mater. Eng. 2021, 306, 2000737. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-Responsive Supramolecular Polymeric Materials. Chem. Soc. Rev. 2012, 41, 6042. [Google Scholar] [CrossRef]
- Cavicchi, K.A.; Pantoja, M.; Cakmak, M. Shape Memory Ionomers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1389–1396. [Google Scholar] [CrossRef]
- Weiss, R.A.; Izzo, E.; Mandelbaum, S. New Design of Shape Memory Polymers: Mixtures of an Elastomeric Ionomer and Low Molar Mass Fatty Acids and Their Salts. Macromolecules 2008, 41, 2978–2980. [Google Scholar] [CrossRef]
- Dong, J.; Weiss, R.A. Shape Memory Behavior of Zinc Oleate-Filled Elastomeric Ionomers. Macromolecules 2011, 44, 8871–8879. [Google Scholar] [CrossRef]
- Xie, T. Tunable Polymer Multi-Shape Memory Effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef]
- Li, J.; Xie, T. Significant Impact of Thermo-Mechanical Conditions on Polymer Triple-Shape Memory Effect. Macromolecules 2011, 44, 175–180. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, C.; Wu, W.; Shen, Q.; Lin, B.; Fu, L. Structure and Performance of Carboxylic Styrene Butadiene Rubber/Citric Acid Composite Films. Ind. Eng. Chem. Res. 2020, 59, 13613–13622. [Google Scholar] [CrossRef]
- Salaeh, S.; Das, A.; Wießner, S. Design and Fabrication of Thermoplastic Elastomer with Ionic Network: A Strategy for Good Performance and Shape Memory Capability. Polymer 2021, 223, 123699. [Google Scholar] [CrossRef]
- González-Jiménez, A.; Malmierca, M.A.; Bernal-Ortega, P.; Posadas, P.; Pérez-Aparicio, R.; Marcos-Fernández, Á.; Mather, P.T.; Valentín, J.L. The Shape-Memory Effect in Ionic Elastomers: Fixation through Ionic Interactions. Soft Matter 2017, 13, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.A.; González-Jiménez, A.; Mora-Barrantes, I.; Posadas, P.; Rodríguez, A.; Ibarra, L.; Nogales, A.; Saalwächter, K.; Valentín, J.L. Characterization of Network Structure and Chain Dynamics of Elastomeric Ionomers by Means of 1H Low-Field NMR. Macromolecules 2014, 47, 5655–5667. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but Strong: A Review of the Mechanical Properties of Carbon Nanotube–Polymer Composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Meng, H.; Li, G. A Review of Stimuli-Responsive Shape Memory Polymer Composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef]
- Chen, J.; Cui, X.; Sui, K.; Zhu, Y.; Jiang, W. Balance the Electrical Properties and Mechanical Properties of Carbon Black Filled Immiscible Polymer Blends with a Double Percolation Structure. Compos. Sci. Technol. 2017, 140, 99–105. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Mondal, T.; Bhowmick, A.K.; Ghosal, R.; Mukhopadhyay, R. Graphene-Based Elastomer Nanocomposites: Functionalization Techniques, Morphology, and Physical Properties; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 267–318. [Google Scholar]
- Yang, Z.; Liu, J.; Liao, R.; Yang, G.; Wu, X.; Tang, Z.; Guo, B.; Zhang, L.; Ma, Y.; Nie, Q.; et al. Rational Design of Covalent Interfaces for Graphene/Elastomer Nanocomposites. Compos. Sci. Technol. 2016, 132, 68–75. [Google Scholar] [CrossRef]
- Araby, S.; Meng, Q.; Zhang, L.; Kang, H.; Majewski, P.; Tang, Y.; Ma, J. Electrically and Thermally Conductive Elastomer/Graphene Nanocomposites by Solution Mixing. Polymer 2014, 55, 201–210. [Google Scholar] [CrossRef]
- Shan, W.; Lu, T.; Majidi, C. Soft-Matter Composites with Electrically Tunable Elastic Rigidity. Smart Mater. Struct. 2013, 22, 085005. [Google Scholar] [CrossRef]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive Polymer Composites with Segregated Structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- O’Halloran, A.; O’Malley, F.; McHugh, P. A Review on Dielectric Elastomer Actuators, Technology, Applications, and Challenges. J. Appl. Phys. 2008, 104, 071101. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. A Review of Vapor Grown Carbon Nanofiber/Polymer Conductive Composites. Carbon 2009, 47, 2–22. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-Responsive Shape Memory Materials: A Review. Mater. Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Bokobza, L. Multiwall Carbon Nanotube Elastomeric Composites: A Review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef]
- Pérez, L.D.; Giraldo, L.F.; Brostow, W.; López, B.L. Poly(Methyl Acrylate) plus Mesoporous Silica Nanohybrids: Mechanical and Thermophysical Properties. e-Polymers 2007, 7, 324–334. [Google Scholar] [CrossRef]
- Zhou, X.-W.; Zhu, Y.-F.; Liang, J. Preparation and Properties of Powder Styrene–Butadiene Rubber Composites Filled with Carbon Black and Carbon Nanotubes. Mater. Res. Bull. 2007, 42, 456–464. [Google Scholar] [CrossRef]
- Choudhury, A.; Bhowmick, A.K.; Ong, C.; Soddemann, M. Effect of Various Nanofillers on Thermal Stability and Degradation Kinetics of Polymer Nanocomposites. J. Nanosci. Nanotechnol. 2010, 10, 5056–5071. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotubes: Science and Applications; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780849321115. [Google Scholar]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Das, N.C.; Maiti, S. Electromagnetic Interference Shielding of Carbon Nanotube/Ethylene Vinyl Acetate Composites. J. Mater. Sci. 2008, 43, 1920–1925. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H.; Shanmugharaj, A.M. Thermal, Mechanical and Electroactive Shape Memory Properties of Polyurethane (PU)/Poly (Lactic Acid) (PLA)/CNT Nanocomposites. Eur. Polym. J. 2013, 49, 3492–3500. [Google Scholar] [CrossRef]
- Costa, P.; Silva, J.; Sencadas, V.; Simoes, R.; Viana, J.C.; Lanceros-Méndez, S. Mechanical, Electrical and Electro-Mechanical Properties of Thermoplastic Elastomer Styrene–Butadiene–Styrene/Multiwall Carbon Nanotubes Composites. J. Mater. Sci. 2013, 48, 1172–1179. [Google Scholar] [CrossRef]
- Kurup, S.N.; Ellingford, C.; Wan, C. Shape Memory Properties of Polyethylene/Ethylene Vinyl Acetate /Carbon Nanotube Composites. Polym. Test. 2020, 81, 106227. [Google Scholar] [CrossRef]
- Hohimer, C.J.; Petrossian, G.; Ameli, A.; Mo, C.; Pötschke, P. 3D printed conductive thermoplastic polyurethane/carbon nanotube composites for capacitive and piezoresistive sensing in soft pneumatic actuators. Addit. Manuf. 2020, 34, 101281. [Google Scholar] [CrossRef]
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Ortega, P.; Bernal, M.M.; González-Jiménez, A.; Posadas, P.; Navarro, R.; Valentín, J.L. New Insight into Structure-Property Relationships of Natural Rubber and Styrene-Butadiene Rubber Nanocomposites Filled with MWCNT. Polymer 2020, 201, 122604. [Google Scholar] [CrossRef]
- Sasikumar, K.; Manoj, N.R.; Mukundan, T.; Khastgir, D. Design of XNBR Nanocomposites for Underwater Acoustic Sensor Applications: Effect of MWNT on Dynamic Mechanical Properties and Morphology. J. Appl. Polym. Sci. 2014, 131, 40752. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Lin, B.; Liang, X.; Chen, Y. Design of Self-Healing Supramolecular Rubbers by Introducing Ionic Cross-Links into Natural Rubber via a Controlled Vulcanization. ACS Appl. Mater. Interfaces 2016, 8, 17728–17737. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, J.; Zhang, L.; Liu, S.; Zan, X.; Nishi, T.; Ning, N. Graphene Encapsulated Rubber Latex Composites with High Dielectric Constant, Low Dielectric Loss and Low Percolation Threshold. J. Colloid Interface Sci. 2014, 430, 249–256. [Google Scholar] [CrossRef]
- Liu, X.; Sun, D.; Wang, L.; Guo, B. Sodium Humate Functionalized Graphene and Its Unique Reinforcement Effects for Rubber. Ind. Eng. Chem. Res. 2013, 52, 14592–14600. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H.; Tang, Y.; Ji, D.; Sun, Y.; Gong, X.; Ding, L. Enhancements of the Mechanical Properties and Thermal Conductivity of Carboxylated Acrylonitrile Butadiene Rubber with the Addition of Graphene Oxide. J. Mater. Sci. 2012, 48, 1571–1577. [Google Scholar] [CrossRef]
- Nair, K.P.; Thomas, P.; Joseph, R. Technical Report. Mater. Des. 2012, 41, 23–30. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1980; ISBN 9780471048947. [Google Scholar]
- Rousseau, I.A. Challenges of Shape Memory Polymers: A Review of the Progress toward Overcoming SMP’s Limitations. Polym. Eng. Sci. 2008, 48, 2075–2089. [Google Scholar] [CrossRef]
- Mora-Barrantes, I.; Malmierca, M.A.A.; Valentin, J.L.; Rodriguez, A.; Ibarra, L. Effect of Covalent Cross-Links on the Network Structure of Thermo-Reversible Ionic Elastomers. Soft Matter 2012, 8, 5201. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ibarra, L.; Mora, I. Effect of Low-Load Reinforcing Nanofillers on Rheological Behavior and Physical Properties of Ionic Elastomers Based on XNBR and MgO. J. Appl. Polym. Sci. 2007, 106, 973–980. [Google Scholar] [CrossRef]
- González, A.E. Viscosity of Ionomer Gels. Polymer 1983, 24, 77–80. [Google Scholar] [CrossRef]
- González, A.E. Viscoelasticity of Ionomer Gels: 2. The Elastic Moduli. Polymer 1984, 25, 1469–1474. [Google Scholar] [CrossRef]
- Leibler, L.; Rubinstein, M.; Colby, R.H. Dynamics of Reversible Networks. Macromolecules 1991, 24, 4701–4707. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S.F. Viscoelastic Properties of Physically Crosslinked Networks. 1. Transient Network Theory. Macromolecules 1992, 25, 1516–1523. [Google Scholar] [CrossRef]
- Rubinstein, M.; Semenov, A.N. Dynamics of Entangled Solutions of Associating Polymers. Macromolecules 2001, 34, 1058–1068. [Google Scholar] [CrossRef]
- Lu, H.; Yin, J.; Xu, B.; Gou, J.; Hui, D.; Fu, Y. Synergistic Effects of Carboxylic Acid-Functionalized Carbon Nanotube and Nafion/Silica Nanofiber on Electrical Actuation Efficiency of Shape Memory Polymer Nanocomposite. Compos. Part B 2016, 100, 146–151. [Google Scholar] [CrossRef]
- Le, H.H.; Kolesov, I.; Ali, Z.; Uthardt, M.; Osazuwa, O.; Ilisch, S.; Radusch, H.-J. Effect of Filler Dispersion Degree on the Joule Heating Stimulated Recovery Behaviour of Nanocomposites. J. Mater. Sci. 2010, 45, 5851–5859. [Google Scholar] [CrossRef]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-Memory Polymers and Their Composites: Stimulus Methods and Applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]







| Sample | XNBR | MgO | DCP | Stearic Acid | Filler | |
|---|---|---|---|---|---|---|
| phr | phr | phr | phr | phr | Type | |
| XNBR-4MgO-0.5DCP (unfilled) | 100 | 4 | 0.5 | 1 | ||
| 5CB | 100 | 4 | 0.5 | 1 | 5 | CB |
| 10CB | 100 | 4 | 0.5 | 1 | 10 | CB |
| 15CB | 100 | 4 | 0.5 | 1 | 15 | CB |
| 20CB | 100 | 4 | 0.5 | 1 | 20 | CB |
| 25CB | 100 | 4 | 0.5 | 1 | 25 | CB |
| 30CB | 100 | 4 | 0.5 | 1 | 30 | CB |
| 5CNT | 100 | 4 | 0.5 | 1 | 5 | CNT |
| 10CNT | 100 | 4 | 0.5 | 1 | 10 | CNT |
| 15CNT | 100 | 4 | 0.5 | 1 | 15 | CNT |
| Sample | Tensile Strength | Elongation at Break | ||
|---|---|---|---|---|
| MPa | % | |||
| Unfilled | 51 | ±3 | 560 | ±10 |
| 5CB | 52 | ±5 | 506 | ±37 |
| 10CB | 49 | ±6 | 493 | ±10 |
| 15CB | 50 | ±2 | 462 | ±17 |
| 20CB | 45 | ±2 | 399 | ±35 |
| 25CB | 44 | ±1 | 353 | ±39 |
| 30CB | 42 | ±2 | 308 | ±23 |
| 5CNT | 45 | ±3 | 493 | ±34 |
| 10CNT | 44 | ±4 | 453 | ±27 |
| 15CNT | 42 | ±2 | 389 | ±18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Jiménez, A.; Bernal-Ortega, P.; Salamanca, F.M.; Valentin, J.L. Shape-Memory Composites Based on Ionic Elastomers. Polymers 2022, 14, 1230. https://doi.org/10.3390/polym14061230
González-Jiménez A, Bernal-Ortega P, Salamanca FM, Valentin JL. Shape-Memory Composites Based on Ionic Elastomers. Polymers. 2022; 14(6):1230. https://doi.org/10.3390/polym14061230
Chicago/Turabian StyleGonzález-Jiménez, Antonio, Pilar Bernal-Ortega, Fernando M. Salamanca, and Juan L. Valentin. 2022. "Shape-Memory Composites Based on Ionic Elastomers" Polymers 14, no. 6: 1230. https://doi.org/10.3390/polym14061230
APA StyleGonzález-Jiménez, A., Bernal-Ortega, P., Salamanca, F. M., & Valentin, J. L. (2022). Shape-Memory Composites Based on Ionic Elastomers. Polymers, 14(6), 1230. https://doi.org/10.3390/polym14061230









