Tribological Properties of Polyamide 46/Graphene Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
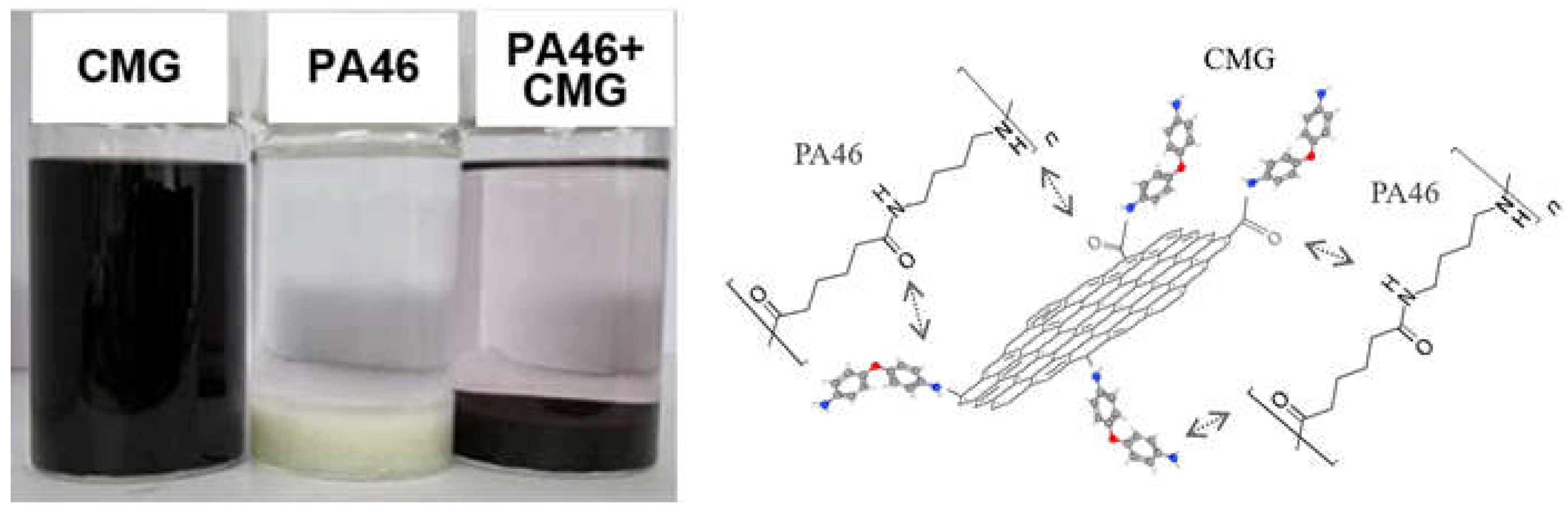
2.2. Preparation of Chemically Modified Graphene (CMG)
2.3. Preparation of Nanocomposites
2.4. Characterization
3. Results
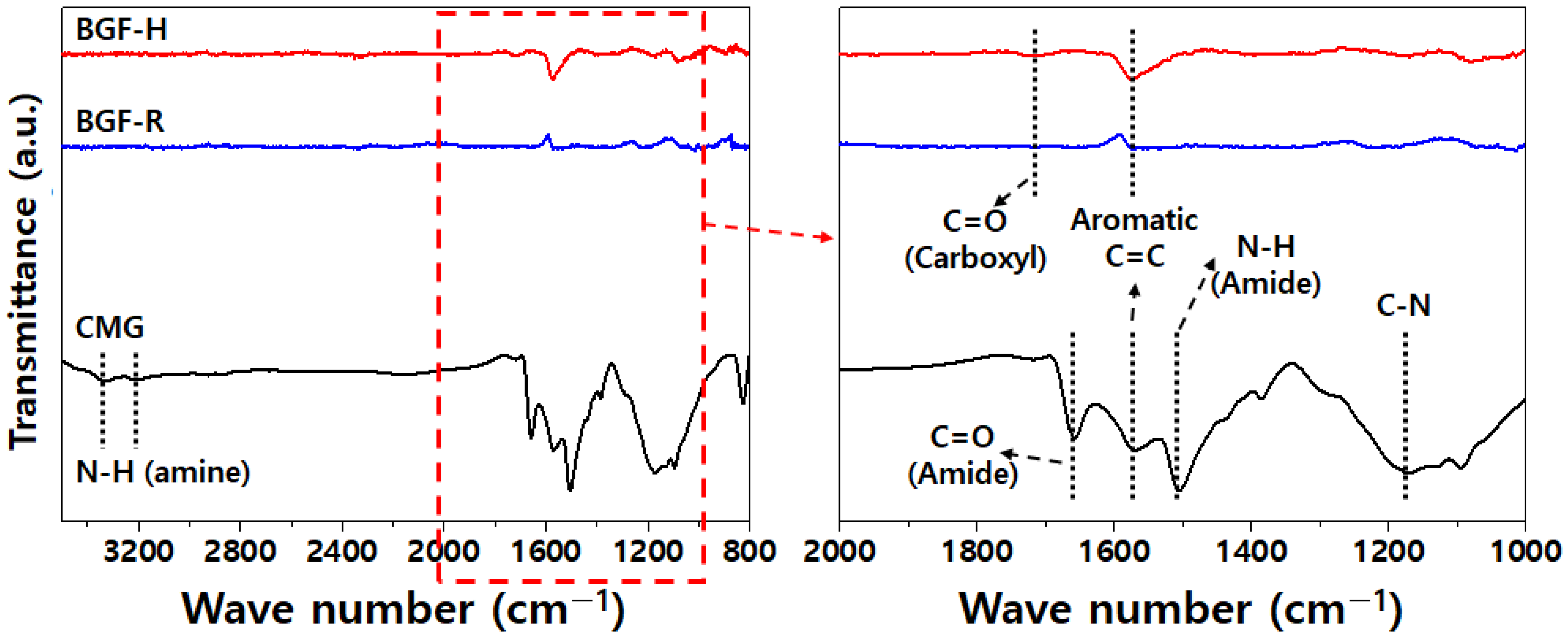
3.1. CMG Analysis
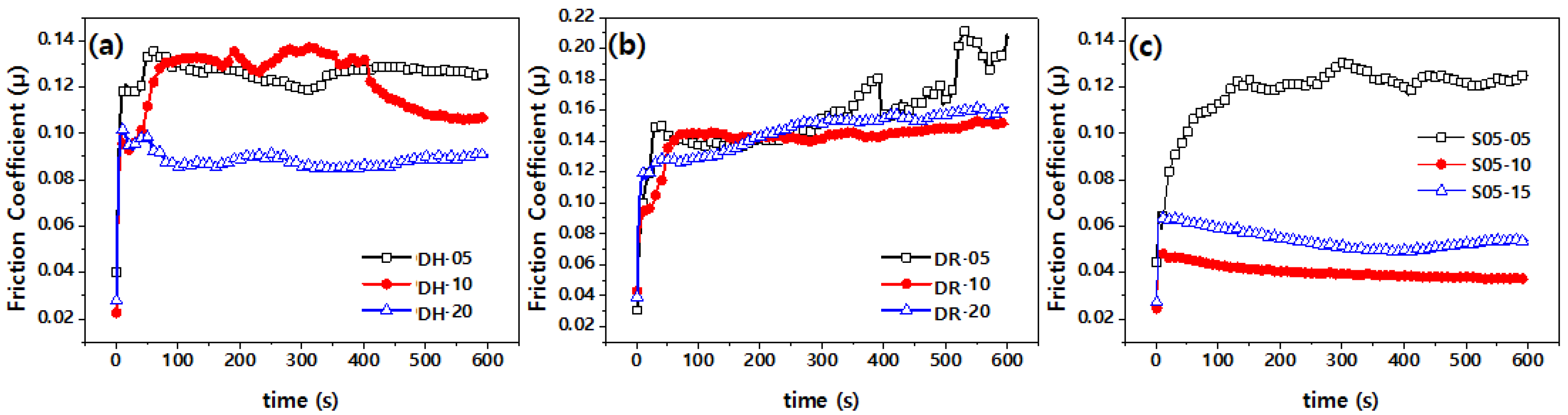
3.2. Frictional Behavior
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Li, D.; Si, G.; Liu, Q.; Chen, Y. Improved thermal and mechanical properties of carbon fiber filled polyamide 46 composites. J. Polym. Eng. 2017, 37, 345–353. [Google Scholar] [CrossRef]
- Cong, P.; Xiang, F.; Liu, X.; Li, T. Morphology and microstructure of polyamide 46 wear debris and transfer film: In relation to wear mechanisms. Wear 2008, 265, 1100–1105. [Google Scholar] [CrossRef]
- Gordon, D.H.; Kukureka, S.N. The wear and friction of polyamide 46 and polyamide 46/aramid-fibre composites in sliding–rolling contact. Wear 2009, 267, 669–678. [Google Scholar] [CrossRef]
- Kurokawa, M.; Uchiyama, Y.; Iwai, T.; Nagai, S. Performance of plastic gear made of carbon fiber reinforced polyamide 12. Wear 2003, 254, 468–473. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. Handbook of Fillers, 5th ed.; ChemTec Publishing: Toronto, ON, Canada, 2021; pp. 139–149. [Google Scholar]
- Kim, D.O.; Lee, J.-H.; Hwang, T.; Oh, J.S.; Hong, J.; Lee, P.-C.; Seferis, J.C.; Nam, J.-D. Nanofillers. In Wiley Encyclopedia of Composites, 2nd ed.; Nicolais, L., Borzacchiello, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1907–1912. [Google Scholar]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, M.; Lu, Z.; Huang, F. Preparation and tribological performance of chemically-modified reduced graphene oxide/polyacrylonitrile composites. Compos. Part A 2013, 54, 153–158. [Google Scholar] [CrossRef]
- Sun, L.; Cao, M.; Xiao, F.; Xu, J.; Chen, Y. POSS functionalized graphene oxide nanosheets with multiple reaction sites improve the friction and wear properties of polyamide 6. Tribol. Int. 2021, 154, 106747. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Castro, M.; Morris, J.A.; Ríos, R.; Ansón-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; Chung, K.-H. Review on molecular simulation of graphene from a tribological perspective. Tribol. Lubr. 2020, 36, 55–63. [Google Scholar]
- Penkov, O.; Kim, H.-J.; Kim, H.-J.; Kim, D.-E. Tribology of graphene: A review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Keresztes, R.; Odrobinz, M.; Nagarajan, R.; Subramanian, K.; Kalacska, G.; Sukumaran, J. Tribological characteristics of cast polyamide 6 (PA6G) matrix and their composites (PA6G SL) under normal and overload conditions using dynamic pin-on-plate system. Compos. Part B 2019, 160, 119–130. [Google Scholar] [CrossRef]
- Gomez, J.; Villaro, E.; Karagiannidis, P.G.; Elmarakbi, A. Effects of chemical structure and morphology of graphene-related materials (GRMs) on melt processing and properties of GRM/polyamide-6 nanocomposites. Results Mater. 2020, 7, 100105. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I.; Pérez-González, J.; Iniestra-Galindo, M.G.; Marín-Santibáñez, B.M. Production and tribological evaluation of polypropylene nanocomposites with reduced graphene oxide (rGO) for using in water-lubricated bearings. Wear 2021, 477, 203860. [Google Scholar] [CrossRef]
- Xin, Y.; Xu, F.; Wang, M.; Li, T. Synergistic effects of carbon nanotube/nano-MoS2 hybrid on tribological performance of polyimide nanocomposite films. Tribol. Lett. 2018, 66, 25. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of nanofillers on tribological properties of polymer nanocomposites: A review on recent development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef] [PubMed]





| Sample | PA46 | BGF-H | BGF-R | MB | Graphene Content (%) |
|---|---|---|---|---|---|
| REF | 100 | - | - | - | - |
| S05-05 | 90 | - | - | 10 | 0.05 |
| S05-10 | 80 | - | - | 20 | 0.10 |
| S05-15 | 70 | - | - | 30 | 0.15 |
| DH-05 | 99.95 | 0.05 | - | - | 0.05 |
| DH-10 | 99.90 | 0.10 | - | - | 0.10 |
| DH-20 | 99.80 | 0.20 | - | - | 0.20 |
| DR-05 | 99.95 | - | 0.05 | - | 0.05 |
| DR-10 | 99.90 | - | 0.10 | - | 0.10 |
| DR-20 | 99.80 | - | 0.20 | - | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.-C.; Kim, S.Y.; Ko, Y.K.; Ha, J.U.; Jeoung, S.K.; Shin, D.; Kim, J.H.; Kim, M.-G. Tribological Properties of Polyamide 46/Graphene Nanocomposites. Polymers 2022, 14, 1139. https://doi.org/10.3390/polym14061139
Lee P-C, Kim SY, Ko YK, Ha JU, Jeoung SK, Shin D, Kim JH, Kim M-G. Tribological Properties of Polyamide 46/Graphene Nanocomposites. Polymers. 2022; 14(6):1139. https://doi.org/10.3390/polym14061139
Chicago/Turabian StyleLee, Pyoung-Chan, Su Young Kim, Youn Ki Ko, Jin Uk Ha, Sun Kyoung Jeoung, Donghyeok Shin, Jung Hoon Kim, and Myeong-Gi Kim. 2022. "Tribological Properties of Polyamide 46/Graphene Nanocomposites" Polymers 14, no. 6: 1139. https://doi.org/10.3390/polym14061139
APA StyleLee, P.-C., Kim, S. Y., Ko, Y. K., Ha, J. U., Jeoung, S. K., Shin, D., Kim, J. H., & Kim, M.-G. (2022). Tribological Properties of Polyamide 46/Graphene Nanocomposites. Polymers, 14(6), 1139. https://doi.org/10.3390/polym14061139







