Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
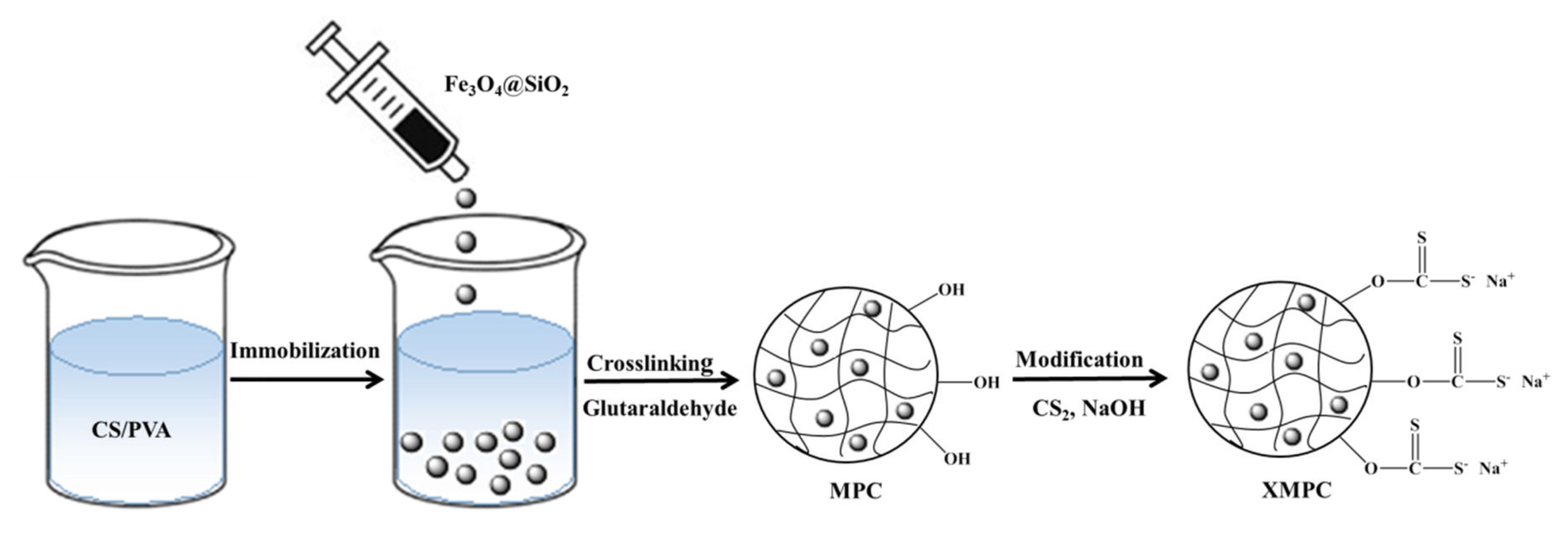
2.2. Preparation of XMPC
2.3. Analytical Methods
3. Results and Discussion
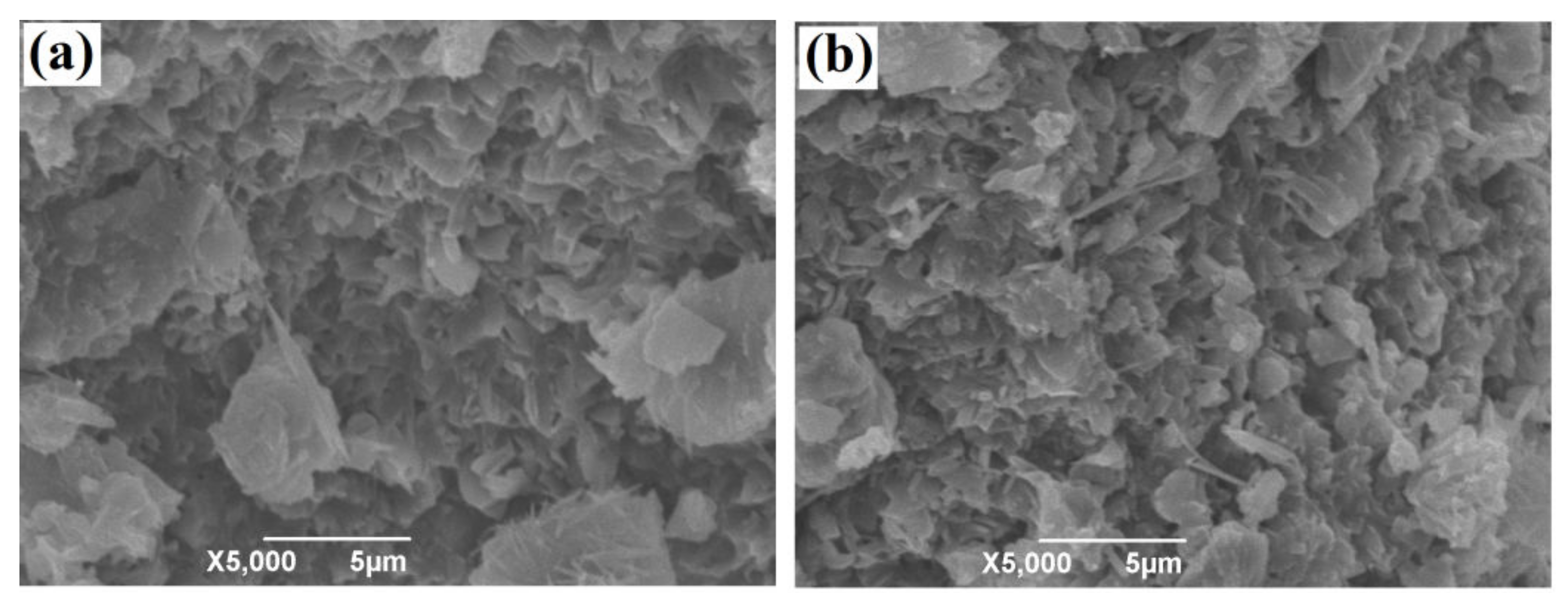
3.1. Preparation and Characterization
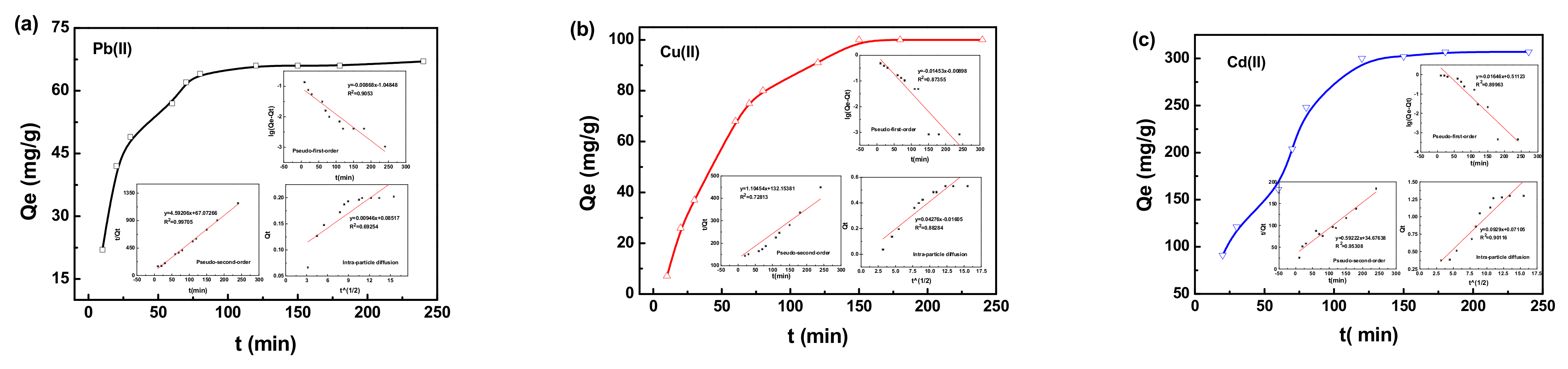
3.2. Evaluation of Adsorption and Desorption Performance
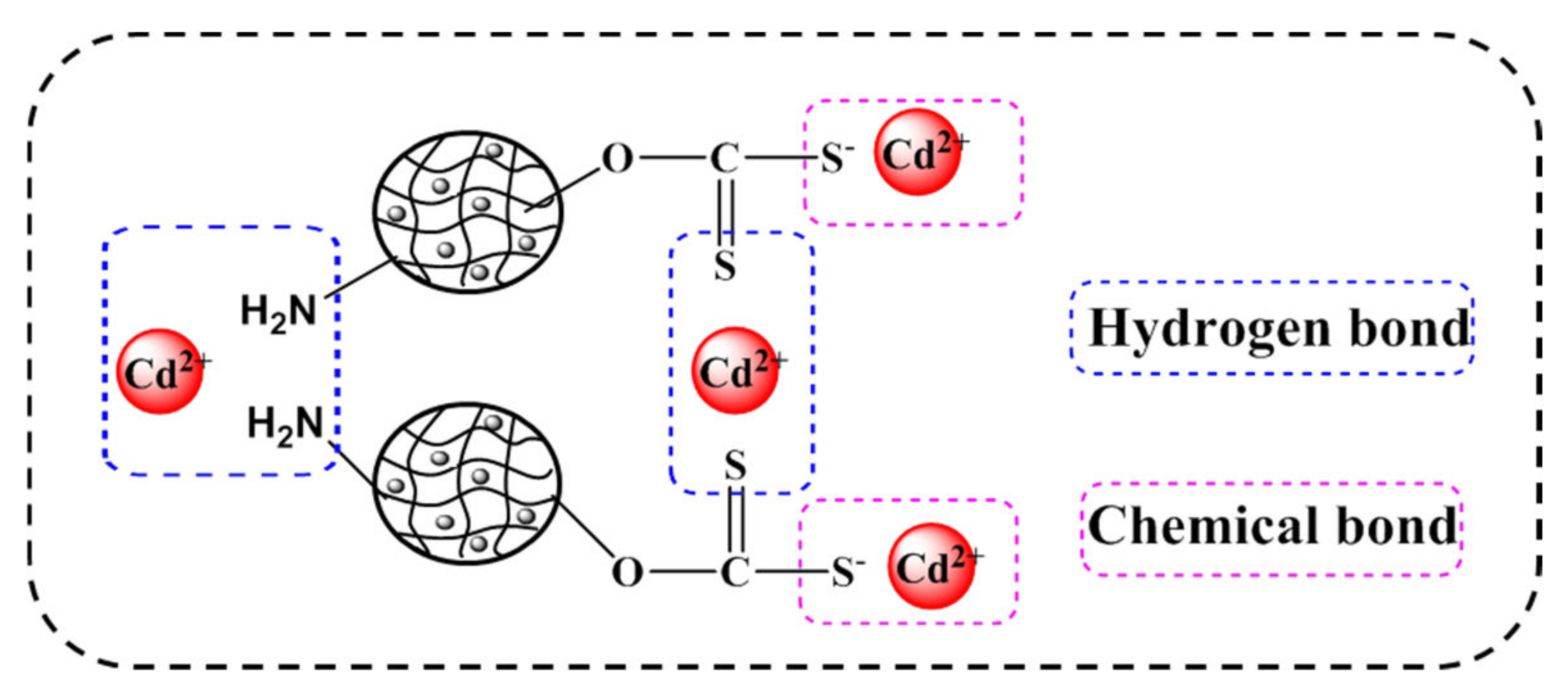
3.3. Selective Adsorption Behaviors of Heavy Metals
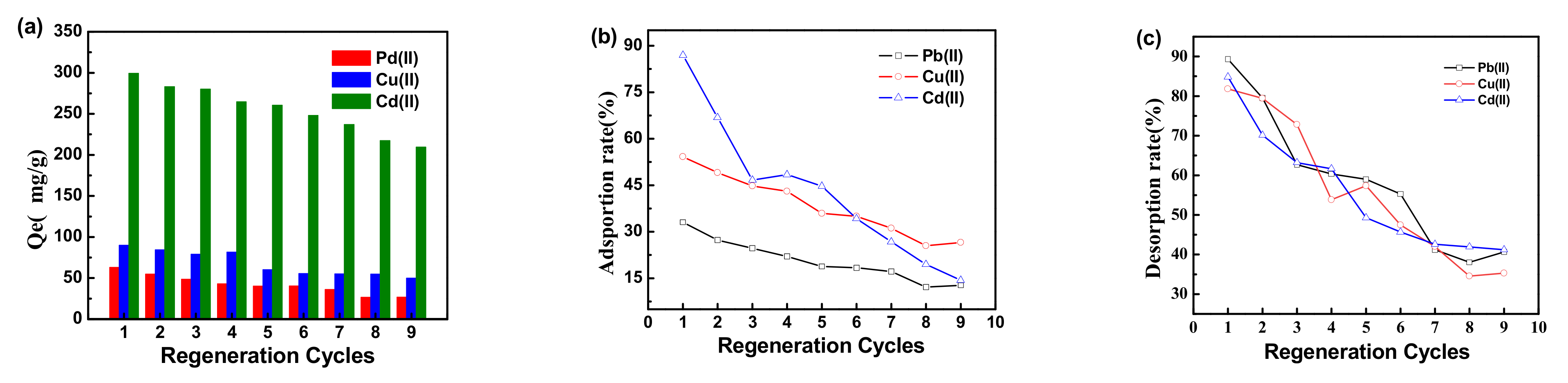
3.4. Desorption and Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ge, F.; Li, M.M.; Ye, H.; Zhao, B.X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211–212, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Căprărescu, S.; Modrogan, C.; Purcar, V.; Dăncilă, A.M.; Orbuleț, O.D. Study of polyvinyl alcohol-SiO2 nanoparticles polymeric membrane in wastewater treatment containing zinc ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Căprărescu, S.; Zgârian, R.G.; Tihan, G.T.; Purcar, V.; Eftimie, T.E.; Modrogan, C.; Chiriac, A.-L.; Nicolae, C.A. Biopolymeric membrane enriched with chitosan and silver for metallic ions removal. Polymers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Sandu, T.; Sârbu, A.; Damian, C.M.; Marin, A.; Vulpe, S.; Budinova, T.; Tsyntsarski, B.; Yardim, M.F.; Sirkecioglu, A. Preparation and characterization of membranes obtained from blends of acrylonitrile copolymers with poly(vinyl alcohol). J. Appl. Polym. Sci. 2014, 131, 41013. [Google Scholar] [CrossRef]
- Sun, G.L.; Reynolds, E.E.; Belcher, A.M. Designing yeast as plant-like hyperaccumulators for heavy metals. Nat. Commun. 2019, 10, 5080. [Google Scholar] [CrossRef]
- Lv, L.; Chen, N.; Feng, C.; Gao, Y.; Li, M. Xanthate-modified magnetic chitosan/poly (vinyl alcohol) adsorbent: Preparation, characterization, and performance of Pb(II) removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 78, 485–492. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality. In Recomendations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008; Volume 1, pp. 390–399. [Google Scholar]
- Sahebjamee, N.; Soltanieh, M.; Mousavi, S.M.; Heydarinasab, A. Removal of Cu2+, Cd2+ and Ni2+ ions from aqueous solution using a novel chitosan/polyvinyl alcohol adsorptive membrane. Carbohydr. Polym. 2019, 210, 264–273. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, R.; Wang, C.; Zhou, G.; Hua, C.; Cao, Y.; Song, Z. Novel environmental-friendly nano-composite magnetic attapulgite functionalized by chitosan and EDTA for cadmium (II) removal. J. Alloys Compd. 2020, 817, 153286. [Google Scholar] [CrossRef]
- Karrari, P.; Mehrpour, O.; Abdollahi, M. A systematic review on status of lead pollution and toxicity in Iran; Guidance for preventive measures. Daru 2012, 20, 2. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Gou, S.; Zhou, L.; Tang, L.; Liu, T.; Liu, L.; Duan, M. Amidoxime-functionalized polyacrylamide-modified chitosan containing imidazoline groups for effective removal of Cu2+ and Ni2+. Carbohydr. Polym. 2021, 252, 117160. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of chromium(III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef]
- Dou, J.; Gan, D.; Huang, Q.; Liu, M.; Chen, J.; Deng, F.; Zhu, X.; Wen, Y.; Zhang, X.; Wei, Y. Functionalization of carbon nanotubes with chitosan based on MALI multicomponent reaction for Cu2+ removal. Int. J. Biol. Macromol. 2019, 136, 476–485. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Saleh, A.S.; Elsharma, E.M.; Metwally, E.; Siyam, T. Chitosan-g-maleic acid for effective removal of copper and nickel ions from their solutions. Int. J. Biol. Macromol. 2019, 121, 1287–1294. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Martino, A.D.; Zednik, J.; Ozaltin, K.; Lovecká, L.; Bergerová, E.D.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; Pongracz, E.; Peramaki, P.; Keiski, R.L. Micellar-enhanced ultrafiltration for the removal of cadmium and zinc: Use of response surface methodology to improve understanding of process performance and optimisation. J. Hazard. Mater. 2010, 180, 524–534. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Z.; Yang, X.; Ren, Z. Xanthate modified magnetic activated carbon for efficient removal of cationic dyes and tetracycline hydrochloride from aqueous solutions. Colloids Surf. A 2021, 615, 126273. [Google Scholar] [CrossRef]
- Zhang, H.; Omer, A.M.; Hu, Z.; Yang, L.Y.; Ji, C.; Ouyang, X.K. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; An, Y.; Yang, L.; Sun, Q.; Ma, J.; Zheng, H. Chitin-biocalcium as a novel superior composite for ciprofloxacin removal: Synergism of adsorption and flocculation. J. Hazard. Mater. 2022, 423, 126917. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, J.; Sun, Q.; An, Y.; Zhao, R.; Zheng, H.; Li, H. Efficient removal of both positively and negatively charged colloidal contaminants using amphoteric starch-based flocculants synthesized by low-pressure UV initiation. Sep. Purif. Technol. 2022, 282, 120120. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Omer, A.M.; El-Aqapa, H.G.; Gaber, N.M.; Attia, N.F.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Abd El-Monaem, E.M. Chitosan based adsorbents for the removal of phosphate and nitrate: A critical review. Carbohydr. Polym. 2021, 274, 118671. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Chen, L.; Wu, P.; Chen, M.; Lai, X.; Ahmed, Z.; Zhu, N.; Dang, Z.; Bi, Y.; Liu, T. Preparation and characterization of the eco-friendly chitosan/vermiculite biocomposite with excellent removal capacity for cadmium and lead. Appl. Clay Sci. 2018, 159, 74–82. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Eltaweil, A.S.; Elshishini, H.M.; Hosny, M.; Abou Alsoaud, M.M.; Attia, N.F.; El-Subruiti, G.M.; Omer, A.M. Sustainable adsorptive removal of antibiotic residues by chitosan composites: An insight into current developments and future recommendations. Arab. J. Chem. 2022, 15, 103743. [Google Scholar] [CrossRef]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Kim, Y.; Schwartz, F.W.; Lee, E.S.; Song, H. A novel chitosan/clay/magnetite composite for adsorption of Cu(II) and As(V). Chem. Eng. J. 2012, 200–202, 654–662. [Google Scholar] [CrossRef]
- Ahmadi, M.; Foladivanda, M.; Jaafarzadeh, N.; Ramezani, Z.; Ramavandi, B.; Jorfi, S.; Kakavandi, B. Synthesis of chitosan zero-valent iron nanoparticles-supported for cadmium removal: Characterization, optimization and modeling approach. J. Water Supply Res. Technol. 2017, 66, 116–130. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Ouyang, X.K.; Yang, L.Y. Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2021, 322, 114523. [Google Scholar] [CrossRef]
- Gao, C.; Wang, X.L.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R. Synergistic preparation of modified alginate aerogel with melamine/chitosan for efficiently selective adsorption of lead ions. Carbohydr. Polym. 2021, 256, 117564. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Xing, H.; Zhao, J.; Li, X.; Huang, Y.; Liu, H. Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chem. Eng. J. 2013, 234, 338–345. [Google Scholar] [CrossRef]
- Xie, Y.; Ren, L.; Zhu, X.; Gou, X.; Chen, S. Physical and chemical treatments for removal of perchlorate from water—A review. Process Saf. Environ. 2018, 116, 180–198. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohyd. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Boominathan, T.; Sivaramakrishna, A. Recent Advances in the Synthesis, Properties, and Applications of Modified Chitosan Derivatives: Challenges and Opportunities. Top. Curr. Chem. 2021, 379, 19. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, F.; Tang, Q.; Du, B.; Ma, D.; Zhao, Z.; Fan, L.; Luo, H.; Zhao, Z.; Huang, X.; et al. Evaluating the performance of bridging-assembly chelating flocculant for heavy metals removal: Role of branched architectures. Chemosphere 2022, 289, 133260. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Xie, Y.H.; Wu, Y.L.; Qin, Y.H.; Yi, Y.; Liu, Z.; Lv, L.; Xu, M. Evaluation of perchlorate removal from aqueous solution by cross-linked magnetic chitosan/poly (vinyl alcohol) particles. J. Taiwan Inst. Chem. Eng. 2016, 65, 295–303. [Google Scholar] [CrossRef]
- Córdova, B.M.; Infantas, G.C.; Mayta, S.; Huamani-Palomino, R.G.; Kock, F.V.C.; Montes de Oca, J.; Valderrama, A.C. Xanthate-modified alginates for the removal of Pb(II) and Ni(II) from aqueous solutions: A brief analysis of alginate xanthation. Int. J. Biol. Macromol. 2021, 179, 557–566. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Caporale, A.G.; Punamiya, P.; Pigna, M.; Violante, A.; Sarkar, D. Effect of particle size of drinking-water treatment residuals on the sorption of arsenic in the presence of competing ions. J. Hazard. Mater. 2013, 260, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Dai, J.; Dai, X.; Yan, Y. Preparation and characterization of chitosan/kaolin/Fe3O4 magnetic microspheres and their application for the removal of ciprofloxacin. Adsorpt. Sci. Technol. 2014, 32, 775–790. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Wang, Y.; Wang, F.; Wang, Y. Adsorption Performance of Amino Functionalized Magnetic Molecular Sieve Adsorbent for Effective Removal of Lead Ion from Aqueous Solution. Nanomaterials 2021, 11, 2353. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hu, C.; An, Y.; Xiong, Z.; Hu, X.; Zhang, G.; Zheng, H. Magnetic phosphorylated chitosan composite as a novel adsorbent for highly effective and selective capture of lead from aqueous solution. J. Hazard. Mater. 2021, 405, 124195. [Google Scholar] [CrossRef]
- Chen, W.; Tang, Q.; Liu, Z.; Luo, F.; Liao, Y.; Zhao, S.; Zhang, K.; Cheng, L.; Ma, D. Fabricating a novel chitosan-based adsorbent with multifunctional synergistic effect for Cu(II) removal: Maleic anhydride as a connecting bridge. Chem. Eng. Res. Des. 2020, 163, 21–35. [Google Scholar] [CrossRef]
- He, M.; Chen, Z.; Xu, C.; Chen, B.; Hu, B. Magnetic nanomaterials as sorbents for trace elements analysis in environmental and biological samples. Talanta 2021, 230, 122306. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, L.; Zhang, D.; Yan, P.; Nie, J.; Sharma, V.K.; Wang, C. Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem. Eng. J. 2019, 358, 253–263. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, H.; Wang, X.; Zhang, J.; Li, W.; Wang, R. Controllable synthesis of bifunctional corn stalk cellulose as a novel adsorbent for efficient removal of Cu2+ and Pb2+ from wastewater. Carbohydr. Polym. 2022, 276, 118763. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, Y.; Kong, L.; Zhang, J.; Zuo, W.; Li, Y.; Cai, G. A novel 3D superelastic polyethyleneimine functionalized chitosan aerogels for selective removal of Cr(VI) from aqueous solution: Performance and mechanisms. Chem. Eng. J. 2021, 425, 131722. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, S.; Wang, T.; Yu, S.; Guo, S.; Du, K. High-strength and low-swelling chitosan/cellulose microspheres as a high-efficiency adsorbent for dye removal. Cellulose 2021, 28, 9323–9333. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar]
- Hu, D.; Lian, Z.; Xian, H.; Jiang, R.; Wang, N.; Weng, Y.; Peng, X.; Wang, S.; Ouyang, X.K. Adsorption of Pb(II) from aqueous solution by polyacrylic acid grafted magnetic chitosan nanocomposite. Int. J. Biol. Macromol. 2020, 154, 1537–1547. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J. The characteristics and mechanism of Co(II) removal from aqueous solution by a novel xanthate-modified magnetic chitosan. Nucl. Eng. Des. 2012, 242, 452–457. [Google Scholar] [CrossRef]
- Chandrakala, H.N.; Ramaraj, B.; Lee, J.H. Polyvinyl alcohol/carbon coated zinc oxide nanocomposites: Electrical, optical, structural and morphological characteristics. J. Alloys Compd. 2013, 580, 392–400. [Google Scholar] [CrossRef]
- Selim, M.S.; Seoudi, R.; Shabaka, A.A. Polymer based films embedded with high content of ZnSe nanoparticles. Mater. Lett. 2005, 59, 2650–2654. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, T.; Chen, N.; Feng, C. Changes in microbial community diversity, composition, and functions upon nitrate and Cr(VI) contaminated groundwater. Chemosphere 2022, 288, 132476. [Google Scholar] [CrossRef]
- Park, S.; Gomez-Flores, A.; Chung, Y.S.; Kim, H. Removal of cadmium and lead from aqueous solution by hydroxyapatite/chitosan hybrid fibrous sorbent: Kinetics and equilibrium studies. J. Chem. 2015, 2015, 396290. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly(vinyl alcohol)/chitosan for selective lead(II) and cadmium(II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef]
- Dinh, V.P.; Le, N.C.; Tuyen, L.A.; Hung, N.Q.; Nguyen, V.D.; Nguyen, N.T. Insight into adsorption mechanism of lead(II) from aqueous solution by chitosan loaded MnO2 nanoparticles. Mater. Chem. Phys. 2018, 207, 294–302. [Google Scholar] [CrossRef]
- Prakash, N.; Soundarrajan, M.; Arungalai Vendan, S.; Sudha, P.N.; Renganathan, N.G. Contemplating the feasibility of vermiculate blended chitosan for heavy metal removal from simulated industrial wastewater. Appl. Water Sci. 2017, 7, 4207–4218. [Google Scholar] [CrossRef] [Green Version]
- Babakhani, A.; Sartaj, M. Removal of Cadmium (II) from aqueous solution using tripolyphosphate cross-linked chitosan. J. Environ. Chem. Eng. 2020, 8, 103842. [Google Scholar] [CrossRef]
- Chen, A.; Zeng, G.; Chen, G.; Hu, X.; Yan, M.; Guan, S.; Shang, C.; Lu, L.; Zou, Z.; Xie, G. Novel thiourea-modified magnetic ion-imprinted chitosan/TiO2 composite for simultaneous removal of cadmium and 2,4-dichlorophenol. Chem. Eng. J. 2012, 191, 85–94. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, D.; Ge, Y.; Koehler, S. Surface-functionalized porous lignin for fast and efficient lead removal from aqueous solution. ACS Appl. Mater. Interfaces 2015, 7, 15000–15009. [Google Scholar] [CrossRef]
- Rajiv Gandhi, M.; Meenakshi, S. Preparation and characterization of silica gel/chitosan composite for the removal of Cu(II) and Pb(II). Int. J. Biol. Macromol. 2012, 50, 650–657. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Wang, A. Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites. J. Hazard. Mater. 2009, 168, 970–977. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdem, A.; Yildiz, U. Synergistic removal of Cu(II) and nitrazine yellow dye using an eco-friendly chitosan-montmorillonite hydrogel: Optimization by response surface methodology. J. Appl. Polym. Sci. 2016, 133, 43664. [Google Scholar] [CrossRef]
- Sabourian, V.; Ebrahimi, A.; Naseri, F.; Irani, M.; Rahimi, A. Fabrication of chitosan/silica nanofibrous adsorbent functionalized with amine groups for the removal of Ni(ii), Cu(ii) and Pb(ii) from aqueous solutions: Batch and column studies. RSC Adv. 2016, 6, 40354–40365. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Li, J.; Ying, D.; Wang, Y.; Jia, J. Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. J. Hazard. Mater. 2017, 326, 211–220. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Zhou, L.; Shang, C.; Liu, Z.; Huang, G.; Adesina, A.A. Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J. Colloids Interface Sci. 2012, 366, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zheng, M.; Chen, N.; Wu, Z.; Xu, N.; Tang, J.; Teng, Z. Uniform magnetic chitosan microspheres with radially oriented channels by electrostatic droplets method for efficient removal of Acid Blue. J. Taiwan Inst. Chem. Eng. 2019, 104, 210–218. [Google Scholar] [CrossRef]
- Shao, Z.; Lu, J.; Ding, J.; Fan, F.; Sun, X.; Li, P.; Fang, Y.; Hu, Q. Novel green chitosan-pectin gel beads for the removal of Cu(II), Cd(II), Hg(II) and Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2021, 176, 217–225. [Google Scholar] [CrossRef] [PubMed]



| Adsorbent | Qm (mg/g) | Conditions | Ref. | |||
|---|---|---|---|---|---|---|
| Cu(II) | Pd(II) | Cd(II) | pH | T (°C) | ||
| Zeolite X/CS hybrid microspheres | 152 | - | - | 5.5 | 25 | [2] |
| CS/PVA/PEI membrane | 86.08 | - | 112.13 | 6 | 25 | [9] |
| Cobalt ferrite@SiO 2–CS/EDTA composite | - | - | 127.79 | 6 | 25 | [10] |
| Amidoxime-functionalized CS | 190.7 | - | - | 5 | 25 | [12] |
| CNTs–CHO–CS composite | 115.84 | - | - | 7 | 25 | [14] |
| CS–g–MA composite | 312.4 | - | - | 6 | 25 | [16] |
| Magnetic Bentonite/carboxymethyl CS/SA hydrogel beads | 56.79 | - | - | 5 | 30 | [20] |
| CS-VMT composite | - | 166.67 | 55.48 | 4 | 30 | [25] |
| MMT/CS | 17.2 | - | - | 9 | 45 | [27] |
| CS@NZVI | - | - | 142.8 | 7 | 25 | [28] |
| Crosslinked carboxylated CS/carboxylated nanocellulose hydrogel beads | - | 334.9 | - | 4 | 35 | [29] |
| Alginate/melamine/CS aerogel | - | 1331.6 | - | 5.5 | 25 | [30] |
| PEI-grafted magnetic CS microspheres | - | 134.9 | - | - | - | [31] |
| Polydopamine modified CS aerogels | - | 441.2 | - | 5 | 45 | [44] |
| Magnetic–CS–PAA nanocomposite | - | 204.9 | - | 6 | 35 | [53] |
| CS–pectin gel beads | 169.4 | 266.5 | 177.64 | 4–9 | 50 | [58] |
| Hydroxyapatite/CS composites | - | 132.1 | 81.1 | 6 | 25 | [59] |
| CS–PVA nanofibers | - | 266.12 | 148.79 | 6, 8 | 25 | [60] |
| MnO2/CS nanoparticles | - | 126.1 | - | 4 | 50 | [61] |
| Sodium tripolyphosphate crosslinked CS beads | - | - | 99.8 | 7 | 55 | [62] |
| Vermiculite blended CS | - | - | 169 | 5.5 | 30 | [63] |
| Thiourea-modified magnetic ion-imprinted CS/TiO2 | - | - | 256.41 | 7 | 25 | [64] |
| Silica/CS composite | 870 | 316 | - | 5 | 30 | [65] |
| CS/PVA/PEI membrane | 86.08 | - | 112.13 | 6 | 25 | [66] |
| CS–g–PAA/APT | 303.03 | - | - | 5.5 | 30 | [67] |
| CS–MMT hydrogel | 132.74 | - | - | 5 | 20 | [68] |
| CS/TEOS/APTES nanofiber | 640.5 | 575.5 | - | 6, 5.5 | 45 | [69] |
| TEPA/CS/CoFe2O4 composite | 168.067 | 228.311 | - | 5 | 30 | [70] |
| XMPC | 100 | 67 | 307 | 5.5 | 30 | This work |
| Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | ||||
|---|---|---|---|---|---|---|
| Metal Ions | K1 (min−1) | R2 | K2 (mg/g min) | R2 | Ki (mg/g min1/2) | R2 |
| Pb(II) | 0.0087 | 0.9053 | 4.5921 | 0.9971 | 0.0095 | 0.6925 |
| Cu(II) | 0.0165 | 0.8996 | 0.5922 | 0.9531 | 0.0929 | 0.9012 |
| Cd(II) | 0.0145 | 0.8736 | 1.1045 | 0.7281 | 0.0428 | 0.8828 |
| Metal Ions | T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | KF | bF | R2 | ||
| Pb(II) | 293 K | 167.4743 | 0.001310 | 0.9960 | 0.4079 | 0.8322 | 0.9221 |
| 303 K | 188.5575 | 0.001742 | 0.9905 | 0.8813 | 0.7483 | 0.9873 | |
| 313 K | 272.3575 | 0.000674 | 0.9997 | 0.3281 | 0.8623 | 0.9958 | |
| Cu(II) | 293 K | 161.6496 | 0.002636 | 0.9821 | 1.3139 | 0.6966 | 0.9703 |
| 303 K | 198.2883 | 0.002166 | 0.9931 | 1.2758 | 0.7186 | 0.9874 | |
| 313 K | 183.9165 | 0.001560 | 0.9958 | 1.0658 | 0.6971 | 0.9936 | |
| Cd(II) | 293 K | 211.2975 | 0.001165 | 0.9996 | 0.4907 | 0.8241 | 0.9971 |
| 303 K | 234.0225 | 0.001546 | 0.9982 | 0.7557 | 0.8033 | 0.9953 | |
| 313 K | 269.0434 | 0.000698 | 0.9982 | 0.3642 | 0.8463 | 0.9981 | |
| Metal Ions | T (K) | K0 | ΔG (KJ·mol−1) | ΔH (KJ·mol−1) | Δ S (J·mol−1·k−1) |
|---|---|---|---|---|---|
| Pb(II) | 293 | 6.4412 | −4.5376 | 6.5584 | 39.2971 |
| 303 | 11.9197 | −6.0369 | |||
| 313 | 7.5612 | −4.9281 | |||
| Cu(II) | 293 | 13.4735 | −6.3354 | 1.1285 | 17.9890 |
| 303 | 14.3811 | −6.4942 | |||
| 313 | 13.0571 | −6.2589 | |||
| Cd(II) | 293 | 8.2486 | −5.1401 | 0.4406 | 19.7548 |
| 303 | 10.7766 | −5.7913 | |||
| 313 | 8.2961 | −5.1541 |
| Metal Ions | Qe (mg/g) | Kd | Ks |
|---|---|---|---|
| Pb(II) | 66.34 | - | - |
| Cu(II) | 91.22 | - | - |
| Co(II) | 84.14 | - | - |
| Ni(II) | 21.21 | - | - |
| Cd(II) | 300.01 | - | - |
| Cd(II) in mixed Cd(II)/Cu(II)) | 120.05 | 255.4 | 2.45 |
| Cu(II) in mixed Cd(II)/Cu(II)) | 50.71 | 104.1 | - |
| Cd(II) in mixed Cd(II)/Co(II)) | 118.79 | 252.6 | 1.92 |
| Co(II) in mixed Cd(II)/Co(II)) | 63.72 | 131.6 | - |
| Cd(II) in mixed Cd(II)/Ni(II)) | 156.19 | 338.8 | 9.67 |
| Ni(II) in mixed Cd(II)/Ni(II)) | 17.36 | 35.0 | - |
| Cd(II) in mixed (Pb(II)/Cd(II)) | 118.79 | 252.6 | 5.37 |
| Pb(II) in mixed (Pb(II)/Cd(II)) | 23.25 | 47.0 | - |
| Pb(II) in five mixed ions | 20.44 | 41.3 | - |
| Cu(II) in five mixed ions | 37.01 | 75.4 | - |
| Co(II) in five mixed ions | 32.58 | 66.2 | - |
| Ni(II) in five mixed ions | 10.11 | 20.3 | - |
| Cd(II) in five mixed ions | 95.43 | 200.4 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, Y.; Yang, A.; Zhu, Q.; Sun, H.; Sun, P.; Yao, B.; Zang, Y.; Du, X.; Dong, L. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers 2022, 14, 1107. https://doi.org/10.3390/polym14061107
Wang S, Liu Y, Yang A, Zhu Q, Sun H, Sun P, Yao B, Zang Y, Du X, Dong L. Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers. 2022; 14(6):1107. https://doi.org/10.3390/polym14061107
Chicago/Turabian StyleWang, Shifan, Yuan Liu, Aiwen Yang, Qi Zhu, Hua Sun, Po Sun, Bing Yao, Yunxiao Zang, Xihua Du, and Liming Dong. 2022. "Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water" Polymers 14, no. 6: 1107. https://doi.org/10.3390/polym14061107
APA StyleWang, S., Liu, Y., Yang, A., Zhu, Q., Sun, H., Sun, P., Yao, B., Zang, Y., Du, X., & Dong, L. (2022). Xanthate-Modified Magnetic Fe3O4@SiO2-Based Polyvinyl Alcohol/Chitosan Composite Material for Efficient Removal of Heavy Metal Ions from Water. Polymers, 14(6), 1107. https://doi.org/10.3390/polym14061107