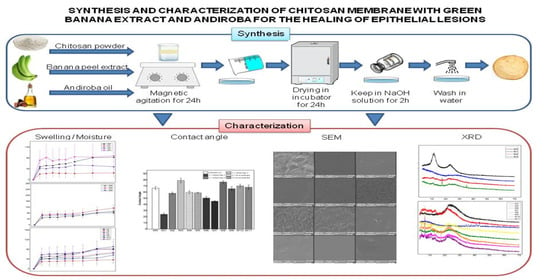
Synthesis and Characterization of Natural Polymeric Membranes Composed of Chitosan, Green Banana Peel Extract and Andiroba Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Green Banana Peel Extracts by Decoction
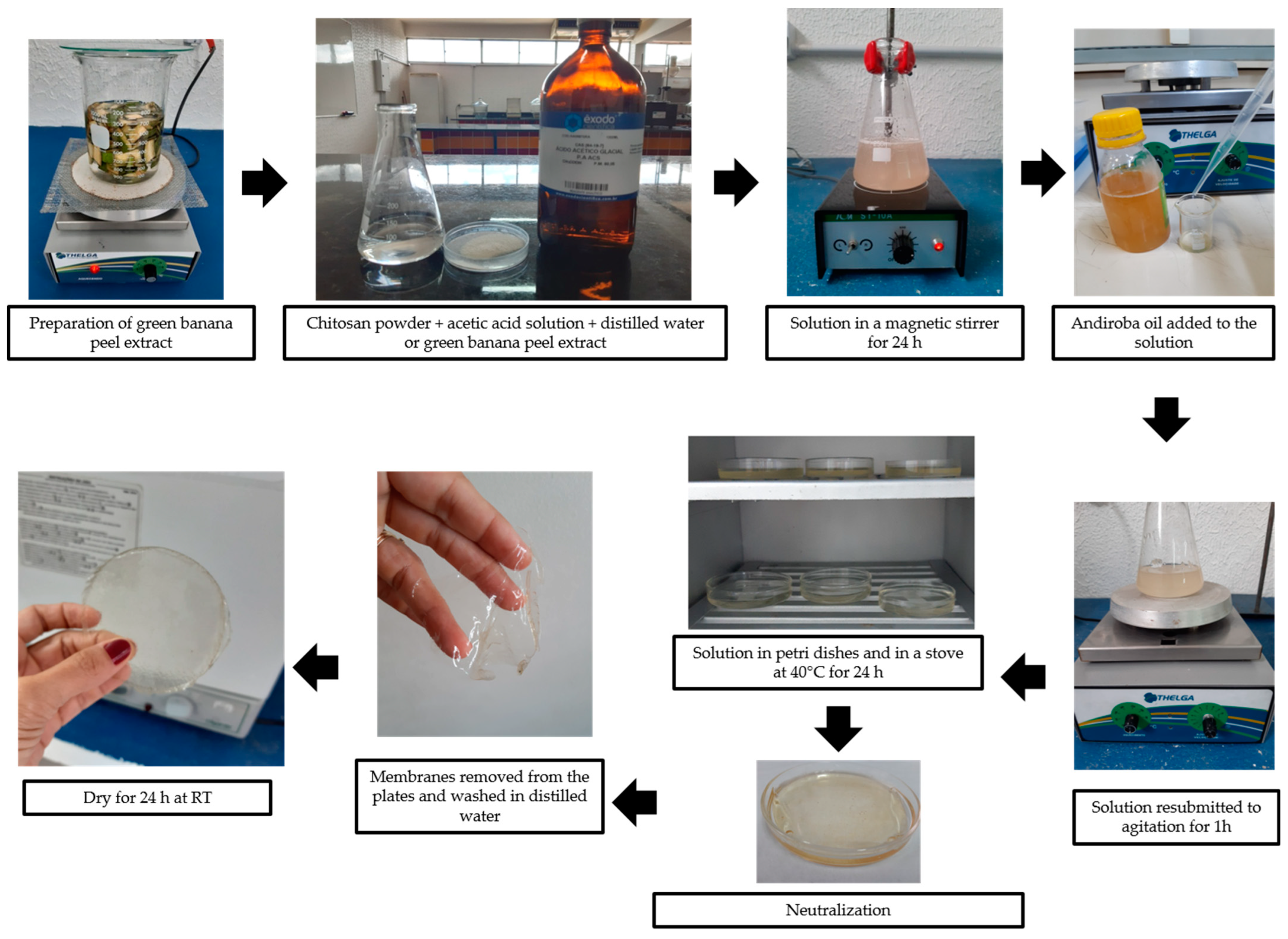
2.3. Synthesis of Membranes
- (i)
- Plain chitosan;
- (ii)
- Chitosan plus green banana peel extract; and
- (iii)
- Chitosan plus andiroba oil (studied as control samples).
2.4. Macroscopic Analysis
2.5. Swelling Test
2.6. Moisture Test
2.7. Contact Angle
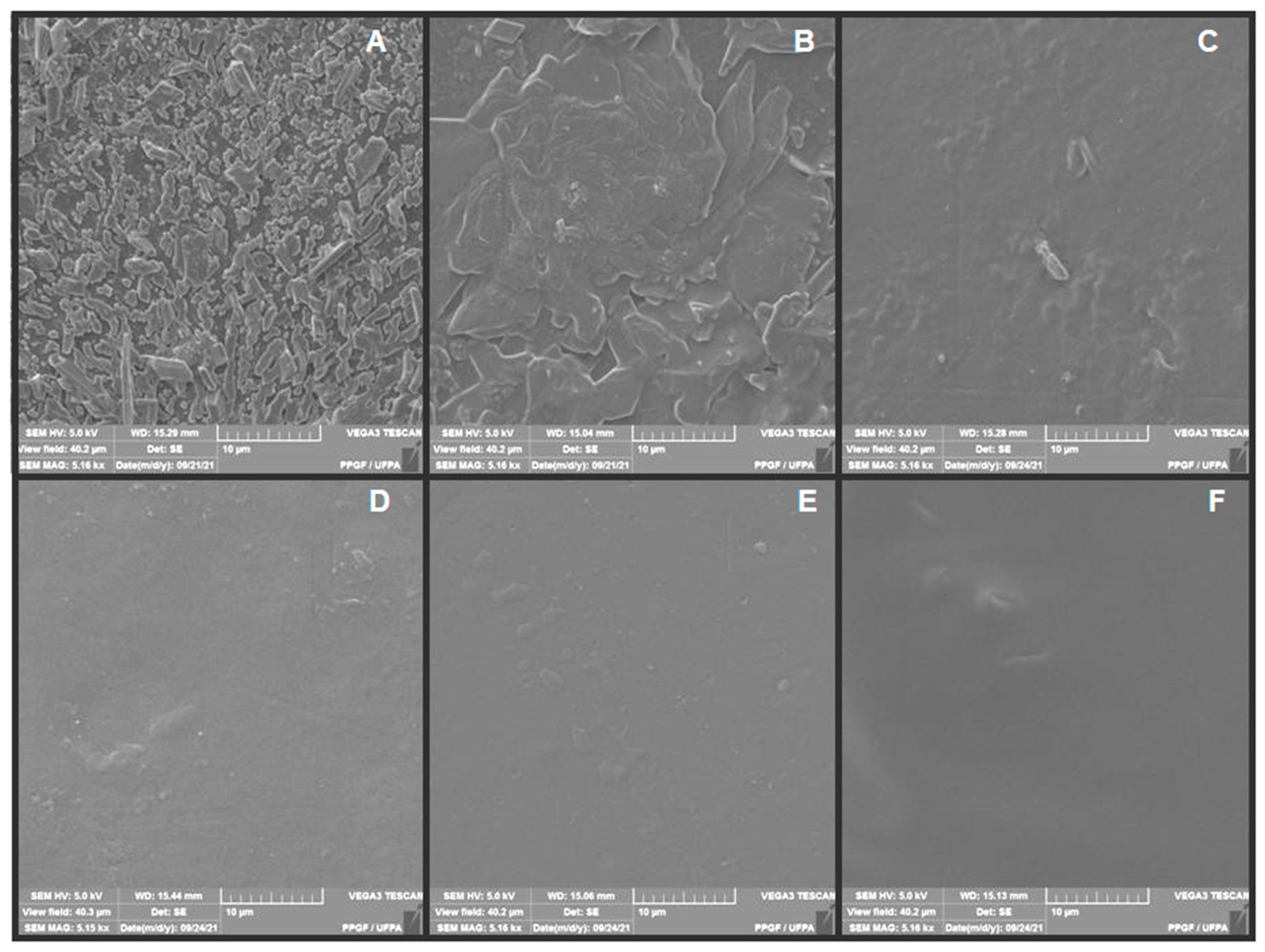
2.8. Scanning Electron Microscopy (SEM)
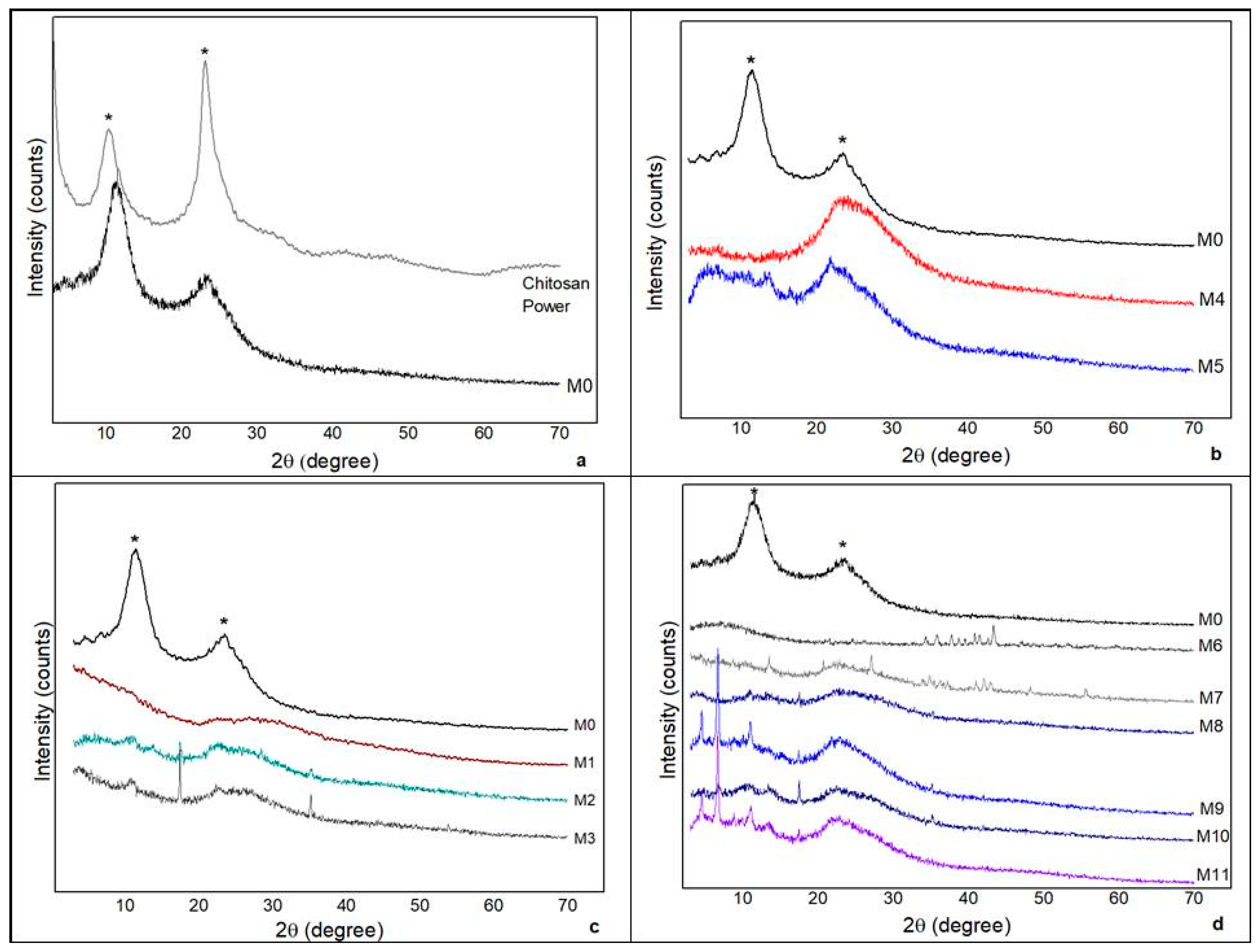
2.9. X-ray Diffraction (XRD)
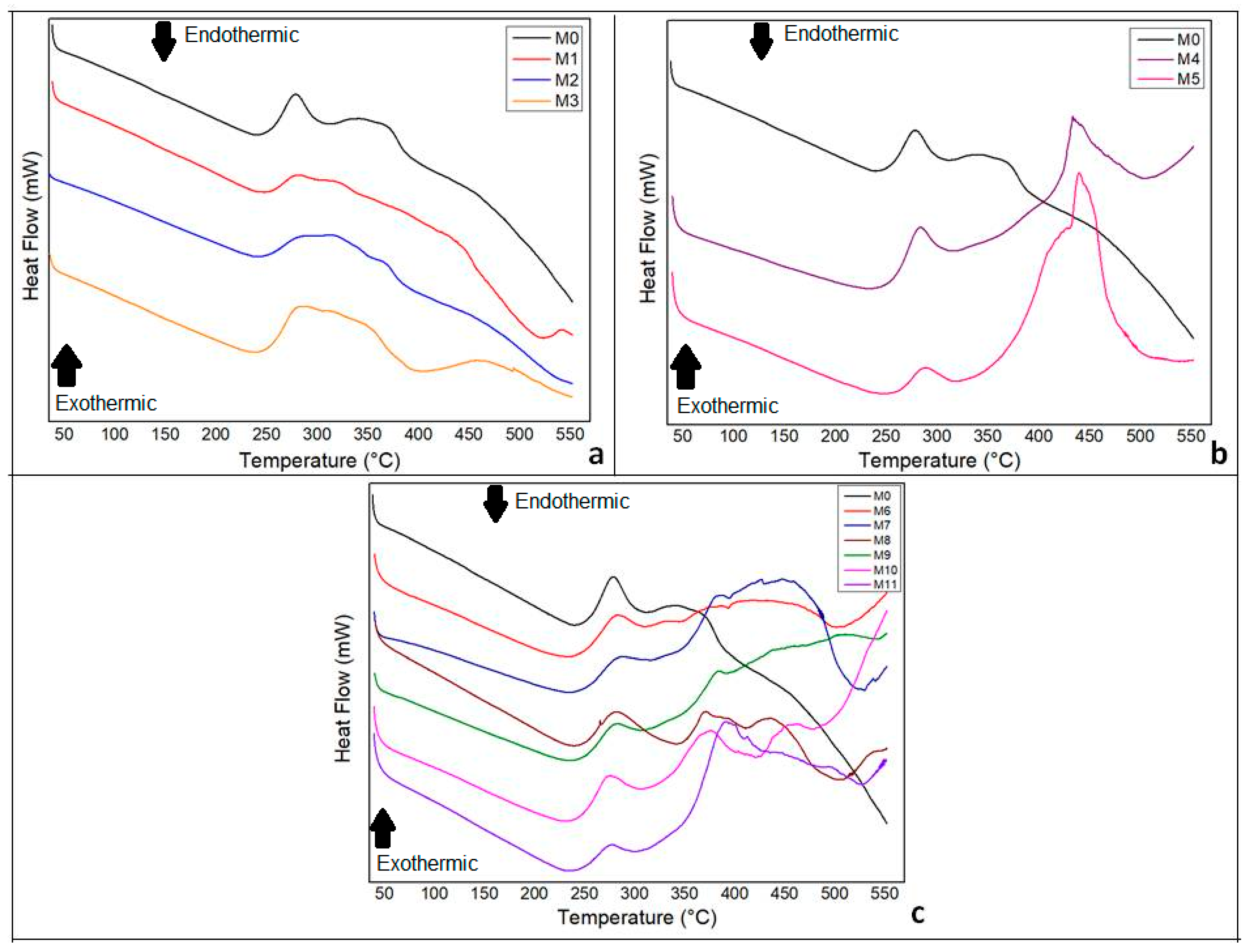
2.10. Differential Scanning Calorimetry (DSC)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Macroscopic Analysis
3.2. Swelling and Moisture Test
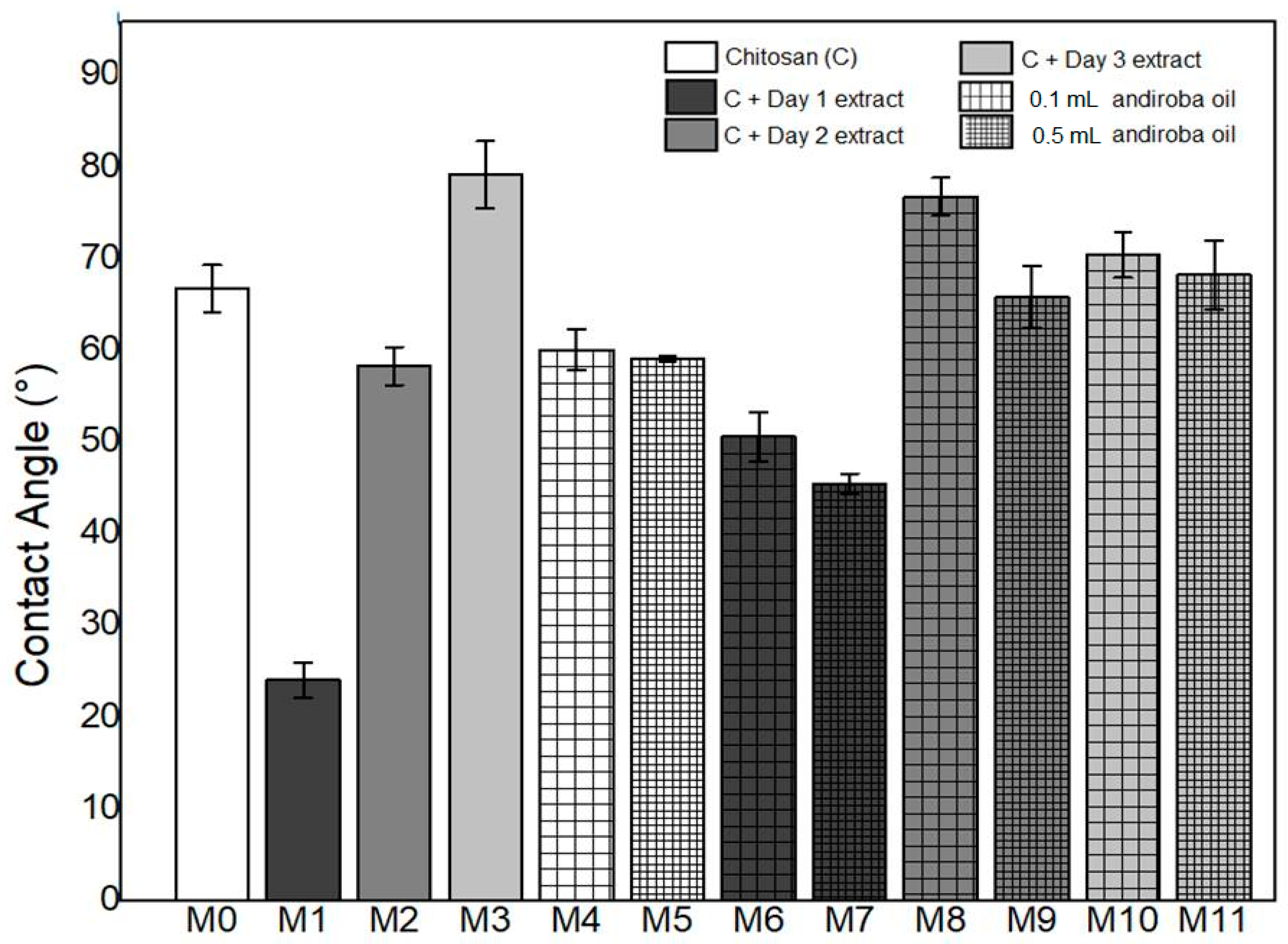
3.3. Contact Angle
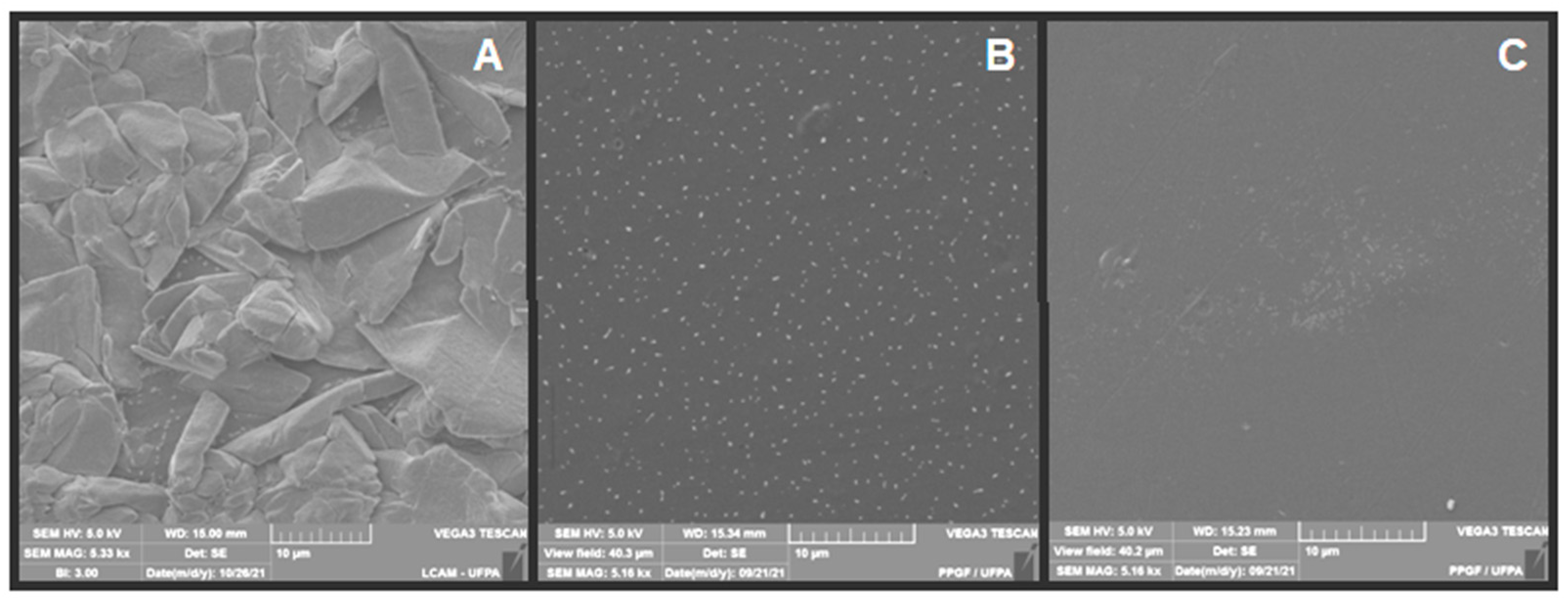
3.4. SEM
3.5. XRD
3.6. DSC
4. Summary and Conclusions
- There was superiority in the percentage of moisture in the membranes composed of green banana peel extract and a higher percentage of swelling in the chitosan membranes with andiroba oil, demonstrating inverse proportionality between the swelling and the moisture of the synthesized membranes.
- The synthesized membranes were hydrophilic. However, the values of the contact angles were lower with green banana peel extracts and higher concentrations of andiroba oil.
- The XRD indicated amorphous material with discrete peaks in some samples and may be related to the presence of the constituents of the plant components present in both the extracts and the oils.
- The DSC demonstrated that the addition of the plant constituents, green banana peel extract and andiroba oil, influenced by the crystallinity of the material.
- Membranes composed of chitosan, green banana peel extract, and andiroba oil have characteristics to make up a biomaterial to treat epithelial lesions, gathering essential properties such as absorption capacity and fluid retention, cellular adhering facilitated by decreased crystallinity and thermal degradation.
- Therefore, the concentrations of the constituents should be adequately studied. In addition, it is necessary to carry out biological tests in the laboratory to evaluate their effectiveness in tissue regeneration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hudecki, A.; Kiryczynski, G.; Los, M.J. Chapter 7: Stem cells and biomaterials for regenerative medicine. In Book Biomaterials, Definition, Overview; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.G.; Lima, I.S.; Ribeiro, A.B.; Lobo, A.O.; Rizzo, M.S.; Osajima, J.A.; Estevinho, L.M.; Silva-Filho, E.C. Biocompatible gels of chitosan-buriti oil for potential wound healing applications. Materials 2020, 13, 1977. [Google Scholar] [CrossRef] [PubMed]
- Ávila Filho, S.H.; Ferreira, P.G.C.; Fares, N.B.; Da Silva, L.A.F. Physical-chemical properties, biological activities and results obtained after medical use of chitosan and its derivatives. Braz. J. Dev. 2020, 6, 95250–99527. [Google Scholar] [CrossRef]
- Kamel, N.A.A.; Abd El-Messieh, S.L.A.; Salehb, N. Chitosan/banana peel powder nanocomposites for wound dressing application: Preparation and characterization. Mater. Sci. Eng. 2020, 72, 543–550. [Google Scholar] [CrossRef]
- Youssef, M.A.; Eman, A.A.; Abeir, M.F.E. Evaluation the bioactive compounds extracted from dried banana (Musa sp.) peels which obtained by different drying methods. Curr. Sci. Int. 2018, 7, 135–148. [Google Scholar]
- Rihayat, T.; Siregar, J.P.; Putra, A.; Fona, Z.; Riskina, S.; Syahputra, W.; Jaafar, J. Wound dressing based on banana peels waste and chitosan by strengthening lignin as wound healing medicine. Mater. Sci. Eng. 2019, 506, 012056. [Google Scholar] [CrossRef]
- Franco, P.B.; Almeida, L.A.; Marques, R.F.C.; Silva, M.A.; Campos, M.G.N. Chitosan associated with the extract of unripe banana peel for potential wound dressing application. Int. J. Polym. Sci. 2017, 2017, 9761047. [Google Scholar] [CrossRef] [Green Version]
- Haslinda, W.H.; Cheng, L.H.; Chong, L.C.; Noor Aziah, A.A. Chemical composition and physicochemical properties of green banana (Musa acuminata × balbisiana Colla cv. Awak) flour. Int. J. Food Sci. Nutr. 2009, 60, 232–239. [Google Scholar] [CrossRef]
- Muñoz-Ruíz, A.; Escobar-García, D.M.; Quintana, M.; Pozos-Guillén, A.; Flores, H. Synthesis and characterization of a new collagen-alginate aerogel for tissue engineering. J. Nanomater. 2019, 2019, 2875375. [Google Scholar] [CrossRef] [Green Version]
- Souza, B.A.A.; Braga, L.A.; Lopes, L.R.O.; Ribeiro, R.F.G., Jr.; Nascimento, L.N.S.; Cavalcante, L.C.C.; Monteiro, A.M.; Couteiro, R.P.; Yasojima, E.Y.; Hamoy, M. Effects of Andiroba oil (Carapa guianensis) on wound healing in alloxan-diabetic rats. Int. Arch. Med. 2017, 10, 263. [Google Scholar] [CrossRef]
- Matsumoto, C.; Koike, A.; Tanaka, R.; Fujimori, K. Limonoid, 7-deacetoxy-7-oxogedunin (CG-1) from Andiroba (Carapa guianensis, Meliaceae) lowers the accumulation of intracellular lipids in adipocytes via suppression of IRS-1/Akt-mediated glucose uptake and a decrease in GLUT4 expression. Molecules 2019, 24, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, A.S.; Wanzeler, A.M.; Cavalcante, G.H.; Barros, E.; Carneiro, R.; Tuji, E.F.M. Therapeutic effects of andiroba (Carapa guianensis Aubl) oil, compared to low power laser, on oral mucositis in children underwent chemotherapy: A clinical study. J. Ethnopharmacol. 2021, 264, 113365. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Lima, C.F.; Fernandes, A.S.; Gomes, E.M.; Oliveira, L.L.; Macedo, A.F.; Antoniassi, R.; Felzenszwalb, I. Antioxidant activity and genotoxic assessment of crabwood (Andiroba, Carapa guianensis Aublet) seed oils. Oxidative Med. Cell. Longev. 2018, 2018, 3246719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brazilian Program for the Modernization of Horticulture & Integrated Fruit Production (PBMH & PIF). Banana Classification Standards; Brazilian Program for the Modernization of Horticulture & Integrated Fruit Production (PBMH & PIF): São Paulo, Brazil, 2006; p. 29. Available online: http://www.ceagesp.gov.br/wp-content/uploads/2015/07/banana.pdf (accessed on 10 October 2021).
- Alminderej, F.M. Study of new cellulosic dressing with enhanced antibacterial performance grafted with a biopolymer of chitosan and myrrh polysaccharide extract. Arab. J. Chem. 2019, 13, 3672–3681. [Google Scholar] [CrossRef]
- Sung, J.H.; Hwang, M.R.; Kim, J.P.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S. Gel characterization and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional biomaterials for treatment of chronic wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Kimura, V.T.; Miyasato, C.S.; Genesi, B.P.; Lopes, P.S.; Yoshida, C.M.P.; da Silva, C.F. The effect of andiroba oil and chitosan concentration on the physical properties of chitosan emulsion film. Polymer 2016, 26, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Leite, I.F.; Wanderley, W.F.; Salviano, A.T.M. Natural chitosan-bioactive membranes for potential use as dressings. In Materials Engineering and Metallurgical; Oliveira, A.C., Holzmann, H.A., Dallamuta, J., Eds.; Athen: Ponta Grossa, Brazil, 2021; Volume 2, pp. 174–185. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51. [Google Scholar]
- Silva, D.F.; Lima, K.T.; Bastos, G.N.T.; Oliveira, J.A.R.; do Nascimento, L.A.S.; Costa, C.E.F.; Filho, G.N.R.; Concha, V.O.C.; Passos, M.F. PCL/Andiroba oil (Carapa guianensis Aubl.) hybrid film for wound healing applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef]
- Oliveira, M.Z.F.S.; Fernandes, T.S.M.; Oak, T.V. Synthesis and characterization of commercial chitosan beads reticulated with glutaraldehyde. Mag. Matter 2021, 26, e12961. [Google Scholar] [CrossRef]
- Dang, Q.; Zhang, Q.; Liu, C.; Yan, J.; Chang, G.; Xin, Y.; Cheng, X.; Cao, Y.; Gao, H.; Liu, Y. Decanoic acid functionalized chitosan: Synthesis, characterization, and evaluation as potential wound dressing material. Int. J. Biol. Macromol. 2019, 139, 1046–1053. [Google Scholar] [CrossRef]
- Kadam, D.; Lele, S.S. Cross-linking effect of polyphenolic extracts of Lepidium sativum seedcake on physicochemical properties of chitosan films. Int. J. Biol. Macromol. 2018, 114, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Barrioni, B.R.; Oliveira, A.C.; de Leite, M.F.; de Pereira, M.M. Sol–gel-derived manganese-releasing bioactive glass as a therapeutic approach for bone tissue engineering. J. Mater. Sci. 2017, 52, 8904–8927. [Google Scholar] [CrossRef]




| Material | Description | Brand/Supplier |
|---|---|---|
| Commercial chitosan | CAS: 9012–76–4) (medium molecular weight-190,000–310,000 Da and deacetylation degree in the range 75–85%) | Sigma Aldrich Laboratory (Sao Paulo, Brazil) |
| Glacial acetic acid | Pure, molecular weight 60.05 g/mol | Scientific Exodus (Sao Paulo, Brazil) |
| Sodium hydroxide solution 5% | Molecular weight 40 g/mol | Scientific Exodus (Sao Paulo, Brazil) |
| Andiroba oil Species: Carapa guianensis | Acidity level < 15.0% weight, density 0.9261 g/mL to 25 °C, iod index 55–80 gI2/100 g, melting point 22 °C, peroxide content < 10.0 (10 meq O2/kg), saponification index 190–210 mg KOH/g, insaponifiable matter (bioactive) 3–5% | Amazon Oil (Belém, Pará, Brazil) |
| Green banana peel | Species Musa spp., category 1 maturation Von Loeseck [15]. | Cultivated in the municipality of Santo Antônio do Tauá, Pará, Brazil. |
| Acronym | Composition |
|---|---|
| M0 | Chitosan + distilled water |
| M1 | Chitosan + green banana peel extract from the 1st day |
| M2 | Chitosan + green banana peel extract from the 2nd day |
| M3 | Chitosan + green banana peel extract from the 3rd day |
| M4 | Chitosana + distilled water + 0.1 mL andiroba oil |
| M5 | Chitosana + distilled water + 0.5 mL andiroba oil |
| M6 | Chitosan + green banana peel extract from the 1st day + 0.1 mL andiroba oil |
| M7 | Chitosan + green banana peel extract from the 1st day + 0.5 mL andiroba oil |
| M8 | Chitosan + green banana peel extract from the 2nd day + 0.1 mL andiroba oil |
| M9 | Chitosan + green banana peel extract from the 2nd day + 0.5 mL andiroba oil |
| M10 | Chitosan + green banana peel extract from the 3rd day + 0.1 mL andiroba oil |
| M11 | Chitosan + green banana peel extract from the 3rd day + 0.5 mL andiroba oil |
| Visual Appearance | M0 | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colour | 0 | 3 | 2 | 1 | 0 | 0 | 2 | 2 | 1 | 1 | 1 | 1 |
| Uniformity | 3 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 3 | 3 |
| Transparency | 3 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 |
| Bubbles/Cracks | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 |
| Brittle aspect | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flexibility | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Detachment of the support | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sample | M0 | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | M11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Swelling (%) | Water | 72.4 ± 15.8 | 11.1 ± 9.9 | 49.3 ± 5.0 | 81.3 ± 16.7 | 133.3 ± 68.3 | 135.0 ± 18.5 | 32.3 ± 2.9 | 34.6 ± 19.8 | 77.8 ± 6.4 | 72.9 ± 39.9 | 98.7 ± 49.7 | 108.3 ± 19.6 |
| PBS | 66.6 ± 16.7 | 22.9 ± 14.8 | 53.6 ± 23.9 | 84.6 ± 44.4 | 82.7 ± 26.1 | 71.1 ± 15.4 | 32.3 ± 9.2 | 43.4 ± 30.2 | 51.8 ± 13.4 | 47.8 ± 19.2 | 68.8 ± 28.9 | 69.0 ± 32.2 | |
| Moisture (%) | 21.2 ± 1.2 | 23.1 ± 1.1 | 21.3 ± 0.3 | 22.2 ± 2.1 | 14.7 ± 1.9 | 14.9 ± 0.9 | 17.6 ± 7.0 | 24.9 ± 3.8 | 16.9 ± 3.0 | 14.9 ± 3.8 | 18.0 ± 2.4 | 23.8 ± 3.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, E.d.S.; Paranhos, S.B.; da Paz, S.P.A.; Canelas, C.A.d.A.; Nascimento, L.A.S.d.; Passos, M.F.; da Silva, A.C.R.; Monteiro, S.N.; Paula, M.V.d.S.; Candido, V.S. Synthesis and Characterization of Natural Polymeric Membranes Composed of Chitosan, Green Banana Peel Extract and Andiroba Oil. Polymers 2022, 14, 1105. https://doi.org/10.3390/polym14061105
Ferreira EdS, Paranhos SB, da Paz SPA, Canelas CAdA, Nascimento LASd, Passos MF, da Silva ACR, Monteiro SN, Paula MVdS, Candido VS. Synthesis and Characterization of Natural Polymeric Membranes Composed of Chitosan, Green Banana Peel Extract and Andiroba Oil. Polymers. 2022; 14(6):1105. https://doi.org/10.3390/polym14061105
Chicago/Turabian StyleFerreira, Elisângela da Silva, Sheila Barbosa Paranhos, Simone Patrícia Aranha da Paz, Caio Augusto de Almeida Canelas, Luís Adriano Santos do Nascimento, Marcele Fonseca Passos, Alisson Clay Rios da Silva, Sergio Neves Monteiro, Marcos Vinícius da Silva Paula, and Verônica Scarpini Candido. 2022. "Synthesis and Characterization of Natural Polymeric Membranes Composed of Chitosan, Green Banana Peel Extract and Andiroba Oil" Polymers 14, no. 6: 1105. https://doi.org/10.3390/polym14061105
APA StyleFerreira, E. d. S., Paranhos, S. B., da Paz, S. P. A., Canelas, C. A. d. A., Nascimento, L. A. S. d., Passos, M. F., da Silva, A. C. R., Monteiro, S. N., Paula, M. V. d. S., & Candido, V. S. (2022). Synthesis and Characterization of Natural Polymeric Membranes Composed of Chitosan, Green Banana Peel Extract and Andiroba Oil. Polymers, 14(6), 1105. https://doi.org/10.3390/polym14061105