The Synergetic Impact of Anionic, Cationic, and Neutral Polymers on VES Rheology at High-Temperature Environment
Abstract
:1. Introduction
2. VES Applications
2.1. VES as Acid Diverter
2.2. VES in Enhanced Oil Recovery (EOR)
2.3. VES in Hydraulic Fracturing
2.4. VES Mixed with Polymers
2.5. Cellulose Functional Groups
3. Materials and Methods
3.1. Materials
3.2. Experimental Design
4. Results and Discussion
4.1. Influence of CMC on VES Fluid
4.2. Influence of Hydroxyethyl Cellulose (HEC) on VES Fluid
4.3. Influence of CaCl2 on VES
4.4. Temperature Impact on VES Systems
4.5. Influence of Poly Diallyldimethylammonium Chloride (DADMAC) on VES Fluid
The Impact of CaCl2 on VES
5. Conclusions
- The study shows that polymers can be used to reduce the VES concentration and improve the rheology.
- Different concentrations of polymers were added to VES and compared to pure VES rheology.
- CaCl2 concentration can regulate the VES viscosity at different temperatures. For instance, at low temperatures, the low CaCl2 concentrations can yield larger viscosities and vice versa for high temperatures.
- At different CaCl2 concentrations, the VES system viscosity became stable after the first 30 min.
- The system containing 9 (v/v) % VES and 0.1% HEC has a superior viscosity than the one containing 10 (v/v) % VES at temperatures lower than 93.3 °C in 30 (w/v) % CaCl2 solution.
- The systems containing 9 (v/v) % VES, 9 (v/v) % VES + 0.1% HEC, and 9 (v/v) % VES + 0.5% HEC have very similar viscosity values above 160 °C.
- A synergistic effect of CMC, HEC, and DADMAC on the viscosity of VES was observed. While HEC in freshwater provides a higher viscosity at lower temperatures; CMC has relatively better thermal stability. The DADMAC polymer showed excellent viscosity and stability in seawater.
- Charge of the polymer apparently has a significant impact in influencing the rheology of the VES fluid.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VES | viscoelastic surfactants |
| CMC | carboxymethyl cellulose |
| HEC | hydroxyethyl cellulose |
| DADMAC | poly diallyl dimethylammonium chloride |
| HPHT | high pressure high temperature |
| PFOS | perfluoro octane sulfonate |
| DOSS | dioctyl sodium sulfosuccinate |
| Triton X-100 | ethylene glycol octyl phenol ethers |
| FMES | fatty methyl ester sulfonate |
| CTAB | cetyltrimethylammonium bromide |
| CTAC | cetyltrimethylammonium chloride |
| SDS | Sodium Dodecyl Sulfate |
| CHAPS | 3-[(3-Cholamidopropyl) dimethyl ammonio]-1-propane sulfonate |
| TEM | transmission electrical microscopy |
| SEM | scanning electron microscope |
| AFM | atomic force microscope |
| DOAPA | N,N-dimethylolamidopropylamine |
| DTPA | diethylene triamine pentaacetic acid |
References
- Economides, M.J.; Nolte, K.G. Reservoir Stimulation; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Das, P.; Rahim, Z. Evaluate Fracturing Fluid Performance for Hydraulic Stimulation in Pre-Khuff Sandstone Reservoirs of Ghawar Gas Field. In SPE Saudi Arabia Section Technical Symposium and Exhibition; Society of Petroleum Engineers: Al-Khobar, Saudi Arabia, 2014; Paper Number: SPE-172217-MS. [Google Scholar] [CrossRef]
- Samuel, M.; Polson, D.; Graham, D.; Kordziel, W.; Waite, T.; Waters, G.; Vinod, P.S.; Fu, D.; Downey, R. Viscoelastic surfactant fracturing fluids: Applications in low permeability reservoirs. In SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition; Society of Petroleum Engineers: Denver, CO, USA, 2000. [Google Scholar] [CrossRef]
- Gandossi, L.; Von Estorff, U. An overview of hydraulic fracturing and other formation stimulation technologies for shale gas production. Eur. Commisison Jt. Res. Cent. Tech. Rep. 2015, EUR 26347. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC98582/an%20overview%20of%20hydraulic%20fracturing%20and%20other%20stimulation%20technologies%20-%20update%202015.pdf (accessed on 25 September 2021). [CrossRef]
- Hoffman, A.; Olsson, G.; Lindström, A. Shale Gas and Hydraulic Fracturing: Framing the Water Issue; Report Nr. 34; SIWI: Stockholm, Sweden, 2014. [Google Scholar]
- Kang, W.; Mushi, S.J.; Yang, H.; Wang, P.; Hou, X. Development of smart viscoelastic surfactants and its applications in fracturing fluid: A review. J. Pet. Sci. Eng. 2020, 190, 107107. [Google Scholar] [CrossRef]
- Anandan, R.; Johnson, S.; Barati, R. Polyelectrolyte complex stabilized CO2 Foam systems for hydraulic fracturing application. Soc. Pet. Eng. 2017-SPE Liq.-Rich Basins Conf.-N. Am. 2017, 187489. [Google Scholar] [CrossRef]
- Faroughi, S.A.; Pruvot, A.J.; McAndrew, J. The rheological behavior of energized fluids and foams with application to hydraulic fracturing: Review. J. Pet. Sci. Eng. 2018, 163, 243–263. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, K. A review of recent advances in foam-based fracturing fluid application in unconventional reservoirs. J. Ind. Eng. Chem. 2018, 66, 45–71. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Harris, P.C.; van Batenburg, D. A Comparison of Freshwater- and Seawater-Based Borate-Crosslinked Fracturing Fluids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 16–19 February 1999. [Google Scholar] [CrossRef]
- Hirsch, M. Lowering Surface Tension–Surfactants in Coating Materials. Available online: https://knowledge.ulprospector.com/3106/pc-surface-active-agents-surfactants (accessed on 3 October 2021).
- Sun, X.; Gao, Z.; Zhao, M.; Gao, M.; Du, M.; Dai, C. Development and evaluation of a novel seawater-based viscoelastic fracturing fluid system. J. Pet. Sci. Eng. 2019, 183, 106408. [Google Scholar] [CrossRef]
- Al-Sadat, W.; Nasser, M.S.; Chang, F.; Nasr-El-Din, H.A.; Hussein, I.A. Rheology of a viscoelastic zwitterionic surfactant used in acid stimulation: Effects of surfactant and electrolyte concentration. J. Pet. Sci. Eng. 2014, 124, 341–349. [Google Scholar] [CrossRef]
- Bruker Nano Surfaces. Using AFM to Measure Viscoelasticity at the Nanoscale. AZO Materials. Article ID=18382. 2014. Available online: https://www.azom.com/article.aspx? (accessed on 3 October 2021).
- Alleman, D.; Qi, Q.; Keck, R. The development and successful field use of viscoelastic surfactant-based diverting agents for acid stimulation. In International Symposium on Oilfield Chemistry; OnePetro: Houston, TX, USA, 2003. [Google Scholar] [CrossRef]
- Advincula, R. Viscoelastic Surfactants (VES) and Oilfield Chemicals, Park Systems, Park Webinar Series [Vedio]. Youtube. Available online: https://www.youtube.com/watch?v=LnM_lkUIyfw&t=1818s (accessed on 20 September 2021).
- Lu, H.; Zheng, C.; Wang, L.; Liu, Y.; Huang, Z. Endowing a cationic hydrophobic associating polyacrylamide solution with CO2 switchable properties using N, N-dimethylolamidopropylamine via the assembly transition between vesicles and spherical micelles by CO2. RSC Adv. 2016, 6, 108440–108447. [Google Scholar] [CrossRef]
- Lu, H.; Wang, L.; Huang, Z. Unusual pH-responsive fluid based on a simple tertiary amine surfactant: The formation of vesicles and worm-like micelles. RSC Adv. 2014, 4, 51519–51527. [Google Scholar] [CrossRef]
- Chang, F.; Qu, Q.; Frenier, W. A novel self-diverting-acid developed for matrix stimulation of carbonate reservoirs. In SPE International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: Houston, TX, USA, 2001. [Google Scholar] [CrossRef]
- Ba Alawi, M.; Hassan, A.; Aljawad, M.S.; Kamal, M.S.; Mahmoud, M.; Al-Nakhli, A. A novel approach to improve acid diversion in carbonate rocks using thermochemical fluids: Experimental and numerical study. Molecules 2020, 25, 2976. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.; Nasr-El-Din, M.A.; Hill, A.D.; Nasr-El-Din, H.A. Diversion and propagation of viscoelastic surfactant-based acid in carbonate cores. In SPE International Symposium on Oilfield Chemistry; OnePetro: The Woodlands, TX, USA, 2009. [Google Scholar]
- Janjua, A.N.; Sultan, A.S.; Saikia, T.; Kamal, M.S.; Mahmoud, M. Experimental Investigation of Noble Viscoelastic Surfactant and Chelating Agent for Heavy Oil Enhanced Oil Recovery in High-Pressure–High-Temperature Carbonate Reservoirs. J. Surfactants Deterg. 2021, 24, 289–300. [Google Scholar] [CrossRef]
- Li, K.X.; Jing, X.Q.; He, S.; Ren, H.; Wei, B. Laboratory study displacement efficiency of viscoelastic surfactant solution in enhanced oil recovery. Energy Fuels 2016, 30, 4467–4474. [Google Scholar] [CrossRef]
- Morvan, M.; Degre, G.; Leng, J.; Masselon, C.; Bouillot, J.; Zaitoun, A.; Moreau, P. New viscoelastic fluid for chemical EOR. In IOR 2009-15th European Symposium on Improved Oil Recovery; European Association of Geoscientists Engineers: Moscow, Russia, 2009. [Google Scholar] [CrossRef]
- Sun, L.; Bai, B.; Wei, B.; Pu, W.; Wei, P.; Li, D.; Zhang, C. Recent advances of surfactant-stabilized N2/CO2 foams in enhanced oil recovery. Fuel 2019, 241, 83–93. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H.; Zhang, W. High performance clean fracturing fluid using a new tri-cationic surfactant. Polymers 2018, 10, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beheshti, N.; Nguyen, G.T.; Kjøniksen, A.L.; Knudsen, K.D.; Nyström, B. Structure and dynamics of aqueous mixtures of an anionic cellulose derivative and anionic or cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 40–49. [Google Scholar] [CrossRef]
- Kästner, U.; Hoffmann, H.; Dönges, R.; Ehrler, R. Interactions between modified hydroxyethyl cellulose (HEC) and surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1996, 112, 209–225. [Google Scholar] [CrossRef]
- Zhang, L.M. New water-soluble cellulosic polymers: A review. Macromol. Mater. Eng. 2001, 286, 267–275. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T. New ionic polymers by cellulose functionalization. Macromol. Chem. Phys. 1998, 199, 2341–2364. [Google Scholar] [CrossRef]
- Kazachenko, A.; Akman, F.; Medimagh, M.; Issaoui, N.; Vasilieva, N.; Malyar, Y.N.; Sudakova, I.G.; Karacharov, A.; Miroshnikova, A.; Al-Dossary, O.M. Sulfation of Diethylaminoethyl-Cellulose: QTAIM Topological Analysis and Experimental and DFT Studies of the Properties. ACS Omega 2021, 6, 22603–22615. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.; Lei, P.; Bing-Liang, L.; Ni, G.; Li-Ping, Z.; Ke-Zhi, L. Influences of molding processes and different dispersants on the dispersion of chopped carbon fibers in cement matrix. Heliyon 2018, 4, e00868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Wandrey, C.; Freitag, R. Mass influences in the performance of oligomeric poly (diallyldimethylammonium chloride) as displacer for cation-exchange displacement chromatography of proteins. J. Chromatogr. A 2002, 944, 149–159. [Google Scholar] [CrossRef]
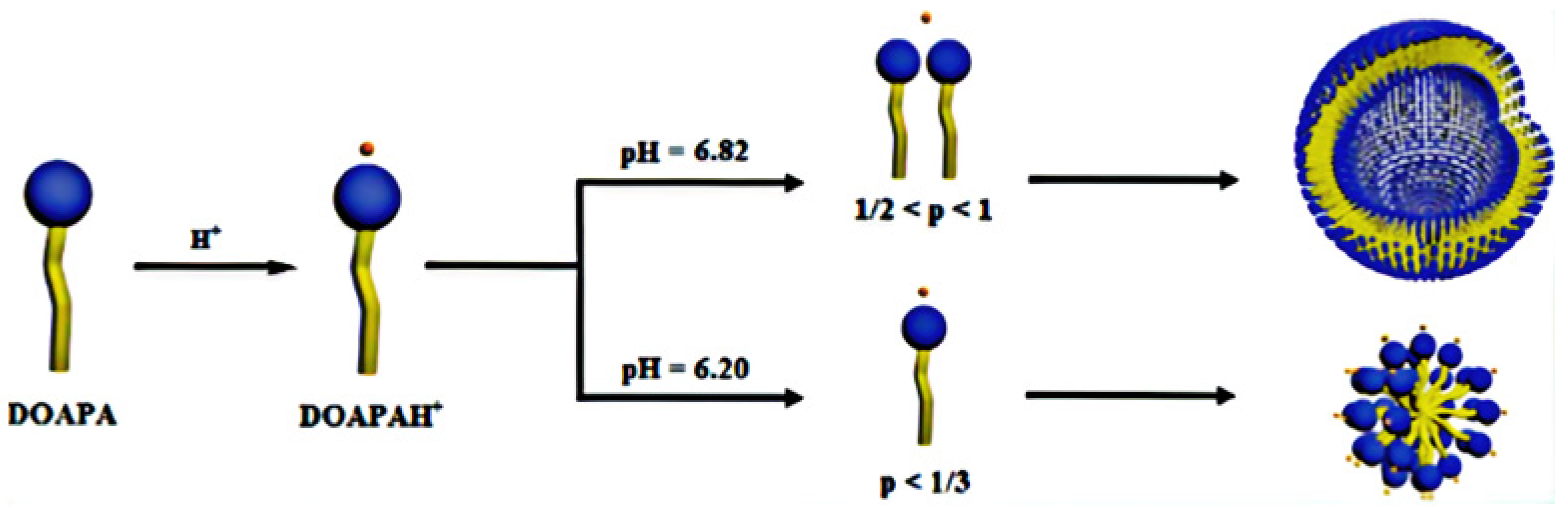
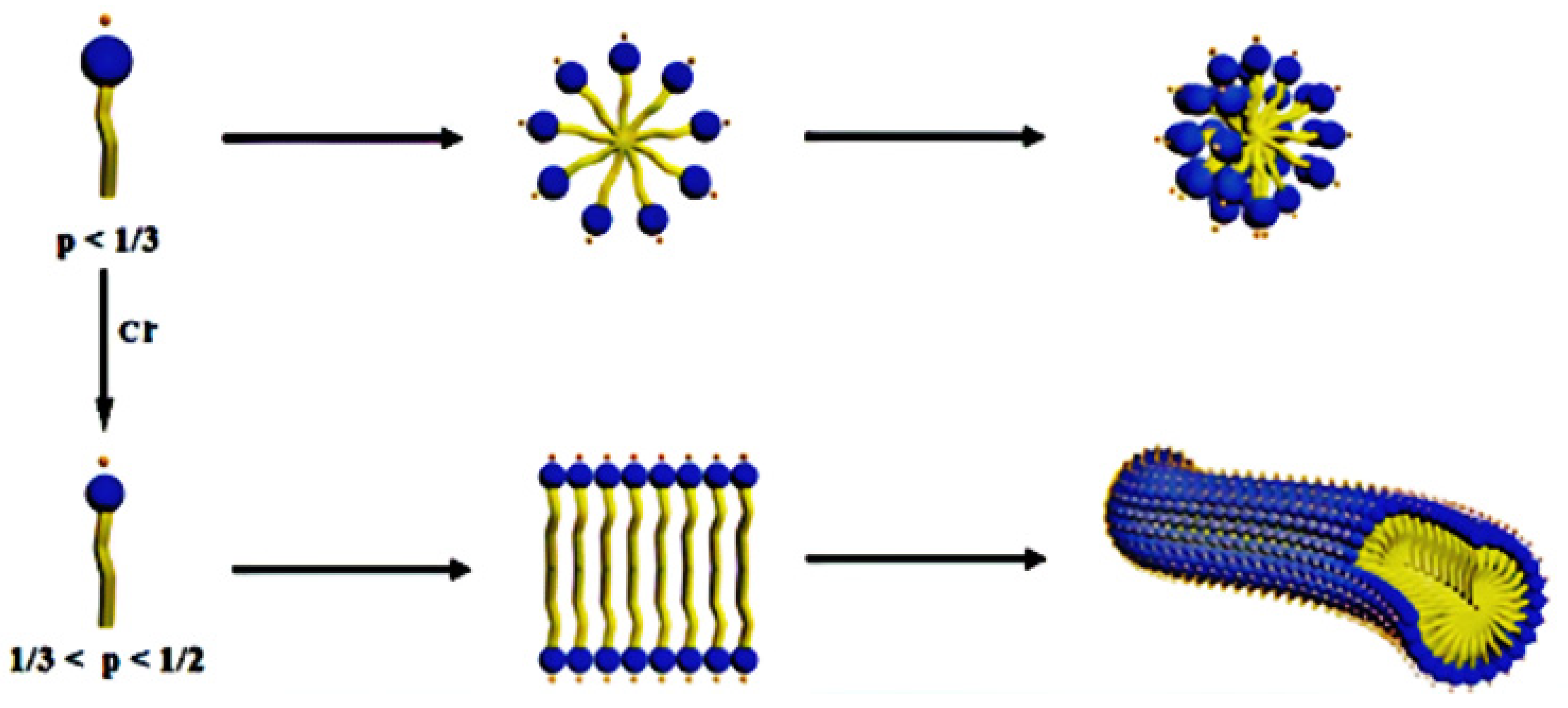



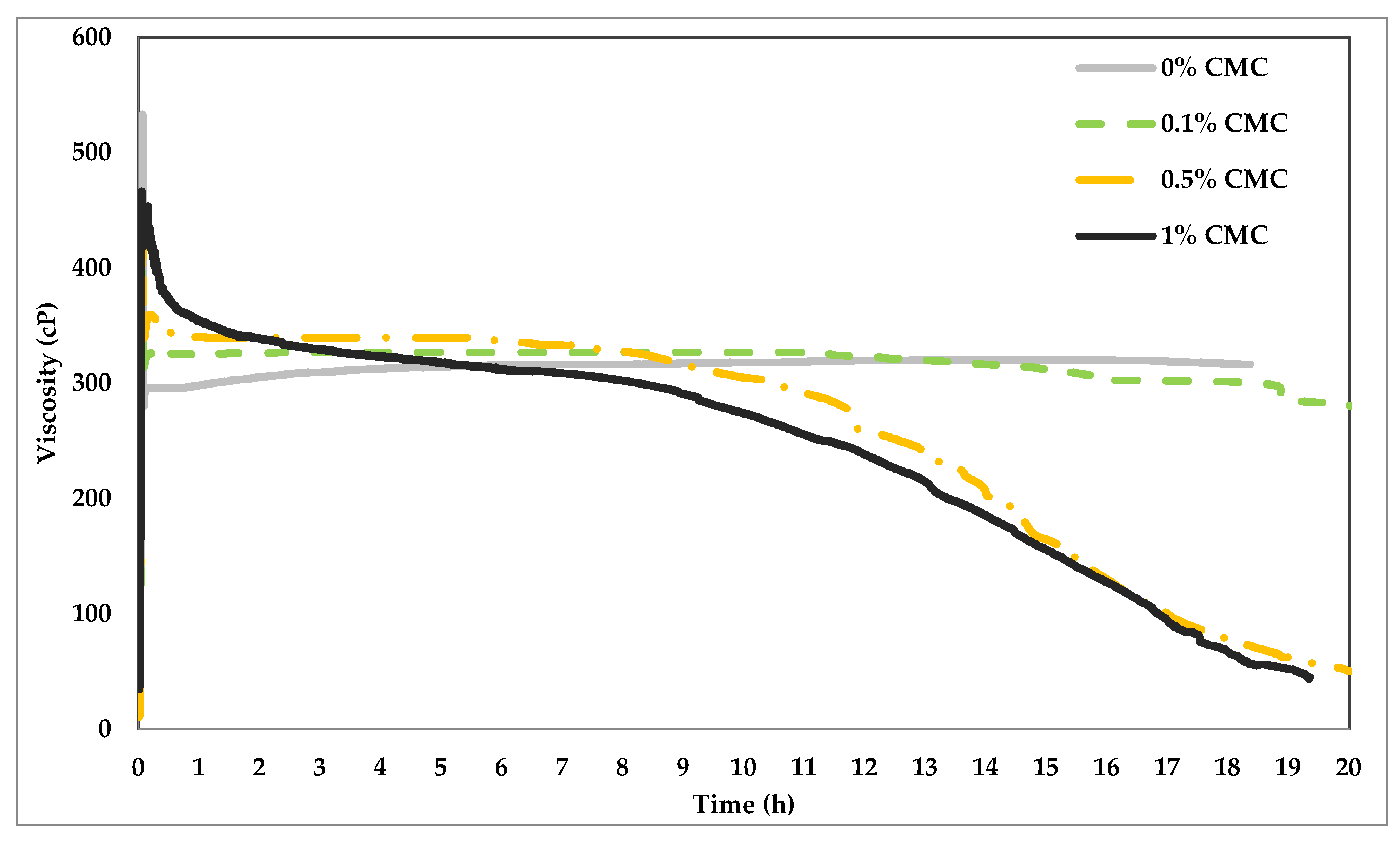

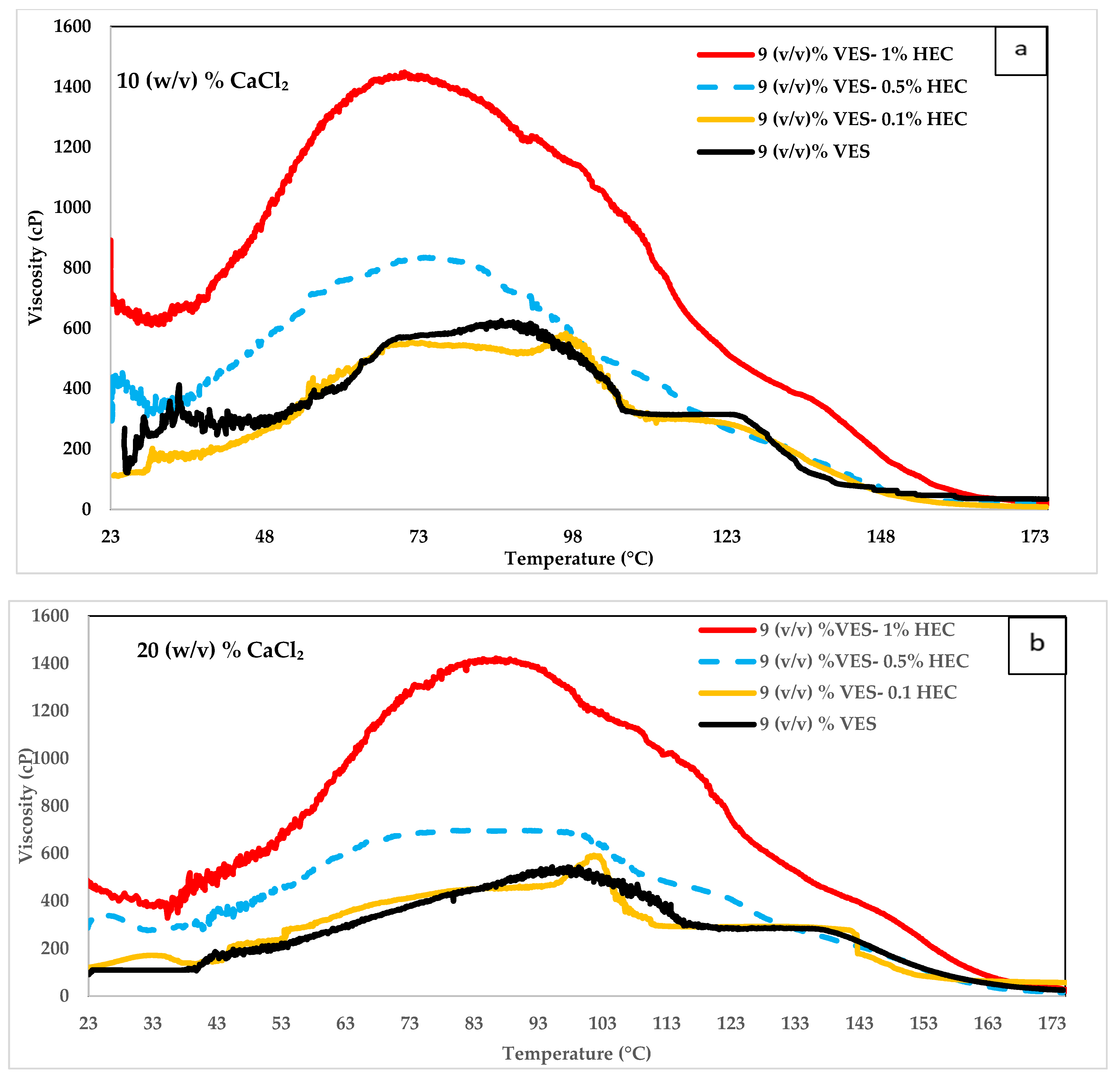
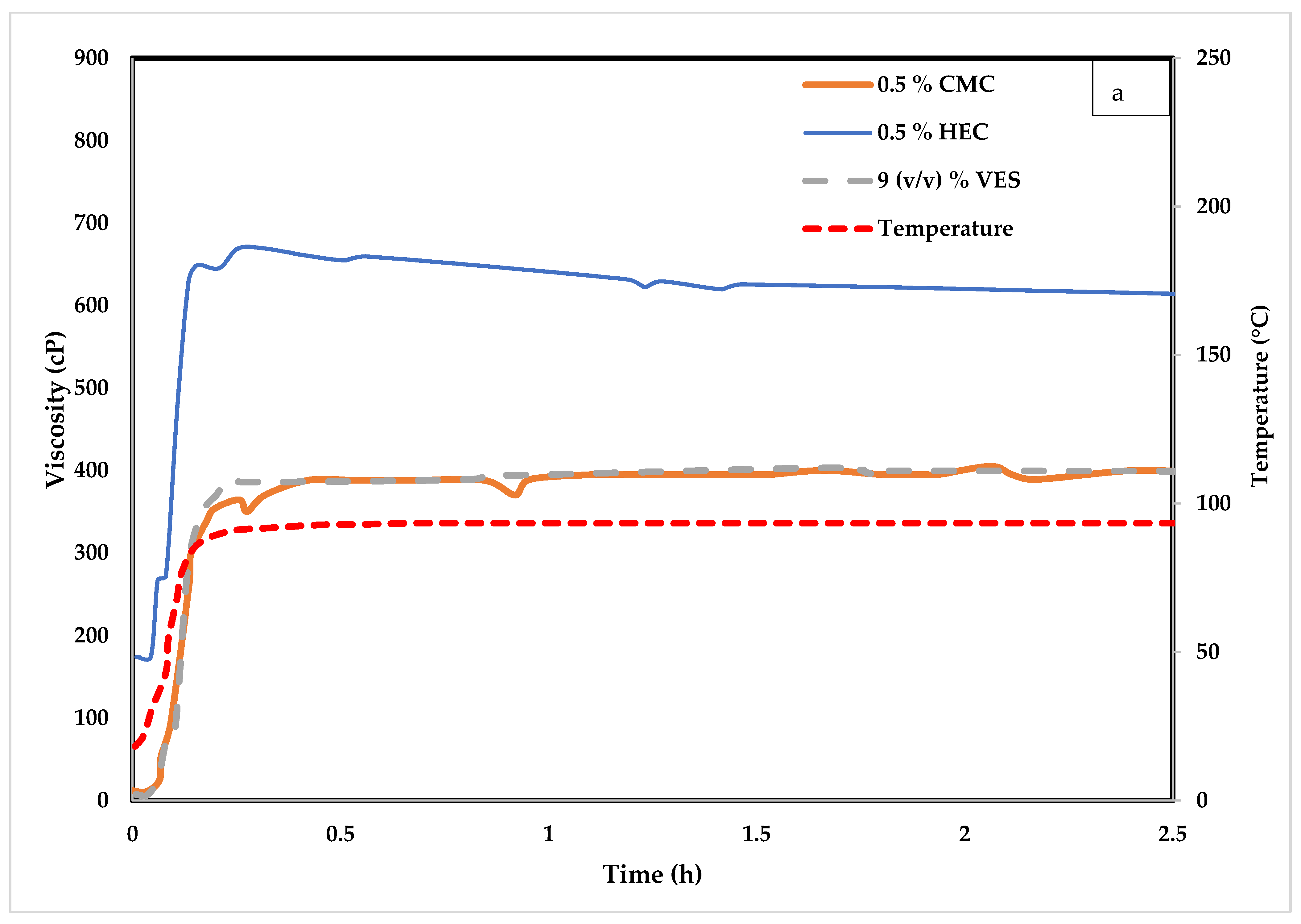

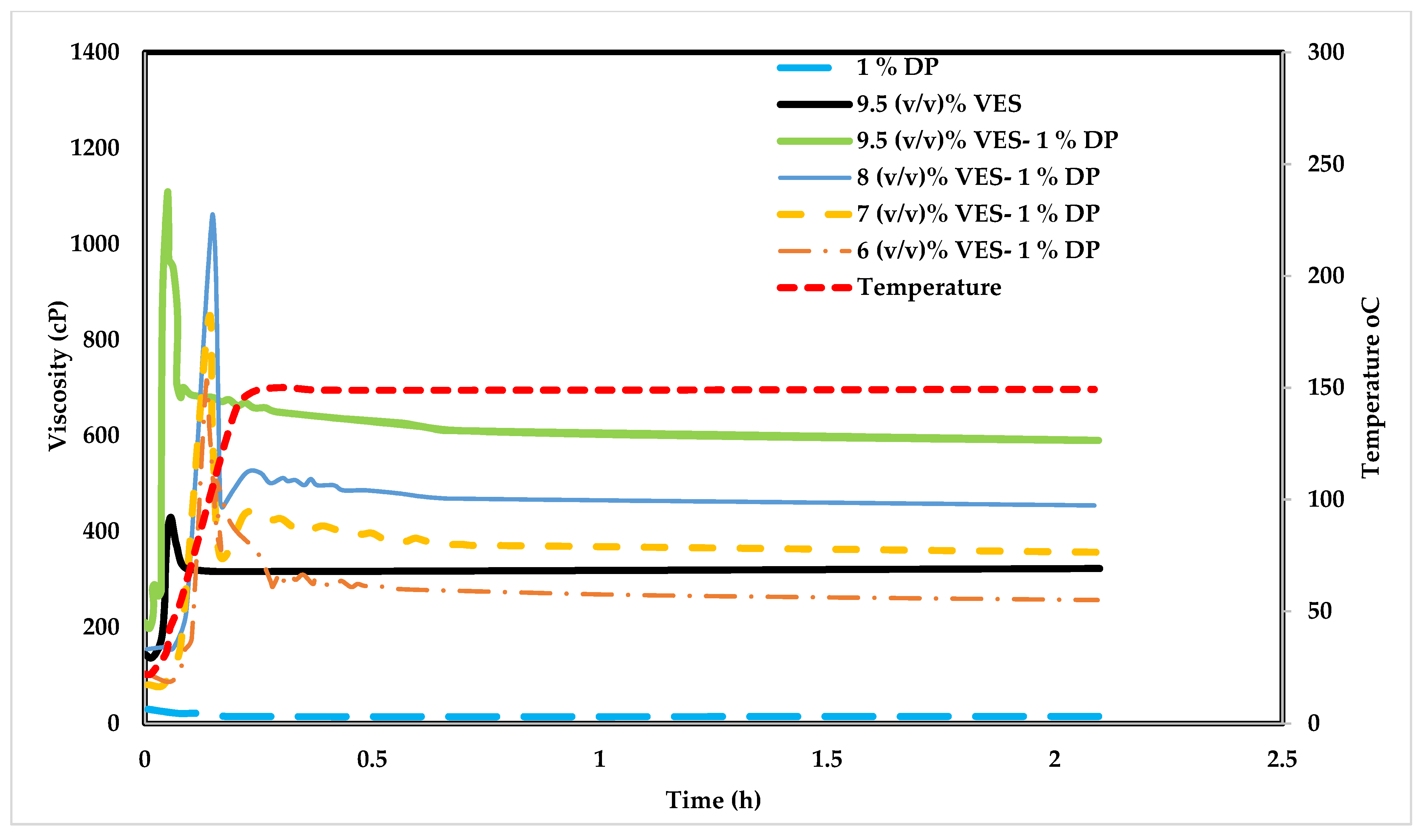


| Test Type | Tested Polymer/Chemical | VES Conc. (v/v) % | Polymer Conc. (v/v) % | CaCl2 Conc. (w/v) % | Temperature °C |
|---|---|---|---|---|---|
| Viscosity vs. Temperature | VES | 9 | 0 | 10 | 26.7–171.1 |
| 9 | 0 | 20 | 26.7–171.1 | ||
| 9 | 0 | 30 | 26.7–171.1 | ||
| 10 | 0 | 30 | 26.7–171.1 | ||
| CMC | 0 | 0.5 | 30 | 26.7–171.1 | |
| 9 | 0.1 | 30 | 26.7–171.1 | ||
| 9 | 0.5 | 30 | 26.7–171.1 | ||
| 9 | 1 | 30 | 26.7–171.1 | ||
| HEC | 9 | 0 | 10 | 26.7–171.1 | |
| 9 | 0.1 | 10 | 26.7–171.1 | ||
| 9 | 0.5 | 10 | 26.7–171.1 | ||
| 9 | 1 | 10 | 26.7–171.1 | ||
| 9 | 0 | 20 | 26.7–171.1 | ||
| 9 | 0.1 | 20 | 26.7–171.1 | ||
| 9 | 0.5 | 20 | 26.7–171.1 | ||
| 9 | 1 | 20 | 26.7–171.1 | ||
| 9 | 0 | 30 | 26.7–171.1 | ||
| 9 | 0.1 | 30 | 26.7–171.1 | ||
| 9 | 0.5 | 30 | 26.7–171.1 | ||
| 9 | 1 | 30 | 26.7–171.1 | ||
| 0 | 1 | 30 | 80–340 | ||
| Viscosity vs. Time (Fixed Temperature) | DP | 0 | 1 | 30 | 93.3 |
| 6 | 1 | 30 | 93.3 | ||
| 7 | 1 | 30 | 93.3 | ||
| 0 | 1 | 30 | 148.9 | ||
| 8 | 1 | 30 | 148.9 | ||
| 7 | 1 | 30 | 148.9 | ||
| 7 | 1 | 20 | 148.9 | ||
| 7 | 1 | 10 | 148.9 | ||
| 6 | 1 | 30 | 148.9 | ||
| 9 | 0 | 30 | 148.9 | ||
| CMC | 9 | 0.5 | 30 | 93.3 | |
| 9 | 0.1 | 30 | 148.9 | ||
| 9 | 0.5 | 30 | 148.9 | ||
| 9 | 1 | 30 | 148.9 | ||
| HEC | 9 | 0.5 | 30 | 93.3 | |
| Viscosity vs. polymer concentration | VES | 10 | 0 | 30 | 148.9 |
| 9 | 0 | 30 | 148.9 | ||
| 8 | 0 | 30 | 148.9 | ||
| 6 | 0 | 30 | 148.9 | ||
| CMC | 9 | 0.1 | 30 | 107.2 & 143.3 | |
| 9 | 0.5 | 30 | 107.2 & 143.3 | ||
| 9 | 1 | 30 | 107.2 & 143.3 | ||
| DP | 9.5 | 1 | 30 | 148.9 | |
| 8 | 1 | 30 | 148.9 | ||
| 7 | 1 | 30 | 148.9 | ||
| 6 | 1 | 30 | 148.9 |
| Fluid Type | Viscosity at 107.2 °C | Viscosity at 143.3 °C | % Increase at 107.2 °C | % Increase at 143.3 °C |
|---|---|---|---|---|
| 0.5% CMC | 0 | 2.6 | -- | -- |
| 9% (v/v) VES | 472 | 290 | -- | -- |
| 9% (v/v) VES + 0.1% CMC | 478 | 320 | 1% | 10% |
| 9% (v/v) VES + 0.5% CMC | 477 | 345 | 1% | 19% |
| 9% (v/v) VES + 1% CMC | 520 | 400 | 9% | 38% |
| 10% (v/v) VES | 580 | 360 | 19% (+) | 24% (X) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, A.; AlSulaimani, M.; Aljawad, M.S.; Sangaru, S.S.; Kamal, M.S.; Mahmoud, M. The Synergetic Impact of Anionic, Cationic, and Neutral Polymers on VES Rheology at High-Temperature Environment. Polymers 2022, 14, 1145. https://doi.org/10.3390/polym14061145
Othman A, AlSulaimani M, Aljawad MS, Sangaru SS, Kamal MS, Mahmoud M. The Synergetic Impact of Anionic, Cationic, and Neutral Polymers on VES Rheology at High-Temperature Environment. Polymers. 2022; 14(6):1145. https://doi.org/10.3390/polym14061145
Chicago/Turabian StyleOthman, Amro, Mohammed AlSulaimani, Murtada Saleh Aljawad, Shiv Shankar Sangaru, Muhammad Shahzad Kamal, and Mohamed Mahmoud. 2022. "The Synergetic Impact of Anionic, Cationic, and Neutral Polymers on VES Rheology at High-Temperature Environment" Polymers 14, no. 6: 1145. https://doi.org/10.3390/polym14061145
APA StyleOthman, A., AlSulaimani, M., Aljawad, M. S., Sangaru, S. S., Kamal, M. S., & Mahmoud, M. (2022). The Synergetic Impact of Anionic, Cationic, and Neutral Polymers on VES Rheology at High-Temperature Environment. Polymers, 14(6), 1145. https://doi.org/10.3390/polym14061145









